Abstract
Background:
Vitamin D has stimulatory and protective effects on melanocytes and acts through its nuclear vitamin D receptor (VDR) on target cells. Various single-nucleotide polymorphisms (SNPs) in VDR genes have been described.
Aims:
The aim was to study and compare the association of SNP of BsmI/Apa-I/TaqI/FokI/Cdx2 in VDR gene as well as the plasma vitamin D levels in vitiligo patients and healthy controls.
Methods:
This was a case-control study, in which 100 patients of vitiligo and an equal number of healthy individuals were studied. The VDR polymorphisms of Bsm I, Apa-I, TaqI, fok I, and cdx2 were investigated, after extraction of genomic DNA by rapid capillary polymerase chain reaction with melting curve analysis, and 25 hydroxy vitamin D levels were measured in cases and controls.
Results:
The frequency of genotypes (SNP FokI and cdx2) was higher in the patient group versus controls (P = 0.002). The genotype frequency (TaqI and Apa-I) was higher in the patients than the controls for the Tt genotype, but not significantly higher (48% vs. 39%, P = 0.1431). The difference between the groups in frequency of the genotype Aa(TaqI and Apa-I) was statistically significant (P = 0.0001 and P = 0.033). Statistically significant difference was also observed in Apa-I-evaluated alleles in cases when compared to controls (P = 0.0001). There was no significant difference in serum vitamin D levels between various genotypes among cases and controls. Out of 100 cases, 10 were found to have vitamin D levels of >30 ng/ml, 15 had levels between 20 and 30 ng/ml, 52 had ≤20 ng/ml, and 23 ≤ 10 ng/ml, respectively.
Limitations:
Since the skin biopsies were not taken from the lesions of vitiligo, the correlation of serum levels with tissue levels of VDR gene was not possible and the role of vitamin D supplementation was not evaluated.
Conclusion:
The single nucleotide gene polymorphisms of various VDR genes as found in the cases might lead to vitamin D deficiency, due to VDR dysfunction, which in turn could increase the susceptibility to develop vitiligo.
Keywords: Genotypes, single nucleotide polymorphism, vitamin D receptor, vitamin D, vitiligo
Introduction
Vitiligo is an acquired pigmentary disorder characterized by areas of depigmented skin resulting from progressive autoimmune loss of functioning melanocytes from the skin and often overlying hair, and mucous membranes. It affects 1–2% of the general population. The etiology of vitiligo is not clear. Although various hypotheses have been proposed, it has been suggested that autoimmunity plays an important role in the pathogenesis of vitiligo.[1,2,3] Although vitiligo is not a life-threatening disease, the psychosocial impact of vitiligo is significant. This signifies the need for preventing and treating the disease.
Vitamin D has both stimulatory and protective effects on melanocytes and it acts through its vitamin D receptor (VDR) on target cells. Many autoimmune diseases have been found to be associated with reduced vitamin D levels. Various single-nucleotide polymorphisms (SNPs) in VDR gene have been described. It has been proposed that genetic variations within the VDR gene could lead to significant receptor dysfunction, which could then further affect the formation of the biologically active vitamin D.[4,5,6]
Several studies have addressed the association between these SNPs and autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, autoimmune diabetes, inflammatory bowel disease, and psoriasis.[7,8] There is evidence that VDR gene polymorphism and the subsequent vitamin D levels influence the development of vitiligo.[9] Vitamin D has been found to be immunoprotective. It inhibits the maturation of dendritic cells and regulates cytokine-mediated shift of Th1 response to Th2 response. It inhibits Th17 cells, increases Treg cells to suppress autoattacks, and maintains self-tolerance. Accordingly, reduced vitamin D levels have been associated with many autoimmune diseases.[6,10] Vitamin D deficiency is believed to act as an environmental trigger for the induction of autoimmunity and subsequently could lead to the development of vitiligo, since autoimmunity is the most widely accepted theory for the development of vitiligo. Thus, high-dose vitamin D supplementation could be preventive for it and may improve the prognosis.[11]
Darker skinned patients and patients with comorbid autoimmune diseases have been observed to have very low 25 hydroxy vitamin D [ 25(OH)D] levels, again pointing to the fact that low vitamin D levels may be associated with vitiligo.[12] According to the international recommended standards, 25(OH)D levels are divided into normal or sufficient (>30 ng/ml), insufficient (20–30 ng/ml), and deficient (20–10 ng/ml). Levels <10 ng/ml are considered as severe deficiency.[13]
Vitamin D analogues are generally considered to be effective topical therapy for cutaneous autoimmune conditions including psoriasis and vitiligo.[14] However, some studies on the role of vitamin D analogues in vitiligo have yielded conflicting results. These results indicated that the variability may result from genetic polymorphism in the VDR, which would allow some patients, but not others to respond to vitamin D analogues.[15,16] The purpose of the present study was to evaluate whether VDR gene polymorphisms could be the susceptibility markers for vitiligo. In addition, the association of serum levels of 25(OH)D and VDR gene polymorphic variants in patients with vitiligo and age- and sex-matched healthy controls were evaluated.
Methods
The present study was a case-control study in which 100 patients of vitiligo were studied and an equal number of healthy age- and sex-matched individuals were taken as controls. Vitiligo patients with no major cardiovascular, hepatic, renal, or gastrointestinal disease were taken as cases. The control subjects were either relatives of subjects (second and third degree but free from the disease), paramedical staff, or patients suffering from unrelated skin diseases such as allergic contact dermatitis. The exclusion criteria included the use of any drug that could alter the outcome of the study such as topical or systemic vitamin D or calcium, systemic steroids, weight loss drugs, cholesterol-lowering drugs, thiazide diuretics, and sunscreen use in the last 1 month. Pregnant and lactating females, smokers, subjects with malabsorption disorders, and other autoimmune diseases were also excluded from the study. The patients (cases and controls) who had received phototherapy were additionally excluded from the study. However, the sun-behavior pattern among cases and controls was not taken into consideration. The patient information, including age, sex, type of vitiligo (generalized, localized, or universal), body surface area involved (BSA) (according to rule of nine), and disease activity determined by the VETI (vitiligo extent tensity index) score, was entered in a predesigned proforma. Ethical clearance was taken from the institutional ethical committee.
The VETI score proposes to measure the extent of vitiligo by a numerical score, combines analysis of extensity and severity of vitiligo, and produces a constant and reproducible number. The percentage of involvement was evaluated using the rule of nine. The body is divided into five sites, namely, head (h), upper limbs (u), trunk (t), lower limbs (l), and genitalia (g), which are separately scored by using five stages of disease tensity (T).[17]
To study and compare the plasma 25(OH)D levels in patients with vitiligo and normal healthy controls, blood samples were taken from both cases as well as controls, and samples were immediately processed by centrifugation at 4000 rpm at room temperature. Plasma 25(OH)D levels were analyzed by chemilumenescence method/kit method (Seimens USA) as per manufacturers' protocol, using Seimens ADVIA Centaur analyzer. Vitamin D deficiency was defined as serum 25(OH)D concentration <30 ng/ml.
To study the genotype differences of VDR among vitiligo patients and controls, the peripheral blood samples of individuals were collected in ethylenediamine tetra acetic acid containing tubes, and genomic DNA was extracted using Genomic DNA Purification Kit (Biotools USA). The FokI4 (T/Crs2228570), BsmI1 (G/A rs1544410), Apa-I2 (G/T rs7975232), and TaqI3 (T/C rs731236) polymorphisms were analyzed by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP). Genomic DNA was amplified for five polymorphic sites on the VDR gene with the forward and reverse primers. All the PCRs were carried out in a total volume of 25 μl. The reaction mixture consisted of 1 μl of 0.5 μg genomic DNA, 1 μl of forward and reverse primers (10 mM), 12.5 μl of PCR master mix (Thermo Scientific, USA), and 9.5 μl DD H2O. The PCR cycle conditions were an initial denaturation cycle at 94°C for 5 min, annealing for 35 cycles at 94°C for 1 min, use of primer specific temperatures for 1 min and a temperature of 72°C for 3 min, and a final one-step extension at 72°C for 5 min. The restriction endonucleases FokI4, BsmI1, Apa-I2, and TaqI3 were used to digest the polymorphic sites of the VDR gene. The enzyme-specific restriction reactions were performed at 37°C for 5 min for the BsmI1, FokI4, and ApaI2 enzymes, and at 65°C for 5 min for the TaqI3 enzyme. The size of the digested fragments was identified by 2% agarose gel electrophoresis, and the results were then visualized under UV light and photographed. The size of the digested PCR products is shown in Figure 1a.
Figure 1.
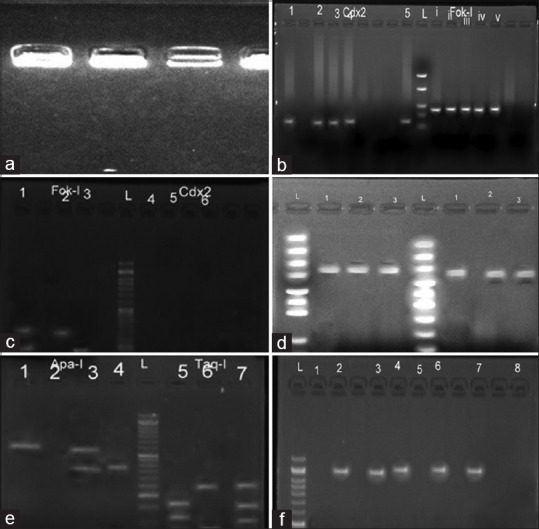
(a) Gel picture of genomic DNA on 0.8% agarose gel. (b) Amplified PCR products of Cdx2 and Fok-I. (c) Digestion results of the 2 polymorphism sites. Fok-I digestion: Ff(lane 1), ff(lane 2); FF {TT}(lane 3), Cdx2 digestion: GA(lane 4), GG(lane 5), AA(lane 6), L: 50 bp DNA ladder. (d) Amplified PCR product of Taq-I and Apa-I. (e) Digestion results of the two polymorphism sites. Apa-I digestion: aa(lane 1), Aa(lane 2); AA(lane 3), Taq-I digestion: Tt(lane 5), TT(lane 6), Tt(lane 7). (f) RFLP digestion results of Bsm-I polymorphism site. BB(lane 1), bb(lane 2); Bb(lane 4); L: 50 bp ladder
To study the Fok-I4 and Cdx25 SNP (RFLP) in vitiligo cases and controls, the PCR cycle conditions were initial denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 1 min, 61°C for 1 min, and one final cycle of extension at 72°C for 5 min. The reaction mixture consisted of 1 μl of 0.5 μg genomic DNA, 1 μl of forward and reverse primers (10 mM) (Eurofins Genomics), 12.5 μl of PCR master mix (Thermo Scientific, USA), and 9.5 μl DD H2O. The PCR products were verified using 2% agarose gel containing ethidium bromide [Figure 1b].
The restriction endonucleases Cdx25 and Fok-I4 were used to digest the polymorphic sites of the VDR gene. PCR products were digested with Cdx2 and Fok-I (Thermo Scientific, USA) restriction enzyme at 65°C for 16 h incubation. The size of the digested fragments was identified by 6% agarose gel electrophoresis, and the results were visualized under UV light and photographed. The size of the digested PCR products is shown in Figure 1c.
To study the SNP of Taq-I3 and Apa-I2 in vitiligo cases and controls, the PCR cycle conditions were initial denaturation at 94°C for 10 min, followed by 35 cycles at 94°C for 1 min, 60°C for 1 min, and 1 final cycle of extension at 72°C for 5 min. The reaction mixture consisted of 1 μl of 0.5 μg genomic DNA, 1 μl of forward and reverse primers (10 mM), 12.5 μl of PCR master mix (Thermo Scientific, USA), and 9.5 μl DD H2O. The PCR products were verified using 2% agarose gel containing ethidium bromide [Figure 1d].
The restriction endonucleases Taq-I3 and Apa-I2 were used to digest the polymorphic sites of the VDR gene. PCR products were digested with Taq-I3 and Apa-I2 (Eurofins Genomics) restriction enzyme at 65°C for 16 h incubation. The size of the digested fragments was identified by 6% agarose gel electrophoresis, and the results were visualized under UV light and photographed. The size of the digested PCR products is shown in Figure 1e.
The restriction endonuclease Bsm-I1 was used to digest the polymorphic sites of the VDR gene. PCR products were digested with Bsm-I1 (Eurofins Genomics) restriction enzyme at 65°C for 16 h incubation. The sizes of the digested fragments were identified by 6% agarose gel electrophoresis, and the results were visualized under UV light and photographed. The size of the digested PCR products is shown in Figure 1f.
Results
The age and gender ratio were not substantially different for each variable among patients with vitiligo (61 females, and 39 males) and healthy controls (60 females, and 40 males). The age group of patients ranged from 4 to 58 years with a mean age of 28.66 ± SD 11.98 years. The duration of the disease ranged from 6 months to 11 years with a mean of 3.81 years ± SD 2.51 years. The vitamin D levels ranged from 3.20 to 38.20 ng/ml with a mean value of 16.17 ± SD 8.62 ng/ml. The VETI score ranged from 0.420 to 26.51 with a mean value of 6.226 ± SD 4.88.
The vitamin D levels in males ranged from 4.0 to 38.2 ng/ml with a mean value of 21.95 ± SD 8.87 ng/ml. The VETI score in males ranged from 0.46 to 26.51 with a mean value of 6.60 ± SD 5.50. The vitamin D levels in females ranged from 3.2 to 32.3 ng/ml with a mean value of 13.35 ± SD 6.61 ng/ml. The VETI score in them ranged from 0.42 to 24 with a mean value of 5.985 ± SD 4.48. Thus, both vitamin D levels as well as the VETI score were more in males as compared to females.
Fifty-nine patients belonged to the rural areas and 41 were from urban areas. The mean vitamin D level was 17.431 ± SD 8.81 ng/ml in rural patients, and the mean VETI score was 6.72 ± SD 5.09. In the urban population, the mean vitamin D level was 15.67 ± SD 8.35 ng/ml, and the mean VETI score was 5.51 ± SD 4.54 [Table 1]. Thus, both vitamin D level as well as the VETI score was slightly more in rural patients as compared to urban patients, although not significantly higher. Vitamin D level and VETI score (as is shown in Table 1) were also noted to have an insignificant inverse correlation.
Table 1.
Comparison of various parameters among cases and controls
|
n=100 | |||||
|---|---|---|---|---|---|
| Overall (100) | Mean | SD | SE | Max | Min |
| Age (years) | 28.66 | 11.98 | 1.2 | 58 | 4 |
| Duration (years) | 3.810 | 2.511 | 0.255 | 11.000 | 0.500 |
| VETI | 6.226 | 4.887 | 0.489 | 26.510 | 0.420 |
| Vit. D levels | 16.710 | 8.629 | 0.863 | 38.200 | 3.200 |
| Descriptive statistics: (gender wise) | |||||
| Males (39) | Mean | SD | SE | Max | Min |
| Age (years) | 29.359 | 11.708 | 1.875 | 55 | 9 |
| Duration (years) | 3.974 | 2.505 | 0.401 | 9 | 0.5 |
| VETI | 6.603 | 5.501 | 0.880 | 26.51 | 0.46 |
| Vit. D levels | 21.956 | 8.870 | 10,420 | 38.2 | 4 |
| Females (61) | Mean | SD | SE | Max | Min |
| Age (years) | 28.213 | 12.234 | 1.566 | 58 | 4 |
| Duration (years) | 3.705 | 2.595 | 0.332 | 11 | 0.5 |
| VETI | 5.985 | 4.482 | 0.574 | 24 | 0.42 |
| Vit. D levels | 13.355 | 6.610 | 0.846 | 32.3 | 3.2 |
| Descriptive statistics: rural/urban | |||||
| Rural (59) | Mean | SD | SE | Max | Min |
| Age (years) | 32.034 | 11.219 | 1.461 | 58 | 12 |
| Duration (years) | 4.144 | 2.555 | 0.333 | 11 | 0.5 |
| VETI | 6.722 | 5.090 | 0.663 | 26.51 | 0.42 |
| Vit. D levels | 17.431 | 8.811 | 1.147 | 38.2 | 4 |
| Urban (41) | |||||
| Age (years) | 23.805 | 11.492 | 1.795 | 48 | 4 |
| Duration (years) | 3.329 | 2.499 | 0.390 | 11 | 0.5 |
| VETI | 5.513 | 4.545 | 0.710 | 24 | 0.46 |
| Vit. D levels | 15.671 | 8.357 | 1.305 | 33.2 | 3.2 |
The vitamin D levels were found to be lower in vitiligo patients than in control group, being 16.170 ± 8.629 ng/ml and 25.49 ± 1.02 ng/ml, respectively (P = 0.0001). Out of 100 cases, 10 had levels >30 ng/ml, 15 were found to have vitamin D levels of ≤30 ng/ml, whereas 52 cases had levels ≤20 ng/ml and 23 cases had ≤10 ng/ml [Table 2].
Table 2.
Comparison of vitamin D level in different age groups between cases and controls
| Vitamin D (ng/ml) | Cases (n=100) mean (16.17±8.62) |
Controls (n=100) mean (25.49±1.02 ng/ml) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| ≥30 | 10 | 10 | 46 | |
| <30.0-20 | 15 | 15 | 35 | 12 |
| <20.0-10 | 52 | 52 | 12 | 7 |
| ≤10.0 | 23 | 23 | 7 | 3 |
Although the levels of vitamin D were low among patients, there was no correlation with the age, sex, disease, family history of vitiligo, or the type of vitiligo. There was also no statistically significant relation between vitamin D levels and the VETI score, as is shown in Table 3. Since vitamin D plays a stimulatory role on melanocytes and melanogenesis, the serum and tissue levels of vitamin D are postulated to play a role in pigmentation through VDR gene expression. Thus, VETI score is expected to show an inverse relation with vitamin D levels, which was found in our study also, although the difference was not statistically significant (P value: 0.882) [Table 3]. Previous studies have also not found any statistical relation between the two variables.
Table 3.
Relation between VETI and vitamin D levels in cases
| Parameter | Mean | SD | SE | Max | Min |
|---|---|---|---|---|---|
| VETI | 6.226 | 4.887 | 0.489 | 26.510 | 0.420 |
| Vitamin D level | 16.710 | 8.629 | 0.863 | 38.200 | 3.200 |
| Correlation coefficient (r) | −0.015 | ||||
| P | 0.882 |
The genotype frequencies were slightly higher but not statistically significant in patients than controls for the Ff genotype (38% vs. 27%, P = 0.112). However, the frequencies of genotypes were higher in the patient group versus controls for the Cc genotype (54.0% vs. 34.0%, P = 0.0023) and cc genotype (P = 0.002) [Table 4]. The differences between the groups regarding the frequency of the genotype Aa were statistically significant (P = 0.0001 and P = 0.033). Statistically significant difference was also observed regarding Apa-I-evaluated alleles in case group when compared to controls (P = 0.0001) [Table 5]. The genotype frequencies were higher but not statistically significant in patients than controls for the Tt genotype (48% vs. 39%, P = 0.1431) [Table 6].
Table 4.
SNP of Fok-I and Cdx2 in both the vitiligo group and the control group (P<0.05)
| Condition of genotype | Frequency in cases (%) | Frequency in controls (%) | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|---|---|
| Fok-I | |||||
| FF (TT) | 55 | 64 | |||
| Ff (TC) | 38 | 27 | 0.6106 | 0.3314-1.125 | 0.112 |
| ff (CC) | 7 | 9 | 1.105 | 0.3860-3.163 | 0.8524 |
| Ff + ff | 45 | 36 | 0.687 | 0.389-1.213 | 0.194 |
| Allele | |||||
| F | 74 | 77.5 | |||
| F | 26 | 22.5 | 0.8132 | 0.4239-1.560 | 0.5334 |
| Cdx2 | |||||
| CC (GG) | 40 | 62 | |||
| Cc (GA) | 54 | 34 | 0.4062 | 0.2263-0.7292 | 0.0023* |
| Cc (AA) | 6 | 4 | 0.4301 | 0.1142-1.620 | 0.2023 |
| Cc + cc | 60 | 38 | 0.4086 | 0.2314-0.7215 | 0.0019* |
| Allele | |||||
| C | 67 | 79 | |||
| C | 33 | 21 | 0.5397 | 0.2855-1.020 | 0.0560 |
*Statistically significant
Table 5.
The allele and genotype frequencies for Taq-I and Apa-I
| Condition of genotype | Frequency in cases (%) | Frequency in controls (%) | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|---|---|
| Apa-I | |||||
| AA (GG) | 39 | 66 | |||
| Aa (GT) | 52 | 27 | 0.3068 | 0.166-0.5651 | 0.0001* |
| aa (TT) | 9 | 7 | 0.4596 | 0.1585-1.332 | 0.145 |
| Aa + aa | 61 | 34 | 0.3294 | 0.1850-0.5864 | 0.0001* |
| Allele frequency | |||||
| A (G) | 65 | 79.5 | |||
| A (T) | 35 | 20.5 | 0.4565 | 0.2537-0.7838 | 0.0125 |
| Taq-I | |||||
| TT (TT) | 36 | 46 | |||
| Tt (TC) | 48 | 39 | 0.6359 | 0.3464-1.167 | 0.1431 |
| Tt (CC) | 16 | 15 | 0.7337 | 0.3203-1.680 | 0.4631 |
| Tt + tt | 64 | 54 | 0.6603 | 0.3745-1.164 | 0.1505 |
| Allele | |||||
| T (T) | 60 | 65.5 | |||
| T (C) | 40 | 34.5 | 0.8077 | 0.4551-1.433 | 0.4652 |
*Statistically significant
Table 6.
Allele and genotype frequencies of Bsm-I among cases and controls
| Condition of genotype | Frequency in cases (%) | Frequency in controls (%) | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|---|---|
| Bsm-I | |||||
| BB (GG) | 49 | 55 | |||
| Bb (GA) | 39 | 35 | 0.7995 | 0.4400-1.453 | 0.4624 |
| bb (AA) | 12 | 10 | 0.7424 | 0.2948-1.870 | 0.5264 |
| Bb + bb | 51 | 45 | 0.7861 | 0.4508-1.371 | 0.3958 |
| Allele frequency | |||||
| G | 68.5 | 72.5 | |||
| A | 31.5 | 27.5 | 0.8232 | 0.4464-1.518 | 0.5331 |
The relation between vitamin D levels and various genotype frequencies is depicted in Table 7 and Graphs 1–5. Thus, there is no significant difference in serum vitamin D levels between various genotypes among cases and controls.
Table 7.
Vitamin D levels in different variants of the VDR gene
| Parameter | Genotype (no.) | Genotype (no.) | Genotype (no.) |
|---|---|---|---|
| Taq-I | |||
| TT (36) | Tt (48) | Tt (16) | |
| 25(OH)D | 15.5±2.5 | 13.2±5.6 | 16.2±1.2 |
| Bsm-I | |||
| BB (49) | Bb (39) | Bb (12) | |
| 25(OH)D | 12.5±8.5 | 15.6±4.20 | 15.2±1.5 |
| Apa-I | |||
| AA | Aa | aa | |
| 25(OH)D | 13.5±8.6 | 16.5±5.5 | 12±2.5 |
| Fok-I | |||
| FF | Ff | ff | |
| 25(OH)D | 15.2±6.5 | 10.2±8.56 | 15.5±1.02 |
| Cdx2 | |||
| CC | Cc | cc | |
| 25(OH)D | 17.2±8.2 | 13.5±5.6 | 10.8±1.25 |
Graph 1.
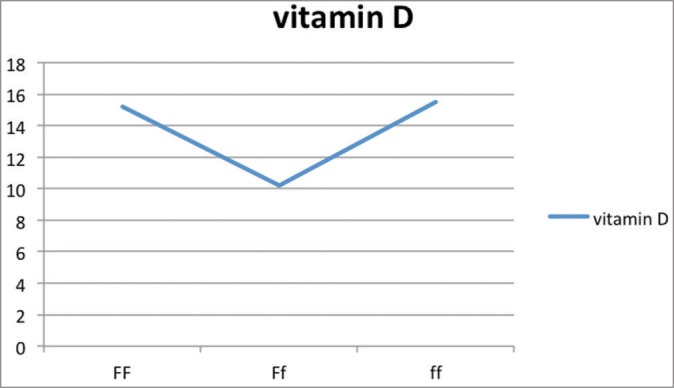
Vitamin D levels as per genotypic distribution of Fok-I SNP
Graph 5.
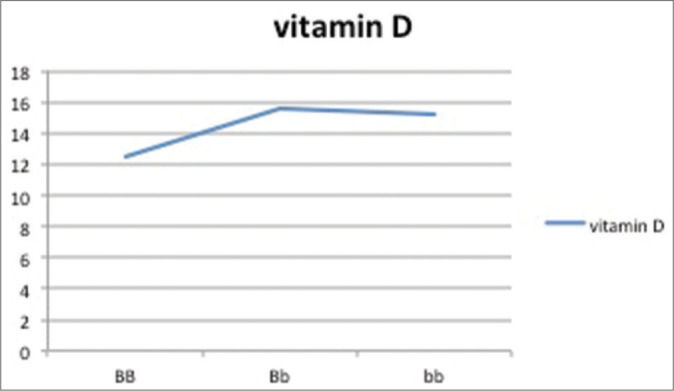
Vitamin D levels as per genotypic distribution of Bsm-I SNP
Graph 2.
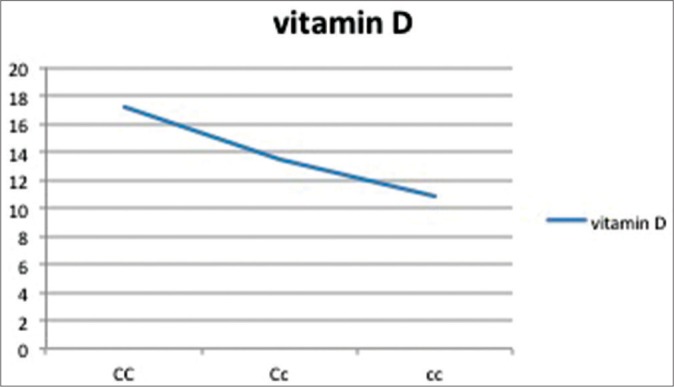
Vitamin D levels as per genotypic distribution of Cd×2 SNP
Graph 3.
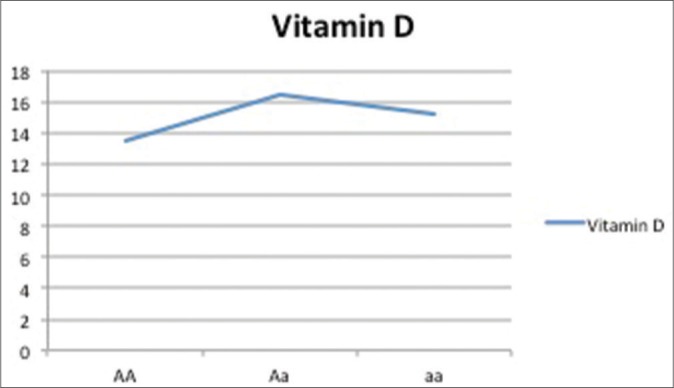
Vitamin D levels as per genotypic distribution of Apa-I SNP
Graph 4.
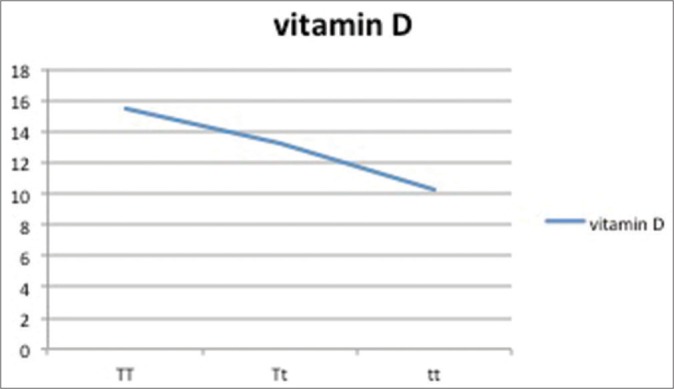
Vitamin D levels as per genotypic distribution of Taq-I SNP
Discussion
Vitiligo is an acquired pigmentary disorder characterized by areas of depigmented skin, resulting from progressive autoimmune loss of functioning melanocytes from the skin and often overlying hair, and mucous membranes. It affects between 1% and 2% of the general population. The exact pathogenesis of vitiligo is unknown, but various studies have suggested that an interplay of multiple factors such as genetic, neural, oxidant–antioxidant imbalance, biochemical, and autoimmunity might induce vitiligo. Vitamin D is synthesized in the epidermal keratinocytes under the influence of UVB light and is known to influence melanogenesis, as has been documented with the efficacy of topical vitamin D analogues, either used as monotherapy or in combination with phototherapy for the treatment of vitiligo.[18]
The number of studies evaluating the role of vitamin D in vitiligo is limited. The levels of 25-hydroxy vitamin D3(25(OH)D3) in serum, which is a widely accepted indicator of overall vitamin D status, were measured in this study. Serum 25(OH)D levels were divided into normal or sufficient (≥30 ng/ml), insufficient (<30–>20 ng/ml), and deficient (≤20 ng/ml) levels.[19]
Our results showed that the mean serum level of 25(OH)D3 was markedly lower among cases than controls. Out of 100 cases, 10 were found to have levels >30 ng/ml, 15 were found to have 25(OH)D levels of ≤30 ng/ml, whereas 52 cases had 25(OH)D levels ≤20 ng/ml and 23 cases had 25(OH)D levels ≤10 ng/ml. The VETI score was slightly higher in patients with 25(OH)D level below 30 ng/ml, which means that the level of vitamin D could influence the extent of disease. In consistent with our results, Silverberg et al.[20] found that more than 68.9% of patients had serum levels of 25(OH)D below 30 ng/ml, and Saleh et al.[21] found that 97.5% of their patients had deficient levels of 25(OH)D. On the contrary, Xu et al.[22] conducted a study over 171 Chinese patients and could detect no significant difference in the 25(OH)D levels between patients and controls.
The low serum levels of vitamin D in vitiligo patients in our study as well as other previous studies could thus be a consequence of the disease, as well as a contributing factor, to the development of the disease, through its immunomodulatory role, as well as its impact on melanogenesis. The mechanism by which vitamin D deficiency could contribute to the pathogenesis of vitiligo is that vitamin D has been found to reduce the expression of major histocompatibility complex class II molecules and inhibit the secretion of pro-inflammatory cytokines including interleukins (IL-1, IL-2, IL-6), interferon gamma, tumor necrosis factor alpha, IL-12, and also inhibit IL-6 and IL-17 secretion, thus impeding Th17 function. An impairment of Th1 and Th17 pathways by low levels of vitamin D could contribute to the process of development of vitiligo.[23,24]
In our study, the serum levels of vitamin D were low in patients as compared to controls and the serum 25(OH)D levels were not significantly different among the different ApaI, Bsm-I, TaqI, Cdx2, and FokI genotypes as is depicted in Table 7.
Sobeih et al.[25] tried evaluating the potential association between VDR gene polymorphisms (ApaI, TaqI, and FokI) and vitiligo susceptibility and detect a correlation between serum 25(OH)D levels and vitiligo, and finally between VDR gene polymorphisms and 25(OH)D levels in vitiligo in an Egyptian study population. It was observed that the serum level of vitamin D was significantly lower in patients than controls. The frequency of the ApaI variant “a” allele, the variant genotype (aa), and the variant genotype (tt) were significantly higher among the vitiligo cases than among controls. Their study also showed that the serum 25(OH)D levels were not significantly different among the different ApaI, TaqI, and FokI genotypes.[25]
Doss et al.[26] in their case-control study on 30 patients and equal number of controls evaluated the role of vitamin D in the pathogenesis of vitiligo through the evaluation of vitamin D serum level and VDR expression in tissue biopsies from the normal and vitiliginous skin. They observed that the mean VDR expression in the tissue biopsies of controls was more than 8 times higher than the lesional tissue biopsies of patients (P < 0.001) and 3 times higher than the nonlesional biopsies from patients (P < 0.001). The mean VDR expression in the nonlesional biopsies was also more than double the mean VDR expression in the lesional biopsies (P < 0.001). Again, they found no relation between patient age, sex, disease duration, family history, stress at onset, vitiligo type, VIDA score, and affected BSA with the VDR expression (both in lesional and nonlesional tissue biopsies), and P value of >0.05 was noted in all. No significant difference existed in VDR expression (in lesional or nonlesional tissue biopsies) between patient group with 25(OH)D levels above 30 ng/ml and the patient group below 30 ng/ml (P > 0.05).[26]
Li et al.[27] conducted a study to evaluate the association of VDR gene polymorphisms and serum 25(OH)D levels in patients with generalized vitiligo. They performed a hospital-based study of 749 patients with vitiligo and 763 matched controls. They investigated four VDR polymorphisms (FokI, BsmI, ApaI, and TaqI) to determine whether they are associated with vitiligo susceptibility in the Chinese population. In addition, the levels of 25(OH)D3 were measured to evaluate possible associations between the VDR polymorphic variants and clinical and laboratory findings of vitiligo. A significantly decreased risk of developing vitiligo was found to be associated with the BsmI-B, ApaI-A, and TaqI-t alleles. According to the genotype distribution, 25(OH)D3 concentrations were significantly higher in patients carrying the Fok I ff or ApaI AA genotypes compared with those carrying the FF or aa genotypes. The findings of their study suggest that these VDR polymorphisms are associated with 25(OH)D levels and that there exists a genetic predisposition for vitiligo in the Chinese population.[27]
Moreover, VDR polymorphism has been implicated in the pathogenesis of other skin diseases, for example, psoriasis. Multiple studies have found an association of polymorphism in the VDR gene with the severity of psoriasis.[28] It has been proven that vitamin D has stimulatory role on melanocytes and plays a role in melanogenesis, as we found SNP of various nucleotides of VDR genes, which could lead to VDR dysfunction, and hence resulting in vitamin D deficiency. Vitamin D deficiency is believed to act as an environmental trigger for the induction of autoimmunity and thus could lead to the development of vitiligo, since autoimmunity is the most widely accepted theory for the development of vitiligo.
Various studies have documented vitamin D deficiency in majority of the Kashmiri population; hence, both our cases as well as controls were deficient in vitamin D.[29]
Thus, we observed that VDR gene polymorphism may have a role in the development of vitiligo. In our study, there was a variation in the serum levels of vitamin D and various VDR genotypes but the correlation was not statistically significant.
The limitation of our study was that skin biopsy samples were not used. But previous studies have found no correlation of serum levels of vitamin D and tissue levels of VDR.
Conclusion
The single nucleotide gene polymorphisms of various VDR genes as found in the cases might lead to vitamin D deficiency, due to alteration of VDR. In turn, this could lead to an increase in the susceptibility of developing vitiligo, due to the stimulatory role of vitamin D in melanogenesis. Since autoimmunity is the most widely accepted theory for the development of vitiligo and low levels of vitamin D are associated with the development of vitiligo, so vitamin D deficiency can be proposed to act as an environmental trigger for the induction of autoimmunity and thus could lead to the development of vitiligo – a hypothesis.
Financial support and sponsorship
The study was financially supported by IADVL L'Oreal Research Grant 2015.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge IADVL and Department of Biochemistry, Government Medical College, Srinagar.
References
- 1.Lerner AB. Vitiligo. J Invest Dermatol. 1959;32:285–310. [PubMed] [Google Scholar]
- 2.Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol. 2001;2:167–81. doi: 10.2165/00128071-200102030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Nath SK, Majumder PP, Nordlund JJ. Genetic epidemiology of vitiligo: Multi locus recessivity cross-validated. Am J Hum Genet. 1994;55:981–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Malek ZA, Ross R, Trinkle L, Swope V, Pike JW, Nordlund JJ. Hormonal effects of vitamin D3 on epidermal melanocytes. J Cell Physiol. 1988;136:273–80. doi: 10.1002/jcp.1041360209. [DOI] [PubMed] [Google Scholar]
- 5.Kira M, Kobayashi T, Yoshikawa K. Vitamin D and the skin. J Dermatol. 2003;30:429–37. doi: 10.1111/j.1346-8138.2003.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 6.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol. 2012;76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 7.Backe F, Takiishi T, Korf H, Gyesmans C, Mathieu C. Vitamin D: Modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Van Belle TL, Gyesmans C, Mathieu C. Vitamin D in autoimmune, I nfectious and allergic diseases: A vital player? Best Pract Res Clin Endocrinol Metab. 2011;25:617–32. doi: 10.1016/j.beem.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Alghamdi K, Kumar A, Moussa N. The role of vitamin D in melanogenesis with an emphasis on vitiligo. Indian J Dermatol Venereol Leprol. 2013;79:750–8. doi: 10.4103/0378-6323.120720. [DOI] [PubMed] [Google Scholar]
- 10.Ortonne J. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd ed. Elsevier; 2008. p. 947. [Google Scholar]
- 11.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erpolat S, Sarifakioglu E, Ayyildiz A. 25-hydroxyvitamin D status in patients with alopecia areata. Postepy Dermatol Alergol. 2017;34:248–52. doi: 10.5114/ada.2017.67847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 14.Karagün E, Ergin C, Baysak S, Erden G, Aktaş H. The role of serum Vitamin D levels in vitiligo. Postepy Dermatol Alergol. 2016;33:300–2. doi: 10.5114/pdia.2016.59507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wacker M, Holick MF. Vitamin D-Effects on skeletal and extraskeltal health and the need for supplementation. Nutrients. 2013;5:111–48. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birlea SA, Costin GE, Norris DA. Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation. Curr Drug Targets. 2008;9:345–59. doi: 10.2174/138945008783954970. [DOI] [PubMed] [Google Scholar]
- 17.Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol. 2006;72:315–21. doi: 10.4103/0378-6323.26722. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami T, Hashimoto T. Disease severity indexes and treatment evaluation criteria in vitiligo. Dermatol Res Pract. 2011;7:50–342. doi: 10.1155/2011/750342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678–88. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg JI, Silverberg AI, Malka E, Silverberg NB. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937–41. doi: 10.1016/j.jaad.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Saleh HM, Abdel Fattah NS, Hamza HT. Evaluation of serum 25-hydroxyvitamin D levels in vitiligo patients with and without autoimmune diseases. Photodermatol Photoimmunol Photomed. 2013;1:34–40. doi: 10.1111/phpp.12016. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Fu WW, Wu WY. Serum 25-hydroxyvitamin D deficiency in Chinese patients with vitiligo: A case-control study. PLoS One. 2012;7:527–78. doi: 10.1371/journal.pone.0052778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang BX, Lin M, Qi XY, Zhang RX, Wei ZD, Zhu J, et al. Characterization of circulating CD8+T cells expressing skin homing and cytotoxic molecules in active non-segmental vitiligo. Eur J Dermatol. 2013;23:331–8. doi: 10.1684/ejd.2013.2011. [DOI] [PubMed] [Google Scholar]
- 24.Van den Wijngaard RM, Aten J, Scheepmaker A, Le Poole IC, Tigges AJ, Westerhof W, et al. Expression and modulation of apoptosis regulatory molecules in human melanocytes: Significance in vitiligo. Br J Dermatol. 2000;143:573–81. doi: 10.1111/j.1365-2133.2000.03712.x. [DOI] [PubMed] [Google Scholar]
- 25.Sobeih S, Mashaly HM, Gawdat H, Amr K, Hamid MF, Shaalan E. Evaluation of the correlation between serum levels of vitamin D and Vitamin D receptor gene polymorphisms in an Egyptian population. Int J Dermatol. 2016;55:1329–35. doi: 10.1111/ijd.13363. [DOI] [PubMed] [Google Scholar]
- 26.Doss RW, El-Rafaie AA, Gohary YM, Rashed LA. Vitamin D receptor expression in vitiligo. Indian J Dermatol. 2015;60:544–8. doi: 10.4103/0019-5154.169123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li K, Shi Q, Yang L, Li X, Liu L, Wang L, et al. The association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with generalized vitiligo. Br J Dermatol. 2012;167:815–21. doi: 10.1111/j.1365-2133.2012.11132.x. [DOI] [PubMed] [Google Scholar]
- 28.Park BS, Park JS, Lee DY, Youn JI, Kim IG. Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol. 1999;112:113–6. doi: 10.1046/j.1523-1747.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- 29.Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, et al. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]


