Abstract
Background
The aim of this study was to detect and molecularly identify Rickettsia spp. in Rhipicephalus sanguineus (sensu lato) collected from free-roaming dogs in 30 communities from five municipalities in the south of Coahuila State, northern Mexico, where Rocky Mountain spotted fever is endemic.
Methods
In total, 60 dogs from each municipality were examined for engorged ticks. DNA was isolated from tick pools and conventional PCR assays targeting the 23S-5S ribosomal RNA intergenic spacer and outer membrane protein (ompA) gene of Rickettsia spp. were performed.
Results
All ticks (n = 1238) were morphologically identified as R. sanguineus (s.l.). Six pools (each with six engorged females) from four municipalities were positive to Rickettsia spp. DNA sequencing and phylogenetic analyses confirmed the presence of R. rickettsii and R. rhipicephali in R. sanguineus (s.l.) in these ticks.
Conclusions
This study confirms the presence of R. rickettsii and R. rhipicephali in R. sanguineus (s.l.) from stray dogs in the south of Coahuila. This suggests that stray dogs may play a role in the inter-municipal dissemination of infected ticks in this region. Further research is required to assess whether ticks from stray dogs could serve as good indicators for the molecular xenomonitoring of R. rickettsii in this region. Considering that R. sanguineus (s.l.) is a proven vector of R. rickettsii in Mexico, increased awareness regarding permanent tick control in dogs is warranted.
Keywords: R. rickettsii, R. rhipicephali, R. sanguineus (s.l.), Coahuila, Mexico
Background
Rickettsia rickettsii is the causative agent of Rocky Mountain spotted fever (RMSF), a tick-borne disease with increasing incidence rate in North, Central and South American countries, including Mexico [1–3]. RMSF is the most common rickettsial disease affecting human beings in Mexico, with devastating outbreaks detected in several communities in the states of Sinaloa, Sonora, Durango and Coahuila [3]. For instance, Coahuila reported an estimated incidence rate of RMSF of three cases per 100,000 inhabitants in 2014, which corresponds to the highest incidence rate reported in Mexico during that year [4]. It is worth mentioning that the possibility of underreporting is high, especially because RMSF cases are often misdiagnosed with other diseases that cause flu-like symptoms, including dengue fever [5].
Recent epidemics of RMSF in south-western USA and northern Mexico have been associated to massive environmental infestations by brown dog ticks, Rhipicephalus sanguineus (sensu lato) [1, 3, 6]. Brown dog ticks are recognized as important vectors of several pathogenic organisms in south-western USA and northern Mexico, including R. rickettsii [1, 3, 6, 7], and are the most frequent ectoparasites of dogs [1, 3, 6–8]. For instance, R. sanguineus (s.l.) is commonly found infesting both privately-owned and stray dogs in urban and rural areas in Mexicali, the capital of Baja California state, northern Mexico [8], where an epidemic of RMSF was documented in 2008, and has since affected approximately 4000 people as of 2018 [6].
In recent outbreaks of RMSF associated with brown dog ticks (e.g. in eastern Arizona, south-western USA [1]), most human cases were diagnosed in communities with large numbers of free-roaming dogs and brown dog ticks. Incidentally, free-roaming dogs are common in northern Mexico and could play a role in disseminating R. rickettsii-infected brown dog ticks between communities and even between municipalities.
Bearing this in mind, the objective of the present study was to detect and molecularly confirm the presence of R. rickettsii in R. sanguineus (s.l.) collected from free-roaming dogs in different municipalities in the south of Coahuila, where a recent outbreak of RMSF was reported.
Methods
Study area and samples
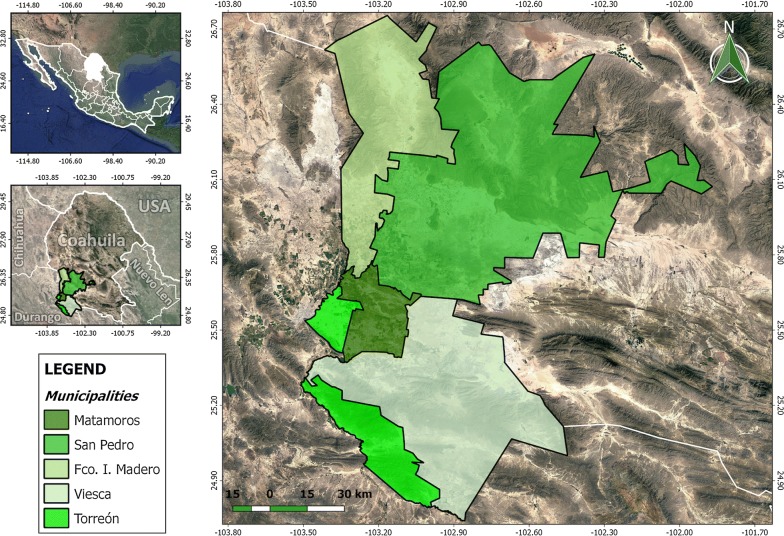
This study was performed from September 2015 to February 2016 in 30 communities belonging to five municipalities (Torreon, San Pedro, Viesca, Francisco I. Madero and Matamoros) in the south of Coahuila (Fig. 1). This region is featured by limited water resources and a warm summer, with an annual temperature ranging between 20–22 °C but reaching around 50 °C during the hottest days of the year. The rainfall ranges between 200–250 mm per year and the average altitude is between 1000 and 1200 m above sea level. From each municipality, 60 free-roaming dogs were captured and restrained with help of people from the local communities, some of them claiming to be the dog owners, although all the dogs were captured in the streets. The sampling procedures were carried out according to the guidelines of the Federal Law for Animal Health [9]. The dogs were examined for engorged ticks, and when present, six ticks per dog were collected from most of the animals. However, the tick infestation level was very low in some cases and less than six ticks were collected. Considering that the R. rickettsii infection rate in ticks is very low, we focused our study on engorged ticks to increase the probability of detection, and also because a previous study showed that 100% of the engorged females became infected upon feeding on dogs with rickettsia [10]. Collected ticks were preserved in vials containing 70% ethanol and morphologically identified in the laboratory [11–13].
Fig. 1.
Map showing the location of the municipalities in Coahuila, Mexico, surveyed in the present study
DNA extraction and PCR assays
To assess the circulation of Rickettsia spp. in the surveyed communities, ticks collected from dogs were pooled per community (maximum six ticks per pool) (Table 1) and stored in 1.5 ml tubes (Eppendorf, Hilden, Germany) at -80 °C. Prior to DNA extraction, ticks were washed three times in distilled water for 10 min to reduce external bacteria and other possible contaminants, then dried using sterile filter paper. DNA from pooled ticks was extracted using the cetyltrimethylammonium bromide method [14].
Table 1.
Number of ticks collected from five municipalities in the south of Coahuila, northern Mexico, according to community of origin and developmental stage. Infestation rates and mean intensity are also reported
| Community | Engorged females | Engorged nymphs | Tick infestation rate (%) | Mean intensity |
|---|---|---|---|---|
| Congregacion Hidalgo | 17 | 3 | 50 | 4.0 |
| Granada | 37 | 14 | 70 | 7.3 |
| Vicente Guerrero | 21 | 12 | 50 | 6.6 |
| Solis | 29 | 6 | 80 | 4.4 |
| Manantial | 28 | 10 | 60 | 6.3 |
| El Cambio | 37 | 2 | 100 | 3.9 |
| La Ventana | 67 | 6 | 70 | 10.4 |
| San Juan de Villanueva | 21 | 8 | 50 | 5.8 |
| San Isidro | 12 | 4 | 80 | 2.0 |
| Villa de Bilbao | 38 | 3 | 70 | 5.9 |
| Gabino Vazquez | 35 | 2 | 100 | 3.7 |
| Zapata | 53 | 2 | 100 | 5.5 |
| El Retiro | 22 | 2 | 80 | 3.0 |
| Luchana | 85 | 2 | 90 | 9.8 |
| Concordia | 20 | 1 | 80 | 2.6 |
| San Miguel | 19 | 2 | 100 | 2.1 |
| Mayrán | 20 | 4 | 100 | 2.4 |
| San Nicolas | 30 | 1 | 80 | 3.9 |
| Lequeitio | 92 | 15 | 100 | 10.7 |
| Jaboncillo | 27 | 4 | 80 | 3.9 |
| Santo Niño | 29 | 1 | 80 | 3.8 |
| Hidalgo | 33 | 16 | 100 | 4.9 |
| El Cántabro | 44 | 5 | 100 | 4.9 |
| El Venado | 72 | 10 | 100 | 8.2 |
| La Flor de Jimulco | 49 | 5 | 100 | 5.4 |
| Ana | 24 | 6 | 100 | 3.0 |
| La Partida | 23 | 15 | 100 | 3.8 |
| Rancho Alegre | 10 | 2 | 60 | 2.0 |
| Juan Eugenio | 25 | 6 | 100 | 3.1 |
| La Trinidad | 44 | 6 | 100 | 5.0 |
| Total | 1063 | 175 | 84.3 | 4.9 |
DNA samples were initially screened by a conventional PCR assay targeting the internal transcribed spacer (ITS) between the 23S and 5S ribosomal RNA genes (23S-5S rRNA ITS) of Rickettsia spp., using the primers ITS-F (5′-GAT AGG TCG GGT GTG GAA G-3′) and ITS-R (5′-TCG GGA TGG GAT CGT GTG-3′), as previously described [15]. Briefly, cycling conditions consisted of 5 min of initial denaturation at 96 °C, 35 cycles at 94 °C for 1 min, 52 °C for 1 min and 72 °C for 1 min, followed by a final extension of 5 min at 72 °C. Distilled water and a previously determined R. rickettsii-positive sample [16] were used as negative and positive controls, respectively.
Positive pools were further tested by a conventional PCR assay targeting the outer membrane protein A (ompA) gene of Rickettsia spp. belonging to the spotted fever group, using the primers Rr190.70p (5′-ATG GCG AAT ATT TCT CCA AAA-3′) and Rr190.701n (5′-GTT CCG TTA ATG GCA GCA TCT-3′), as described elsewhere [17, 18]. PCR thermal conditions were as follows: 3 min of initial denaturation at 95 °C, 35 cycles at 95 °C for 20 s, 46 °C for 30 s and 63 °C for 1 min, followed by a holding at 72 °C for 7 min. All amplifications were carried out in a Px2 Thermal Cycler (ThermoFisher Scientific, Waltham, MA, USA).
DNA sequencing and phylogenetic analysis
PCR products were purified with ExoSAP-IT kit (Affymetrix, Cleveland, OH, USA), following the manufacturer’s instructions, and purified products sequenced at the Macrogen DNA Sequencing Service (Rockville, MD, USA). For phylogenetic analysis, maximum likelihood (ML) phylogenetic trees based on Rickettsia 23S-5S rRNA ITS and ompA gene sequences were inferred using Molecular Evolutionary Genetics Analysis (MEGA) software, v.6 [19]. Sequences were collected in GenBank to represent different Rickettsia species (using 23S-5S rRNA ITS and ompA) and strains (using ompA) as shown in results. Sequences were aligned with MAFFT (v.7) configured for the highest accuracy using the scoring matrix 200PAM/kD2, alignment strategy MAFFT-FFT-NS-I, gap opening penalty 1.53 and offset value 0.123. The best-fit model of sequence evolution was selected based on corrected Akaike information criterion and Bayesian information criterion implemented in MEGA. The Tamura 3-parameter model [20], which showed the lowest values of corrected Akaike information criterion and Bayesian information criterion (for both 23S-5S rRNA ITS and ompA), was chosen for the tree reconstruction. Initial trees for the heuristic search were obtained automatically in MEGA by applying neighbour-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood approach, and then selecting the topology with superior log likelihood value. Reliability of internal branches was assessed using the bootstrapping method with 1000 bootstrap replicates [21].
Minimum infection rate
The minimum infection rate (MIR) was calculated assuming that each positive pool contained at least one positive tick, using the formula: MIR = (number of positive pools / total number of tested ticks) × 100.
Results
Out of 300 free-roaming dogs enrolled in the present study, 253 were infested by ticks, which corresponds to an overall infestation rate of 84.3% (95% CI: 80.2–88.4%). All ticks collected were morphologically identified as R. sanguineus (s.l.). In total, 1238 specimens were collected, of which 1063 (85.9%) were engorged females and 175 (14.1%) engorged nymphs. Out of 30 pools tested, six (each six females) were found to be positive to Rickettsia spp. DNA (Table 2), with an MIR of 3.3% (95% CI: 1.2–7.1%).
Table 2.
Rickettsia spp. identified in Rhipicephalus sanguineus (sensu lato) ticks collected from dogs from five municipalities in the south of Coahuila, northern Mexico
| Municipality | Tick-infested dogs/examined dogs | Tick infestation rate (%) | Pools positive/tested to 23S-5S rRNA ITS (GenBank ID) | Pools positive/tested to ompA (GenBank ID) | Species identification |
|---|---|---|---|---|---|
| Francisco I. Madero | 56/60 | 93.3 | 1/6 (MF925412) | 1/1 (MF925420) | R. rickettsii |
| Torreon | 56/60 | 93.3 | 2/6 (MF925413, MF325415) | 1/2 (MF925418) | R. rickettsii and R. rhipicephali |
| Viesca | 47/60 | 78.3 | 1/5 (MF925414) | 1/1 (MF925419) | R. rickettsii |
| San Pedro | 53/60 | 88.3 | 2/7 (MF925416, MF925417) | 0/2 | R. rhipicephali |
| Matamoros | 41/60 | 68.3 | 0/6 | 0/0 | na |
| Total | 253/300 | 84.3 | 6/30 | 3/6 | na |
Abbreviation: na, not applicable
By BLAST analysis, the sequences obtained from ticks collected in Francisco I. Madero, Torreon and Viesca presented 99% identity to different sequences of R. rickettsii from the USA (GenBank: U11022, CP003311 and CP018914). Two sequences from San Pedro and one from Torreon showed 99% identity to Rickettsia rhipicephali from south-eastern Brazil (GenBank: KT340606), New Mexico (USA) (GenBank: CP013133) and North Carolina (USA) (GenBank: CP003342). No positive samples were found in the municipality of Matamoros. GenBank accession numbers for the sequences generated in this study are provided in Table 2 and Fig. 2.
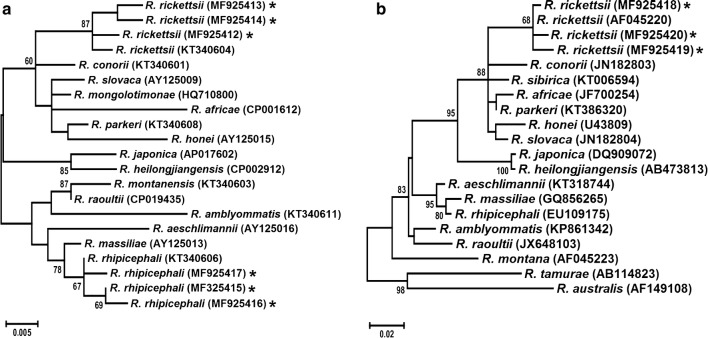
Fig. 2.
Phylogenetic analysis of Rickettsia spp. identified in Mexico. Maximum likelihood phylogenetic trees were inferred using 23S-5S rRNA ITS (a) and ompA sequences (b) of Rickettsia spp. identified in this study (asterisks) and other bacteria of the order Rickettsiales. The 23S-5S rRNA ITS and ompA sequences of Mexico clustered with R. rickettsii and R. rhipicephali reference sequences. Reliability of internal branches was assessed using the bootstrap test (1000 replicates) and only values higher than 60% are shown
The phylogenetic analysis confirmed that the sequences generated in this study formed well-defined groups with other sequences from R. rhipicephali and R. rickettsii, which were supported by high bootstrap values (Fig. 2).
Discussion
In the present study, we detected the presence of Rickettsia spp. DNA in R. sanguineus (s.l.) collected from free-roaming dogs in all investigated municipalities except Matamoros. Rickettsia rickettsii was detected in ticks from the municipalities of Francisco I. Madero, Torreon and Viesca. The absence of positive samples from Matamoros is in contrast with a previous study, which reported the presence of R. rickettsii DNA in R. sanguineus (s.l.) [16]. Nonetheless, this finding is not unexpected as the prevalence of infection in ticks is usually very low [1, 22]. In fact, high infection rates may be due to ticks collected from dogs with bacteraemia, as discussed elsewhere [23].
In two pools from Torreon and San Pedro, sequences with 99% of identity to R. rhipicephali were obtained, representing the first record of this rickettsial organism in northern Mexico. Rickettsia rhipicephali was originally detected in R. sanguineus (s.l.) in Mississippi, USA [24], then in several other tick species and regions of the world [25]. Because no co-infection with other species from the spotted fever group (e.g. R. rickettsii) has been found in ticks infected with R. rhipicephali, it has been speculated that this rickettsial organism of unknown pathogenicity may protect ticks from subsequent infections with pathogenic rickettsiae [25].
Although Rickettsia spp. DNA was detected in tick pools, the possibility that this DNA was contained in the dog’s blood ingested by the tick during feeding cannot be ruled out, particularly considering that females with varying degrees of engorgement were included in the pools. Nonetheless, it is interesting to note that the bacteraemia produced by R. rickettsii in dogs is transient, lasting from 3 to 13 days under experimental conditions [10, 26]. Therefore, there is also a possibility that some of these females were already infected before feeding on these dogs. As previously mentioned, R. sanguineus (s.l.) is a recognized vector of R. rickettsii in some epidemic foci of RMSF [27], including in northern Mexico and south-western USA [1, 3, 5], where this tick is the primary vector of this pathogen.
Although brown dog ticks are considered to display low affinity for humans [28], previous studies indicate that human parasitism may be more common than previously recognized in some regions [29–31]. Moreover, R sanguineus (s.l.) constitutes a complex of species [11, 12, 32], whose degree of affinity to humans and other non-canine hosts should be better investigated.
Even though all dogs enrolled in this study were found free roaming in the streets of the investigated municipalities, most of them are in daily contact with people. In some cases, people from the local communities declared themselves as owners of the dogs and reported that they were typically living outdoors during the day and indoors during the night. This behaviour may contribute to the introduction to ticks into the houses, potentially increasing the risk of R. rickettsii transmission. This highlights the need for One Health education initiatives in the RMSF-endemic regions in Mexico, to inform dog owners and the general population about responsible ownership as well as to increase awareness regarding ticks and tick control in dogs. The community-wide application of long-acting tick collars may be effective in controlling focal outbreaks [33], but should be promoted by public-private partnerships, considering that many dog owners may not be able to handle the costs of permanent tick control. Other interventions such as dog spay and neuter programs and treatment of houses against ticks may also be needed [33].
Conclusions
The present study confirms the presence of R. rickettsii and R. rhipicephali circulating among R. sanguineus (s.l.) collected from free-roaming dogs in Coahuila, where RMSF is endemic. This suggests that free-roaming dogs may play a role in the dissemination of R. rickettsii-infected ticks in this region. Further research is required to assess whether ticks from free-roaming dogs could serve as indicators for the molecular xenomonitoring of R. rickettsii in areas witnessing epidemics of RMSF associated with massive R. sanguineus (s.l.) infestation.
Acknowledgements
We thank the residents of the municipalities where this study was performed. We are also in debt to Alfonso Lomelí-Juárez for his assistance during tick collections and to Lucas C. de Sousa-Paula (Aggeu Magalhães Institute, Fiocruz, Brazil) for elaborating Fig. 1. Publication of this paper was sponsored by Bayer Animal Health in the framework of the 14th CVBD World Forum Symposium.
Funding
EN-R was supported by a postdoctoral fellowship by the Mexican National Council for Science and Technology (CONACYT, grant number: 290941-UAAAN) and VHG-Á was supported by a graduate scholarship from CONACYT. FDT is the recipient of a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 313118/2018-3).
Availability of data and materials
All data that support the findings reported in this study are included in the article. Sequences generated herein have been deposited in the GenBank database under the accession numbers MF925412-MF925417.
Authors’ contributions
AOM and EN-R designed the study. EN-R, VAR, VHG-Á, ACM and QSR performed the experiments. AOM, ACC, CA and FDT analysed the data. AOM, CA and FDT wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Animal Ethics committee of the Universidad Autonoma Agraria Antonio Narro, Unidad Laguna, Coahuila, Mexico.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- RMSF
Rocky Mountain spotted fever
- 23S-5S rRNA ITS
internal transcribed spacer between the 23S and 5S ribosomal RNA genes
- ompA
outer membrane protein A gene
- MIR
minimum infection rate
- s.l.
sensu lato
- PCR
polymerase chain reaction
Contributor Information
Aldo I. Ortega-Morales, Email: agrortega@hotmail.com
Erika Nava-Reyna, Email: erinava27@gmail.com.
Verónica Ávila-Rodríguez, Email: vavilar@gmail.com.
Vicente H. González-Álvarez, Email: homerogonzalez.itssmo@gmail.com
Antonio Castillo-Martínez, Email: acm_sultan@hotmail.com.
Quetzaly K. Siller-Rodríguez, Email: qksr@hotmail.com
Alejandro Cabezas-Cruz, Email: alejandro.cabezas@vet-alfort.fr.
Filipe Dantas-Torres, Email: filipe.dantas@cpqam.fiocruz.br.
Consuelo Almazán, Email: consuelo.almazan@anses.fr.
References
- 1.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 2.Bermúdez CSE, Troyo A. A review of the genus Rickettsia in Central America. Res Rep Trop Med. 2018;9:103–112. doi: 10.2147/RRTM.S160951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Álvarez-Hernández G, Roldán JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis. 2017;17:e189–e196. doi: 10.1016/S1473-3099(17)30173-1. [DOI] [PubMed] [Google Scholar]
- 4.Barton-Behravesh C, Álvarez Hernández G. Panorama epidemiológico de la Fiebre Manchada en Estados Unidos y México. 2016. https://cursofiebremanchada2016.files.wordpress.com/2016/04/2-panorama-epidemiologico-curso-fmrr.pdf. Accessed 11 Aug 2018.
- 5.Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724–732. doi: 10.1016/S1473-3099(07)70261-X. [DOI] [PubMed] [Google Scholar]
- 6.Tinoco-Gracia L, Lomelí MR, Hori-Oshima S, Stephenson N, Foley J. Molecular confirmation of Rocky Mountain spotted fever epidemic agent in Mexicali, Mexico. Emerg Infect Dis. 2018;24:1723–1725. doi: 10.3201/eid2409.171523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martínez MT, Rentería-Evangelista TB, González-Medina Y, Barreras-Serrano A, et al. Prevalence of Rhipicephalus sanguineus ticks on dogs in a region on the Mexico-USA border. Vet Rec. 2009;164:59–61. doi: 10.1136/vr.164.2.59. [DOI] [PubMed] [Google Scholar]
- 9.Secrataria de Agricultura, Ganaderia, Desarrollo Rural, Pesca y Alimentacion. Ley Federal de Sanidad Animal. 2007. http://www.diputados.gob.mx/LeyesBiblio/ref/lfsa/LFSA_orig_25jul07_ima.pdf. Accessed 13 Nov 2018.
- 10.Piranda EM, Faccini JL, Pinter A, Pacheco RC, Cançado PH, Labruna MB. Experimental infection of Rhipicephalus sanguineus ticks with the bacterium Rickettsia rickettsii, using experimentally infected dogs. Vector Borne Zoonotic Dis. 2011;11:29–36. doi: 10.1089/vbz.2009.0250. [DOI] [PubMed] [Google Scholar]
- 11.Walker JB, Keirans JE, Horak IG. Genus Rhipicephalus (Acari, Ixodidae). A guide to the brown ticks of the world. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 12.Dantas-Torres F, Latrofa MF, Annoscia G, Giannelli A, Parisi A, Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors. 2013;6:213. doi: 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nava S, Beati L, Venzal JM, Labruna MB, Szabó MPJ, Petney T, et al. Rhipicephalus sanguineus (Latreille, 1806): neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick Borne Dis. 2018;9:1573–1585. doi: 10.1016/j.ttbdis.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 15.Vitorino L, Zé-Zé L, Sousa A, Bacellar F, Tenreiro R. rRNA intergenic spacer regions for phylogenetic analysis of Rickettsia species. Ann New York Acad Sci. 2003;990:726–733. doi: 10.1111/j.1749-6632.2003.tb07451.x. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Martínez A, Cueto-Medina SM, Hernández-Rodríguez S, Gallegos-Robles MA, Valdés-Perezgasga MT, Sánchez-Ramos FJ, et al. Detección de Rickettsia sp. en la garrapata café del perro Rhipicephalus sanguineus (Acari: Ixodidae) en Matamoros, Coahuila, México. Acta Zool. 2015;31:80–83. [Google Scholar]
- 17.Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 22.Moraes-Filho J, Marcili A, Nieri-Bastos FA, Richtzenhaina LJ, Labruna MB. Genetic analysis of ticks belonging to the Rhipicephalus sanguineus group in Latin America. Acta Trop. 2011;117:51–55. doi: 10.1016/j.actatropica.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Pacheco RC, Moraes-Filho J, Guedes E, Silveira I, Richtzenhain LJ, Leite RC. Rickettsial infections of dogs, horses and ticks in Juiz de Fora, southeastern Brazil, and isolation of Rickettsia rickettsii from Rhipicephalus sanguineus ticks. Med Vet Entomol. 2011;25:148–155. doi: 10.1111/j.1365-2915.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 24.Burgdorfer W, Sexton DJ, Gerloff RK, Anacker RL, Philip RN, Thomas LA. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975;12:205–210. doi: 10.1128/iai.12.1.205-210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, et al. The eco-epidemiology of Pacific Coast tick fever in California. PLoS Negl Trop Dis. 2016;10:e0005020. doi: 10.1371/journal.pntd.0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piranda EM, Faccini JL, Pinter A, Saito TB, Pacheco RC, Hagiwara MK, et al. Experimental infection of dogs with a Brazilian strain of Rickettsia rickettsii: clinical and laboratory findings. Mem Inst Oswaldo Cruz. 2008;103:696–701. doi: 10.1590/S0074-02762008000700012. [DOI] [PubMed] [Google Scholar]
- 27.Bustamante ME, Varela GIV. Studies on spotted fever in Mexico: the role of Rhipicephalus sanguineus in the transmission of spotted fever in the Mexican Republic. Rev Inst Salubr Enferm Trop. 1947;8:139–141. [Google Scholar]
- 28.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 29.Zavala-Castro JE, Zavala-Velázquez JE, Walker DH, Ruíz-Arcila EE, Laviada-Molina H, Olano JP, et al. Fatal human infection with Rickettsia rickettsii, Yucatán, Mexico. Emerg Infect Dis. 2006;12:672–674. doi: 10.3201/eid1204.051282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dantas-Torres F, Figueredo LA, Brandao-Filho SP. Rhipicephalus sanguineus (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rev Soc Bras Med Trop. 2006;39:64–67. doi: 10.1590/S0037-86822006000100012. [DOI] [PubMed] [Google Scholar]
- 31.Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Cascio A, Cazzin S, et al. Ticks infesting humans in Italy and associated pathogens. Parasit Vectors. 2014;7:328. doi: 10.1186/1756-3305-7-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dantas-Torres F. Species concepts: what about ticks? Trends Parasitol. 2018;34:1017–1026. doi: 10.1016/j.pt.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Drexler N, Miller M, Gerding J, Todd S, Adams L, Dahlgren FS, et al. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One. 2014;9:e112368. doi: 10.1371/journal.pone.0112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings reported in this study are included in the article. Sequences generated herein have been deposited in the GenBank database under the accession numbers MF925412-MF925417.