Abstract
Background
A possible strategy for increasing smoking cessation rates could be to provide smokers with feedback on the current or potential future biomedical effects of smoking using, for example, measurement of exhaled carbon monoxide (CO), lung function, or genetic susceptibility to lung cancer or other diseases.
Objectives
The main objective was to determine the efficacy of providing smokers with feedback on their exhaled CO measurement, spirometry results, atherosclerotic plaque imaging, and genetic susceptibility to smoking‐related diseases in helping them to quit smoking.
Search methods
For the most recent update, we searched the Cochrane Tobacco Addiction Group Specialized Register in March 2018 and ClinicalTrials.gov and the WHO ICTRP in September 2018 for studies added since the last update in 2012.
Selection criteria
Inclusion criteria for the review were: a randomised controlled trial design; participants being current smokers; interventions based on a biomedical test to increase smoking cessation rates; control groups receiving all other components of intervention; and an outcome of smoking cessation rate at least six months after the start of the intervention.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We expressed results as a risk ratio (RR) for smoking cessation with 95% confidence intervals (CI). Where appropriate, we pooled studies using a Mantel‐Haenszel random‐effects method.
Main results
We included 20 trials using a variety of biomedical tests interventions; one trial included two interventions, for a total of 21 interventions. We included a total of 9262 participants, all of whom were adult smokers. All studies included both men and women adult smokers at different stages of change and motivation for smoking cessation. We judged all but three studies to be at high or unclear risk of bias in at least one domain. We pooled trials in three categories according to the type of biofeedback provided: feedback on risk exposure (five studies); feedback on smoking‐related disease risk (five studies); and feedback on smoking‐related harm (11 studies). There was no evidence of increased cessation rates from feedback on risk exposure, consisting mainly of feedback on CO measurement, in five pooled trials (RR 1.00, 95% CI 0.83 to 1.21; I2 = 0%; n = 2368). Feedback on smoking‐related disease risk, including four studies testing feedback on genetic markers for cancer risk and one study with feedback on genetic markers for risk of Crohn's disease, did not show a benefit in smoking cessation (RR 0.80, 95% CI 0.63 to 1.01; I2 = 0%; n = 2064). Feedback on smoking‐related harm, including nine studies testing spirometry with or without feedback on lung age and two studies on feedback on carotid ultrasound, also did not show a benefit (RR 1.26, 95% CI 0.99 to 1.61; I2 = 34%; n = 3314). Only one study directly compared multiple forms of measurement with a single form of measurement, and did not detect a significant difference in effect between measurement of CO plus genetic susceptibility to lung cancer and measurement of CO only (RR 0.82, 95% CI 0.43 to 1.56; n = 189).
Authors' conclusions
There is little evidence about the effects of biomedical risk assessment as an aid for smoking cessation. The most promising results relate to spirometry and carotid ultrasound, where moderate‐certainty evidence, limited by imprecision and risk of bias, did not detect a statistically significant benefit, but confidence intervals very narrowly missed one, and the point estimate favoured the intervention. A sensitivity analysis removing those studies at high risk of bias did detect a benefit. Moderate‐certainty evidence limited by risk of bias did not detect an effect of feedback on smoking exposure by CO monitoring. Low‐certainty evidence, limited by risk of bias and imprecision, did not detect a benefit from feedback on smoking‐related risk by genetic marker testing. There is insufficient evidence with which to evaluate the hypothesis that multiple types of assessment are more effective than single forms of assessment.
Plain language summary
Does giving people feedback on the effects of smoking on their body help them quit smoking?
Background
Biomedical risk assessment is the process of giving people feedback on the effects of smoking on their body. The physical effects of smoking can be assessed using various measurements, and some people think this could be used as a tool to encourage people to quit smoking. We reviewed the evidence about whether giving adult smokers feedback on the effects of smoking on their body helps them quit smoking.
Study characteristics
This review includes 20 studies using a variety of measurements. One study included two measurements, for a total of 21 measurements assessed. The main feedback measurements we assessed were the level of carbon monoxide in people's breath (a sign of current smoking), measures of lung function (a sign of lung damage from smoking), genetic tests to provide individual risk of cancer, and ultrasound of major arteries in the neck to measure the amount of plaque (a risk factor for stroke). We grouped studies into three categories according to the type of feedback people were given: feedback on exposure to smoking (five studies); feedback on a person's risk for smoking‐related diseases (five studies); and feedback on the harms of smoking (11 studies). The studies included a total of 9262 people. All participants were adult smokers, and both men and women were included (although one study performed in a clinic for army veterans included only 4% women). Most studies were conducted in general practices or ambulatory clinics. All of the studies lasted at least six months. The reported evidence is current as of March 2018.
Key results
We did not find evidence that giving smokers feedback on their smoking exposure, their genetic risk of smoking‐related disease, or the effects of smoking on their body helps them quit smoking. The most promising results were for giving people feedback on the harm smoking does to their bodies. The studies did not report on harms or side effects of providing feedback. However, given the nature of the measurements (lung or blood tests), we would expect the risk of harms to be low.
Certainty of evidence
Because of problems with the way some of the studies were conducted, we think that further research is likely to change our conclusions.
Summary of findings
Summary of findings for the main comparison. Biomedical risk assessment compared with standard care or minimal intervention for smoking cessation.
| Biomedical risk assessment compared with standard care or minimal intervention for smoking cessation | |||||
| Patient or population: people who smoke Setting: mixed Intervention: biomedical risk assessment Comparison: standard care or minimal intervention | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with biomedical risk assessment | ||||
| Feedback on smoking exposure Smoking cessation at longest follow‐up over 6 months |
Study population |
RR 1.00 (0.83 to 1.21) |
2368 (5 studies) | ⊕⊕⊕⊝1 moderate |
|
| 153 per 1000 |
153 per 1000 (127 to 185) |
||||
| Feedback on smoking‐related risk Smoking cessation at longest follow‐up over 6 months |
Study population |
RR 0.80 (0.63 to 1.01) |
2064 (5 studies) | ⊕⊕⊝⊝1,2 low |
|
| 130 per 1000 |
104 per 1000 (82 to 131) |
||||
| Feedback on smoking‐related harm Smoking cessation at longest follow‐up over 6 months |
Study population |
RR 1.26 (0.99 to 1.61) |
3314 (11 studies) | ⊕⊕⊕⊝3 moderate |
|
| 117 per 1000 |
147 per 1000 (116 to 188) |
||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias: three out of five studies at high risk of bias, and remaining studies at unclear risk of bias. 2Downgraded one level for imprecision: fewer than 300 events. 3Downgraded one level for imprecision and risk of bias: sensitivity analysis removing studies at high risk of bias produced a statistically significant benefit.
Background
Description of the condition
Smoking is the leading cause of preventable mortality and morbidity in industrialised countries (CDC 2014; Phillips 1995; Sargeant 2001; Yach 2000), and increasingly the smoking epidemic is affecting low‐ and middle‐income countries. This is particularly true for vascular and respiratory diseases, as well as for cancer (DHHS 2004; Doll 2004; Thun 2013). Stopping smoking prolongs life and reduces morbidity (DHHS 1990; Jha 2013). Despite increasing scientific knowledge about health hazards due to cigarette consumption, there is still an increase in prevalence in many countries (Bilano 2015). The gap between knowledge and smoking cessation has been attributed, in part, to smokers' underestimation of their personal risks of smoking‐related illness (Krosnick 2017; Lerman 1993; Romer 2001). Although many smokers who are successful in quitting do so on their own (Livingstone‐Banks 2019; Schwartz 1987), an increasing proportion use specific aids to support a quit attempt.
Description of the intervention
Evidence is growing on how to help smokers to quit (Fiore 2008; Hartmann‐Boyce 2018; Kottke 1988; Lancaster 2017; Stead 2013; West 2000). Interventions that have been shown to help quitting smoking include individual or group counselling, pharmacological therapies, and possibly some forms of self‐help materials. Another possible strategy for increasing quit rates is to provide feedback on the physical effects of smoking by physiological measurements. We can conceptually distinguish, in this respect, three different types of feedback: the first one explores biomarkers of smoking exposure (cotinine, carbon monoxide); the second one gives information on smoking‐related disease risk (e.g. lung cancer susceptibility according to cytochrome P450 2D6 (CYP2D6) genotyping) (Audrain 1997); and the third one depicts smoking‐related harm (e.g. atherosclerotic plaque, impaired lung function) (Buist 2002).
How the intervention might work
The rationale for such interventions is to provide personalised motivational feedback to promote risk awareness and to accelerate smoking behaviour change (Curry 1993; Miller 1991). Recognition of personal susceptibility to the adverse effects of smoking may be an important step in the pathway to smoking cessation (McClure 2001; Weinberger 1981; Young 2010). Indeed, most theories related to health behaviour changes (e.g. self‐determination theory, theory of planned behaviour, health belief model, transtheoretical model, social cognitive theory, social ecological model, common‐sense model) have in common that they recognise an important role to the person's understanding of the potential negative consequences of a given behaviour (Hale 2007; Joseph 2016).
Why it is important to do this review
Individual studies have produced conflicting data on the effect of physiological feedback (Bovet 2002; Buffels 2006; Lerman 1993; McBride 2000; Parkes 2008; Rodondi 2012). This review systematically examined data on smoking cessation rates from randomised controlled trials using feedback on the physiological effect of smoking or on genetic susceptibility to smoking‐related diseases.
Objectives
The main objective was to determine the efficacy of providing smokers with feedback on their exhaled carbon monoxide (CO) measurement, spirometry results, atherosclerotic plaque imaging, and genetic susceptibility to smoking‐related diseases in helping them to quit smoking.
The hypotheses to be examined were as follows.
Feedback on personal characteristics indicating that the effects of smoking, or susceptibility to smoking‐related illness, increases rates of smoking cessation.
Multiple types of measurement (e.g. spirometry and exhaled CO measurement used together) are more effective for smoking cessation than a single form of measurement.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials.
Types of participants
We included studies of any individuals who smoked and who participated in smoking cessation programmes, or in screening for respiratory disease, or in health checkups. There were no restrictions on participant age, motivation to quit, or whether participants were pregnant, were hospitalised, or were suffering from coexistent illness.
Types of interventions
We included studies of any intervention in which a physical measurement, such as exhaled carbon monoxide (CO), spirometry, atherosclerotic plaque imaging, or genetic testing, was used as a way to increase smoking cessation rate. We considered studies in which reporting of these measurements was the only component of an intervention, or was tested as an adjunct to another intervention such as counselling, where the control group received all other components except for the reporting of such measurements. We excluded trials in which the effect of biological measurements was confounded by the use of other components in the active intervention.
Types of outcome measures
The main outcome measure was abstinence from smoking, measured at least six months after the start of the intervention. We used the most conservative measure of quitting at the longest follow‐up, preferring biochemically validated results where available. We counted participants lost to follow‐up as continuing smokers. We excluded studies that did not provide data on cessation but instead measured intermediate outcomes such as withdrawal symptoms.
Search methods for identification of studies
For this update we searched the Cochrane Tobacco Addiction Group Specialized Register on 27 March 2018 and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) on 3 September 2018 for studies that involved any use of a biomedical test as part of an intervention. At the time of the search, the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), Issue 1, 2018; MEDLINE (via Ovid) to update 20180209; Embase (via Ovid) to week 201807; and PsycINFO (via Ovid) to update 20180212. See the Cochrane Tobacco Addiction Group website for full search strategies and a list of other resources.
We used the following topic‐related keywords and text terms to identify potentially relevant studies:
patient education, patient compliance, patient counselling, persuasive communication, spirometry, respiratory function, bronchospirometry, carbon monoxide, forced expiratory flow rates, obstructive lung diseases, genetic testing, genetic susceptibility, genetic predisposition, biomarker, feedback.
See Appendix 1 for the full strategy.
Data collection and analysis
Two review authors (out of CC, RB, YM, KS) independently prescreened all search results (abstracts) for possible inclusion or as useful background. Studies were selected for full‐text assessment if retained by at least one of the review authors. Two review authors (out of CC, RB, YM, KS) then independently assessed the selected articles for inclusion, resolving any discrepancies by consensus. We have recorded reasons for the non‐inclusion of studies in the Characteristics of excluded studies table.
Each review author then extracted and compared the data from the selected studies. This stage included an evaluation of risk of bias. Four review authors independently assessed each study according to the presence and quality of the randomisation process, whether or not trialists and assessors were 'blinded', whether the analysis was appropriate to the study design, and the description of withdrawals and dropouts. We used Covidence for the screening and data extraction (Covidence).
We extracted data, where available, on the following.
Country and setting (e.g. primary care, community, hospital outpatient/inpatient)
Recruitment
Method of selection of participants (e.g. willingness to make a quit attempt)
Definition of smoker used
Method of randomisation
Allocation concealment
Smoking and demographic characteristics of participants (e.g. average age, sex, average number of cigarettes smoked per day)
Description of the experimental and control interventions (provider, length, number of visits, etc.)
Outcomes, including definition of abstinence used, and biochemical validation of cessation
Proportion of participants with follow‐up data
Whether or not data were analysed on an intention‐to‐treat basis
Appropriateness of statistical approach
Declaration of interest and source of funding
Data synthesis
We expressed results as a risk ratio (RR) for smoking cessation with 95% confidence intervals (CI). Where appropriate, we pooled studies using a Mantel‐Haenszel random‐effects method. We assessed heterogeneity using the I2 statistic.
'Summary of findings' table
We created a 'Summary of findings' table for our primary outcome in accordance with standard Cochrane methodology. We assessed the certainty of evidence using the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias).
Results
Description of studies
Results of the search
For this update we found 1096 records for screening, from which we identified five new trials, for a total of 20 trials of 9262 people that met our inclusion criteria. The flow of studies is reported in a PRISMA flow diagram (Figure 1). We found 15 studies that were potentially eligible and either ongoing (ACTRN12618000291280; IRCT2017080435257N1; NCT02658032; NCT02840513; NCT02991781; NCT03521141; NCT03583203), or finished but with no published data and no information retrievable through contacting study authors (Martin Lujan 2011; Martin Lujan 2016; NCT01186016; NCT02431611; NCT03377738; Pita Fernandez 2015; Ripoll 2012; Muhammad 2015). Details of these studies can be found in the Characteristics of ongoing studies table. One study was ongoing, but there was insufficient information to assess its eligibility, therefore we assessed it as awaiting classification (NCT02351167).
1.
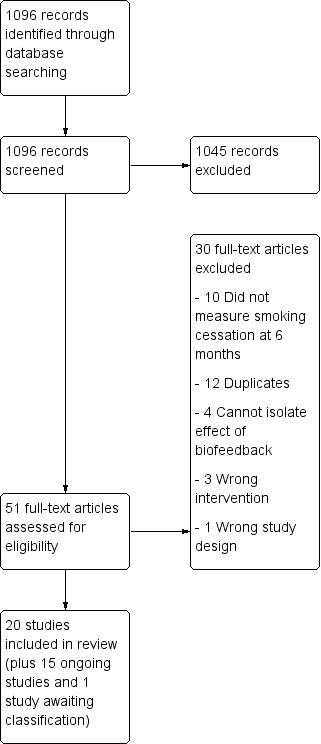
Study flow diagram for 2019 update.
Included studies
One included study tested two interventions (Audrain 1997). Out of the 21 interventions, five tested feedback on smoking exposure, each measuring the effect of exhaled CO measurements (Audrain 1997; Brunette 2013; Jamrozik 1984; Sanders 1989; Shahab 2011). Five studies tested feedback on smoking‐related disease risk; of these, four tested feedback about genetic susceptibility to cancer (Audrain 1997; Hishida 2010; Ito 2006; Nichols 2014), and one tested feedback about genetic susceptibility to Crohn's disease (Hollands 2012). In Audrain 1997, the intervention was feedback about genetic susceptibility combined with CO measurement, which could either be compared to a control group of CO measurement alone, or to a control group without biomarker feedback, thereby testing the combination of the two interventions. Eleven studies assessed feedback on smoking‐related harm: four tested the combination of exhaled CO measurement and spirometry (McClure 2009; Risser 1990; Sippel 1999; Walker 1985); five tested the effect of spirometry alone (Buffels 2006; Irizar Aramburu 2013; Segnan 1991), or with the addition of feedback on lung age (Drummond 2014; Parkes 2008); two tested the effect of undergoing an ultrasonography of carotid arteries (and femoral arteries for Bovet 2002) with photographic demonstration of atherosclerotic plaques when present (Bovet 2002; Rodondi 2012).
The trials were conducted in different settings: 11 trials took place in general practice (Buffels 2006; Irizar Aramburu 2013; Jamrozik 1984; Nichols 2014; Parkes 2008; Sanders 1989; Segnan 1991), or outpatient clinics (Bovet 2002; Ito 2006; Rodondi 2012; Sippel 1999); one study took place in the community, recruiting first‐degree relatives of probands with Crohn's disease (Hollands 2012); two took place in smoking cessation clinics (Audrain 1997; Walker 1985); one was conducted in a health promotion clinic for army veterans (Risser 1990); one was conducted in a mental health clinic (Brunette 2013); one was conducted in a company (Hishida 2010); and three took place in research institutions (Drummond 2014; McClure 2009; Shahab 2011). Seven trials took place in the USA (Audrain 1997; Brunette 2013; Drummond 2014; McClure 2009; Risser 1990; Sippel 1999; Walker 1985), six in the UK (Hollands 2012; Jamrozik 1984; Nichols 2014; Parkes 2008; Sanders 1989; Shahab 2011), two in Japan (Hishida 2010; Ito 2006), and one trial each took place in Italy (Segnan 1991), Belgium (Buffels 2006), the Seychelles Islands (Bovet 2002), Switzerland (Rodondi 2012), and Spain (Irizar Aramburu 2013).
Methods of recruitment were heterogeneous between studies. Among the six studies conducted in general practice, one recruited patients at their first visit (Jamrozik 1984); another screened outpatients on specific days (Segnan 1991); two screened patients during the recruitment period (Buffels 2006; Sanders 1989); and two invited known smokers by post (Parkes 2008; Nichols 2014). One study recruited smokers among outpatients in primary care clinics (Sippel 1999); one study recruited outpatients at a cancer centre hospital (Ito 2006); and one study recruited patients referred to a mental health treatment clinic (Brunette 2013). Four studies recruited smokers by media advertisement (Audrain 1997; Rodondi 2012; Shahab 2011; Walker 1985). The remaining studies recruited smokers participating in a health survey (Bovet 2002); employees who identified themselves as smokers in a questionnaire used for an annual workplace checkup (Hishida 2010); veterans that responded to a mailed invitation to attend a health promotion clinic (Risser 1990); and participants of a cohort study of people with a history of injecting drugs (Drummond 2014). Two studies combined diverse methods of recruitment. One study used media advertisement, outpatient recruitment, data from health plan records, a quitline register, and a purchased e‐mail list of smokers (McClure 2009), and another asked probands to identify first‐degree relatives through three routes, first approaching probands receiving care through hospital services, secondly contacting them by mail using Crohn's disease databases at 42 participating hospitals, and finally placing advertisements in the newsletters of associations (Hollands 2012). One study did not specify the method of recruitment (data were obtained after contacting the study author; the study is not yet published) (Irizar Aramburu 2013). Participation rates (i.e. the proportion of those approached who agreed to take part in the trial) were seldom recorded.
All studies included male and female adults who were smokers at the time of inclusion. Only 10 studies provided a definition of being a smoker at the time of inclusion (Audrain 1997; Bovet 2002; Drummond 2014; Hollands 2012; Irizar Aramburu 2013; Ito 2006; McClure 2009; Nichols 2014; Rodondi 2012; Shahab 2011). The mean age of the participants when given varied between 31.7 and 53.0 years. The proportion of women in the trials varied between 4% and 65%. The mean number of cigarettes smoked per day varied between 11.9 and 29.2. The mean number of cigarettes smoked per day tended to be highest in the trials set in a smoking cessation clinic (29.2 per day in Walker 1985 and 22.7 per day in Audrain 1997) or among veterans (23.5 per day in Risser 1990). Levels of nicotine addiction as assessed by the Fagerström score were provided in three studies (Heatherton 1991), and ranged from 3.5 to 5.4 (Drummond 2014; Nichols 2014; Rodondi 2012); proportions of participants in the various stages of change according to Prochaska and Di Clemente were only provided in five studies (Prochaska 1983), with the proportion of participants in the preparation stage ranging from 17% to 37.5% (Audrain 1997; Ito 2006; McClure 2009; Parkes 2008; Sippel 1999).
The therapist delivering the intervention was a physician in five trials (Bovet 2002; Buffels 2006; Irizar Aramburu 2013; Jamrozik 1984; Segnan 1991); a nurse in four trials (Hishida 2010; Risser 1990; Rodondi 2012; Sanders 1989); a specific study staff member in seven trials (Audrain 1997; Drummond 2014; Ito 2006; Parkes 2008; Shahab 2011; Sippel 1999; Walker 1985); a trained health educator or research counsellor in two trials (Hollands 2012; McClure 2009); the principal investigator with help from trained smoking cessation practitioners in one study (Nichols 2014); and the intervention and feedback was web based in one study (Brunette 2013). Further details on the included studies can be found in the Characteristics of included studies tables.
Excluded studies
For this update, we excluded 31 studies at full‐text screening, and listed a total of 61 excluded studies. The primary reasons for exclusion were because the effect of the biomedical assessment could not be evaluated separately from the rest of the cessation intervention; the study had less than six months follow‐up; or the study was not a true randomised trial. The excluded studies along with reasons for their exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
We judged all but three included studies to be at high or unclear risk of bias in at least one domain (Parkes 2008; Rodondi 2012; Segnan 1991). 'Risk of bias' judgements for each study are displayed in Figure 2.
2.
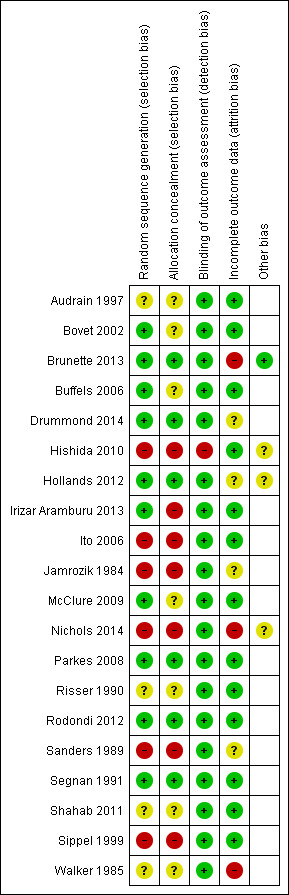
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only six of the 20 included trials reported an adequate procedure for both randomisation and allocation concealment (Brunette 2013; Drummond 2014; Hollands 2012; Parkes 2008; Rodondi 2012; Segnan 1991). Eight studies did not report the method of randomisation, or provided insufficient information to assume that allocation was adequately concealed (Audrain 1997; Bovet 2002; Buffels 2006; McClure 2009; Nichols 2014; Risser 1990; Shahab 2011; Walker 1985). Five studies reported inadequate allocation sequences, with allocation by day, week, or month of attendance (Hishida 2010; Ito 2006; Jamrozik 1984; Sanders 1989), or by odd‐/even‐numbered questionnaire at the time of check‐in (Sippel 1999). In one of these studies, 120 participants were allocated to the wrong group and were excluded from further analysis (Sanders 1989).
Due to the demonstrative nature of the intervention, participants and those delivering the intervention could not be blinded to allocation, therefore we did not assess performance bias, and assessed detection bias independent of blinding. We judged the risk of detection bias to be low if abstinence was biochemically verified, or if the intervention and control groups received similar amounts of face‐to‐face contact. If abstinence was not biochemically verified and the intervention group received more face‐to‐face contact than the control group, then we judged the risk of detection bias to be high because the results may be prone to differential misreport. We judged only one study to be at high risk of detection bias based on these criteria (Hishida 2010); we assessed the remaining studies as at low risk of bias.
Three trials used urinary cotinine level to validate smoking cessation at follow‐up (Parkes 2008; Sanders 1989; Segnan 1991). One study used the same validation procedure but only on a subsample of self‐reported ex‐smokers (Jamrozik 1984). Two studies used expired air carbon monoxide (Risser 1990; Walker 1985). One study used serum cotinine level (Rodondi 2012); one study used saliva cotinine (Hollands 2012); and two studies used both exhaled CO and salivary cotinine (Drummond 2014; Nichols 2014). Nine studies did not use any biochemical validation (Audrain 1997; Bovet 2002; Brunette 2013; Buffels 2006; Hishida 2010; Ito 2006; McClure 2009; Shahab 2011; Sippel 1999). Only seven studies explicitly mentioned that assessors were blinded to allocation at the time of outcome determination (Bovet 2002; Brunette 2013; Parkes 2008; Risser 1990; Rodondi 2012; Shahab 2011; Sippel 1999).
In three studies (Audrain 1997; Nichols 2014; Walker 1985), it was not possible to determine the initial allocation of the participants who were subsequently lost to follow‐up, and the analysis had to be performed per protocol. This may have overestimated cessation rates for the individual studies, but impact on meta‐analysis results were probably limited given the limited number of participants in those three studies. In one study (Hishida 2010), those who were allocated to the intervention group but who subsequently declined to participate in biomarker testing were included in the baseline characteristics table, but were excluded from further analyses.
Effects of interventions
See: Table 1
Results are given as risk ratios (RRs) for smoking cessation at the latest recorded follow‐up time (six to 12 months) between intervention and control groups, with 95% confidence intervals (CI). An RR greater than one favours the intervention group. We classified trials into three analyses by type of intervention: those that provided feedback on smoking exposure, those that provided feedback on smoking‐related disease risk, and those that provided feedback on smoking‐related harms. Results of the analyses are reproduced in Figure 3, Figure 4, and Figure 5.
3.
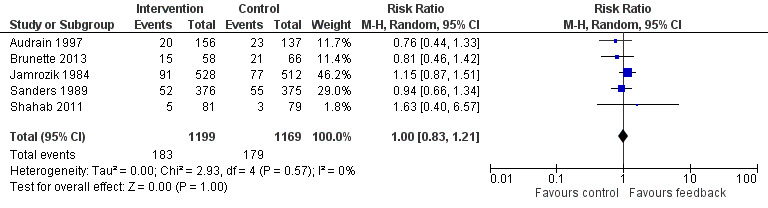
Forest plot of comparison: 1 All interventions, outcome: 1.1 Smoking cessation ‐ feedback on smoking exposure.
4.
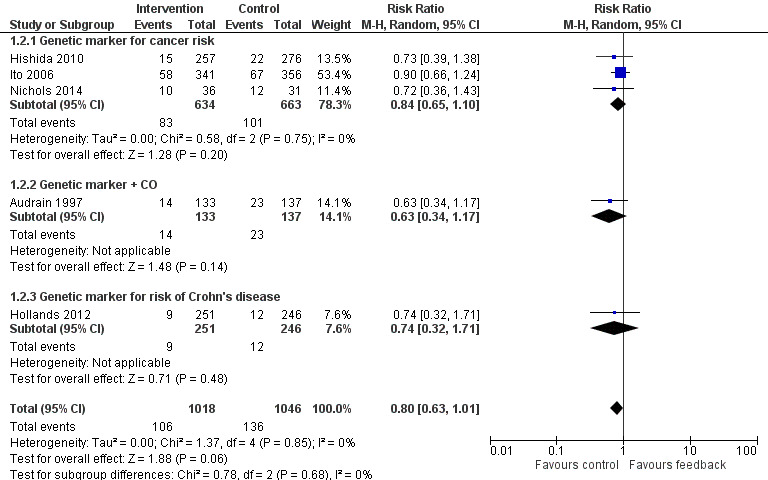
Forest plot of comparison: 1 All interventions, outcome: 1.2 Smoking cessation ‐ feedback on smoking‐related disease risk.
5.
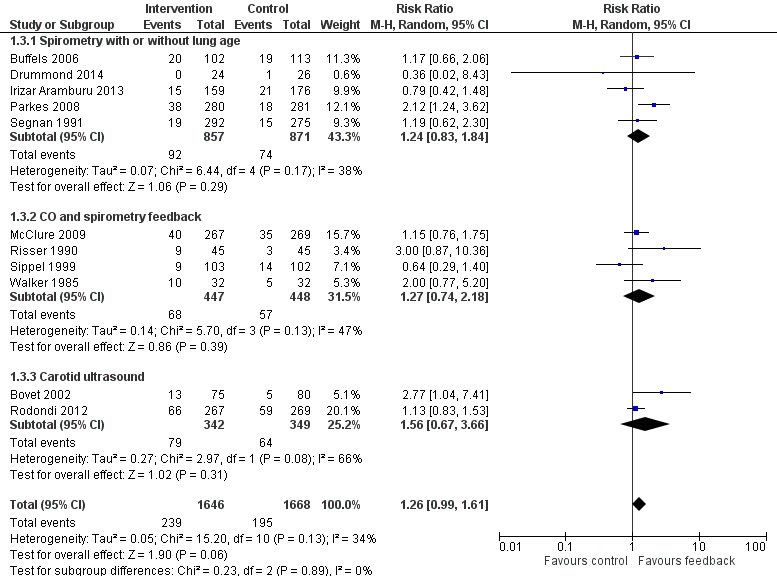
Forest plot of comparison: 1 All interventions, outcome: 1.3 Smoking cessation ‐ feedback on smoking‐related harm.
Feedback on smoking exposure
Five studies isolated the effect of demonstrating levels of exhaled CO on smoking cessation rate (Audrain 1997; Brunette 2013; Jamrozik 1984; Sanders 1989; Shahab 2011). There was no evidence of a significant benefit from these pooled studies (RR 1.00, 95% CI 0.83 to 1.21; I2 = 0%; n = 2368; Analysis 1.1; Figure 3).
1.1. Analysis.
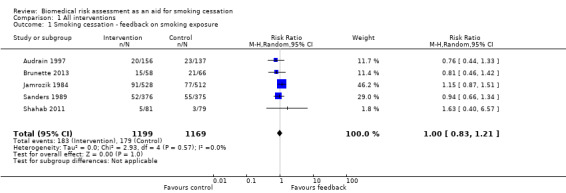
Comparison 1 All interventions, Outcome 1 Smoking cessation ‐ feedback on smoking exposure.
Feedback on smoking‐related disease risk
Three trials studied measurements of genetic susceptibility to smoking‐related cancer (Hishida 2010; Ito 2006; Nichols 2014), and one measured the genetic susceptibility to Crohn's disease (Hollands 2012). We pooled all five trials and found no evidence of a significant benefit from these pooled studies (RR 0.80, 95% CI 0.63 to 1.01; I2 = 0%; n = 2064; Analysis 1.2; Figure 4).
1.2. Analysis.
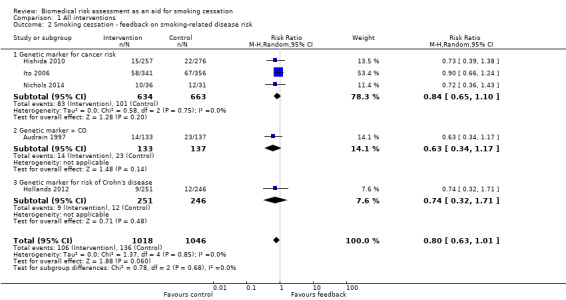
Comparison 1 All interventions, Outcome 2 Smoking cessation ‐ feedback on smoking‐related disease risk.
We pooled the three trials testing feedback on the genetic susceptibility to smoking‐related cancers and found no evidence of a significant benefit from these pooled studies (RR 0.84, 95% CI 0.65 to 1.10; I2 = 0%; n = 1297; Analysis 1.2.1). Hollands 2012 did not detect a benefit from feedback on genetic susceptibility to Crohn's disease (in relatives of individuals suffering from Crohn's disease) (RR 0.74, 95% CI 0.32 to 1.71; n = 497).
Feedback on smoking‐related harms
Eleven studies provided feedback on smoking‐related harm. Pooling all 11 studies narrowly missed a significant effect on smoking cessation (RR 1.26, 95% CI 0.99 to 1.61; I2 = 34%; n = 3314; Analysis 1.3; Figure 5). However, a sensitivity analysis removing the three studies at high risk of bias did detect an effect (RR 1.36, 95% CI 1.07 to 1.74; I2 = 24%; n = 2710; analysis not shown).
1.3. Analysis.
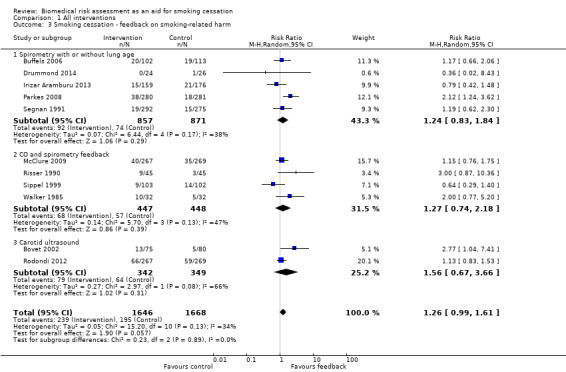
Comparison 1 All interventions, Outcome 3 Smoking cessation ‐ feedback on smoking‐related harm.
Five studies provided feedback based on spirometry results (Buffels 2006; Drummond 2014; Irizar Aramburu 2013; Parkes 2008; Segnan 1991). There was no evidence of a significant benefit from spirometry with or without feedback on lung age when the five studies were pooled (RR 1.24, 95% CI 0.83 to 1.84; I2 = 38%; n = 1728; Analysis 1.3.1). Four trials used exhaled CO measurement and spirometry together (McClure 2009; Risser 1990; Sippel 1999; Walker 1985). None of these trials detected a significant effect independently, and there was no evidence of a significant benefit when these studies were pooled (RR 1.27, 95% CI 0.74 to 2.18; I2 = 47%; n = 895; Analysis 1.3.2). Two trials tested ultrasonography of carotid (and femoral) arteries (Bovet 2002; Rodondi 2012). There was no evidence of a significant benefit from these pooled studies (RR 1.56, 95% CI 0.67 to 3.66; I2 = 66%; n = 691; Analysis 1.3.3).
Multiple versus single forms of measurement
Only one study directly compared multiple forms of measurement with a single form of measurement (Audrain 1997). The study did not detect a significant difference in effect between measurement of CO plus genetic susceptibility to lung cancer and measurement of CO only (RR 0.82, 95% CI 0.43 to 1.56; n = 189; Analysis 2.1). The other included studies were too clinically heterogenous to allow indirect comparisons of multiple versus single forms of measurement.
2.1. Analysis.
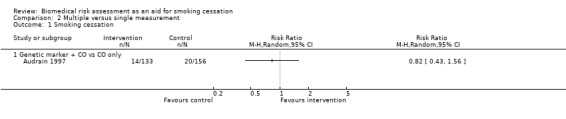
Comparison 2 Multiple versus single measurement, Outcome 1 Smoking cessation.
Discussion
Summary of main results
Due to the scarcity of evidence of sufficient quality, we cannot make definitive statements about the effectiveness of biomedical risk assessment as an aid for smoking cessation. Most studies were small and did not detect significant effects. We pooled studies based on the type of feedback they provided. The most promising results relate to spirometry and carotid ultrasound, where moderate‐certainty evidence, limited by imprecision and risk of bias, did not detect a statistically significant benefit, but confidence intervals very narrowly missed one, and the point estimate favoured the intervention. A sensitivity analysis removing those studies at high risk of bias did detect a benefit. Moderate‐certainty evidence limited by risk of bias did not detect an effect of feedback on smoking exposure by CO monitoring. Low‐certainty evidence, limited by risk of bias and imprecision, did not detect a benefit from feedback on smoking‐related risk by genetic marker testing. There is insufficient evidence with which to evaluate the hypothesis that multiple types of assessment are more effective than single forms of assessment. Results are summarised in Table 1.
Only two studies detected statistically significant intervention effects. One of these was a trial in primary care that used spirometry and immediate feedback of the results using a graphical display (Parkes 2008). Intervention participants were told their "lung age" if this was older than their chronological age. The control group also had spirometry and feedback, but the results were given by letter in terms of standard measures of lung function, with no mention of lung age. All participants were advised to quit and offered a referral to intensive support. Contact time was longer in the intervention group because of the verbal feedback. Giving the spirometry results in terms of lung age resulted in an increase in the cessation rate at 12 months from 6.4% to 13.6%. In considering the strength of evidence and generalisability of this trial, it should also be noted that the outcome was limited to point‐prevalence abstinence, and information was lacking about the comparability of the study sample with the entire recruitment population. Another study, retrieved in the current update, also assessed spirometry with lung age feedback (compared with spirometry results in a standardised written format) but in a research cohort of intravenous drug users (Drummond 2014). The sample size was small (50 participants), and the study did not show a significant benefit of this approach.
The other study with a statistically significant positive effect used pictures of ultrasound photographs of atherosclerotic plaques (Bovet 2002). Participants were randomised to undergo ultrasonography (n = 74) or not (n = 79). Those with ultrasonographic demonstration of atherosclerotic plaques (n = 20) were given photographs of their plaques with a relevant explanation, whereas the others did not benefit from any further procedure. This study's external validity can be questioned, as the sample was made up predominantly of male light smokers (average 10 to 12 cigarettes a day). Another study using a similar feedback method had a larger sample (536 smokers), a larger proportion of women (45%), and heavier smokers as participants (an average of 20 cigarettes smoked per day) (Rodondi 2012). This study did not show a statistically significant effect of the intervention. The authors noted in their discussion that they included only smokers with a motivation to quit and that they used a very intensive smoking cessation programme in both the intervention and control groups.
While the evidence does not directly support the use of biomedical risk assessment for smoking cessation, more research is needed before we can be confident in this result.
Overall completeness and applicability of evidence, and limitations of the review
Cochrane methods are designed to minimise bias in the review process. However, due to the nature of the intervention, there were some difficulties in finding suitable evidence. In most of the included studies, the biomedical testing component was added to intensive quit‐smoking sessions, with counselling lasting up to 60 minutes and completed by written material and reinforcement sessions or follow‐up phone calls. The incremental effect of biomedical risk assessment might have been diluted by the high intensity of the 'standard' care used. It is also possible that the changes in motivational stages induced by biomedical risk assessment are too subtle to be characterised as directly leading to a successful quit attempt. Data from those studies do not permit verification of this hypothesis. However, given the increasing evidence that the transtheoretical model of behaviour change is not a mandatory prerequisite for a smoking cessation intervention to be effective (Cahill 2010; Riemsma 2003), we do not consider this lack of intermediate data to be a major concern.
Biomedical risk assessment was also conceptualised as a part of multicomponent interventions (with components other than biomedical risk assessment) by some authors of studies identified by our search strategy (Borrelli 2002; Borrelli 2005; Ferketich 2012; Hajek 2002; Humerfelt 1998; Kotz 2009; Martucci 2010; McBride 2002; McClure 2013; Prokhorov 2008; Richmond 1986). Even though four of these studies demonstrated effects significantly favouring intervention versus control groups, they did not isolate the specific effect of biomedical feedback. The retrieved studies also did not provide us with sufficient data to adequately examine our second research hypothesis, that feedback with different types of measurements is more effective for smoking cessation than feedback of a single measurement.
Another possible explanation for the absence of effectiveness of biomedical risk assessment provided in addition to counselling could be the potentially counterproductive effect of communicating normal results to smokers. Six included studies provided some insight about smoking cessation rates in subgroups according to test results. Sippel 1999, Buffels 2006, and Parkes 2008 did not find any correlation between smoking cessation and abnormal spirometry results. Bovet 2002, which overall detected significantly higher cessation rates in participants offered ultrasonography, found a statistically non‐significant lower smoking cessation rate among the subgroup of participants without plaques at ultrasonography than among participants in the control group. Rodondi 2012 found that, in the ultrasonography group, cessation rates did not differ according to the presence or absence of atherosclerotic plaques. Parkes 2008 noted that anecdotally some smokers were motivated by having normal results because it helped them feel it was "not too late" to quit. Ito 2006 found some evidence that among participants without cancer, those told they had increased susceptibility to cancer were more likely to quit. This particular question, and the way to handle and communicate normal results, has yet to be answered.
Trials demonstrating smoking‐related harms using spirometry (especially when combined with lung age) or carotid ultrasound tended to show a stronger effect on smoking cessation, though this effect was not significant. This could be explained by the fact that direct and concrete demonstration of manifest harm might be more likely to motivate smokers to quit smoking, according to the Leventhal Common Sense Model (Hale 2007). It might be more straightforward for a smoker to establish a causal link between smoking and a direct and "noticeable" harm such as lung damage (increased lung age) or carotid plaques. Inversely, feedback on smoking exposure, using indirect and more obtuse measures such as CO, might not be sufficient to motivate a change in behaviour. Genetic feedback might be even more difficult to understand and counterproductive in the sense that people might believe that risks are not modifiable due to the deterministic aspect of genetics.
Two other trials used demonstration of smokers' children's exposure to environmental tobacco smoke by measuring the child's urinary cotinine level (abstinence was a secondary outcome) and demonstration of Latino caregivers' asthmatic children's exposure to secondhand CO (Borrelli 2010; Wakefield 2002), with RRs of 0.26 (95% CI 0.02 to 2.33) and 0.90 (95% CI 0.72 to 1.12), respectively. A third study evaluated the efficacy of personalised feedback during foetus ultrasound on pregnant smokers (Stotts 2009), and again did not detect a benefit (RR 1.31, 95% CI 0.66 to 2.58). We excluded these studies from our analysis because our research hypothesis was that smokers, although adept at estimating the risks of smoking in terms of morbidity and mortality, are prone to underestimate their own risk with regard to smoking (Lerman 1993; Romer 2001). It therefore seemed to us that providing biomarker feedback about someone else's health (even one's own children or foetus) would act differently and may not contribute to counteracting this personal optimistic bias. In any event, these trials did not detect benefits for the interventions tested.
A promising new approach could be for dental practitioners to use a point‐of‐care test for salivary nicotine metabolites. A short‐term randomised controlled trial compared smoking cessation after eight weeks among participants who received either counselling with immediate or delayed (at the end of the study) feedback on salivary nicotine level (Barnfather 2005). The risk ratio for smoking cessation is close to being statistically significantly in favour of those who received immediate feedback: RR of 3.40 (95% CI 1.00 to 11.61). Trials with longer‐term follow‐up are needed to confirm this effect (Coleman 2005).
Despite broad inclusion criteria regarding the type of participants, we found only scarce data exploring the effect of biomedical risk assessment on hospitalised patients or acutely ill individuals. It is possible that such a specific context and the presence of coexistent illnesses could facilitate a modification of risk perception. One study included participants both with and without cancer (Ito 2006). Reported subgroup analyses did not detect benefits either for participants with cancer (RR 0.65, 95% CI 0.43 to 1.02) or for participants without cancer (RR 1.22, 95% CI 0.63 to 2.36).
Some recent studies used connected devices such as mobile‐phone applications (ACTRN12618000291280; NCT02840513; NCT03583203), or provided web‐based feedback (Brunette 2013). Biofeedback on lung damage or risk of lung cancer, or both, is a well‐represented intervention in ongoing studies (NCT02658032; NCT03521141; NCT03583203).
Certainty of the evidence
The certainty of the evidence using GRADE criteria was low to moderate, depending on feedback category. The evidence on interventions providing feedback on risk exposure was of moderate certainty, limited by risk of bias because three of the five studies were at high risk of bias, and the remaining studies were at unclear risk of bias. The evidence on interventions providing feedback on smoking‐related disease risk was of low certainty, limited by risk of bias and imprecision because fewer than 300 events were recorded. The evidence on interventions providing feedback on smoking‐related harm through spirometry or carotid ultrasound was of moderate certainty, limited by imprecision and risk of bias because the confidence intervals only narrowly missed a statistically significant benefit, and a sensitivity analysis excluding studies at high risk of bias did detect one.
Agreements and disagreements with other studies or reviews
An earlier non‐systematic review was conducted on the use of biomarkers in smoking cessation (McClure 2001). This work aimed to review the theoretical rationale and empirical evidence regarding this practice, and therefore did not specifically focus on the assessment of the efficacy of biomarker feedback as a way to increase rates of long‐term smoking cessation. The review therefore included non‐randomised trials (Haddow 1991; Kilburn 1990; Loss 1979; Scott 1990); trials providing multicomponent interventions that precluded the isolation of the specific effect of biomarker feedback (Bauman 1983; Lerman 1997; Richmond 1986); trials comparing the effect of abnormal test results versus normal test results rather than test versus no test (Li 1984); and trials reporting outcomes other than smoking cessation. One study identified by McClure as "in press" appears never to have been published (Hoffman 1998), and we were unable to obtain further information despite several attempts to contact the authors. Four studies mentioned by McClure were also included in our review (Audrain 1997; Jamrozik 1984; Risser 1990; Walker 1985). Our review includes 16 trials not in the McClure review (Bovet 2002; Brunette 2013; Buffels 2006; Drummond 2014; Hishida 2010; Hollands 2012; Irizar Aramburu 2013; Ito 2006; McClure 2009; Nichols 2014; Parkes 2008; Rodondi 2012; Sanders 1989; Segnan 1991; Shahab 2011; Sippel 1999). When focusing on efficacy data, McClure 2001 concluded that biomarker feedback may enhance the likelihood of cessation because a trend for increased abstinence was found in three randomised trials (Hoffman 1998; Risser 1990; Walker 1985). The fact that two of these trials, Walker 1985; Risser 1990, are subject to major methodological limitations (small samples, inadequate randomisation procedures), and that the report of Hoffman 1998 remains unpublished, calls for great caution in drawing such conclusions.
Authors' conclusions
Implications for practice.
We found little evidence about the effects of biomedical risk assessment as an aid for smoking cessation. However, the current evidence base does not support the use of biomedical risk assessment as an aid for smoking cessation. The most promising results relate to spirometry and carotid ultrasound, where moderate‐certainty evidence, limited by imprecision and risk of bias, did not detect a statistically significant benefit, but confidence intervals very narrowly missed one, and the point estimate favoured the intervention. A sensitivity analysis removing those studies at high risk of bias did detect a benefit. Moderate‐certainty evidence limited by risk of bias did not detect an effect of feedback on smoking exposure by carbon monoxide monitoring. Low‐certainty evidence, limited by risk of bias and imprecision, did not detect a benefit from feedback on smoking‐related risk by genetic marker testing. There is insufficient evidence with which to evaluate the hypothesis that multiple types of assessment are more effective than single forms of assessment.
Implications for research.
There is room for improvement in the methodological quality of studies aimed at evaluating the efficacy of biomedical risk assessment as an aid to smoking cessation. In particular, further studies assessing feedback on smoking‐related harm could be more beneficial than those assessing feedback on smoking exposure or smoking‐related disease risk. Future studies should aim to have adequate sample sizes; conceal allocation following the randomisation procedure; use a standard definition of a smoker and of abstinence from smoking; systematically use biochemical validation of smoking abstinence; and include of all those lost to follow‐up in the denominator of each group (on an intention‐to‐treat basis).
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2019 | Amended | Addition to acknowledgments |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 16 November 2018 | New citation required but conclusions have not changed | New searches run: 27 March 2018. Conclusions unchanged. |
| 15 October 2018 | New search has been performed | Updated, 5 new studies included (1 with incomplete data), conclusions unchanged |
| 20 August 2012 | New citation required but conclusions have not changed | Updated with 4 new included studies, conclusions unchanged |
| 20 August 2012 | New search has been performed | Updated search run, 4 new studies included, text updated |
| 28 January 2009 | New citation required and conclusions have changed | 1 new study shows a significant effect. |
| 27 January 2009 | New search has been performed | 3 new studies identified in update for Issue 2, 2009. |
| 21 April 2008 | Amended | Converted to new review format |
| 11 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Olivier Terraz and Thomas Brauchli of the Center for Primary Care and Public Health for their assistance in retrieving and selecting references identified by our search strategy, and Alvine Bissery of the same institution for her statistical expertise on earlier drafts of this review. We also thank Jon Britton and Jonathan Foulds for helpful suggestions on the protocol, and Andy McEwen and Lion Shahab for constructive comments on earlier drafts of this review. Our thanks also to Lee Bromhead and Sandra Wilcox for reviewing our plain language summary.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. Specialized Register search strategy
For Cochrane Register of Studies
#1 patient education:EMT,MH,KW,TI,AB #2 patient compliance:EMT,MH,KW #3 patient counsel?ing:EMT,MH,KW,TI,AB #4 persuasive communication:EMT,MH,KW #5 spirometry:EMT,MH,KW,TI,AB #6 respiratory function:EMT,MH,KW #7 bronchospirometry:EMT,MH,KW,TI,AB #8 carbon monoxide:EMT,MH,KW,TI,AB #9 (forced expiratory volume or forced expiratory flow):EMT,MH,KW,TI,AB #10 FEV?:TI,AB #11 obstructive lung disease*:EMT,MH,KW,TI,AB #12 genetic testing:EMT,MH,KW #13 genetic susceptibility:EMT,MH,KW #14 genetic predisposition:EMT,MH,KW #15 (genetic NEAR (test* OR risk*)):TI,AB #16 biomarker*:EMT,MH,KW,TI,AB #17 screening:TI,AB #18 feedback:EMT,MH,KW,TI,AB #19 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18
Data and analyses
Comparison 1. All interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation ‐ feedback on smoking exposure | 5 | 2368 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2 Smoking cessation ‐ feedback on smoking‐related disease risk | 5 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.01] |
| 2.1 Genetic marker for cancer risk | 3 | 1297 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.10] |
| 2.2 Genetic marker + CO | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.34, 1.17] |
| 2.3 Genetic marker for risk of Crohn's disease | 1 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.71] |
| 3 Smoking cessation ‐ feedback on smoking‐related harm | 11 | 3314 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.99, 1.61] |
| 3.1 Spirometry with or without lung age | 5 | 1728 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.83, 1.84] |
| 3.2 CO and spirometry feedback | 4 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.74, 2.18] |
| 3.3 Carotid ultrasound | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.67, 3.66] |
Comparison 2. Multiple versus single measurement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Genetic marker + CO vs CO only | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. All interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spirometry and/or lung age | 5 | 1728 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.83, 1.84] |
| 1.1 Spirometry with lung age | 2 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.62, 5.05] |
| 1.2 Spirometry without lung age | 3 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.48] |
3.1. Analysis.
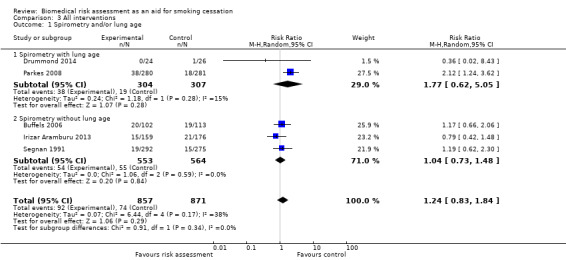
Comparison 3 All interventions, Outcome 1 Spirometry and/or lung age.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Audrain 1997.
| Methods | Setting: "smoking clinic", USA Recruitment: press Selected: advertisement: "free smoking‐cessation study" | |
| Participants | 550 smokers (defined as >= 5 cpd for >= 1 year) out of 1104 eligible; mean age 44 years, 62.8% female, 83.9% white, mean cpd: 22.7 Stage of change: Preparation stage: 37.5%, mean Fagerström score: 5.4 Therapist: trained health educator | |
| Interventions |
Control: QSC group: 60‐minute Quit‐Smoking Consultation (QSC) (quit plan, gaining support). Intervention 1: Exposure Biomarker (CO) Feedback (EBF) plus 10‐minute motivational counselling before QSC. Intervention 2: Susceptibility Biomarker (CYP2D6) Feedback plus 10‐minute motivational intervention plus EBF plus QSC |
|
| Outcomes | Definition of abstinence: 30‐day abstinence Duration of follow‐up: 12 months Biochemical validation of non‐smokers: none | |
| Identification | ||
| Notes | Source of funding: Grant ROI CA63562 from the National Institutes of Health, National Cancer Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised between 2 interventions and control, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence not biochemically validated, but similar amounts of face‐to‐face contact between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis, 33% lost to follow‐up. Distribution of baseline 550 participants among the 3 groups not reported. "There were no significant differences between treatment groups with respect to loss to follow‐up." |
Bovet 2002.
| Methods | Setting: Seychelles Heart Study II Recruitment: age‐ and sex‐stratified sample drawn from general population of Mahé, invited by letter to a cardiovascular risk factor survey Selected: last 155 participants to the Seychelles Heart Study II who reported smoking. | |
| Participants | 155 smokers (defined as >= 1 cpd during previous week); mean age 46 years, 15% female, mean cpd: 11.9 Therapist: physician | |
| Interventions | Intervention: ultrasonography of carotid and femoral arteries + quit‐smoking counselling. Smokers with >= 1 plaque given 2 photographs of their plaque plus explanation. Control: quit‐smoking counselling | |
| Outcomes | Definition of abstinence: 7‐day abstinence Duration of follow‐up: 6 months Biochemical validation of non‐smokers: none (assessor blinded) | |
| Identification | ||
| Notes |
Source of funding: This study was supported by the Ministry of Health of Seychelles, the University Institute of Social and Preventive Medicine of Lausanne (Switzerland), and the Division of Cardiology of the University Hospital of Lausanne. P Bovet benefited from a grant from the Swiss National Science Foundation (No. 3233‐038792.93). F Paccaud benefited from a grant from the Swiss National Science Foundation (No. 3233‐038792.93). F Paccaud benefited from grants from the "Fondation Vaudoise de Cardiologie" and the "Fondation Emma Muschamp" (Switzerland). Sonotron Ltd (Switzer‐products) provided the echographic system for the study, and Air Seychelles transported this equipment free of charge. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pre‐established random sequences of numbers matched to rank of arrival |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether allocation was concealed or not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation, but follow‐up assessors blinded to allocation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants lost to follow‐up, not included for analysis |
Brunette 2013.
| Methods |
Setting: large mental health treatment organisation, Chicago, USA Study design: randomised controlled trial Study grouping: parallel group Screened participants: 279 referred participants Included participants: 124 Intention‐to‐treat analyses: not specified |
|
| Participants |
Inclusion criteria: adult, English‐speaking, daily smoker, in treatment for severe mental illness (defined as mood or psychotic disorder with persisting functional disability) at the mental health program Exclusion criteria: current other substance dependence; used smoking cessation treatment to try to quit in the past month Baseline characteristics: mean age 46.5 years, 28.2% female, 48.4% African‐American, 15.0 mean number of cigarettes per day |
|
| Interventions |
Intervention characteristics Intervention: Decision support system (DSS) with CO
Control: DSS without CO
|
|
| Outcomes | Smoking cessation: 7‐day point‐prevalence abstinence at 2 and 6 months, self‐reported | |
| Identification |
Country: USA Setting: Thresholds, a large mental health treatment organisation in Chicago Author's name: Mary F Brunette, MD Institution: Geisel School of Medicine at Dartmouth and Dartmouth Psychiatric Research Center, Concord, NH 03301, USA Email: Mary.F.Brunette@Dartmouth.Edu Address: Geisel School of Medicine at Dartmouth College, 46 Centerra Parkway, EverGreen Center, Suite 315, Lebanon, NH 03766, USA |
|
| Notes | 6‐month outcome data by arm obtained directly from main author. Source of funding: This research is funded in part by the US Department of Education, National Institute on Disability and Rehabilitation Research and the Substance Abuse and Mental Health Services Administration, Center for Mental Health Services and Consumer Affairs Program, under Cooperative Agreement No. H133B100028. This research was also supported by the Bristol‐Myers Squibb Foundation. The views expressed in this manuscript do not reflect the policy or position of any federal agency. Declaration of interest: The authors do not have any competing interests to report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program (using blocks of 10) assigned participants to use the multicomponent decision support system with or without the CO feedback. |
| Allocation concealment (selection bias) | Low risk | Computer based. Research assistant adherence to the decision support system study visit protocol was assessed with a 10‐item checklist. Adherence to the visit protocol was high (mean score 9.6 out of 10) and did not differ between treatment groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence not biochemically validated, but similar amounts of face‐to‐face contact between groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants did not complete the 2‐month assessment. Numbers not completing 6‐month assessment not given. |
| Other bias | Low risk | 6‐month outcome not reported by allocation arm, but information was retrieved directly after contacting the authors. |
Buffels 2006.
| Methods | Setting: 14 general practices, Belgium Recruitment: screening of all attending patients above 15 years of age Selected: smokers in the motivation or action stage | |
| Participants | 215 randomised out of 1206 screened smokers. 182 analysed. Smoker definition not given. Mean age: 47.0, 47.3% female, mean pack‐years: 24.6 Therapist: physicians | |
| Interventions | Intervention: spirometry plus control intervention Control: minimal intervention based on the 5A model: ask, advise, assess, assist, arrange plus quit date plus NRT or bupropion plus follow‐up contact | |
| Outcomes | Definition of abstinence: continuous abstinence from quit date Duration of follow‐up: 12 months (24‐month data not used in analysis because of increasing loss to follow‐up) Biochemical validation of non‐smokers: none at the 12‐month follow‐up. Very partial at 24 months | |
| Identification | ||
| Notes | Additional data provided by author. Source of funding: This study was realised with an unconditional grant by Voorzorgskas voor Geneesheren, Brussels, Belgium. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by coin toss |
| Allocation concealment (selection bias) | Unclear risk | No statement that random sequence generated by coin toss was concealed at time of enrolment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation of abstinence requested, but few completed. Similar amounts of face‐to‐face contact between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 (13%) intervention, 20 (18%) control lost before 6 months, all losses included as smokers. |
Drummond 2014.
| Methods |
Setting: AIDS Linked to the Intravenous Experience (ALIVE) study, a prospective, longitudinal cohort of people with a history of injecting drugs, Baltimore, USA Study design: randomised controlled trial Study grouping: parallel group Comment: factorial design Duration of inclusion: from 16 March 2011 to 3 February 2012 = 1 year How sample size is determined: not specified Duration of follow‐up: 6 months Intention‐to‐treat analyses: yes Number of participants screened for eligibility: 265 Number of participants included: 100 |
|
| Participants |
Inclusion criteria: injection drug users (current and former) from ALIVE cohort; smoking at least 100 cigarettes in their lifetime as well as reporting any cigarette smoking in the last month; interested in involvement in a smoking cessation trial; ability to perform spirometry Exclusion criteria: current involvement in a smoking cessation programme; current use of nicotine replacement therapy or other smoking cessation pharmacological treatments (bupropion, varenicline) Definition of smokers: history of smoking at least 100 cigarettes in their lifetime and reporting any cigarette smoking in the last month Study population: residents of Baltimore with a history of injecting drugs Baseline characteristics (range of medians or proportions across arms)
|
|
| Interventions |
Intervention characteristics Experimental intervention A: lung age
Experimental intervention B: contingency management
Experimental intervention C: lung age plus contingency management
Control intervention: usual care
|
|
| Outcomes |
Smoking cessation: 7‐day point‐prevalence abstinence, validated
Smoking cessation: 7‐day point‐prevalence abstinence self‐reported |
|
| Identification |
Country: USA Setting: AIDS Linked to the Intravenous Experience (ALIVE) study, a prospective, longitudinal cohort of people with a history of injecting drugs followed in Baltimore, Maryland since 1988 Author's name: Michael B Drummond Institution: Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, MD, USA Email: mdrummo3@jhmi.edu Address: Johns Hopkins University, Baltimore, MD, USA |
|
| Notes |
Source of funding: This study was funded primarily by a Tobacco Dependence Research Fund from the American Thoracic Society (Principal Investigator: Drummond). This work was also supported in part by the National Institutes of Health (grants R01‐HL‐90483, R01‐DA‐04334, and R01‐DA‐12568). The funding sources had no role in study design, collection, analysis, or interpretation of data, writing the manuscript, or decision to submit for publication. Declaration of interest: All authors have no financial conflict of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was computer generated using a block randomisation approach with randomly ordered 4 and 8 sample blocks. |
| Allocation concealment (selection bias) | Low risk | 120 sequentially numbered, opaque, sealed envelopes that included random assignment to 1 of 4 interventions were externally prepared. Study staff were provided the next sequential envelope which assigned the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear |
Hishida 2010.
| Methods |
Setting: a bank in Japan Recruitment/selection: employees who indicated they were smokers on a questionnaire used at an annual workplace health checkup |
|
| Participants | All 562 eligible participants were included. No inclusion or exclusion criteria apart from being an employee of the bank and identifying as a smoker in the questionnaire. Smoker definition not given. 6.2% female. Stage of quitting smoking at initiation: "no concern" 20.3% in intervention group vs 17.0% in control group; "no intention" 73.4% vs 79.3%; "wish to quit" 5.9% vs 3.3%; "no answer" 0.3% vs 0.4% Therapist: public nurses |
|
| Interventions | All participants received a booklet on tobacco‐related diseases, concept of tobacco carcinogen susceptible genotypes, and benefits of smoking cessation. Intervention group: participants who agreed to genotype testing had a blood sample taken. The staff handed a report of the results with a written explanation to each of the participants at 3 months. In the reports, an explanation was attached that smoking elevates the risk of oesophageal cancer substantially among those with genotypes SS (OR = 8) and LS (OR = 7), while OR = 2 among those with LL genotype, and similar for lung cancer. The staff added general comments on the genotype for each participant, and provided specific comments when participants asked for details. 29 participants allocated to genotyping group refused genotype testing. They were later excluded from analysis. Control group: no further intervention |
|
| Outcomes | Abstinence assessed by postal questionnaire (no validation). Duration of follow‐up: 12 months No definition of abstinence provided. |
|
| Identification | ||
| Notes | New for 2012 update Source of funding: This study was supported in part by a Grant‐in‐Aid for Cancer Research from the Japanese Ministry of Health, Labour and Welfare. Declaration of interest: The authors have no conflicts of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random allocation by month of attendance |
| Allocation concealment (selection bias) | High risk | Allocation known at time of enrolment may lead to selection bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Abstinence not biochemically validated, and difference in face‐to‐face contact between intervention arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up (no response to follow‐up questionnaire) 16.1% in intervention group, 11.2% in control group. |
| Other bias | Unclear risk | A law against smoking in the workplace was issued in August 2002 in Japan, which may be a confounding factor. |
Hollands 2012.
| Methods |
Setting: family clusters of relatives of people with Crohn's disease Study design: cluster‐randomised controlled trial Study grouping: parallel group Duration of follow‐up: 6 months Duration of inclusion: April 2007 to September 2010 How sample size is determined: the sample size was originally set at 540 participants (270 per arm) with an anticipated follow‐up of 430 participants (projected 80% follow‐up rate). We allowed for clustering of participants in families by assuming an intracluster correlation no greater than 0.6 and a mean cluster size of 1.13 derived from a pilot study. Intention‐to‐treat analyses: yes Type of recruitment:
Consecutive recruitment: no Number of participants screened for eligibility: 1890 Number of participants included: 497 |
|
| Participants |
Inclusion criteria:
Exclusion criteria: having a diagnosis of Crohn's disease or ulcerative colitis Definition of smokers: smoking 5 or more cigarettes daily Baseline characteristics Intervention:
Control
|
|
| Interventions |
Intervention characteristics Intervention: DNA arm
Control: non‐DNA arm
|
|
| Outcomes |
Smoking cessation: 7‐day point‐prevalence abstinence validated
Smoking cessation: 7‐day point‐prevalence abstinence self‐reported |
|
| Identification |
Country: UK Setting: First‐degree relatives of probands with Crohn's disease Comments: NA Author's name: Theresa M Marteau Institution: Department of Psychology (at Guy's), Section of Health Psychology Email: theresa.marteau@kcl.ac.uk Address: King's College London, London SE1 9RT, UK |
|
| Notes | Source of funding: This study was funded as part of a grant from the Medical Research Council, UK (Risk communication in preventive medicine: optimising the impact of DNA risk information; G0500274). NJP and CGM are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at St Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London. AF is supported by NIHR through the Comprehensive Biomedical Research Centre at UCLH/UCL. The trial protocol was peer reviewed by the Council. Other than as indicated, the funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Declaration of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial statistician prepared the randomisation sequence using randomly selected block sizes of 6, 8, and 10 with randomly permuted allocations within each block, and with an equal allocation ratio. Participants were randomised by family cluster. Each participant was allocated by using the next assignment in the sequence, with clusters of participants required to be allocated to the same arm along the sequence. |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was concealed from the trial co‐ordination team and research counsellor, and the statistical team was only given study data needed for randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completed primary outcome measure: 209/251 (83%) for the DNA risk assessment group and 217/246 (88%) for the non‐DNA risk assessment group |
| Other bias | Unclear risk | Table 3 reports a total number of participants in the DNA arm of n = 232, whereas only n = 226 received the intervention according to Figure 2. |
Irizar Aramburu 2013.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Comment: sample size not reached; clustering by practice not taken into account in analysis. Duration of follow‐up: 1 year Duration of inclusion: not specified How sample size is determined: based on assuming a level of significance of 5%, a quit rate after brief antismoking intervention only (control group) of 5%, an expected quit rate in the experimental group of 14%, and a 1:1 ratio of control to experimental participants. Given these premises, to obtain a power of 80% to detect differences in the test of the null hypothesis Ho: p1 = p2 using a 2‐tailed Chi2 test for 2 independent samples, it would be necessary to include 166 experimental units in the reference group and a further 166 in the experimental group, making a total of 332 units for the study. Assuming a loss to follow‐up of 25%, it would be necessary to recruit 222 participants for the reference group and a further 222 for the experimental group, summing to the aforementioned total of 444 participants for the study. Intention‐to‐treat analyses: yes Number of participants included: 335 Number of participants screened for eligibility: 1775 |
|
| Participants |
Baseline characteristics Overall
Inclusion criteria: active smokers; older than 40 years of age; more than 10 pack‐year history of smoking; have not been diagnosed with COPD Exclusion criteria: older than 80 years of age or institutionalised or having a life expectancy of less than 1 year; having undergone spirometry testing within the previous 2 years; previously diagnosed with respiratory diseases (asthma, interstitial lung diseases, or COPD) that cause pattern changes in spirometry tests; having contraindications for spirometry testing Pretreatment: no full text available Definition of smokers: > 10 pack‐years Study population: primary care active smokers > 40 years without COPD |
|
| Interventions |
Intervention characteristics Experimental intervention
Control intervention
|
|
| Outcomes | Smoking cessation: 7‐day point‐prevalence abstinence validated by exhaled CO < 10 ppm | |
| Identification |
Sponsorship source: International Centre of Research Excellence in Chronicity, Kronikgune, and the Department of Health of the Basque Government Country: Spain Setting: primary care (general practitioners from health centres) Comments: 39 health professionals work in 22 health centres Author's name: María Isabel Irizar‐Aramburu Institution: Idiazabal Primary Care Medical Centre, Idiazabal, Gipuzkoa, Spain Email: MARIAISABEL.IRIZARARAMBURU@osakidetza.net Address: Idiazabal Primary Care Medical Centre, Idiazabal, Gipuzkoa, Spain |
|
| Notes | Results provided by authors upon request.
Source of funding: We would also like to thank the International Centre of Research Excellence in Chronicity, Kronikgune and the Department of Health of the Basque Government's decision to fund this project. Declaration of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Doctors will send data on the patients recruited to the Primary Care Research Unit of the Gipuzkoa Health Region where the patients will be randomly assigned to the intervention or control group. The randomization sequence will be generated by computer and kept in the research unit. The list of patients assigned to each group will be sent to the nurses in charge of the first appointment. At this appointment, once written informed consent has been given, the interventions detailed below will be performed for each group." |
| Allocation concealment (selection bias) | High risk | Nurse in charge of organising appointment aware of allocation before confirming consent. Imbalance between size of groups (159 vs 176). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated. Assessment of outcome made by health professionals blinded to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 lost to follow‐up out of 335 individuals. |
Ito 2006.
| Methods | Setting: first visit at Aichi Cancer Center Hospital (ACCH), Japan Recruitment: screening of first‐visit outpatients (whether consecutive or not is unknown) | |
| Participants | 697 included out of 859 eligible smokers. Smoker defined as smoking at least 1 cigarette on the previous day. Mean age 46.5, 40.5% female, mean cpd: 22.2, pre‐contemplator/contemplator: 70% Therapist: trained interviewer | |
| Interventions | Intervention: information at baseline on the effect of L‐myc polymorphism on modulating the risk of cancer due to smoking (5 to 10 minutes). Sent genotype report at 3 months, with same information about effect of polymorphism (65% got genotype information) Control: just followed‐up for smoking status | |
| Outcomes | Definition of abstinence: point prevalence at 9 months (continuous abstinence, not smoking at both the 3‐ and 9‐month follow‐ups also reported, but genotype only provided after 3 months follow‐up) Duration of follow‐up: 9 months Biochemical validation of non‐smokers: none (attempt made but none agreed to return for CO measurement) | |
| Identification | ||
| Notes | Not given genotype until 3 months. Most participants in both groups had already quit at 3 months, small proportion of new quitters after 3 months. Source of funding: This work was supported in part by a Grant‐in‐Aid for Cancer Research from the Japanese Ministry of Health, Labor and Welfare (17‐1). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random allocation by week of attendance |
| Allocation concealment (selection bias) | High risk | Allocation known at time of enrolment, so potential for selection bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by postal questionnaire, biochemical validation attempted but refused. Similar amount of contact between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data for 52.9% (369), did not differ significantly between groups |
Jamrozik 1984.
| Methods | Setting: 6 general practices, the UK Recruitment: clinic, first visit Selected: outpatients | |
| Participants | 2110 smokers (defined as a person admitting to smoking cigarettes) out of 6052 screened. 1040 in relevant arms 61% female, no detailed patient characteristics given. Significant difference of social classes between groups Therapist: physician | |
| Interventions |
Intervention: demonstration of participant exhaled CO plus verbal advice plus booklet
Control: verbal advice plus booklet 2 study arms were not relevant to this review |
|
| Outcomes | Definition of abstinence: point prevalence Duration of follow‐up: 12 months Biochemical validation of non‐smokers: urinary cotinine in a sample (41%) of self‐reported non‐smokers | |
| Identification | ||
| Notes | Outcome based on unvalidated data. Between 24% and 40% may have misrepresented smoking status, but no evidence of differential misreporting between groups. Sources of funding: Nuffield Dominions Trust and the Health Education Council. National Institute on Drug Abuse, DA 2507, D A0007 for cotinine assays. Helen Van Vunakis is the recipient of a US Public Health Service Career Award 5K6/A 12372. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised according to day of attendance, balanced over 4 weeks |
| Allocation concealment (selection bias) | High risk | Allocation known at enrolment, possibility of selection bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome based on unvalidated data. Between 24% and 40% may have misrepresented smoking status, but no evidence of differential misreporting between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 28% lost to follow‐up across all 4 arms, treated as smokers. No information on differential loss |
McClure 2009.
| Methods | Setting: Group Health Center for Health Studies, Seattle, Washington, USA (research institution) Recruitment: health plan records, data from the Washington State Quitline, purchased mailing list of smokers, ads in local media, public clinics, and other local venues | |
| Participants | 536 smokers (with elevated expired CO levels >= 10 ppm and a mean of 15 cpd for the past year or smoked >= 10 cpd but had smoked for >= 10 years), mean age 50.8 years, 53.2% female, 24.8% pre‐contemplation, 50.8% contemplation, 24.2% preparation, mean cpd: 20.7 Therapists: health educators |
|
| Interventions |
Intervention: brief (20 minutes), personally tailored counselling sessions based on lung functioning (spirometry), CO exposure, and smoking‐related health conditions Control: generic smoking risk information and personalised counselling about diet, body mass index, and physical activity All were advised to quit smoking and were offered access to a free phone‐counselling programme. |
|
| Outcomes |
Definition of abstinence: 7‐day point‐prevalence abstinence Duration of follow‐up: 12 months Biochemical validation of non‐smokers: none |
|
| Identification | ||
| Notes | New for 2012 update Source of funding: This study was supported by the National Cancer Institute (R01 CA100341) and the Group Health Center for Health Studies in Seattle. We thank Amy Mohelnitzky, Richard Hert, MD, Ralph Stumbo, RRT, CPFT, Rick Bloss, Zoe Bermet, Mary Shea, Lisa Shulman, Emily Westbrook, Mona Deprey, Free & Clear Inc., the Washington State Quitline, and the staff of the Center for Health Studies' Survey Research Programme for their help with this research. Declaration of interest: No financial disclosures were reported by the authors of this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible smokers were randomised to treatment using an automated randomisation algorithm. |
| Allocation concealment (selection bias) | Unclear risk | Concealment not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence not biochemically validated, but similar amounts of face‐to‐face contact between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data missing for 13% of participants in each group at 12 months. |
Nichols 2014.
| Methods |
Setting: large general practice, Surrey, UK Study design: randomised controlled trial Study grouping: parallel group Comment: sample size based on previous research = 602 Quasi‐randomisation: clinics were run in parallel at the same venue but on different weekdays for the tests and control groups. Study period: 2011 to 2013 Number of participants screened: 1775 Number of participants included: 109 |
|
| Participants |
Included criteria: aged 20 to 70 years; smoking more than 10 cigarettes daily Excluded criteria: history of major depression and other psychiatric conditions, dementias, and serious or terminal illness (cancers, etc.). Individuals on warfarin. Individuals who do not wish to have a genetic test or do not wish to take part in a research study. Recruitment setting: primary care premises of a group general practitioner practice in an English suburb southwest of London Baseline characteristics Test group (genetic test and risk score)
Control group
|
|
| Interventions |
Intervention characteristics Intervention: genetic test and risk score (Respiragene)
Both groups:
|
|
| Outcomes | Smoking cessation at 6 months, validated (CO breath test and salivary cotinine) | |
| Identification |
Country: UK Setting: United Kingdom National Health Services (NHS) smoking cessation clinic Comments: NA Authors name: John A A Nichols Institution: Department of Clinical and Experimental Medicine, University of Surrey, Guildford Email: drjaan@ntlworld.com Address: 60 Manor Way, Onslow Village, Guildford, Surrey GU2 7RR, UK |
|
| Notes |
Source of funding: Lab 21, Cambridge, UK; Synergenz BioScience Ltd, Evanston, IL, USA Declaration of interest: JN and PG are in receipt of research grants from Lab 21, Cambridge, who are marketing the Respiragene test in the UK, and Synergenz BioScience Ltd, who financed the development of the test from its origins in New Zealand. We initially purchased SmokeScreen kits (for salivary cotinine estimation) from GFC Diagnostics Ltd, but they subsequently supplied 30 kits free of charge. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 2 clinics were run in parallel at the same venue but on different weekdays for test and control groups. Participants who replied stating that they wished to stop smoking by attending our clinic were randomised by the principal investigator. |
| Allocation concealment (selection bias) | High risk | Participants were allocated by the principal investigator. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses are not intention‐to‐treat, and lost to follow‐up excluded from analyses. |
| Other bias | Unclear risk | Selective reporting = unclear: Initially defined secondary endpoints (intention to stop smoking, cigarette consumption, uptake of invitation to cessation, adherence to cessation course, and self‐reported smoking cessation) not all reported. Self‐reported cessation also considered as primary endpoint in final analysis. |
Parkes 2008.
| Methods | Setting: primary care (5 general practices, UK) Recruitment: by letter to registered patients Selection: not selected by motivation | |
| Participants | 561 current smokers (not defined) aged > 35, 54% female, average age 53, mean cpd: 17, 29% pre‐contemplative, 32% contemplative, 17% preparation, 21% action Therapists: study staff member | |
| Interventions | All participants had lung function assessed by spirometry before randomisation. Strongly encouraged to give up and given written contact details of National Health Service (NHS) smoking cessation services. If evidence of asthma or restrictive lung disease, advised to see GP. Told they would be re‐tested at 12 months. Intervention 1: immediate verbal feedback of lung age with explanation. Described as normal if lung age less than or equal to chronological age. Intervention 2: letter giving test results without lung age | |
| Outcomes | Definition of abstinence: unspecified Duration of follow‐up: 12 months Biochemical validation of non‐smokers: exhaled CO, cotinine < 14.2 ng/mL | |
| Identification | ||
| Notes |
Source of funding: Leading Practice Through Research award from the Health Foundation Declaration of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A clerk (who then took no further part in the study) prepared 600 sequentially numbered opaque sealed envelopes, each containing a card with allocation group determined by computer generated random number (odd = intervention)” |
| Allocation concealment (selection bias) | Low risk | “If the participant met the inclusion criteria and gave consent, he or she was entered into the study and underwent baseline spirometry. The next numbered envelope in the series was then opened to determine allocation group” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessors blind to allocation group, biochemical validation used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% lost to follow‐up in each arm, included as smokers. |
Risser 1990.
| Methods | Setting: US Veterans Administration Demonstration Project Recruitment: veterans attending a health promotion clinic Selected: responding to mailed invitations for health promotion. Some recruited at second visit. | |
| Participants | 90 smokers (not defined); mean age 53.7 years (55.5 vs 51.7), 4% female, mean cpd: 23.5, mean pack‐year: 60.4 Initial cessation intent: 51% vs 44% Therapist: nurse‐practitioner | |
| Interventions | Intervention: spirometry, exhaled CO, discussion of pulmonary symptoms + control intervention Control: 50‐minute educational intervention, review of self‐help manual, invitation to a 9‐session 1‐to‐1 counselling programme | |
| Outcomes | Definition of abstinence: point prevalence Duration of follow‐up: 12 months Biochemical validation of non‐smokers: exhaled CO <= 10 ppm | |
| Identification | ||
| Notes | Funding source: supported by VA Health Services Research and Development Funds | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessors blind to allocation group, biochemical validation used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 (29%) intervention, 6 (13%) control lost to follow‐up at 12 months, included as smokers. |
Rodondi 2012.
| Methods |
Setting: Department of Ambulatory Care and Community Medicine, University of Lausanne Recruitment: newspaper advertisement (lay press) in the French‐speaking part of Switzerland Selected: smokers from the general population |
|
| Participants | 536 smokers randomised (267 in the intervention group and 269 in the control group) out of 1036 participants screened for eligibility; 265 and 267 analysed in the intervention group and control groups, respectively; smokers defined as more than 10 cpd with no period of smoking abstinence of at least 3 months in the past year. Mean age: 51.1 years; 43.5% female; median cpd: 20; 17 years age at initiation (median); 5.2 Fagerström score (mean); 2 previous quit attempts (median) Therapist: nurse |
|
| Interventions | Intervention: carotid plaque screening (ultrasound) + control intervention (smokers with at least 1 carotid plaque received a 7‐minute standardised brief explanation on the significance of atherosclerotic plaques) Control: brief advice for smoking cessation; 7‐minute explanation on the risks associated with tobacco smoking; 6 individual counselling sessions, 1 telephone call at 6 months, NRT patches tailored to individual needs; brochures on smoking cessation | |
| Outcomes | Definition of abstinence: 1‐week smoking abstinence (point prevalence) Duration of follow‐up: 12 months Biochemical validation of non‐smokers: exhaled CO < 10 ppm and serum cotinine level < 25 ng/mL | |
| Identification | ||
| Notes | New for 2012 update Source of funding: This study was supported by research grant 3200B0‐116097 from the Swiss National Science Foundation, by the Swiss Heart Foundation, by the Lausanne University Hospital Strategic Plan, and by grant FPT 08.002282 from the Swiss Tobacco Prevention Funds, Federal Office of Public Health. Declaration of interest: None declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to 1 of the 2 groups using a computer‐generated randomisation scheme in a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 participants were lost to follow‐up; 7 who withdrew their participation were classified as current smokers. |
Sanders 1989.
| Methods | Setting: 11 UK general practices Recruitment: screening of all outpatients Selected: outpatients plus made appointment for health check | |
| Participants | 751 participants out of 4330 identified smokers (self‐defined) Mean age 38.5 years. Other characteristics not mentioned. Therapist: practice nurse | |
| Interventions | Intervention: exhaled CO measure plus discussion of significance plus control intervention Control: counselling by practice nurse plus written material given plus offer of a follow‐up appointment | |
| Outcomes | Definition of abstinence: point prevalence Duration of follow‐up: 12 months Biochemical validation of non‐smokers: urinary cotinine for sample, cut‐off not reported Outcomes used are self‐report. | |
| Identification | ||
| Notes | Sources of funding: We are grateful to the doctors, nurses, receptionists and others in the practices participating in this study, to Elaine Fullard for her advice, to Valerie Stone for clerical assistance, and to the department of preventive medicine at St Bartholomews Hospital for performing the cotinine assays. We also thank the British Heart Foundation and Health Education Authority for financial support. David Mant and Lesley Jones are supported by the Imperial Cancer Research Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised by day of attendance on a 1:2 basis. Desktop card reminding doctors of right allocation. 120 wrongly allocated participants, excluded from further analysis. Second step randomised health check attenders for the CO intervention, method not described |
| Allocation concealment (selection bias) | High risk | First‐stage randomisation had potential for selection bias; only attenders eligible for second stage, no information on concealment at this stage. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used only for a sample, results used in analysis not validated, but similar amounts of face‐to‐face contact between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 63.8% followed up, but percentage by group not provided; unclear if differential attrition present. Non‐responders included as smokers. |
Segnan 1991.
| Methods |
Setting: 44 general practices, Italy
Recruitment: screening of outpatients on specific days Selected: outpatients |
|
| Participants | 923 included out of 1009 screened. Smoker definition not given. Age: 20.1% < 31 years; 28.0% 31 to 40 years; 26.8% 41 to 50 years; 25.0% > 50 years. 38% female, cpd: 16.7% <= 10 cpd; 55.2% 11 to 20 cpd; 28.1% > 20 cpd. 51% reporting symptoms. Therapist: physician | |
| Interventions | Intervention: spirometry prescription plus control intervention Control: repeated counselling with reinforcement sessions (2 other groups not used in our comparison: minimal intervention and repeated counselling plus nicotine gum) | |
| Outcomes | Definition of abstinence: 7 days abstinence Duration of follow‐up: 12 months Biochemical validation of non‐smokers: urinary cotinine < 100 ng/mg | |
| Identification | ||
| Notes | In the intervention group, 124 participants out of 292 reported having a spirometry test. Funding sources/declaration of interest: Serono SPA provided the nicotine gum used in the study. The Anti‐Smoking Committee of the Municipality, Torino, and ACI (Automobile Club Italiano) also helped in various ways. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised; "blocked treatment allocation was based on a sequence of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Closed, numbered envelopes"; "The envelopes were indistinguishable from the outside"; "The research staff checked physicians' compliance with the procedure for assignment by comparing envelope numbers and dates of recruitment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% lost to follow‐up at 12 months, included as smokers |
Shahab 2011.
| Methods | Setting: Laboratory at University College London (research institution) Recruitment: smokers were recruited from the general population through advertisements in local newspapers, flyers, e‐mails, posters on public bulletin boards at and around University College London Selected: smokers who responded to the advertisements | |
| Participants | 160 smokers (> 5 cpd for the past year) aged between 18 and 35 years, 43.7% female, average age 31.7 years, mean cpd: 13.8 Therapists: postdoctoral researchers | |
| Interventions | Intervention: expired air CO level + generic leaflet about lung disease + brief targeted feedback about their CO levels in relation to the development of cardiovascular and respiratory diseases Control: expired air CO measurement (results not shown) + generic leaflet about lung disease + standardised brief advice to quit smoking | |
| Outcomes | Definition of abstinence: 7‐day point‐prevalence abstinence Duration of follow‐up: 6 months Biochemical validation of non‐smokers: none | |
| Identification | ||
| Notes | New for 2012 update. Participants received an incentive of GBP 50 for their time. Source of funding: This study was funded by the Department of Health and the charity Cancer Research United Kingdom. These bodies bear no responsibility for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We thank Erdem Pulcu and Onupama Roy for their help with collecting data for this study. Declaration of interest: Lion Shahab has received an honorarium for a talk and travel expenses from Pfizer. Robert West undertakes research and consultancy for the following developers and manufacturers of smoking cessation treatments; Pfizer, J7J, McNeil, GSK, Nabi, Novartis and Sanofi‐Aventis. Robert West also has a share in the patent of a novel nicotine delivery device. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number‐generated group allocation. No description about the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope containing the random number‐generated group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence not biochemically validated. Participants were contacted by a researcher blinded to group allocation to complete the follow‐up. Similar amounts of face‐to‐face contact between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers lost to follow‐up in both groups (23/79 control, 28/81 intervention ‐ reason given for all: "could not be contacted"). All lost to follow‐up included as smokers in analysis. |
Sippel 1999.
| Methods | Setting: 2 primary care clinics, USA Recruitment: all smokers among outpatients Selected: outpatients | |
| Participants | 205 included out of 360 smokers (self‐defined); mean age 38.5 years, 62.5% female, mean cpd: 20.0, mean pack‐years: 28.9. Stage of change: 36% in preparation stage Therapist: study staff | |
| Interventions | Intervention: spirometry and exhaled CO + control intervention Control: counselling according to transtheoretical model stage + written material + NRT encouraged if prepared to stop | |
| Outcomes | Definition of abstinence: sustained quitting rate Duration of follow‐up: 9 months Biochemical validation of non‐smokers: none | |
| Identification | ||
| Notes | Source of funding: This project was funded by the American Lung Association of Oregon and the American Academy of Family Practice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomised; questionnaires numbered consecutively, and participants enrolled in chronological order based on time of check‐in. Odd numbered = intervention |
| Allocation concealment (selection bias) | High risk | Not concealed, potential selection bias, although nurses performing participant check‐in were blinded to the questionnaire numbers. "As 4 to 6 nurses conducted patient check‐in independently and simultaneously at each clinic, it is unlikely that any given patient would be preferentially enrolled into either study arm." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessors blind to allocation group. No biochemical validation used, but similar amounts of face‐to‐face contact between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18.6% intervention and 12.6% control lost to follow‐up, included as smokers. |
Walker 1985.
| Methods | Setting: "stop‐smoking clinic", USA Recruitment: public service announcement + media advertising Selected: those responding to advertising, paying USD 45 | |
| Participants | 64 out of 141 eligible (self‐defined smokers) Mean age: 35.5 years, 59% female, mean cpd: 29.2, mean 3.4 previous quit attempts Therapist: first author | |
| Interventions | Intervention: exhaled CO and spirometry feedback plus Taste Satiation (TS) (in 50%) or Focused Smoking (FS) (in 50%) and booster sessions for half of each subgroup Control: 50% TS sessions or 50% FS sessions, booster sessions for half of each subgroup | |
| Outcomes | Definition of abstinence: 10 days abstinence Duration of follow‐up: 6 months Biochemical validation of non‐smokers: exhaled CO < 8 ppm | |
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in 2x2x2 factorial design, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants reached at 6 months included in analysis. "Five subjects dropped out of treatment and 3 additional subjects were randomly eliminated to facilitate data analysis." |
CO: carbon monoxide
COPD: Chronic Obstructive Pulmonary Disease cpd: cigarettes per day
FEV1: forced expiratory volume in one second
FVC: forced vital capacity
ICMJE: International Committee of Medical Journal Editors
IDU: injecting drug user NRT: nicotine replacement therapy ppm: parts per million
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anthonisen 1994 | Effect of spirometry cannot be isolated. Spirometry performed in all participants. Randomisation to 3 groups: smoking intervention (12‐session programme) + bronchodilatator, smoking intervention + placebo, no intervention |
| Ashraf 2014 | Cannot isolate effect of biofeedback |
| Barnfather 2005 | Only 8 weeks follow‐up. Intervention was immediate feedback from a point‐of‐care test for salivary nicotine metabolites. |
| Borrelli 2002 | Effect of CO cannot be isolated. No results available. Intervention: Precaution Adoption Model: CO feedback + environmental tobacco smoke to the child feedback + motivational counselling Control: Behavioral Action Model: self efficacy‐enhancing counselling |
| Borrelli 2005 | Effect of CO cannot be isolated. Intervention: motivational interviewing and CO feedback Control: standard care |
| Borrelli 2010 | Feedback about secondhand CO in asthmatic children of the participants. Too‐short follow‐up (3 months) |
| ChiCTR‐TRC‐13003255 | Trial not completed. |
| Cope 2003 | Not a true randomised trial |
| Cox 2003 | No control group. All participants received chest CT scan screening for lung carcinoma. |
| Ferketich 2012 | Effect of CT scan on the motivation to quit cannot be isolated. |
| Foulds 2015 | No measure of smoking cessation at 6 months |
| Frank 1999 | Full text not available. Intervention: urinary cotinine feedback to prenatal patients |
| Gulliver 2008 | Spirometry was not used to give feedback on lung function. |
| Hajek 2001 | Effect of CO cannot be isolated. Intervention: counselling, written materials, CO Control: standard antismoking leaflets |
| Hajek 2002 | Effect of CO cannot be isolated. Intervention: CO feedback, booklet, quiz, "buddy", declaration of commitment to give up, sticker in patient's notes Control: verbal advice + different booklet |
| Humerfelt 1998 | Effect of spirometry cannot be isolated. Intervention: spirometry + written counselling + pamphlet Control: spirometry |
| Kaminsky 2011 | Follow‐up at 1 month only |
| Kanner 1996 | Effect of spirometry cannot be isolated. Spirometry performed in all participants. Randomisation to 3 groups: smoking intervention (12‐session programme) + bronchodilatator, smoking intervention + placebo, no intervention |
| Kanner 1999 | Effect of spirometry cannot be isolated. Spirometry performed in all participants. Randomisation to 3 groups: smoking intervention (12‐session programme) + bronchodilatator, smoking intervention + placebo, no intervention |
| Kotz 2009 | Effect of spirometry feedback cannot be isolated. Intervention group received spirometry/COPD feedback but also an intervention to challenge irrational beliefs about smoking. They were further instructed to use a smoking cessation diary to monitor smoking behaviour and beliefs about smoking. The latter interventions were not offered to the control group. |
| Lipkus 2007 | Outcomes were perceived smoking‐related health risks, worries, and desire to quit, not smoking cessation. |
| Marteau 2012 | Study of behavioural impact of pharmacogenomics. Outcome was adherence to smoking cessation medication. Measures effect of informing smokers of genetic test results on responsiveness to smoking cessation medication |
| Martucci 2010 | Effect of bronchoscopy results on smoking cessation cannot be isolated. |
| McBride 2002 | Effect of genetic biomarker feedback cannot be isolated. Intervention: feedback on glutathione‐S‐transferase M1 (GSTM1) + 4 phone calls + control intervention Control: self help manual +/‐ NRT. No phone calls |
| McClure 2013 | No measure of smoking cessation at 6 months |
| McIntosh 1994 | Smoking cessation is not considered as an outcome. |
| Mols 2015 | Cannot isolate effect of biofeedback |
| NCT00862368 | Cannot isolate effect of biofeedback |
| NCT00991081 | No measure of smoking cessation at 6 months |
| NCT01145729 | No measure of smoking cessation at 6 months |
| NCT02206971 | No measure of smoking cessation at 6 months |
| NCT02837809 | Wrong intervention |
| NCT02922790 | No measure of smoking cessation at 6 months |
| Nuesslein 2006 | Only 6 weeks' follow‐up Intervention: notification of urine cotinine levels + control Control: written advice to quit from a paediatrician |
| Ojedokun 2013 | No measure of smoking cessation at 6 months |
| Paek 2014 | Wrong intervention |
| Pistelli 2007 | Conference abstract. Post hoc analysis Intervention: participation in a lung cancer screening + control Control: biofeedback (lung CT) + smoking cessation programme |
| Powers 2011 | Only 3 months' follow‐up. Coronary heart disease risk communication. Participants were smokers and non‐smokers (being a smoker was not a criterion for inclusion). |
| Prokhorov 2008 | Effect of the health feedback (expired CO) cannot be isolated. The aim of the study was to compare standard care with computer‐assisted care. |
| Rennie 2012 | Participants assigned to hypothetical risk scenarios; outcome was motivation, not cessation. |
| Richmond 1986 | Effect of biomarker feedback cannot be isolated. Spirometry, blood CO, urinary cotinine in both groups. Intervention: 4 visits and discussion of manual |
| Rodriguez 2011 | Effect of biomarker feedback cannot be isolated. "Both arms will receive brief structured advice and a detailed discussion of the spirometry results at visit 0. The control group will only be given brief structured advice about giving up smoking on the follow up" |
| Sanderson 2005 | Short‐term study of effect of genetic testing for lung cancer susceptibility. Follow‐up at 10 weeks. Main outcomes were motivation to quit, perceived risk, depression and anxiety. |
| Sanderson 2007 | Hypothetical scenario tested (not real life) |
| Sanderson 2008 | Follow‐up too short (2 months) |
| Sejourne 2010 | No measure of smoking abstinence at 6 months or later after the start of the intervention |
| Shahab 2007 | Pilot study of visual personalised biomarker feedback with follow‐up at 4 weeks. Main outcomes were perception of susceptibility, engagement in cessation behaviours, and intentions to stop. |
| Shi 2013 | Wrong intervention |
| Shoptaw 2002 | Effect of CO feedback cannot be isolated. CO feedback given to all groups. 2x2 design: 1. relapse‐prevention counselling + NRT 2. contingency management: vouchers given for validated abstinence + NRT Control: NRT |
| Stotts 2009 | Assessments at baseline (16 to 26 weeks of gestation) and at the end of pregnancy. Too‐short follow‐up |
| Stratelis 2006 | Not a true randomised trial, no control without biomedical risk assessment. Smokers with normal lung function at baseline received different frequencies of spirometry (annually or in 3 years). |
| Tammemagi 2014 | Wrong study design |
| Taylor 2007 | No control group. Study of impact of screening on smoking cessation and readiness to stop smoking among participants in trials of lung screening |
| Terazawa 2001 | Effect of biomarker feedback (CO and urinary cotinine) cannot be isolated, combined with 1 counselling session and 4 follow‐up calls. |
| Townsend 2005 | No control group. Longitudinal study of smoking behaviour in people receiving lung cancer screening |
| van der Aalst 2011 | Post hoc analysis. Current and former smokers. Screening of lung cancer by low‐dose thoracic CT in intervention arm. Only positive or indeterminate test results (lung nodules) were managed according to a protocol (feedback was not standardised, and not about smoking cessation but lung cancer). |
| Wakefield 2002 | Biomarker feedback given on the health of the participant's child, not on the participant's own health. The motivational component here differs from the approach in the other included studies. |
| Warner 2012 | Long‐term abstinence not an outcome. CO levels were measured preoperatively. |
| Wilt 2007 | Review |
| Wright 2006 | Outcome was motivation to quit based on hypothetical risk information. |
| Zullig 2014 | No measure of smoking cessation at 6 months |
CO: carbon monoxide COPD: chronic obstructive pulmonary disease CT: computed tomography NRT: nicotine replacement therapy
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02351167.
| Methods | Randomised controlled trial with parallel assignment (triple‐blinded) |
| Participants | 822 participants aged 21 years and older Inclusion criteria:
Exclusion criteria:
|
| Interventions | The investigators propose a prospective, genotype‐based stratified randomisation trial to compare 2 smoking cessation medications (combination NRT (patch and lozenge), varenicline vs placebo) for 3 months in 720 smokers with known genotypes. |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Notes | 7‐day point‐prevalence abstinence at 6 months should be available. Genotype‐based randomisation, but apparently no biofeedback |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618000291280.
| Trial name or title | Effectiveness of quit smoking motivation with carbon monoxide feedback module (COQUIT) among college smoker in Selangor |
| Methods | Single‐blinded cluster‐randomised controlled trial at 10 tertiary colleges |
| Participants | 160 students at Community College in Selangor, Malaysia |
| Interventions | Standard brief 5R motivational strategy and an additional CO motivational feedback module. Expired carbon monoxide is measured by the principal investigator on a 1‐to‐1 basis. The CO reading is recorded directly into the iPad (using a mobile phone application) together with explained feedback about the result and interpretation. The result of the CO is then e‐mailed to the participants. Participants in the control group only receive the standard brief 5R motivational strategy. |
| Outcomes | Primary: stage of changes based on the Transtheoretical Model that will be measured using validated questionnaire at 1, 3, and 6 months postintervention Secondary: any quit attempt defined as 24 hours of not smoking, number of cigarettes smoked per day, and smoking habit at 1, 3, and 6 months |
| Starting date | Anticipated 16 April 2018 |
| Contact information | Dr Muhammad Adil Zainal Abidin adilzainal@gmail.com |
| Notes |
IRCT2017080435257N1.
| Trial name or title | Effectiveness of spirometry as a motivational tool for smoking cessation based on health belief model |
| Methods | Single‐blinded, parallel‐group randomised controlled trial |
| Participants | 160 participants Inclusion criteria: patient satisfaction; smoking history at least 10 pack/year; lack of non‐cigarette related pulmonary disease; new spirometry test; ages between 20 and 90 Exclusion criteria: patient dissatisfaction |
| Interventions | Intervention group: participants will attend a speech meeting to increase information about cigarettes and make them susceptible to smoking cessation. At this meeting, educational pamphlets will be distributed on health, economic, and social disadvantages. Then an individual motivational educational counselling exercise will be carried out for each participant, with an emphasis on testing the spirometry of the participant and comparing it with spirometry of a healthy person of the same age and sex, and providing relevant statistics. In the next step, the intervention group will be divided into groups of 10, discussing the benefits and barriers to smoking cessation in the group discussion. Then the questionnaire will be filled by self‐reporting to determine the perceived sensitivity, perceived severity, perceived benefits, and perceived barriers. At intervals at the end of the second week, 3 and 6 months, information about the rate of smoking cessation will be collected using the follow‐up questionnaire. |
| Outcomes | "Action to smoking cessation"? at 2 weeks, 3 months and 6 months "keep smoking cessation"? at 2 weeks, 3 months and 6 months |
| Starting date | 20 March 2016 |
| Contact information | Saeid Ghasemian ghasemian@shmu.ac.ir |
| Notes |
Martin Lujan 2011.
| Trial name or title | Effectiveness of Smoking Cessation Advice Combined With Spirometric Results in Adult Smokers (ESPITAP) |
| Methods | Randomised controlled trial, parallel assignment, single‐blinded (participants) |
| Participants | 596 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention arm: spirometry and smoking cessation advice. Participants will be given a brief but structured smoking cessation advice (according to the standards of the Tobacco Study Group of the Catalan Society of Family Medicine) together with a detailed and structured discussion of the spirometric results. Control arm: smoking cessation advice. Brief but structured smoking cessation advice (according to the standards of the Tobacco Study Group of the Catalan Society of Family Medicine). |
| Outcomes | Smoking abstinence: self‐reported abstinence (12 or more months). Time Frame: 12 months Smoking abstinence confirmed by an expired air carbon monoxide |
| Starting date | June 2008 |
| Contact information | Principal Investigator: Francisco Martín‐Luján, MD, Catalan Institute of Health |
| Notes | Last update posted on ClinicalTrials.gov: 7 April 2011 Authors contacted but no answer received. |
Martin Lujan 2016.
| Trial name or title | Multicentric Randomized Clinical Trial to Evaluate the Long‐term Effectiveness of a Motivational Intervention Against Smoking, Based on the Information Obtained From Spirometry in Primary Care. (RESET‐ESPITAP2) |
| Methods | Randomised controlled trial, parallel assignment, open‐label |
| Participants | 1100 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention arm: spirometry. Will be given brief but structured smoking cessation advice (according to the standards of the Tobacco Study Group of the Catalan Society of Family Medicine) together with a detailed and structured 20‐minute visit with details of the spirometry data (values of respiratory capacity and volumes referring on the theoretical) Control arm: brief smoking cessation advice. Will be given brief but structured smoking cessation advice (according to the standards of the Tobacco Study Group of the Catalan Society of Family Medicine) |
| Outcomes | Cessation of tobacco consumption at 12 months. Time Frame: 12 months |
| Starting date | November 2011 (estimated study completion date: November 2014) |
| Contact information | Principal Investigator: Antoni Santigosa‐Ayala, MD, Catalan Institute of Health |
| Notes | Last update posted on ClinicalTrials.gov: 2 June 2014 Authors contacted but no answer received. Protocol published in 2016, but no results published. |
Muhammad 2015.
| Trial name or title | A pilot randomized controlled trial on the effectiveness of a 'lung age' intervention on smoking cessation. Effectiveness of a 'lung age' intervention on smoking cessation rate in a Singaporean community |
| Methods | Pilot randomised controlled trial |
| Participants | 108 participants (convenience sample) recruited from population health screenings in Singapore Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention arm: lung age intervention A spirometry test will be conducted to determine the lung age of participants in the intervention group. These participants will then receive an educational intervention on their lung age in addition to standard smoking cessation counselling. Control arm: usual smoking education |
| Outcomes | Smoking cessation rates at 3 and 6 months |
| Starting date | 4 September 2014 (end date 3 September 2015) |
| Contact information | Wenru Wang, PhD, RN nurww@nus.edu.sg |
| Notes | Study completed but data not published. Author contacted but no answer received. |
NCT01186016.
| Trial name or title | Developing genetic education for smoking cessation |
| Methods | Randomised controlled trial with parallel assignment, open‐label |
| Participants | 103 participants. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: Genetic Education Session (GES) The intervention includes receiving education about genetics and smoking. The content is basic genetics and education about the multifactorial nature of smoking; research findings about genetic contributions to smoking, potential applications of this research for cessation treatment, and legal, ethical, and social implications of future use of genotyping for cessation. All participants also receive a 5‐week standard cognitive‐behavioural smoking cessation intervention with 6 weeks of over‐the‐counter transdermal nicotine replacement therapy. Active comparator: Nutrition Education Session (NES) To control for an attention placebo effect, the control group will receive information about nutritional guidelines as established by the US Department of Agriculture (USDA) and the US Food and Drug Administration. The attention control group will be referred to as the Nutritional Education Session (NES) group. The content of NES sessions 1 and 2 are use of the USDA (MyPyramid) dietary and food safety guidelines. All participants also receive a 5‐week standard cognitive‐behavioural smoking cessation intervention with 6 weeks of over‐the‐counter transdermal nicotine replacement therapy. |
| Outcomes | Smoking‐related behaviour when experimental and attention control groups are compared. Time Frame: 6 months after the end of the Smoking Cessation Intervention Use of cessation strategies, abstinence, and interest in genotyping |
| Starting date | February 2010 |
| Contact information | Professor Julia Houfek, PhD, APRN‐CNS, University of Nebraska |
| Notes | Last update posted on ClinicalTrials.gov: 21 January 2013 Authors contacted but no answer received. |
NCT02431611.
| Trial name or title | Biomarker Feedback to Motivate Tobacco Cessation in Pregnant Alaska Native Women (MAW) ‐ Phase 3 Pilot Clinical Trial |
| Methods | Randomised controlled trial, parallel assignments, open‐label |
| Participants | 60 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: biomarker feedback intervention (phone‐based smoking cessation counselling). Feedback on maternal cotinine and likely infant NNAL. Phone‐based behavioural smoking cessation counselling Active comparator: control condition (phone‐based smoking cessation counselling). Phone‐based behavioural smoking cessation counselling |
| Outcomes | Smoking abstinence in late pregnancy (self‐reported abstinence verified with cotinine). Time Frame: at week 36 gestation or greater up to the time of delivery Self‐reported abstinence verified with cotinine. |
| Starting date | March 2015 (study completion date 2017) |
| Contact information | Christi A Patten, PhD, Mayo clinic |
| Notes | Last update posted: 19 December 2017 No data published, author contacted but no answer received. |
NCT02658032.
| Trial name or title | Personalized Intervention Program: Tobacco Treatment for Patients at Risk for Lung Cancer (PIP) |
| Methods | Single‐blind (participants) randomised trial with factorial assignment (2 interventions) |
| Participants | 276 adults 50 years of age and older Inclusion criteria:
Exclusion criteria:
|
| Interventions | The purpose of this study is to test the efficacy of 2 separate, sequential interventions to promote tobacco cessation/reduction in individuals who are screened for lung cancer or who are eligible for lung cancer screening. Each intervention will be compared to standard of care. The first intervention will be a personalised‐message intervention; the second intervention will consist of a biofeedback‐based intervention. The aim of the second intervention is to evaluate the efficacy of a novel, biofeedback‐based intervention that provides personalised individual‐level feedback on biomarkers of lung cancer risk and how they improve in response to cessation, delivered in a gain‐framed way. The biomarkers include skin carotenoid status, spirometry, and plasma bilirubin, all of which improve with cessation. The study team will examine whether the biofeedback prevents relapse in those who quit and leads to reductions in smoking in lung nodule patients who failed to quit. |
| Outcomes | Primary outcome:
Secondary outcomes
|
| Starting date | January 2016 |
| Contact information | Brenda Cartmel, PhD brenda.cartmel@yale.edu |
| Notes | Unclear whether 6‐month smoking cessation outcome will be available |
NCT02840513.
| Trial name or title | Smartphone App and CO Self‐monitoring for Smoking Cessation (SMART‐CO) |
| Methods | Parallel‐group, open‐label randomised controlled trial |
| Participants | 510 participants, 16 years of age and older Inclusion criteria:
Exclusion criteria:
|
| Interventions | The app offers a coaching function where users receive personalised messages to encourage smoking cessation and advice for behavioural changes. For the first 4 weeks of the intervention, individuals will also be asked to blow daily into a breath carbon monoxide monitor before going to sleep. Depending on the results of the breath test, individualised messages will be delivered by the Smokelyzer feedback app to either enhance maintenance of abstinence or to increase the motivation to quit. After the first 4 weeks, participants will use the breath carbon monoxide monitor at least twice a week until the end of the 6‐month study. The app will react with positive feedback in individuals doing well with smoking cessation and messages to encourage individuals with difficulties quitting smoking. |
| Outcomes | Primary outcome measures: Self‐reported abstinence biochemically verified by a carbon monoxide test in‐person. (Time Frame: 6 months) Secondary outcome measures: Differences in the number of daily cigarettes smoked from baseline to 6‐month follow‐up and point prevalence of abstinence (i.e. no smoking in the past 7 days) at 6‐month follow‐up. (Time Frame: 6 months) |
| Starting date | 1 June 2017 |
| Contact information | Dmitry Gryaznov dmitry.gryaznov@usb.ch |
| Notes | Estimated study completion date: 1 July 2019 |
NCT02991781.
| Trial name or title | Combined bio‐ and neuro‐ feedback vs. varenicline use for smoking cessation |
| Methods | Randomised, parallel‐group, open‐label trial |
| Participants | 140 participants: Inclusion criteria:
Exclusion criteria:
|
| Interventions | This study will develop and experimentally test the efficiency of a neurofeedback (NF) training protocol for smoking cessation. As non‐pharmacological, non‐invasive, and painless brainwave technique, NF contributes to teach individuals how they can take control of their mind through operant conditioning. NF regulates brain function in a natural way. The protocol will consist of 5 sessions of skin temperature biofeedback and 20 sessions of a neurofeedback training protocol that will consist of Alpha‐Theta ratio up‐training. The aim of the training is to reach a cross‐over state, were initially Alpha activity will increase and then in a deeper state, the Theta activity will take over. This state is associated with a reverie and disidentification with problems, stress, or traumatic experiences. Participants will therefore will learn how to increase their Theta/Alpha ratio. The neurofeedback intervention will be compared with a different group of participants receiving an intervention based on varenicline use for approximately 3 months. The electrophysiological evaluation of the efficacy of the intervention will include EEG resting state and a sleep polysomnography measurement. Questionnaires and clinical evaluation include the same measurements as the neurofeedback intervention but only at 3 time points: prior, during, after the completion of the study. |
| Outcomes | Standardised percentage of participants that give up smoking (Time Frame 2 years) |
| Starting date | January 2017 |
| Contact information | Professor Panos Bamidis bamidis@auth.gr |
| Notes | Not sure if it will be possible to isolate the effect of biofeedback |
NCT03377738.
| Trial name or title | Effectiveness of the spirometry test as a motivational tool for quitting tobacco in primary care |
| Methods | Randomised controlled trial, parallel assignment, open‐label |
| Participants | 90 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: diagnostic test: spirometry All participants will be given an intervention for tobacco cessation that will depend on the individual's cessation phase and will be given a spirometry test as a motivational element for dishabituation. Control: no intervention: antismoking therapy All participants will be given only an intervention for tobacco cessation that will depend on the individual's cessation phase. |
| Outcomes |
|
| Starting date | May 2011 |
| Contact information | A Lopez‐santiago, MD |
| Notes | Last update posted on ClinicalTrials.gov: 17 December 2017 Authors contacted but no answer received. |
NCT03521141.
| Trial name or title | PRecision Interventions for SMoking in the SCCS (PRISM‐SCCS) |
| Methods | Single‐blinded randomised trial with parallel groups |
| Participants | 68 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | 3 intervention arms (only arm 3 (group 2) is provided with biofeedback) 1. Guideline‐based care (GBC) GBC participants are 1) referred to the state quitline, 2) provided the National Cancer Institute Clearing the Air smoking cessation programme, and 3) asked to talk to their healthcare provider about potential lung cancer screening. Medication assignment is guided by standard guidelines and a conversation between the study tobacco counsellor and the participant. Groups 1 and 2 also receive GBC counselling. 2. Active comparator: Nicotine metabolite ratio (PC‐NMR) Group 1, nicotine metabolism. Medication is guided by nicotine metabolism. 3. Active comparator: Respiragene (PC‐Respiragene) Group 2, genetically informed lung cancer risk score. Medication assignment is guided by standard guidelines and a conversation between the study nurse and the participant. |
| Outcomes | Primary outcome measures: Intervention Feasibility: ability to retain participants. Time Frame: 6 months. Feasibility of delivering precision care interventions in the SCCS, as evidenced by ability to recruit, engage, and retain participants through the end of the study. Secondary outcome measures: Cessation History ‐ Quit attempts (Time Frame: 6 months) Quit attempt (yes/no) Cessation History ‐ Medication use (Time Frame: 6 months) Medication use (duration of use) Cessation History ‐ Quitline (Time Frame: 6 months) Self‐reported quitline utilisation Cessation History ‐ Self‐reported abstinence (Time Frame: 6 months) Self‐reported abstinence Cessation History ‐ Validated abstinence (Time Frame: 6 months) Biochemically verified (salivary cotinine, >= 10 ng/mL) abstinence |
| Starting date | 18 May 2018 |
| Contact information | Hilary Tindle, Associate Professor of Medicine, Vanderbilt University Medical Center |
| Notes |
NCT03583203.
| Trial name or title | Tobacco intensive motivational and estimate risk |
| Methods | Randomised, open‐label, prospective study |
| Participants | 204 participants, aged 40 to 70 years old Inclusion criteria:
Exclusion criteria:
|
| Interventions | The primary objective evaluates the effectiveness of an intensive antismoking programme that informs participants of their individual risk of lung damage and the possibilities of prevention. The intervention group will undergo spirometry, and the presence and degree of respiratory obstruction will be assessed. Participants will be given individual information to generate a motivational message about the possibilities of prevention, and the information will be maintained for 3 months by sending text messages (SMS) to their mobile phones. This group will include personalised information about lung damage. After evaluating their COPD, participants will be informed about its existence and staging. Depending on the damage found, the generation of motivation will focus on the different prevention methods. Likewise, after the motivation level is set, participants will be offered the option of treatment and regular follow‐up. The intervention will be strengthened by motivational messages, half of which are linked to the possibilities of preventing respiratory damage, sent to the participant's mobile phone via SMS during the 3 months after the face‐to‐face intervention. Participants without mobile phones will receive a call on their phone to convey the same messages. The control intervention lasts 30 minutes and will be structured around the 5 A's technique (Ask, Advice, Assess, Assist and Arrange). |
| Outcomes | Primary outcome measures: smoking cessation (Time Frame: 12 months) self‐reported abstinence over the previous 7 days, confirmed by co‐oximetry with expired CO < 10 ppm |
| Starting date | 12 July 2018 |
| Contact information | Fernando Sarramea Crespo fscferro69@gmail.com |
| Notes |
Pita Fernandez 2015.
| Trial name or title | Effectiveness of co‐oximetry and minimum advice for smoking cessation in kidney transplant recipients |
| Methods | Randomised controlled trial (open, with blinded evaluation) |
| Participants | 122 participants Smoking kidney transplant recipients, in preparation stage, pre‐contemplation, and contemplation stage of change, who consent to participate |
| Interventions | Intervention group: brief advice + exhaled CO measurement Control group: brief advice for smoking cessation |
| Outcomes | Smoking cessation at 6 and 12 months confirmed by nicotine test |
| Starting date | 1 December 2012 (end date 31 December 2015) |
| Contact information | Dr Salvador Pita‐Fernández |
| Notes | Study completed but data not published. Author contacted but no answer received. |
Ripoll 2012.
| Trial name or title | Clinical trial on the efficacy of exhaled carbon monoxide measurement in smoking cessation in primary health care |
| Methods | Parallel randomised controlled trial with blind evaluation (single) |
| Participants | 942 participants Inclusion criteria:
|
| Interventions | Intervention group: brief advice plus exhaled CO measure Control group: brief face‐to‐face antismoking advice from the physician during patient consultation |
| Outcomes | Sustained abstinence (at 6 and 12 months) validated by urine cotinine test |
| Starting date | 15 October 2010 (end date 15 October 2012) |
| Contact information | Miss Joana Ripoll jripoll@ibsalut.caib.es |
| Notes | Study no longer recruiting, no data published. Author contacted but no answer received. |
CO: carbon monoxide
COPD: chronic obstructive pulmonary disease
EEG: electroencephalogram
NNAL: 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol
NRT: nicotine replacement therapy
Differences between protocol and review
Following changes to the Cochrane Tobacco Addiction Group's recommended method of data analysis since this review was first prepared, we have changed the way in which we summarise the effects of treatment. From the 2009 update onwards, we use the risk ratio rather than the odds ratio for summarising individual trial outcomes and for estimates of pooled effect. Treatment effects will seem smaller when expressed as risk ratios than when expressed as odds ratios, unless the event rates are very low. For example, if 20 out of 100 participants have quit in the intervention group, and 10 out of 100 in the control group, the risk ratio is 2.0 ((20/100)/(10/100)), while the odds ratio is 2.25 ((20/80)/(10/90)). While there are circumstances in which odds ratios may be preferable, there is a danger that they will be interpreted as if they are risk ratios, making the treatment effect seem larger (Higgins 2011). We estimated a pooled weighted average of risk ratios using a Mantel‐Haenszel random‐effects method, with 95% confidence intervals.
From the 2019 update onwards, we decided to pool studies using similar biofeedback strategies even if they were performed in diverse settings. Given the fact that there were more studies included than in previous reviews, we considered that the clinical heterogeneity was acceptable even if the setting and staff were different.
From the 2019 update onwards, we changed the 'Risk of bias' assessment from assessing blinding of participants, study personnel, and outcome assessment (both performance and detection bias) to assessing outcome assessment (detection bias only) alone. This is because interventions of this type cannot be blinded, and is in accordance with Cochrane Tobacco Addiction Group guidance.
Contributions of authors
CC: (for 2019 update) screening search results, organising retrieval of papers, screening papers for inclusion, quality appraisal of papers, data extraction, data management, data entry into Review Manager 5, data analysis and interpretation, writing to authors of papers for additional information, update of the review.
YM: screening search results, organising retrieval of papers, screening papers for inclusion, quality appraisal of papers, data extraction, data management, data entry into Review Manager 5, data analysis and interpretation, writing the review, writing to authors of papers for additional information.
JLB: (for 2019 update) conducted the GRADE evaluation and added the 'Summary of findings' table, and contributed to the writing of the review.
BB: conception and design of the review, developing search strategy, screening papers for inclusion, quality appraisal of papers, interpretation of data, methodological perspective, general advice on the review, securing funding.
JYC: (for 2012 update) screening papers for inclusion, quality appraisal of papers, data extraction, data management, data entry into Review Manager 5, data analysis and interpretation, update of the review.
JC: conception and design of the review, developing search strategy, screening search results, screening papers for inclusion, quality appraisal of papers, interpretation of data, clinical and methodological perspective, general advice on the review.
MRW: (for 2012 update) screening papers for inclusion, quality appraisal of papers, data extraction, data management, data entry into Review Manager 5.
KS: (for 2019 update) screening papers for inclusion, quality appraisal of papers, data extraction.
RB : co‐ordinating the review, conception and design of the review, data collection, developing search strategy and undertaking searches, screening search results, organising retrieval of papers, screening papers for inclusion, quality appraisal of papers, data extraction, data management, data entry into Review Manager 5, data analysis and interpretation, writing the review, writing to authors of papers for additional information, providing a clinical perspective.
Sources of support
Internal sources
-
Center for Primary Care and Public Health, University of Lausanne, Switzerland.
Core salaries of CC, YM, KS, and RB
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
External sources
National Institute for Health Research (NIHR) Cochrane Programme Grant, UK.
Declarations of interest
CC: no interest to declare
YM: no interest to declare
JLB: no interest to declare
BB: no interest to declare
JYC: no interest to declare
JC: was the co‐author of one of the studies included in the review.
MRW: no interest to declare
KS: no interest to declare
RB: no interest to declare
Edited (no change to conclusions)
References
References to studies included in this review
Audrain 1997 {published data only}
- Audrain J, Boyd NR, Roth J, Main D, Caporaso NF, Lerman C. Genetic susceptibility testing in smoking‐cessation treatment: one‐year outcomes of a randomized trial. Addictive Behaviors 1997;22:741‐51. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, et al. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking‐related cognitions, emotions, and behavior change. Health Psychology 1997;16:87‐99. [DOI] [PubMed] [Google Scholar]
Bovet 2002 {published data only}
- Bovet P, Perret F, Cornuz J, Quilindo J, Paccaud F. Improved smoking cessation in smokers given ultrasound photographs of their own atherosclerotic plaques. Preventive Medicine 2002;34(2):215‐20. [DOI] [PubMed] [Google Scholar]
Brunette 2013 {published data only}
- Brunette MF, Ferron JC, Drake RE, Devitt TS, Geiger PT, McHugo GJ, et al. Carbon monoxide feedback in a motivational decision support system for nicotine dependence among smokers with severe mental illnesses. Journal of Substance Abuse Treatment 2013;45(4):319‐24. [DOI: 10.1016/j.jsat.2013.04.005] [DOI] [PubMed] [Google Scholar]
Buffels 2006 {published data only}
- Buffels J, Degryse J, Decramer M, Heyrman J. Can spirometry improve the success rate of smoking cessation in general practice [abstract]. European Respiratory Journal 2005;26(Suppl 49):670s. [Google Scholar]
- Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respiratory Medicine 2006;100(11):2012‐7. [DOI] [PubMed] [Google Scholar]
Drummond 2014 {published data only}
- Drummond MB, Astemborski J, Lambert AA, Goldberg S, Stitzer ML, Merlo CA, et al. A randomized study of contingency management and spirometric lung age for motivating smoking cessation among injection drug users. BMC Public Health 2014;14:761. [DOI: 10.1186/1471-2458-14-761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01334736. A study of novel smoking cessation interventions in current and former injection drug users. https://clinicaltrials.gov/ct2/show/NCT01334736 first received 13 April 2011. [NCT01334736]
Hishida 2010 {published data only}
- Hishida A, Terazawa T, Mamiya T, Ito H, Matsuo K, Tajima K, et al. Efficacy of genotype notification to Japanese smokers on smoking cessation ‐ an intervention study at workplace. Cancer Epidemiology 2010;34(1):96‐100. [DOI] [PubMed] [Google Scholar]
Hollands 2012 {published data only}
- Hollands GJ, Whitwell SC, Parker RA, Prescott NJ, Forbes A, Sanderson J, et al. Effect of communicating DNA based risk assessments for Crohn's disease on smoking cessation: randomised controlled trial. BMJ (Clinical research ed.) 2012;345:e4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell SC, Mathew CG, Lewis CM, Forbes A, Watts S, Sanderson J, et al. Trial protocol: communicating DNA‐based risk assessments for Crohn's disease: a randomised controlled trial assessing impact upon stopping smoking. BMC Public Health 2011;11(44):1‐8. [DOI: 10.1186/1471-2458-11-44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Irizar Aramburu 2013 {published data only}
- Irizar‐Aramburu MI, Martinez‐Eizaguirre JM, Pacheco‐Bravo P, Diaz‐Atienza M, Aguirre‐Arratibel I, Pena‐Pena MI, et al. Effectiveness of spirometry as a motivational tool for smoking cessation: a clinical trial, the ESPIMOAT study. BMC Family Practice 2013;14:185. [DOI: 10.1186/1471-2296-14-185] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01821885. Effectiveness of spirometry as a motivational tool to quit smoking. Effectiveness of spirometry and the report of spirometric test results by a primary care physician on smoking cessation rate in adult smokers: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01821885 first received 01 April 2013.
Ito 2006 {published data only}
- Ito H, Matsuo K, Wakai K, Saito T, Kumimoto H, Okuma K, et al. An intervention study of smoking cessation with feedback on genetic cancer susceptibility in Japan. Preventive Medicine 2006;42(2):102‐8. [DOI] [PubMed] [Google Scholar]
Jamrozik 1984 {published data only}
- Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Vunakis H. Controlled trial of three different antismoking interventions in general practice. British Medical Journal (Clinical research ed.) 1984;288(6429):1499‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2009 {published data only}
- McClure JB, Ludman E, Grothaus L, Pabiniak C, Richards J, Mohelnitzky A. Immediate and short‐term impact of a brief motivational smoking intervention using a biomedical risk assessment: the Get PHIT trial. Nicotine & Tobacco Research 2009;11(4):394‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Ludman EJ, Grothaus L, Pabiniak C, Richards J. Impact of a brief motivational smoking cessation intervention the Get PHIT randomized controlled trial. American Journal of Preventive Medicine 2009;37(2):116‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Lundman EJ, Grohause L, Pabiniak C, Richards J. Impact of spirometry feedback and brief motivational counseling on long‐term smoking outcomes: a comparison of smokers with and without lung impairment. Patient Education & Counseling 2010;80(2):280‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 2014 {published data only}
- NCT01176383. Impact of a gene test for susceptibility to lung cancer in smokers. clinicaltrials.gov/ct2/show/NCT01176383 first received 6 August 2010.
- Nichols JA, Grob P, Lusignan S, Kite W, Williams P. Genetic test to stop smoking (GeTSS) trial protocol: randomised controlled trial of a genetic test (Respiragene) and Auckland formula to assess lung cancer risk. BMC Pulmonary Medicine 2014;14:77. [DOI: 10.1186/1471-2466-14-77] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parkes 2008 {published data only}
- Deane K, Stevermer JJ, Hickner J. Help smokers quit: tell them their "lung age." [comment]. Journal of Family Practice 2008;57(9):584‐6. [PMC free article] [PubMed] [Google Scholar]
- Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: The Step2quit randomised controlled trial. BMJ 2008;336(7644):598‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Risser 1990 {published data only}
- Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. Journal of General Internal Medicine 1990;5(1):16‐22. [DOI] [PubMed] [Google Scholar]
Rodondi 2012 {published data only}
- Rodondi N, Bovet P, Hayoz D, Cornuz J. The impact of CAROtid plaque Screening on Smoking (CAROSS) cessation and control of other cardiovascular risk factors: rationale and design of a randomized controlled trial. Contemporary Clinical Trials 2008;29(5):767‐73. [DOI] [PubMed] [Google Scholar]
- Rodondi N, Collet T‐H, Nanchen D, Locatelli I, Depairon M, Aujesky D, et al. Impact of carotid plaque screening on smoking cessation and other cardiovascular risk factors. Archives of Internal Medicine 2012;172:344‐52. [DOI] [PubMed] [Google Scholar]
Sanders 1989 {published data only}
- Sanders D, Fowler G, Mant D, Fuller A, Jones L, Marzillier J. Randomized controlled trial of anti‐smoking advice by nurses in general practice. Journal of the Royal College of General Practitioners 1989;39(324):273‐6. [PMC free article] [PubMed] [Google Scholar]
Segnan 1991 {published data only}
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes & Control 1991;2(4):239‐46. [DOI] [PubMed] [Google Scholar]
Shahab 2011 {published data only}
- Shahab L, West R, McNeill A. A randomized, controlled trial of adding expired carbon monoxide feedback to brief stop smoking advice: evaluation of cognitive and behavioral effects. Health Psychology 2011;30(1):49‐57. [DOI] [PubMed] [Google Scholar]
- Shahab L, West R, McNeill A. Adding expired air‐carbon‐monoxide feedback to brief stop smoking advice ‐ evaluation of cognitive and behavioral effects. Annals of Behavioral Medicine 2008;35(Suppl 1):S205. [DOI] [PubMed] [Google Scholar]
Sippel 1999 {published data only}
- Sippel JM, Osborne ML, Bjornson W, Goldberg B, Buist AS. Smoking cessation in primary care clinics. Journal of General Internal Medicine 1999;14(11):670‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1985 {published data only}
- Walker WB, Franzini LR. Low‐risk aversive group treatments, physiological feedback, and booster sessions for smoking cessation. Behavior Therapy 1985;16:263‐74. [Google Scholar]
References to studies excluded from this review
Anthonisen 1994 {published data only}
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994;272(19):1497‐505. [PubMed] [Google Scholar]
Ashraf 2014 {published data only}
- Ashraf H, Saghir Z, Dirksen A, Pedersen JH, Thomsen LH, Døssing M, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low‐dose CT: final results after a 5‐year screening programme. Thorax 2014;69(6):574‐9. [DOI: 10.1136/thoraxjnl-2013-203849] [DOI] [PubMed] [Google Scholar]
- Ashraf H, Tonnesen P, Holst PJ, Dirksen A, Thorsen H, Dossing M. Effect of CT screening on smoking habits at 1‐year follow‐up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64(5):388‐92. [DOI] [PubMed] [Google Scholar]
- Clark MM, Jett JR. Change in smoking status after low‐dose spiral chest CT screening for lung cancer: opportunity for smoking intervention. Thorax 2009;64(5):371‐2. [DOI] [PubMed] [Google Scholar]
Barnfather 2005 {published data only}
- Barnfather KD, Cope GF, Chapple IL. Effect of incorporating a 10 minute point of care test for salivary nicotine metabolites into a general practice based smoking cessation programme: randomised controlled trial. BMJ 2005;331(7523):979‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borrelli 2002 {published data only}
- Borrelli B, McQuaid EL, Becker B, Hammond K, Papandonatos G, Fritz G, et al. Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Education Research 2002;17(5):659‐69. [DOI] [PubMed] [Google Scholar]
Borrelli 2005 {published data only}
- Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community‐nurse Assisted Research and Education on Smoking). Preventive Medicine 2005;41:815‐21. [DOI] [PubMed] [Google Scholar]
Borrelli 2010 {published data only}
- Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: a randomized trial. Journal of Consulting & Clinical Psychology 2010;78(1):34‐43. [DOI] [PubMed] [Google Scholar]
ChiCTR‐TRC‐13003255 {published data only}
- ChiCTR‐TRC‐13003255. Effect on smoking quit rate of telling patients their lung age. www.chictr.org.cn/hvshowproject.aspx?id=5866 first received: unknown.
Cope 2003 {published data only}
- Cope GF, Nayyar P, Holder R. Feedback from a point‐of‐care test for nicotine intake to reduce smoking during pregnancy. Annals of Clinical Biochemistry 2003;40(6):674‐9. [DOI] [PubMed] [Google Scholar]
Cox 2003 {published data only}
- Cox LS, Clark MM, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer 2003;98(11):2495‐501. [DOI] [PubMed] [Google Scholar]
Ferketich 2012 {published data only}
- Ferketich A, Otterson G, King M, Browning K, Wewers M. A pilot study to examine the feasibility of delivering a tobacco dependence treatment program at the time of lung cancer screening. Journal of Thoracic Oncology 2010;5(12 Suppl 7):S549. [Google Scholar]
- Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer 2012;76:211‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foulds 2015 {published data only}
- Foulds J, Veldheer S, Hrabovsky S, Yingst J, Sciamanna C, Chen G, et al. The effect of motivational lung age feedback on short‐term quit rates in smokers seeking intensive group treatment: a randomized controlled pilot study. Drug and Alcohol Dependence 2015;153:271‐7. [DOI: 10.1016/j.drugalcdep.2015.05.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01980485. The Get Quit‐Stay Quit study: a randomized trial of health risk feedback and relapse prevention for treatment‐seeking smokers. clinicaltrials.gov/ct2/show/NCT01980485 first received 11 November 2013.
Frank 1999 {published data only}
- Frank JE, Hoffman DS, Flanagan V. Use of repeated cotinine determinations as a motivational and educational tool in smoking cessation counseling for pregnant women. Pediatric Research 1999;45:1153. [Google Scholar]
Gulliver 2008 {published data only}
- Gulliver SB, Kamholz BW, Helstrom AW, Morissette SB, Kahler CW. A preliminary evaluation of adjuncts to motivational interviewing for psychiatrically complex smokers. Journal of Dual Diagnosis 2008;4(4):394‐413. [Google Scholar]
Hajek 2001 {published data only}
- Hajek P, West R, Lee A, Foulds J, Owen L, Eiser J, et al. Randomized controlled trial of a midwife‐delivered brief smoking cessation intervention in pregnancy. Addiction 2001;96(3):485‐94. [DOI] [PubMed] [Google Scholar]
Hajek 2002 {published data only}
- Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ 2002;324(7329):87‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humerfelt 1998 {published data only}
- Humerfelt S, Eide GE, Kvale G, Aaro LE, Gulsvik A. Effectiveness of postal smoking cessation advice: a randomized controlled trial in young men with reduced FEV1 and asbestos exposure. European Respiratory Journal 1998;11(2):284‐90. [DOI] [PubMed] [Google Scholar]
Kaminsky 2011 {published data only}
- Kaminsky DA, Marcy T, Dorwaldt A, Pinckney R, DeSarno M, Solomon L, et al. Motivating smokers in the hospital pulmonary function laboratory to quit smoking by use of the lung age concept. Nicotine & Tobacco Research 2011;13(11):1161‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky DA, Marcy TW, Solomon LJ, Dorwaldt AL, DeSarno M, Pinckney RG. Spirometry to motivate quit smoking attempts: use in the pulmonary function testing lab [abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A5444. [Google Scholar]
Kanner 1996 {published data only}
- Kanner RE. Early intervention in chronic obstructive pulmonary disease. A review of the Lung Health Study results. Medical Clinics of North America 1996;80(3):523‐47. [DOI] [PubMed] [Google Scholar]
Kanner 1999 {published data only}
- Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. American Journal of Medicine 1999;106(4):410‐6. [DOI] [PubMed] [Google Scholar]
Kotz 2009 {published data only}
- ISRCTN64481813. The efficacy and cost‐effectiveness of confrontational counselling for smoking cessation in smokers with previously undiagnosed COPD: a randomised controlled trial. www.isrctn.com/ISRCTN64481813 first received 28 April 2006. [NTR627]
- Kotz D, Huibers MJH, West RJ, Wesseling G, Schayck OCP. The effect of confrontational counseling on smoking cessation in smokers with COPD [Abstract]. European Respiratory Journal 2008;52:E444. [Google Scholar]
- Kotz D, Huibers MJH, West RJ, Wesseling G, Schayck OCP. What mediates the effect of confrontational counselling on smoking cessation in smokers with COPD?. Patient Education & Counseling 2009;76(1):16‐24. [DOI] [PubMed] [Google Scholar]
- Kotz D, Vos R, Huibers MJ. Ethical analysis of the justifiability of labelling with COPD for smoking cessation. Journal of Medical Ethics 2009;35(9):534‐40. [DOI] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confronting smokers with airflow limitation for smoking cessation. European Respiratory Journal 2009;33(4):754‐62. [DOI] [PubMed] [Google Scholar]
- Soriano JB, Parkes G. Remember elephants and icebergs... "Your lung function should be here, but it is there!" [comment]. European Respiratory Journal 2009;33(4):715‐6. [DOI] [PubMed] [Google Scholar]
Lipkus 2007 {published data only}
- Lipkus IM, Prokhorov AV. The effects of providing lung age and respiratory symptoms feedback on community college smokers' perceived smoking‐related health risks, worries and desire to quit. Addictive Behaviors 2007;32(3):516‐32. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Schwartz‐Bloom R, Kelley MJ, Pan W. A preliminary exploration of college smokers' reactions to nicotine dependence genetic susceptibility feedback. Nicotine & Tobacco Research 2015;17(3):337‐43. [DOI: 10.1093/ntr/ntu155] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marteau 2012 {published data only}
- Marteau TM, Aveyard P, Munafo MR, Prevost AT, Hollands GJ, Armstrong D, et al. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PLOS ONE 2012;7:e35249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Munafo MR, Aveyard P, Hill C, Whitwell S, Willis TA, et al. Trial protocol: using genotype to tailor prescribing of nicotine replacement therapy: a randomised controlled trial assessing impact of communication upon adherence. BMC Public Health 2010;10:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martucci 2010 {published data only}
- Martucci P, Sestini P, Canessa PA, Brancaccio L, Guarino C, Barbato N, et al. Smoking cessation in patients requiring bronchoscopy: the Bronchoscopy AntiSmoking Study (BASIS). Respiratory Medicine 2010;104(1):61‐6. [DOI] [PubMed] [Google Scholar]
McBride 2002 {published data only}
- McBride CM, Bepler G, Lipkus IM, Lyna P, Samsa G, Albright J, et al. Incorporating genetic susceptibility feedback into a smoking cessation program for African‐American smokers with low income. Cancer Epidemiology, Biomarkers & Prevention 2002;11(6):521‐8. [PubMed] [Google Scholar]
McClure 2013 {published data only}
- David SP, McClure JB, Fauver R, Mohelnitzky A, Pabiniak C, Shulman L, et al. Prospective, genetically tailored treatment for smoking cessation mixed‐methods feasibility trial (POS4‐93). Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13‐16 Houston Texas 2012;152 2012. [Google Scholar]
- McClure JB, Swan GE, John J, Fauver R, Javitz HS, Bergen AW, et al. Pharmacogenetic smoking cessation intervention in a health care setting: a pilot feasibility study. Nicotine & Tobacco Research 2013;15(2):518‐26. [DOI: 10.1093/ntr/nts173] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 1994 {published data only}
- McIntosh NA, Clark NM, Howatt WF. Reducing tobacco smoke in the environment of the child with asthma: a cotinine‐assisted, minimal‐contact intervention. Journal of Asthma 1994;31(6):453‐62. [DOI] [PubMed] [Google Scholar]
Mols 2015 {published data only}
- Mols RE, Jensen JM, Sand NP, Fuglesang C, Bagdat D, Vedsted P. Visualization of coronary artery calcification: influence on risk modification. American Journal of Medicine 2015;128(9):1023 e23‐31. [DOI: 10.1016/j.amjmed.2015.03.033] [DOI] [PubMed] [Google Scholar]
NCT00862368 {published data only}
- NCT00862368. Sustaining smoking cessation in smokers with kids with asthma. clinicaltrials.gov/ct2/show/NCT00862368 first received 16 March 2012.
NCT00991081 {published data only}
- NCT00991081. Behaviorally enhanced counseling on nicotine dependence (BEACON) trial. clinicaltrials.gov/ct2/show/NCT00991081 first received 7 October 2009.
NCT01145729 {unpublished data only}
- NCT01145729. Clinical response to biomarker documentation of child secondhand smoke exposure. clinicaltrials.gov/ct2/show/NCT01145729 first received 17 June 2010.
NCT02206971 {published data only}
- NCT02206971. Biomarker feedback for smoking cessation. clinicaltrials.gov/ct2/show/NCT02206971 first received 1 August 2014.
NCT02837809 {published and unpublished data}
- NCT02837809. Early lung cancer detection in high risk individuals. clinicaltrials.gov/ct2/show/NCT02837809 first received 20 July 2016.
NCT02922790 {published data only}
- NCT02922790. Feasibility and smokers' reactions to DNA feedback. clinicaltrials.gov/ct2/show/NCT02922790 2017; Vol. first received 4 October 2016.
Nuesslein 2006 {published data only}
- Nuesslein TG, Struwe A, Maiwald N, Rieger C, Stephan V. Maternal tobacco consumption can be reduced by simple intervention of the paediatrician. Klinische Padiatrie 2006;218(5):283‐6. [DOI] [PubMed] [Google Scholar]
Ojedokun 2013 {published data only}
- Ojedokun J, Keane S, O'Connor K. The effect of lung age feedback with brief smoking cessation advice during routine consultations on smoking habit ‐ Know2quit multicenter randomized control trial. European Journal of General Practice 2013;19(1):39. [DOI: 10.3109/13814788.2012.759936] [DOI] [Google Scholar]
Paek 2014 {published data only}
- Paek YJ, Lee S, Kim YH, Lee KS, Yim HW, Kim MS, et al. Effect on smoking quit rate of telling smokers their health risk appraisal in terms of health age: a randomized control trial. Asian Pacific Journal of Cancer Prevention 2014;15(12):4963‐8. [DOI] [PubMed] [Google Scholar]
Pistelli 2007 {published data only}
- Pistelli F, Aquilini F, Tavanti L, Cini S, Spinelli C, Falaschi F, et al. Smoking cessation over the first year of follow up in a lung cancer screening with spiral chest CT scan (Italung CT study) [abstract]. European Respiratory Journal 2007;30(Suppl 51):503s. [Google Scholar]
Powers 2011 {published data only}
- Powers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. American Heart Journal 2011;161(4):673‐80. [DOI] [PubMed] [Google Scholar]
Prokhorov 2008 {published data only}
- Prokhorov AV, Marani S, Vost T, Luca M, Mullen M. Project SUCCESS: students using computerized coaching to end smoking successfully [PA2‐3]. Society for Research on Nicotine & Tobacco 17th Annual Meeting, February 16‐19, Toronto 2011. [Google Scholar]
- Prokhorov AV, Warneke C, Fouladi R, Moor C, Mullin Jones M, Rosenblum C, et al. Smoking cessation among community college students: program outcomes (POS4‐24). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 New Orleans, Louisiana 2003. [Google Scholar]
- Prokhorov AV, Yost T, Mullin‐Jones M, Moor C, Ford KH, Marani S, et al. "Look At Your Health": Outcomes associated with a computer‐assisted smoking cessation counseling intervention for community college students. Addictive Behaviors 2008;33(6):757‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rennie 2012 {published data only}
- Rennie CA, Stinge A, King EA, Sothirachagan S, Osmond C, Lotery AJ. Can genetic risk information for age‐related macular degeneration influence motivation to stop smoking? A pilot study. Eye 2012;26:109‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richmond 1986 {published data only}
- Richmond R, Austin A, Webster I. Three year evaluation of a programme by general practitioners to help patients to stop smoking. BMJ 1986;292:803‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R, Webster I. A smoking cessation program for use in general practice. Medical Journal of Australia 1985;142:190‐4. [PubMed] [Google Scholar]
Rodriguez 2011 {published data only}
- Rodriguez Alvarez MM, Zurilla Lonarte E, Munoz Ortiz I, Martinez Gonzalez S, Montero Alia JJ, Negrete Palma A, et al. Role of the spirometry in the decrease of smoking habit. Preliminary results [abstract]. Primary Care Respiratory Journal 2008;17(2):123. [Google Scholar]
- Rodriguez‐Alvarez M, Toran‐Monserrat P, Munoz‐Ortiz L, Negrete‐Palma A, Montero‐Alia JJ, Jimenez‐Gonzalez M, et al. Effectiveness of regular reporting of spirometric results combined with a smoking cessation advice by a primary care physician on smoking quit rate in adult smokers: a randomized controlled trial. ESPIROTAB study. BMC Family Practice 2011;12:61. [ClinicalTrials.gov: NCT01296295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanderson 2005 {published data only}
- Sanderson SC, Wardle J. Will genetic testing for complex diseases increase motivation to quit smoking? Anticipated reactions in a survey of smokers. Health Education and Behavior 2005;32(5):640‐53. [DOI] [PubMed] [Google Scholar]
Sanderson 2007 {published data only}
- Sanderson SC, Michie S. Genetic testing for heart disease susceptibility: potential impact on motivation to quit smoking. Clinical Genetics 2007;71(6):501‐10. [DOI] [PubMed] [Google Scholar]
Sanderson 2008 {published data only}
- Sanderson SC, Humphries SE, Hubbart C, Hughes E, Jarvis MJ, Wardle J. Psychological and behavioural impact of genetic testing smokers for lung cancer risk: a phase II exploratory trial. Journal of Health Psychology 2008;13(4):481‐94. [DOI] [PubMed] [Google Scholar]
Sejourne 2010 {published data only}
- Sejourne C, Parot‐Schinckel E, Rouquette A, Pare F, Delcroix M, Fanello S. Impact of exhaled CO measurement. A randomised study among 578 smoking patients in general practice. Revue des Maladies Respiratoires 2010;27(3):213‐8. [DOI] [PubMed] [Google Scholar]
Shahab 2007 {published data only}
- Shahab L, Hall S, Marteau T. Showing smokers with vascular disease images of their arteries to motivate cessation: a pilot study. British Journal of Health Psychology 2007;12(2):275‐83. [DOI] [PubMed] [Google Scholar]
Shi 2013 {published data only}
- Shi Y, Ehlers S, Hinds R, Baumgartner A, Warner DO. Monitoring of exhaled carbon monoxide to promote preoperative smoking abstinence. Health Psychology 2013;32(6):714‐7. [DOI: 10.1037/a0029504] [DOI] [PubMed] [Google Scholar]
Shoptaw 2002 {published data only}
- Shoptaw S, Rotheram‐Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, et al. Smoking cessation in methadone maintenance. Addiction 2002;97(10):1317‐28. [DOI] [PubMed] [Google Scholar]
Stotts 2009 {published data only}
- Stotts AL, Groff JY, Velasquez MM, Benjamin‐Garner R, Green C, Carbonari JP, et al. Ultrasound feedback and motivational interviewing targeting smoking cessation in the second and third trimesters of pregnancy. Nicotine & Tobacco Research 2009;11(8):961‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratelis 2006 {published data only}
- Stratelis G, Molstad S, Jakobsson P, Zetterstrom O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scandinavian Journal of Primary Health Care 2006;24(3):133‐9. [DOI] [PubMed] [Google Scholar]
Tammemagi 2014 {published data only}
- Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. Journal of the National Cancer Institute 2014;106(6):dju084. [DOI: 10.1093/jnci/dju084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2007 {published data only}
- Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer 2007;56(1):125‐34. [DOI] [PubMed] [Google Scholar]
Terazawa 2001 {published data only}
- Terazawa T, Mamiya T, Masui S, Nakamura M. The effect of smoking cessation counselling at health checkup. Sangyo Eiseigaku Zasshi 2001;43(6):207‐13. [DOI] [PubMed] [Google Scholar]
Townsend 2005 {published data only}
- Townsend CO, Clark MM, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer 2005;103(10):2154‐62. [DOI] [PubMed] [Google Scholar]
van der Aalst 2011 {published data only}
- Aalst CM, Klaveren RJ, Bergh KA, Willemsen MC, Koning HJ. The impact of a lung cancer computed tomography screening result on smoking abstinence. European Respiratory Journal 2011;37(6):1466‐73. [DOI] [PubMed] [Google Scholar]
- Aalst CM, Bergh KA, Willemsen MC, Koning HJ, Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow‐up data from the Dutch‐Belgian randomised controlled lung cancer screening trial. Thorax 2010;65(7):600‐5. [DOI] [PubMed] [Google Scholar]
Wakefield 2002 {published data only}
- Wakefield M, Banham D, McCaul K, Martin J, Ruffin R, Badcock N, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low‐income parents of children with asthma: a controlled trial. Preventive Medicine 2002;34(1):58‐65. [DOI] [PubMed] [Google Scholar]
Warner 2012 {unpublished data only}
- Warner DO, Shi Y, Ehlers SL, Baumgartner A, Hinds RF. Monitoring of exhaled carbon monoxide to promote pre‐operative smoking abstinence (POS3‐111). Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13‐16 Houston Texas 2012. [Google Scholar]
Wilt 2007 {published data only}
- Wilt TJ, Niewoehner D, Kane RL, MacDonald R, Joseph AM. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine & Tobacco Research 2007;9:21‐32. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright AJ, French DP, Weinman J, Marteau TM. Can genetic risk information enhance motivation for smoking cessation? An analogue study. Health Psychology 2006;25:740‐52. [DOI] [PubMed] [Google Scholar]
Zullig 2014 {published data only}
- Zullig LL, Sanders LL, Shaw RJ, McCant F, Danus S, Bosworth HB. A randomised controlled trial of providing personalised cardiovascular risk information to modify health behaviour. Journal of Telemedicine and Telecare 2014;20(3):147‐52. [DOI: 10.1177/1357633X14528446] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02351167 {unpublished data only}
- NCT02351167. Genetically informed smoking cessation trial. clinicaltrials.gov/ct2/show/NCT02351167 first received 30 January 2015.
References to ongoing studies
ACTRN12618000291280 {unpublished data only}
- ACTRN12618000291280. Effectiveness of quit smoking motivation with carbon monoxide feedback module (COQUIT) among college smoker in Selangor. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372786 first received 27 October 2017.
IRCT2017080435257N1 {published data only}
- IRCT2017080435257N1. The effect of motivational counseling on smoking cessation. en.irct.ir/trial/26698 first received 11 September 2017.
Martin Lujan 2011 {unpublished data only}
- Martin‐Lujan F, Pinol‐Moreso JL, Martin‐Vergara N, Basora‐Gallisa J, Pascual‐Palacios I, Sagarra‐Alamo R, et al. Effectiveness of a structured motivational intervention including smoking cessation advice and spirometry information in the primary care setting: the ESPITAP study. BMC Public Health 2011;11(1):859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01194596. Effectiveness of smoking cessation advice combined with spirometric results in adult smokers (ESPITAP). clinicaltrials.gov/ct2/show/NCT01194596 first received 3 September 2010.
Martin Lujan 2016 {published data only}
- Martin‐Lujan F, Santigosa‐Ayala A, Pinol‐Moreso JL, Sorli‐Aguilar M, Flores‐Mateo G, Blade‐Creixenti J, et al. Multicentric randomized clinical trial to evaluate the long‐term effectiveness of a motivational intervention against smoking, based on the information obtained from spirometry in primary care: the RESET study protocol. BMC Family Practice 2016;17:15. [DOI: 10.1186/s12875-016-0415-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorli‐Aguilar M, Martin‐Lujan F, Flores‐Mateo G, Arija‐Val V, Basora‐Gallisa J, Sola‐Alberich R. Dietary patterns are associated with lung function among Spanish smokers without respiratory disease. BMC Pulmonary Medicine 2016;16(1):162 MISC3 ‐ BPMMB. [DOI: 10.1186/s12890-016-0326-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muhammad 2015 {published data only}
- Muhammad I, Mok W, Toh HM, Sii D, Wang W. A pilot randomized controlled trial on the effectiveness of a 'lung age' intervention on smoking cessation: study protocol. Journal of Advanced Nursing 2015;71(10):2426‐34. [DOI: 10.1111/jan.12689] [DOI] [PubMed] [Google Scholar]
NCT01186016 {published data only}
- NCT01186016. Developing genetic education for smoking cessation. clinicaltrials.gov/ct2/show/NCT01186016 first received 20 August 2010.
NCT02431611 {published data only}
- NCT02431611. Biomarker feedback to motivate cessation in pregnancy (MAW Phase 3). clinicaltrials.gov/ct2/show/NCT02431611 first received 1 May 2015.
NCT02658032 {published data only}
- NCT02658032. Personalized intervention program: tobacco treatment for patients at risk for lung cancer (PIP). clinicaltrials.gov/ct2/show/NCT02658032 first received 14 January 2019.
NCT02840513 {published data only}
- NCT02840513. Smartphone App and CO Self‐monitoring for Smoking Cessation (SMART‐CO). clinicaltrials.gov/ct2/show/NCT02840513 first received 21 July 2016.
NCT02991781 {published data only}
- NCT02991781. Combined bio‐ and neuro‐ feedback vs. varenicline use for smoking cessation. clinicaltrials.gov/ct2/show/NCT02991781 first received 14 December 2016.
NCT03377738 {published data only}
- NCT03377738. Effectiveness of the spirometry test as a motivational tool for quitting tobacco in primary care. clinicaltrials.gov/ct2/show/NCT03377738 first received 19 December 2017.
NCT03521141 {published data only}
- NCT03521141. PRecision Interventions for SMoking in the SCCS (PRISM‐SCCS). clinicaltrials.gov/ct2/show/NCT03521141 first received 10 May 2018.
NCT03583203 {published data only}
- NCT03583203. Tobacco intensive motivational and estimate risk. clinicaltrials.gov/ct2/show/NCT03583203 first received 11 July 2018.
Pita Fernandez 2015 {published data only}
- Pita‐Fernández S, Seijo‐Bestilleiro R, Pértega‐Díaz S, Alonso‐Hernández Á, Fernández‐Rivera C, Cao‐López M, et al. A randomized clinical trial to determine the effectiveness of CO‐oximetry and anti‐smoking brief advice in a cohort of kidney transplant patients who smoke: study protocol for a randomized controlled trial. Trials 2016;17:174. [DOI: 10.1186/s13063-016-1311-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita‐Fernández S, Valdés‐Cañedo F, Seijo‐Bestilleiro R, Seoane‐Pillado T, López‐Calviño B, Pértega‐Díaz S. Effectiveness of co‐oximetry and minimum advice for smoking cessation in kidney transplant recipients. European Journal of Epidemiology 2015;30(8):843‐4. [DOI: 10.1007/s10654-015-0072-z] [DOI] [Google Scholar]
Ripoll 2012 {published data only}
- Ripoll J, Girauta H, Ramos M, Medina‐Bombardo D, Pastor A, Alvarez‐Ossorio C, et al. Clinical trial on the efficacy of exhaled carbon monoxide measurement in smoking cessation in primary health care. BMC Public Health 2012;12:322. [DOI: 10.1186/1471-2458-12-322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bauman 1983
- Bauman KE, Bryan ES, Dent CW, Koch GG. The influence of observing carbon monoxide level on cigarette smoking by public prenatal patients. American Journal of Public Health 1983;73:1089‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilano 2015
- Bilano V, Gilmour S, Moffiet T, Tursan d'Espaignet E, Stevens GA, Commar A, et al. Global trends and projections for tobacco use, 1990‐2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 2015;385:966‐76. [DOI] [PubMed] [Google Scholar]
Buist 2002
- Buist AS. Guidelines for the management of chronic obstructive pulmonary disease. Respiratory Medicine 2002;96(Suppl C):S11‐6. [DOI] [PubMed] [Google Scholar]
Cahill 2010
- Cahill K, Lancaster T, Green N. Stage‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD004492.pub4] [DOI] [PubMed] [Google Scholar]
CDC 2014
- U.S. Department of Health and Human Services. The health consequences of smoking ‐ 50 years of progress: a report of the surgeon general. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
Coleman 2005
- Coleman T. Near patient tests for smoking cessation. BMJ 2005;331:979‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 27 March 2018.
Curry 1993
- Curry SJ. Self‐help interventions for smoking cessation. Journal of Consulting and Clinical Psychology 1993;61:790‐803. [DOI] [PubMed] [Google Scholar]
DHHS 1990
- U.S. Department of Health and Human Services. The health benefits of smoking cessation: a report of the Surgeon General. Rockville (MD): Public Health Service, Office on Smoking and Health; 1990. DHHS Publication No. (CDC) 90‐8416.
DHHS 2004
- U.S. Department of Health and Human Services. Health consequences of smoking. Public Health Service, Office on Smoking and Health 2004.
Doll 2004
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004;328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service 2008.
Haddow 1991
- Haddow JE, Knight GJ, Kloza EM, Palomaki GE, Wald NJ. Cotinine‐assisted intervention in pregnancy to reduce smoking and low birth‐weight delivery. British Journal of Obstetrics and Gynaecology 1991;98:859‐65. [DOI] [PubMed] [Google Scholar]
Hale 2007
- Hale ED, Treharne GJ, Kitas GD. The Common‐Sense Model of self‐regulation of health and illness: how can we use it to understand and respond to our patients' need?. Rheumatology 2007;46:904‐6. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2018
- Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heatherton 1991
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction 1991;86(9):1119‐27. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 1998
- Hoffman DW, Flanagan VA, Frank JE. Use of repeated cotinine determinations as a motivational and educational tool in smoking‐cessation counseling for pregnant women. Unpublished final grant report, Robert Wood Johnson Foundation, 1998. Cited as Ref. 31 in McClure JB, Behavioral Medicine 2001, 27:37‐41.
Jha 2013
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine 2013;368(4):341‐50. [DOI: 10.1056/NEJMsa1211128] [DOI] [PubMed] [Google Scholar]
Joseph 2016
- Joseph RP, Daniel CL, Thind H, Benitez TJ, Pekmezi D. Applying psychological theories to promote long‐term maintenance of health behaviors. American Journal of Lifestyle Medicine 2016;10:356‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilburn 1990
- Kilburn KH, Warshaw RH. Effects of individually motivating smoking cessation in male blue‐collar workers. American Journal of Public Health 1990;80:1334‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kottke 1988
- Kottke TE, Battista RN, DeFriese GH, Brekke ML. Attributes of successful smoking cessation interventions in medical practice. A meta‐analysis of 39 controlled trials. JAMA 1988;259:2883‐9. [DOI] [PubMed] [Google Scholar]
Krosnick 2017
- Krosnick JA, Malhotra N, Hyunjung Mo C, Bruera EF, Chang L, Pasek J, et al. Perceptions of health risks of cigarette smoking: a new measure reveals widespread misunderstanding. PLOS ONE 2017;12(8):e0182063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
Lerman 1993
- Lerman C, Orleans CT, Engstrom PF. Biological markers in smoking cessation treatment. Seminars in Oncology 1993;20:359‐67. [PubMed] [Google Scholar]
Lerman 1997
- Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, et al. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking‐related cognitions, emotions, and behavior change. Health Psychology 1997;16:87‐99. [DOI] [PubMed] [Google Scholar]
Li 1984
- Li VC, Kim YJ, Ewart CK, Terry PB, Cuthie JC, Wood J, et al. Effects of physician counseling on the smoking behavior of asbestos‐exposed workers. Preventive Medicine 1984;13:462‐76. [DOI] [PubMed] [Google Scholar]
Livingstone‐Banks 2019
- Livingstone‐Banks J, Ordóñez‐Mena JM, Hartmann‐Boyce J. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD001118.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loss 1979
- Loss RW, Hall WJ, Speers DM. Evaluation of early airway disease in smokers: cost effectiveness of pulmonary function testing. American Journal of the Medical Sciences 1979;278:27‐37. [DOI] [PubMed] [Google Scholar]
McBride 2000
- McBride CM, Halabi S, Bepler G, Lyna P, McIntyre L, Lipkus I, et al. Maximizing the motivational impact of feedback of lung cancer susceptibility on smokers' desire to quit. Journal of Health Communication 2000;5:229‐41. [DOI] [PubMed] [Google Scholar]
McClure 2001
- McClure JB. Are biomarkers a useful aid in smoking cessation? A review and analysis of the literature. Behavioral Medicine 2001;27:37‐41. [DOI] [PubMed] [Google Scholar]
Miller 1991
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: Guilford, 1991. [Google Scholar]
Phillips 1995
- Phillips A, Savigny D, Law MM. As Canadians butt out, the developing world lights up. Canadian Medical Association Journal 1995;153:111‐4. [PMC free article] [PubMed] [Google Scholar]
Prochaska 1983
- Prochaska JO, DiClemente CD. Stages and processes of self‐change of smoking: toward an integrative model of change. Journal of Consulting and Clinical Psychology 1983;51(3):390‐5. [DOI] [PubMed] [Google Scholar]
Riemsma 2003
- Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al. Systematic review of the effectiveness of stage based interventions to promote smoking cessation. BMJ 2003;326(7400):1175‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romer 2001
- Romer D, Jamieson P. Do adolescents appreciate the risks of smoking? Evidence from a national survey. Journal of Adolescent Health 2001;29(1):12‐21. [DOI] [PubMed] [Google Scholar]
Sargeant 2001
- Sargeant JD, Tickle JJ, Beach ML, Dalton MA, Ahrens MB, Heatherton TF. Brand appearances in contemporary cinema films and contribution to global marketing of cigarettes. Lancet 2001;357:29‐32. [DOI] [PubMed] [Google Scholar]
Schwartz 1987
- Schwartz JL. Review and evaluation of smoking cessation methods: the United States and Canada, 1978‐1985. Washington, DC: National Institutes of Health, Division of Cancer Prevention and Control, National Cancer Institute; 1987. U.S. DHHS no. 87‐2940.
Scott 1990
- Scott RR, Mayer JA, Denier CA, Dawson BL, Lamparski D. Long‐term smoking status of cardiac patients following symptom‐specific cessation advice. Addictive Behaviors 1990;15:549‐52. [DOI] [PubMed] [Google Scholar]
Stead 2013
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thun 2013
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50‐year trends in smoking‐related mortality in the United States. New England Journal of Medicine 2013;368(4):351‐64. [DOI: 10.1056/NEJMsa1211127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 1981
- Weinberger M, Greene JY, Mamlin JJ, Jerin MJ. Health beliefs and smoking behavior. American Journal of Public Health 1981;71:1253‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2000
- West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Health Education Authority. Thorax 2000;55:987‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yach 2000
- Yach D, Bettcher D. Globalisation of tobacco industry influence and new global responses. Tobacco Control 2000;9:206‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2010
- Young RP, Hopkins RJ, Smith M, Hogarth DK. Smoking cessation: the potential role of risk assessment tools as motivational triggers. Postgraduate Medical Journal 2010;86:26‐33. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bize 2009
- Bize R, Burnand B, Mueller Y, Rège Walther M, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004705.pub3] [DOI] [PubMed] [Google Scholar]
Bize 2012
- Bize R, Burnand B, Mueller Y, Rège‐Walther M, Camain JY, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004705.pub4] [DOI] [PubMed] [Google Scholar]


