Abstract
Lichen planus is a chronic inflammatory disease, which involves skin, mucous membrane, and nails. Prevalence of oral lichen planus varies between 0.5% and 2.6% of adult population worldwide with overall female preponderance. It is considered as a potentially malignant disorder with rate of transformation to oral cancer varying between 0.5% and 2%. Oral lichen planus may either be unilateral or bilateral, or may involve multiple sites. Although the exact etio-pathogenesis of this condition is unknown, it is believed that stress, use of medications, dental fillings, genetics, immunity, and hypersensitivity reactions may contribute to its pathogenesis. It is a T-cell-mediated autoimmune disorder in which CD8+ T cells are involved which release various cytokines such as tumor necrosis factor-α and interleuking-12 leading to disruption of basement membrane integrity. Zinc activates caspase-3 and DNA fragmentation, resulting in the apoptosis of keratinocytes. By prevention of matrix metalloproteinase (MMP)-1 activation, it inhibits T-cell accumulation in oral lichen planus, and by inhibiting MMP-9 it prevents cleavage of collagen IV resulting in maintaining the integrity of the basement membrane. The present case series describes the use of oral zinc acetate (50 mg) in patients having symptomatic oral lichen planus with favorable outcome in terms of size of lesion and global index score.
Keywords: Global index score, oral lichen planus, zinc
Introduction
Lichen planus is a chronic inflammatory disease which involves skin, mucous membrane, and nails with an estimated prevalence rate of 0.5%–2.6% in the general population.[1] It is believed that factors such as stress, use of systemic medications, dental materials, genetics, immunity, and hypersensitivity reactions and viruses such as human papilloma virus (HPV 16 and 18), human herpes virus, and hepatitis C may contribute in the pathogenesis of lichen planus.[2,3]
It is a T-cell-mediated autoimmune disease in which the auto-cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium. T cells (CD8+ cells) recognize the antigen associated with major histocompatibility complex (MHC)-1 on keratinocyte. Activated CD4+ lymphocytes increase the number of Langerhans cells along with upregulation of MHC-II expression leading to enhanced antigen recognition. The activated CD8+ T cells release cytokines which damage the basal keratinocytes through tumor necrosis factor (TNF)-α and granzyme B leading to keratinocytes apoptosis. Through the disrupted epithelium, cytokines attract additional lymphocytes into developing lesion.[4]
Corticosteroids are the mainstay of treatment due to their role in dampening cell-mediated immune activity thereby modulating the immune function.[5,6] To reduce the adverse effects, corticosteroid-sparing drugs such as tacrolimus, cyclosporine, and retinoids are used.[2]
Zinc plays an important role in maintaining the proper reproductive function, immune function, and wound repair through regulation of DNA and RNA polymerases, thymidine kinase, and ribonuclease.[7] It has an anti-inflammatory action through inhibiting the activity of cytokines and causing induction of apoptosis-enhancing macrophage and neutrophil functions. Zinc has been shown to have anti-inflammatory effect by reducing the keratinocyte activation markers such as expression of intercellular adhesion molecule 1 and production of TNF.[8]
Basement membrane disruption in lichen planus is mainly due to mast cell proteases and keratinocyte apoptosis. Zinc chelation activates caspase-3 and DNA fragmentation, resulting in the apoptosis of keratinocytes.[9] Matrix metalloproteinase (MMP)-1 activates T-cell accumulation in oral lichen planus, whereas MMP-9 and its activators cleave collagen IV, which are normally required for intact basement membrane. It also upregulates the OLP lesional T cells, resulting in increased basement membrane disruption.[10,11]
This case series describes 3 patients having symptomatic oral lichen planus who had significant improvement after oral zinc therapy.
Report of Case 1
A 52-year-old male patient presented with burning sensation in both buccal mucosa for a year precipitated by intake of food. He had history of smoking and gutka chewing for last 3 years and history of alcohol consumption for 10 years. He had no other co-morbidities. On intraoral examination, erosive lesions interspersed with keratotic striations adherent to the right buccal mucosa measuring approximately 2 × 1 cm were present, which were extending from the mesial aspect of 45 to the distal aspect of 47 posteriorly and extending superiorly along the vestibule to gingival margin [Figure 1a]. On palpation, the lesions were tender and non-scrapable. The burning sensation assessed by visual analog scale (VAS) was 10/10, and the size of the lesion was assessed by global index scale. Histopathologically, it was diagnosed as erosive lichen planus involving the right buccal mucosa [Figure 1b], and the patient was advised treatment with oral zinc acetate 50 mg two times a day for 8 weeks along with 0.1% triamcinolone acetonide oral paste for 1 week at first visit. The steroid oral paste was discontinued after 1 week of application, whereas tablet ascazin 50 mg was continued for 8 weeks with monthly follow up till 2-months after stopping treatment.
Figure 1.
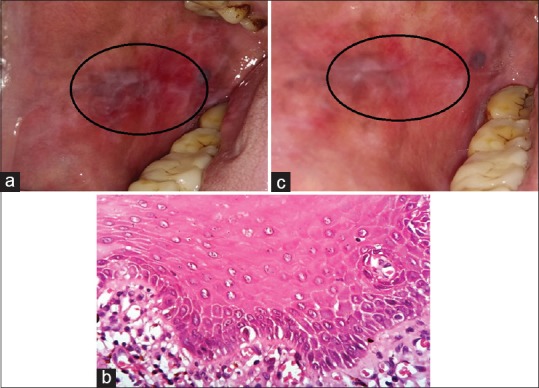
(a) Pretreatment erosive lichen planus lesions on buccal mucosa of Case 1. (b) ×400 view of H and E–stained soft tissue section of Case 1 with hyperkeratotic epithelium along with underlying fibrocellular connective tissue stroma with spongiosis and acanthosis at focal areas. The underlying connective tissue is fibrocellular with dense inflammatory cells chiefly lymphocytes and cells at subepithelial connective tissue and scattered. (c) Posttreatment reduction in erosive pattern of the lesions after zinc therapy on buccal mucosa in Case 1
The patient's VAS was 10/10 during the first visit which gradually decreased to 1/10 in a span of 8 weeks [Table 1]. The size and number of the lesions were assessed by global index scale in terms of percentage values by a single observer at each visit, and a 90% decrease in the size of the lesion was noticed after 8 weeks of treatment. At follow up visit of 2 months after stopping treatment, there was no recurrence of burning sensation and complete resolution of lesion [Figure 1c] was observed.
Table 1.
Burning sensation scores by visual analog scale (VAS)
| 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks | 6 weeks | 7 weeks | 8 weeks | Post-treatment follow-up visit at 1 month | Post-treatment follow-up visit at 2 months | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 10 | 8 | 6 | 5 | 4 | 3 | 1 | 1 | 1 | 0 |
| Patient 2 | 10 | 9 | 8 | 6 | 5 | 3 | 2 | 1 | 1 | 0 |
| Patient 3 | 10 | 4 | 8 | 4 | 4 | 1 | 1 | 1 | 1 | 0 |
Case 2
A 45-year-old female patient with no comorbidities complained of burning sensation in the gums for last 6 months which was aggravated by intake of food. On intraoral examination, erosive lesions were present on the upper marginal and attached gingivae extending from the distal aspect of the right upper canine to the mesial aspect of the left upper premolars. The burning sensation score was 10/10 on VAS. Histopathologically, it was diagnosed as erosive lichen planus involving the gingiva. The patient was treated on a similar lines as case 1, and posttreatment follow-up demonstrated good healing. It was noticed that there was 80% decrease in size of the lesion during the 8 weeks follow up according to global index scale [Figure 2a and b].
Figure 2.
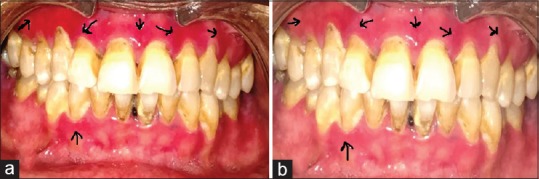
(a) Pretreatment erosive lichen planus lesions on gingiva in Case 2. (b) Posttreatment reduction lesions on gingiva in Case 2
Case 3
A 48-year-old female patient complained of pain and burning sensation in the buccal mucosa after intake of food for last 6 months. On intraoral examination, keratotic plaques was present on the right and left buccal mucosae measuring approximately 5 × 3 cm, extending anterior posteriorly 1 cm away from the corner of the mouth to the retromolar region [Figure 3a]. On palpation, it was rough in texture, tender, and nonscrapable. Histopathologically, it was diagnosed as plaque-type lichen planus involving the right and left buccal mucosa [Figure 3b]. The patient was treated using same protocol as case 1.
Figure 3.
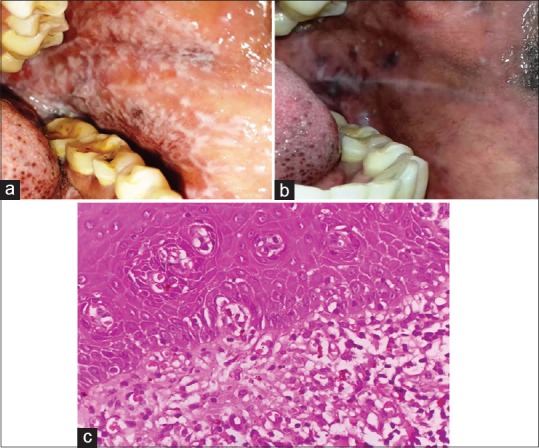
(a) Pretreatment lesions of reticulopapular lichen planus in Case 3. (b) ×100 view of H and E–stained soft tissue section of Case 3 with hyperkeratotic epithelium with underlying fibrovascular connective tissue stroma with basal cell hyperplasia at focal areas and basal cell degeneration. (c) Noticeable change in the reticular pattern of the lesions with reduction after zinc therapy in Case 3
The patient's VAS 10/10 at first visit which decreased to 4/10 at second visit. There was a sudden increase in the VAS to 8/10 at third visit due to discontinuation of treatment [Table 1]. It was noticed that the size of the lesion was decreased by 90% as calculated by global index scales after 8 weeks [Figure 3c].
There was a significant decrease in VAS and the size of the lesion in all the three patients. No recurrence of lesions or burning sensation was observed at follow up visits till 2 months after stopping treatment.
Discussion
The present case series revealed that oral zinc acetate 50 mg twice daily may be an effective treatment modality in patients of oral lichen planus. Significant decrease in burning sensation, pain, and lesion size was observed which may be explained by the anti-inflammatory and wound-healing properties.
Mehdipour et al. compared the efficacy of combination of 0.2% zinc mouthwash with fluocinolone with fluocinolone monotherapy in the treatment of oral lichen planus and found that combination of 0.2% zinc mouthwash with fluocinolone was more effective in decreasing the lesion surface area, pain, and burning sensation probably owing to the role of zinc in healing the disrupted epithelium.[2] Zinc helps in regeneration and repair of epithelium, strengthens the local defense system thus leading to reduction in inflammation and bacterial growth. Zinc also has a role as a cofactor in numerous transcription factors which may explain its regenerative properties.[12]
Thomas J et al. compared the efficacy of combination of topical zinc sulfate 2.5% with 0.05% clobetasol propionate cream with 0.05% clobetasol propionate cream monotherapy in the treatment of subacute and chronic eczema, lichen planus, and psoriasis, and found that the combination was effective in reducing the severity of the lesions owing to the anti-inflammatory action of zinc.[13]
Some animal studies have demonstrated that 0.3 mg/cm2 zinc oxide cream promotes epithelialization by enhancing endogenous growth factors and enzymes important for epithelial proliferation and migration.[14] Baroni A et al. added zinc oxide to human dermal fibroblasts and observed increased secretion of fibroblast growth factor suggesting that zinc in granulating wounds is also possibly capable of upregulating growth factors other than insulin-like growth factor-I.[15]
Gholizadeh N et al. evaluated the serum zinc level in 22 patients with erosive lichen planus and 22 patients with non erosivelichen planus and concluded that serum zinc levels were reduced in patients with erosive lichen planus, and probably by its deficiency it may lead to inhibition and stimulation of lymphocytic reaction, subsequently having an effect on the reduction in the number of T-cells.[16]
On an average, adult zinc content is between 1.4 and 2.3 g, with the highest tissue concentration in the prostate, seminal fluid, uveal tissue, and skin.[7] There are various biological forms of zinc for therapeutic use. Zinc sulfate has many adverse effects such as nausea, stomach upset, and heartburn and rare adverse effects include fever, sore throat, mouth sores, weakness, and fatigue, compared with zinc acetate. Hence, in our present cases, zinc acetate was given to patients for treatment of oral lichen planus.
Steroids were used only for a week to treat the burning sensation in the patients. Thereafter, they were discontinued and only zinc supplements were maintained. It is noteworthy that there were reports with rebound effect of lesions with steroids. In the present case series, there was no recurrence of lesion and no rebound effect was noticed. The major limitation of this case series, was that zinc serum levels was not monitored or correlated with response.
Conclusion
Oral zinc therapy was associated with significant reduction in the burning sensation and lesion size in the symptomatic oral lichen planus. Further prospective studies with larger sample are recommended.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors acknowledge Dr. Parmeshwar Naishadam, oral pathologist and microbiologist, for helping us in the histopathological examination of biopsy specimen.
References
- 1.Ismail S, Kumar S, Zain R. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 2.Mehdipour M, TaghaviZenouz A, Bahramian A, Yazdani J, Pouralibaba F, Sadr K. Comparison of the effect of mouthwashes with and without zinc and fluocinolone on the healing process of erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects. 2010;4:25–8. doi: 10.5681/joddd.2010.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razavi SM, Ghalayani P, Salehi MR, Attarzadeh H, Shahmoradi M. Human papilloma virus as a possible factor in the pathogenesis of oral lichen planus. Dent Res J (Isfahan) 2009;6:82–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqubal M, Khan M, Chaudhary UC, Swetarchi, Farhat B, Akhtar N. Oral lichen planus: An update. Int J Curr Res Rev. 2016;8:30–3. [Google Scholar]
- 6.Thongprasom K, Dhanuthai K. Steroids in the treatment of lichen planus: A review. J Oral Sci. 2008;50:377–85. doi: 10.2334/josnusd.50.377. [DOI] [PubMed] [Google Scholar]
- 7.Gupta M, Mahajan V, Mehta K, Chauhan P. Zinc therapy in dermatology: A review. Dermatol Res Pract. 2014;2014:1–11. doi: 10.1155/2014/709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasad AS. Zinc in human health: Effect of zinc on immune cells. Mol Med. 2008;14:353–7. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa Y, Kinoshita M, Shimada S, Kawamura T. Zinc and skin disorders. Nutrients. 2018;10:199. doi: 10.3390/nu10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira P, Carneiro S, Ramos-e-Silva M. Oral lichen planus: An update on its pathogenesis. Int J Dermatol. 2015;54:1005–10. doi: 10.1111/ijd.12918. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Jawanda MK. Oral lichen planus: An update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222–9. doi: 10.4103/0019-5154.156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagherani N, R Smoller B. An overview of zinc and its importance in dermatology – Part II: The association of zinc with some dermatologic disorders. Glob Dermatol. 2016;3:337–50. [Google Scholar]
- 13.Thomas J, Kandhari S, Oberoi C, Jayaseelan E, Yogi Raj K. A double-blind randomized multicentre controlled study of topical 0.05% clobetasol propionate with 2.5% zinc sulphate preparation. Indian J Dermatol Venereol Leprol. 2001;67:135–7. [PubMed] [Google Scholar]
- 14.Magnus S, Hendrick H, Agren Zinc oxide augments endogenous expression of insulin like growth factor I (IGF-I) and activates matrix metalloproteinases (MMPS) in wounds. EMWA J. 2001;1:1–3. [Google Scholar]
- 15.Baroni A, Perfetto B, Buttini G, Catalanotti P, Gorga F, Tufano MA. Topical amikacin formulation induces fibroblast growth factor and cytokine release from human dermal fibroblasts. Arch Dermatol Res. 1999;291:296–9. doi: 10.1007/s004030050411. [DOI] [PubMed] [Google Scholar]
- 16.Gholizadeh N, Mehdipour M, Najafi Sh, Bahramian A, Garjani SH, Khoeini Poorfar H. Evaluation of the serum zinc level in erosive and non-erosive oral lichen planus. J Dent (Shiraz) 2014;15:52–6. [PMC free article] [PubMed] [Google Scholar]


