Abstract
Infections with Bartonella bacilliformis result in Carrion’s disease in humans. In the first phase of infection, the pathogen causes a hemolytic fever (“Oroya fever”) with case-fatality rates as high as ~90% in untreated patients, followed by a chronical phase resulting in angiogenic skin lesions (“verruga peruana”). Bartonella bacilliformis is endemic to South American Andean valleys and is transmitted via sand flies (Lutzomyia spp.). Humans are the only known reservoir for this old disease and therefore no animal infection model is available. In the present review, we provide the current knowledge on B. bacilliformis and its pathogenicity factors, vectors, possible unknown reservoirs, established and potential infection models and immunological aspects of the disease.
Keywords: Bartonella bacilliformis, Carrion’s disease, Vector-borne disease, Lutzomyia, South America, Neglected tropical disease
Background
Carrion’s disease is a vector-borne biphasic illness restricted to the South American Andes including Peru, Ecuador and Colombia and is endemic in Andean valleys at an altitude of 600–3200 m above sea level; it has also been described in the coastal areas of Guayas and Manabi in Ecuador [1, 2]. The causative agent of this neglected disease is Bartonella bacilliformis, which is a motile, aerobic, facultative intracellular alpha-2-proteobacterium. It infects human erythrocytes first causing a serious acute hemolytic anemia called “Oroya fever” followed by a chronic infection of endothelial cells resulting in vasculo-endothelial proliferations called “verruga peruana” as the result of the continuous angiogenic stimulus by B. bacilliformis. These two syndromes typically occur sequentially but sometimes independently. An infection with B. bacilliformis can result in a variety of different clinical manifestations such as severe illness, mild or asymptomatic illness or chronic asymptomatic bacteremia [3]. The exact factors which define the clinical course of Carrion’s disease are still unknown but it is assumed that the interplay of virulence factors of the strain, the inoculum and the fitness and individual predisposition of the host determine the severity of the clinical manifestation [4]. The existence of less virulent bacterial strains that cause mild atypical bartonellosis has been suggested, meaning Carrion’s disease is under-reported [1]. Bartonella bacilliformis is transmitted to humans by female phlebotomine sand flies (Lutzomyia spp.) which are present in high-altitude regions. Climatic changes favor the expansion of B. bacilliformis infections through sand fly proliferation [5, 6].
Oroya fever (characterized by an intraerythrocytic anemia) (Fig. 1) is more common in children than in adults and it is characterized by a plethora of symptoms including fever, hemolytic anemia, pallor, myalgia, headache, anorexia, tachycardia and hepatomegaly [5] with an immune-compromised state that facilitates secondary infections such as Toxoplasma gondii myocarditis or bacteremia with Staphylococcus aureus or Salmonella enterica [4]. In this early phase of infection, B. bacilliformis spreads into the circulatory system invading erythrocytes and leading a hemolytic anemia due to the splenic depletion of infected erythrocytes. Case-fatality rates as high as 88% have been described in the Oroya fever phase in untreated patients, meanwhile around 10% case-fatality rates have been reported for patients receiving timely antibiotic treatment [7].
Fig. 1.
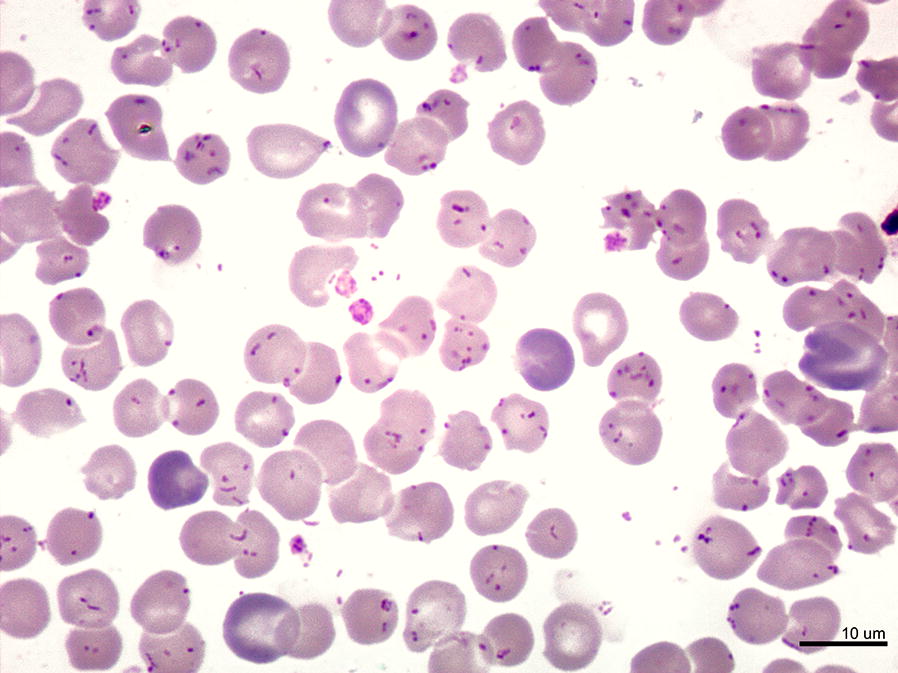
Overwhelming parasitism of erythrocytes by B. bacilliformis. Giemsa-stained blood smear from a patient with Oroya fever, showing parasitism of all erythrocytes, with bacillary and coccoid forms of B. bacilliformis. Scale-bar: 10 µm (courtesy of P. Ventosilla and M. Montes, Universidad Peruana Cayetano Heredia, Lima, Peru)
The life-cycles of Bartonella spp. in their respective vectors are better known for many of the species other than B. bacilliformis. Those studies propose that Bartonella is present in the midgut of arthropod vectors and is released onto the mammalian skin in feces in order to pass to the dermal niche after erosion of the skin. The lymphatic system seems to be responsible for spreading the pathogen into the circulatory system and an intracellular presence of the bacteria (here in erythrocytes) avoids clearance by the host immune system [8, 9]. In the case of B. bacilliformis, it remains unknown if there is a dermal inoculation prior the blood spreading since the only known vectors to date are sand flies (Lutzomyia spp.) which might transmit the bacteria directly into the bloodstream. Moreover, as there are currently no animal infection models, the exact mechanisms underlying the pathobiology of this early infection state cannot be analyzed in detail in an experimental setting.
If Oroya fever is survived, the chronic verruga peruana phase can occur impressing as blood-filled nodular hemangioma-like lesions in the skin (Fig. 2). Under all human pathogenic bacteria, only the family of Bartonella has the ability to trigger angiogenic disease entities (B. bacilliformis: verruga peruana; B. henselae, B. quintana: bacillary angiomatosis, peliosis hepatis [10]). It is suggested that the abnormal endothelial cell proliferation is induced by a chronic Bartonella-infection in which the bacteria are included into vacuoles inside the capillary endothelium. Peruvian warts are mostly found on the head and extremities persisting from weeks to months. These lesions were described in the 16th century by Spanish conquerors [5, 7] (Fig. 3).
Fig. 2.
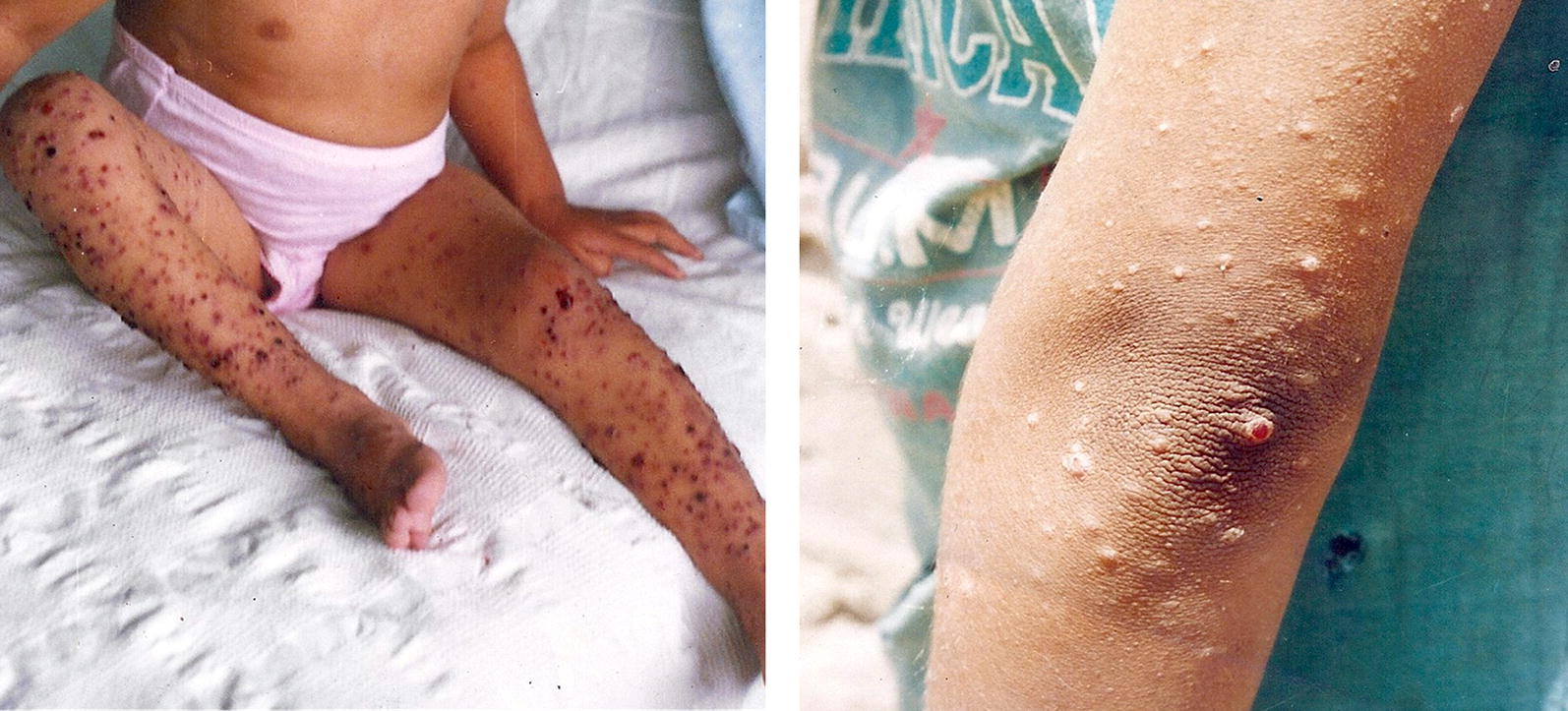
Patients with Verruga peruana caused by B. bacilliformis. Left: 9-year-old girl with numerous bleeding verrugas on her legs; Huaraz, Ancash, 1993. Right: 17-year-old girl (facing left) showing multiple verrugas close to her left elbow; a single verruga has broken the overlying epidermis, and may later bleed; Huari, Ancash, 2002 (courtesy of C. Maguiña, Universidad Peruana Cayetano Heredia, Lima, Peru)
Fig. 3.

Ceramic masks (400 B.C.–400 A.D.). Two masks discovered in Ecuador displaying the facial symptoms of verruga peruana. Citation: Sotomayor-Tribín HA. Pensamiento analógico mítico en la interpretación del arte prehispánico de interés para la arqueomedicina y la paleopatología. Repert Med Cir. 2016;25:50–71 [94]. With permission of Elsevier
In general, Carrion’s disease has been only poorly investigated; a PubMed query in December 2018 with the terminus “Bartonella bacilliformis” revealed only 258 publications, many of them from Peru where the pathogen is endemic [in contrast: Staphylococcus aureus, 112,157 publications; Trypanosoma cruzi (endemic in South America), 14,936 publications). The field suffers from a significant lack of data about many aspects of Carrion’s disease, a limited knowledge about confirmed vectors or reservoirs of B. bacilliformis and the absence of feasible animal infection models. The assumed general strategy underlying a Bartonella infection is (i) the avoidance of the host immune response and the infection of a primary niche (if this exists); (ii) the invasion of erythrocytes; and (iii) an intraerythrocytic replication [11] resulting in erythrocyte rupture [12]. Exact mechanisms involved in all these steps are not studied in detail. It is known that flagella of B. bacilliformis are not recognized by Toll-like receptor 5 (TLR5) avoiding a broad activation of the innate immune system [13] and it is assumed that adhesins might mediate autoaggregation [14] to prevent phagocytosis [11]. On the other hand, adhesins, flagellin, hemolysin, deformin or the invasion associate locus proteins A and B are some factors that have been associated with erythrocyte infections. In this review we summarize the current knowledge for B. bacilliformis with regard to vectors, pathogenicity factors and infection models.
Vectors and reservoirs for B. bacilliformis
Sand flies belonging to the genus Lutzomyia (Fig. 4) are considered the only vector for B. bacilliformis. The first evidence for the transmission of B. bacilliformis was found in 1913 when Charles Townsend captured sand flies in the train station where workers suffered from Carrion’s disease [15]. In 1929, the pioneer in analyzing Oroya fever, Hideyo Noguchi, determined which insects are responsible of the transmission of the disease by exposing Macacus rhesus monkeys to bat flies, bedbugs, buffalo gnats, fleas, horse flies, lice, mites, midges, mosquitoes, sheep ticks, ticks, and three species of sand flies (L. verrucarum, L. peruensis and L. noguchii). He injected crushed arthropods intradermally and blood cultures were analyzed for the presence of B. bacilliformis. The only vectors whose injections resulted in an infection were L. verrucarum and L. noguchii [16]. From literature, the following Lutzomyia species are suggested vectors for B. bacilliformis: L. ayacuchensis [2], L. columbiana [17], L. gomezi [17], L. maranonensis [18], L. noguchii [16], L. panamensis [17], L. peruensis [19, 20], L. pescei [5], L. robusta [21], L. serrana [2] and L. verrucarum [22]. However, the presence of B. bacilliformis DNA in these insects has only been demonstrated for L. verrucarum [22], L. peruensis [20], L. robusta [23] and L. maranonensis [18].
Fig. 4.

Adult Lutzomyia verrucarum sand flies. Left: male. Right: blood-fed female. Colony-bred adults. Length of each between 2 and 3 mm (courtesy of E. Pérez, Universidad Peruana Cayetano Heredia, Lima, Peru)
Colonization experiments with artificially-infected L. verrucarum (competent vector) and L. longipalpis (non-competent vector) showed that green-fluorescent protein (GFP)-expressing B. bacilliformis bacteria remain in the midgut and are digested with time in L. longipalpis (non-competent vector) meanwhile the pathogen is able to persist in L. verrucarum [24]. The molecular mechanisms for persistence in L. verrucarum have not yet been elucidated.
There is a clear correlation between the distribution of Carrion’s disease and the presence of vectors in endemic areas. The main sand fly species in northern, southern and central Peru are L. verrucarum and L. peruensis. These sand fly species are predominant at altitudes between 1100 and 3200 m above sea level in the Andean mountain valleys of South America [25, 26]. The epidemiological presence of Carrion’s disease in other areas, however, suggests the existence of other Lutzomyia vectors. Lutzomyia serrana was detected in an outbreak in Monzon Valley, L. robusta in outbreaks taking place in Jaen, San Ignacio and Utcubamba, and L. pescei in Huancavelica, Churcampa, Tayacaja, Urubamba, Calca and Quispicanchis (all Peru) during outbreaks [27]. In Colombia, the potential vector for Carrion’s disease is L. columbiana. During 2009–2013, a total of 1389 cases of bartonellosis were reported in Colombia from which 16% were assigned to Carrion’s disease (~3% Oroya fever and ~13% verruga peruana). Reports demonstrated that it was not only the typical endemic areas such as Nariño, Cauca and Valle del Cauca that were affected, but also Antioquia, Caldas, Huila, La Guajira and Risaralda which were not previously considered to be endemic [28].
Noguchi suggested already in 1926 that ticks might represent possible vectors for B. bacilliformis as he demonstrated that B. bacilliformis was transmitted by bites of Dermacentor andersoni from two experimentally-infected to two healthy Macacus rhesus monkeys [29]. In a recent study, B. bacilliformis DNA was detected in ticks (Amblyomma spp. and Rhipicephalus microplus) collected from Tapirus terrestris and Pecari tajacu from Madre de Dios (Peru) suggesting that ticks might be at least considered as potential vectors for B. bacilliformis [30]. It is important to critically discuss some points of this study the possibility of false positive results due to the DNA extraction method (from crushed insects) or due to the high number of cycles (n = 55) and the missing amplicon sequencing procedures. A recent study identified a novel “Candidatus Bartonella rondoniensis” from kissing bugs (Eratyrus mucronatus) in French Guiana [31]. This novel strain is phylogenetically related to B. bacilliformis and B. ancashensis, both known to be human pathogenic [32]. More studies are needed to clarify whether B. bacilliformis and closely related species can be transmitted through other vectors to humans which are not assigned today.
Currently, apart from humans, there is no confirmed reservoir for B. bacilliformis. No solid evidence exists that Tapirus terrestris and Pecari tajacu might serve as reservoirs for B. bacilliformis because no serum/blood was collected from these two wild mammals from which B. bacilliformis DNA-positive ticks were removed [30]. On the other hand, the broad distribution of Tapirus terrestris, Pecari tajacu and ticks is not in concordance with the distribution of Carrion’s disease; therefore, further studies are needed to confirm or discard this possibility. In the hypothetical case that these wild animals did not suffer from a B. bacilliformis infection, ticks might have become infested via blood meals from other, so far unknown animals or even from humans since only 3 out of 43 ticks (6.97%) collected from three Tapirus terrestris and 12 out of 67 ticks (17.91%) collected from three Pecari tajacu were positive for B. bacilliformis DNA [30]. In the case that an animal is found to be bacteremic with Bartonella spp., one could assume that the majority of these blood-sucking ticks would harbor B. bacilliformis DNA as this has been demonstrated for feeding Ixodes ricinus ticks collected from a B. henselae-seropositive cat [33].
Many Bartonella species have various specific animal reservoirs (e.g. cats, deer, foxes, rodents, cattle [34]). For B. bacilliformis, some animal and plant reservoir candidates have been proposed in the past. Here, it is important to know that both male and female sand flies feed on plants, but only females feed on blood since blood meals are required for the maturation of eggs. [9]. A total of 50 animals were tested from households whose children were suffering from Carrion’s disease and only four out of nine non-domesticated rodents were found to be positive for Bartonella-like bacteria; unfortunately, no species determination was undertaken, so it remains unknown if an unexplored animal reservoir for B. bacilliformis might exist [35]. On the other hand, several human pathogens are able to infect or to persist on plant reservoirs such as Salmonella enterica, Pseudomonas aeruginosa, Burkholderia cepacia, Erwinia spp., Staphylococcus aureus, Escherichia coli and Listeria monocytogenes [36]. With this scenario, another possibility might be that B. bacilliformis survives in a plant environment and sand flies become infested after feeding from plants. Bacterial type III and type IV secretion systems are usually involved in plant infection processes. However, B. bacilliformis lacks these secretion systems [37]. In 1953, Herrer [38] tried to recover B. bacilliformis from euphorb plants distributed in the same areas where there had been recent cases of Carrionʼs disease where Carrion’s disease took place but without success.
Pathogenicity factors of B. bacilliformis
The genus Bartonella can be classified into three clades which are formed by Bartonella apis, Bartonella tamiae and the eubartonellea. [39]. The most ancestral Bartonella spp., B. apis, is a honey bee gut symbiont. It is the only non-pathogenic representative of the genus Bartonella and the closest known relative of pathogenic Bartonella species. The genome of the intraerythrocytic pathogen B. tamiae shows many ancestral characteristics but lacks the most of the eubartonellea specific virulence factors. It is believed that this species presents the evolutionary transition state from a gut symbiont towards an intraerythrocytic pathogen [39]. The clade of the eubartonellea itself is subdivided in four major lineages (L1-L4). L1 is formed by B. bacilliformis and B. ancashensis and it is supposed that these Bartonella spp. infect exclusively humans. L2 species are restricted to ruminants and L3 and L4 species infect a variety of different reservoir hosts with the most commonly recognized human pathogenic species B. henselae and B. quintana (both members of L4). All members of the clade eubartonella harbour type IV secretion systems (T4SS) (VirB/VirD4, Vbh/TraG and/or Trw) for, e.g. cellular invasion. The only exception is B. bacilliformis which is the most ancestral species of this clade identified from phylogenetic studies. Genome evolution in Bartonella at species level shows that a high dynamic genomic expansion exists in some species (e.g. B. tribocorum: 2.64 Mb) and genome reduction in others as (e.g. B. bacilliformis: 1.45 Mb) [40].
Confirmed pathogenicity factors of B. bacilliformis
Adhesin
Trimeric autotransporter adhesins (TAA) are found in many Gram-negative bacteria. TAAs mediate autoaggregation, adherence to host cells and matrix proteins, are immunodominant and involved in triggering a specific host cell response after infection [14]. The essential role of TAAs in bacterial pathogenicity has been shown for several TAAs, such as Yersinia adhesin A (YadA) from Y. enterocolitica [41] or Neisseria adhesin A (NadA) from N. meningitidis [42]. As known today, TAAs are encoded in the genomes of all Bartonella spp. [10] and the best studied TAA is Bartonella adhesin A (BadA) of B. henselae [43–45]. Genes homologous to badA have also been found in the genomes of B. bacilliformis [10]. Here, three putative B. bacilliformis adhesins were identified (NCBI accession numbers WP_005766217.1, WP_005766221.1, WP_005767360.1) with a deduced TAA domain structure similar to other TAAs from species of the genus Bartonella. The exact role of Bartonella bacilliformis adhesin A (BbadA) in the infection process is not clear, own ongoing work is aimed to elucidate this in detail (Fig. 5).
Fig. 5.
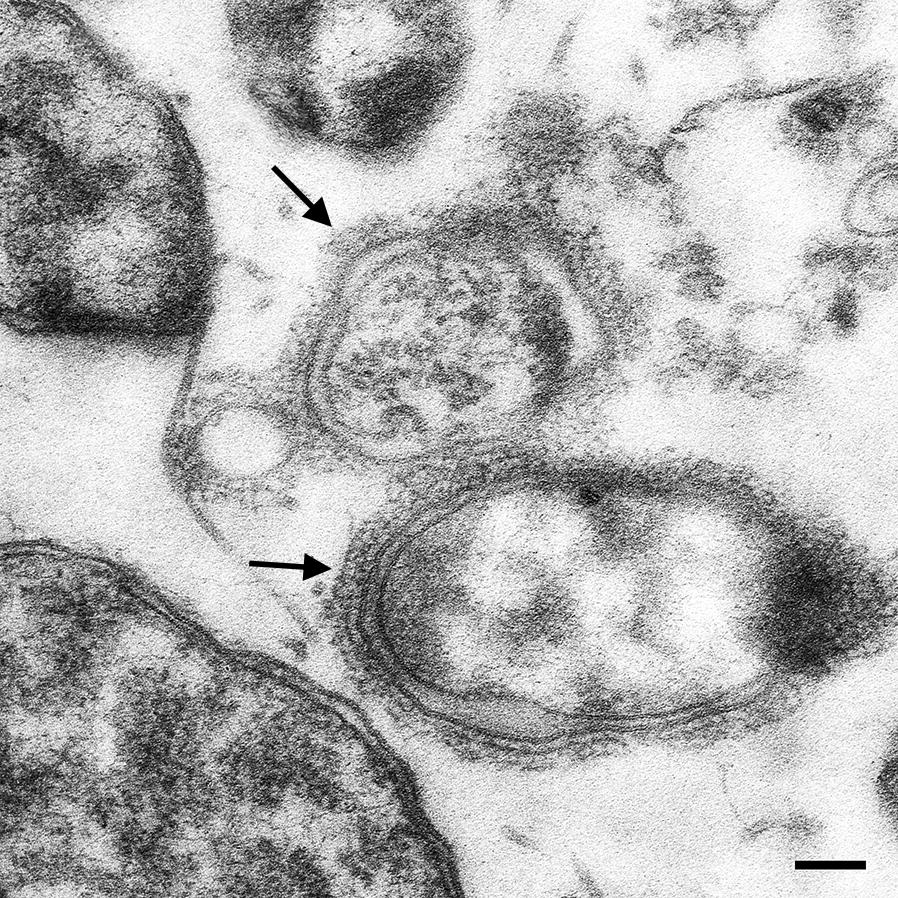
Bartonella bacilliformis adhesin A (BbadA) expressed on the surface of B. bacilliformis. Electron microscopy of B. bacilliformis ATCC 35686 (grown for four days at 28 °C in Bartonella liquid medium [95]). Arrows indicate the presumptive BbadA expression on the bacterial surface. Scale-bar: 100 nm (courtesy of M. Schaller and B. Fehrenbacher, Eberhard Karls-University, Tuebingen, Germany)
Flagellin
Flagella mediate the motility of B. bacilliformis and are composed of 42 kDa flagellin subunits (NCBI accession number WP_011807398) [3]. Typically, B. bacilliformis expresses 2–16 unipolar flagella [3] ~3–10 µm in length (Fig. 6). Adherence of bacteria to erythrocytes correlates with their ability to be motile; however, it is not known whether flagella are directly involved in erythrocyte adhesion or if the bacterial motility increases the probability of encountering erythrocytes. Mutants lacking flagellin expression have been demonstrated to exhibit less erythrocyte adherence compared with wild type bacteria [46] and were unable to enter erythrocytes [47]. In accordance, it was reported that expression of flagella is decisive for erythrocyte invasion since the presence of anti-flagellin antibodies reduced in vitro the erythrocyte invasion of B. bacilliformis [48]. In contrast to other flagellated bacteria (e.g. E. coli, P. aeruginosa or Legionella pneumophila), flagellin from B. bacilliformis is not recognized by Toll-like receptor 5 (TLR5) due to an amino acid exchange in the N-terminal D1 domain and this avoids a NF-κB-regulated inflammatory host cell activation [13].
Fig. 6.
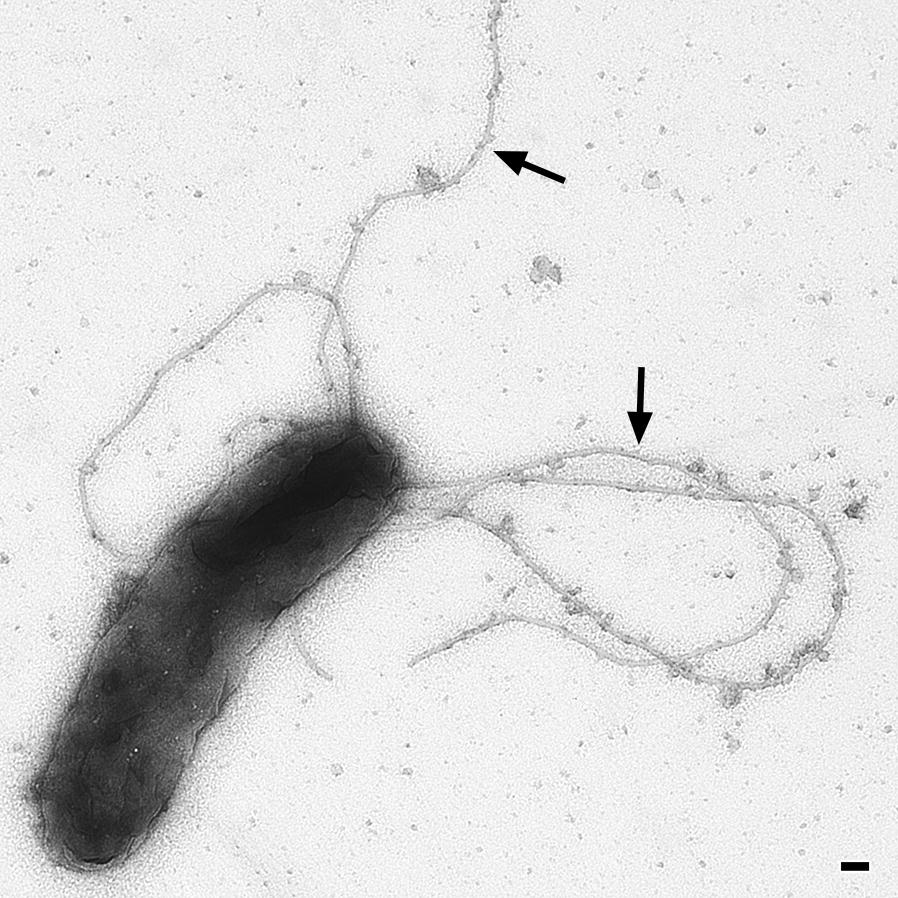
Flagella of B. bacilliformis. Electron microscopy of B. bacilliformis ATCC 35686 (grown for four days at 28 °C in Bartonella liquid medium [95]). Arrows indicate the presumptive BbadA expression on the bacterial surface. Scale-bar: 100 nm (courtesy of M. Schaller and B. Fehrenbacher, Eberhard Karls-University, Tuebingen, Germany)
GroEL
GroEL is a housekeeping protein found nearly in all prokaryotic cells. This heat-shock chaperone is highly conserved and its encoding sequence has been used for multi-locus sequence typing (MLST) [49] and for the analysis of phylogenetic relationships in Bartonella species [50]. The protein (NCBI accession number WP_005767840.1) is also immunodominant in humans [51]. GroEL is present in the inner and outer membrane of B. bacilliformis but it has also been reported to be secreted and involved in establishing an angiogenic phenotype of endothelial cells in vitro [52]. It remains unknown if GroEL is a mitogenic factor by itself or whether it interferes with the expression or stability of other angiogenic B. bacilliformis proteins. Secretion of GroEL has also been described in Helicobacter pylori to protect secreted ureases [53, 54]. The groESL operon is upregulated in response to thermal stress resulting in a ~4-fold induction of groEL expression by a temperature upshift from 30 °C to 37 °C comparable to the temperature shift occurring at the transmission event from sand fly vectors to the human host [55]. GroEL of B. bacilliformis increases apoptosis of human umbilical vein endothelial cells (HUVEC) [56] thereby possibly regulating the growth of endothelial cells.
Hemin-binding proteins
The genome of B. bacilliformis encodes three hemin binding protein (hbp) genes [57] that are homologous to the Pap31 protein of B. henselae [58] (NCBI accession numbers ABA60112.1, KZN22406.1, KZM38396.1, EKS45023.1, ABM44681.1). So far, no functional data of Hbps exist although experiments suggest that these proteins react with patient sera (with unclear specificity). Pap31 of B. bacilliformis seems to be an immunodominant protein [57] and, therefore, it was proposed as a candidate for potential vaccine development strategies [59]. In line with this, owl monkeys (Aotus nancymaae) experienced a four-fold increase of anti-Pap31 (anti-Hbp) IgM levels after infection with B. bacilliformis [60].
Invasion-associated locus proteins A and B
Invasion-associated locus proteins A and B (IalA, IalB; NCBI accession numbers P35640.1 and P35641.1) are important for the invasion of B. bacilliformis into erythrocytes. Heterologous expression of these proteins in E. coli resulted in a strong (up to 39-fold) increase of human erythrocyte invasion in vitro [61]. Homologous proteins have been found in other invasive bacteria (e.g. Ail of Y. enterocolitica mediating invasion into epithelial cells [62, 63]). The exact biological function of IalA, a (di)nucleoside polyphosphate hydrolase, is not clear [64]. The ialB gene encoding a membrane protein is highly conserved among other human-infecting Bartonella and an ialB-deficient mutant exhibits a decreased invasion in human erythrocytes [65]. The highest levels of ialB mRNA and IalB expression were found at 20 °C and acidic pH and the lowest levels were found at 37 °C and basic pH. These observations suggest that in chronic infections (verruga peruana), a further invasion of B. bacilliformis in circulating erythrocytes (which would result in hemolytic anemia) is avoided [66].
Non-confirmed pathogenicity factors
Deformin
An infection with B. bacilliformis induces morphological changes of erythrocytes that finally result in Bartonella invagination (Fig. 7). This deformation seems to be induced by extracellular molecules potentially secreted by B. bacilliformis (called “deformation factors” or “deformins” [47]). This effect was also detectable when erythrocytes were exposed to unknown compounds filtrated from B. bacilliformis culture supernatants. The nature of these compounds seems to be aminoacidic as heating of the supernatants prohibits this effect. To date, there is no consensus in the weight of the hypothetical molecule [67]. Moreover, in the recently published genomes, no clear hit for a “deformin” has been found.
Fig. 7.
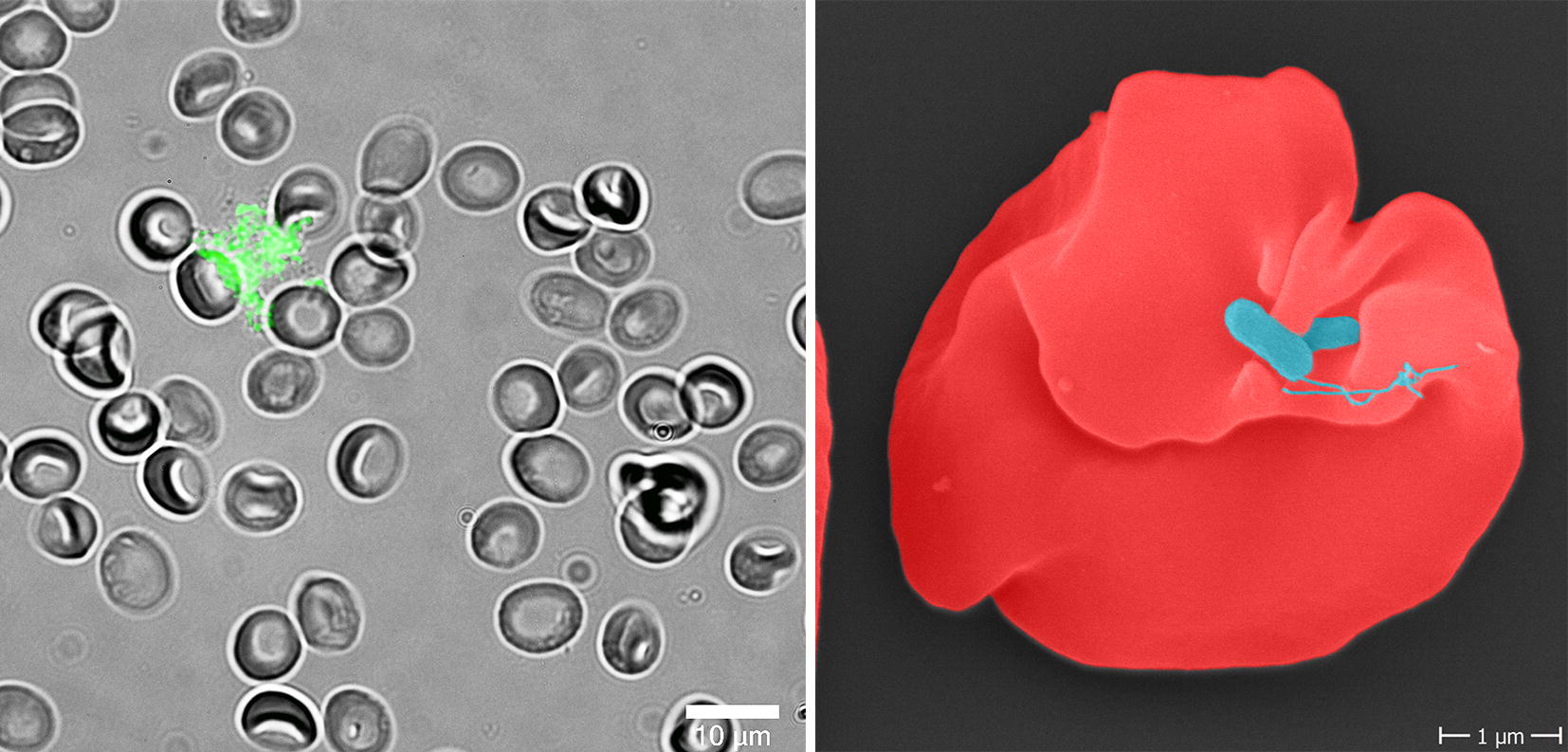
Human erythrocytes infected with B. bacilliformis. Left: Fluorescence microscopy of human erythrocytes infected with GFP-expressing B. bacilliformis ATCC 35686 (6 h). Note the deformation of the erythrocyte cell surface (Aepfelbacher and Kempf, 2018). Scale-bar: 10 µm. Right: Scanning electron microscopy of infected human erythrocytes (24 h). Note the deformation of the erythrocyte. Scale-bar: 1 µm (courtesy of C. Sittmann, Goethe University, Frankfurt am Main, Germany and K. Hipp, Max Planck-Institute for developmental Biology, Tuebingen, Germany)
Hemolysins
The first deeper analysis of the hemolytic activity of B. bacilliformis revealed that for the hemolytic activity a proteinaceous compound might be responsible which increases red blood fragility but the author failed in an exact identification of the presumed compound [68]. Different accession numbers for hemolysin A and D are given (NCBI accession numbers KZN22078.1, KZM38023.1, EKS44973.1, KZN22169.1, KZN21496.1, KZM38155.1, KZM37455.1, ABM44735.1); however, these entries have not been supported by any functional data.
Non-identified outer membrane proteins
Outer membrane proteins (OMPs) of B. bacilliformis were investigated for the ability to bind directly to actin. Six major proteins with molecular weights of 100, 92, 84, 46, 37 and 12 kDa, respectively, bind possibly to actin [69]. These experiments were limited by the fact that they were performed under SDS-denaturing conditions and no further functional assays have been published in course, neither were these proteins further identified. On the other hand,
B. bacilliformis was demonstrated to be able to bind human erythrocyte proteins such as spectrin, band 3 protein, and glycophorin A and B [70] which are components of the erythrocyte cytoskeleton.
Cellular B. bacilliformis infection models
To date, no reliable small animal infection model exists for B. bacilliformis. Therefore, “cellular microbiology” seems to be the tool of choice to understand the underlying pathogenicity mechanisms occurring in B. bacilliformis infections. To study the biphasic Carrion’s disease, various in vitro infection models have been established employing erythrocytes and endothelial cells.
Erythrocyte infection models
Bartonella bacilliformis infection experiments with human erythrocytes allow the analysis of bacterial adhesion and invasion in greater detail. For this, standard techniques were mainly employed [46, 71] as follows (or similar): after removing unbound bacteria by washing, erythrocyte-bound bacteria are visualized and quantified by Giemsa staining and light microscopy or via electron microscopy. By this, it was shown that B. bacilliformis leads to substantial and long-lasting deformations in erythrocyte membranes where bacteria are localized [46, 48] and this resulted in the hypothesis of a so-called “deformin” protein (see above). The entry of B. bacilliformis into erythrocytes has also been monitored by fluorescence microscopy and by transmission electron microscopy [46]. Moreover, invasion kinetics were determined using gentamicin-protection assays killing the extracellular bacteria prior the lysis of erythrocytes and subsequent cultivation of the intracellular (aminoglycoside-protected) bacteria [48]. Various studies revealed that non-motile or flagella-function-inhibited bacteria are drastically reduced in their association with erythrocytes. Furthermore, treatment with enzymes (affecting outer proteins) or incubation with respiratory chain inhibitors was also demonstrated to influence bacterial erythrocyte adherence [71].
Endothelial cell infection models
Bartonella bacilliformis invades endothelial cells and induces cellular proliferation (similar to angiogenesis events) causing the formation of verruga peruana. To identify potential pathogeny factors, live bacteria, bacterial lysates or conditioned media were co-cultivated with human endothelial cells.
By using 35S-methionine-labeled bacteria, it has been shown that B. bacilliformis invades several cell types in vitro (e.g. human dermal fibroblasts, HEp-2 and HeLa-229 cells and HUVECs). From this it was hypothesized that the in vivo preference for endothelial cell infection might be based on the dissemination route (bloodstream) rather than on cell tropism [72]. Electron microscopy revealed that bacteria invade endothelial cells rapidly (1 h) forming large vacuolic inclusions after 12 hours of infection similar to Rocha-Lima inclusions [73]. Bartonella bacilliformis stimulates its entry into endothelial cells by activating Rho-family GTPases (Rho, Rac, Cdc42) leading to morphological changes of infected endothelial cells [74–76]. These small GTP-binding proteins are key regulators in the organization of the actin cytoskeleton and their activation results in the formation of filopodia and lamellopodia facilitating bacterial entry into host cells [76].
The addition of B. bacilliformis culture extracts stimulates HUVEC proliferation ~3-fold and this phenomenon was attributed to a heating-sensitive compound of around 12–14 kDa [77]. In addition, B. bacilliformis activates the release of the tissue plasminogen activator (t-PA) from endothelial cells in vitro and this process is known to be involved in angiogenic processes. These authors also demonstrated that infection with B. bacilliformis results in endothelial proliferation and that a direct contact between bacteria and host cells results in higher proliferation rates compared with settings where bacteria and host cells were physically separated [73]. The increase of endothelial proliferation (6- to 20-fold) was confirmed in a later study by exposing endothelial cells to B. bacilliformis culture supernatants and this phenomenon was dependent on a bacteria-derived proteinaceous mitogen [52].
Other experiments demonstrated that a B. bacilliformis infection results in a strong induction of angiopoietin-2 in endothelial cells [78]. These findings are in-line with the observations made by in situ hybridizations of clinical human verruga peruana specimens where high expression levels of angiopoietin-2 and vascular endothelial growth factor (VEGF) receptors were detected in the endothelium. As the major source of VEGF, the overlying epidermis of the verruga peruana was identified suggesting an angiogenic loop mechanism between infected endothelium and the overlying epidermis [78].
Animal B. bacilliformis-infection models
Animal infection models are crucial to understanding bacterial pathogenicity mechanisms in vivo. Besides humans, only rhesus macaques are known to be susceptible to Carrion’s disease. In a study of Noguchi and Battistini from 1926, Macacus rhesus monkeys suffered from Oroya fever and verruga peruana illnesses after being infected with B. bacilliformis [79]. However, to date there is no reliable small animal B. bacilliformis infection model available. As a trade-off, particular laboratory parameters and the underlying immune response are determined by using blood and serum samples from infected patients. Not surprisingly, these samples are difficult to obtain and strongly limited by nature. Therefore, a suitable animal infection model is urgently needed.
The intravenous injection of B. bacilliformis in rhesus monkeys induced a prolonged irregular remittent fever. The pathogen was cultivatable from peripheral blood for a long period (58 days) [80] and was detected within erythrocytes, reproducing the precise appearances observed in human cases of Oroya fever. However, in all tested subjects the intensity of the anemia was less severe than in humans. The intradermal injection of B. bacilliformis resulted in nodular formations rich in new blood vessels where the bacteria were found within endothelial cells and could be re-isolated. Complete convalescence of the infected animals occurred after a period from two to five months [81]. Further experiments on rhesus monkeys showed that virulence of B. bacilliformis was enhanced by passaging the pathogen through susceptible animals. Here, a severe anemia with reduction of erythrocyte counts was observed but the number of invaded erythrocytes was still lower compared to Oroya fever in humans [80]. Furthermore, a high variety in the course of disease was observed: rhesus monkeys developed from mild (mild anemia, mild course of verruga peruana-like lesions) to severe (see above) symptoms after B. bacilliformis infections [80]. The variation of the course of infection suggested that the severity of symptoms of Carrion’s disease was primarily attributed to the virulence of the particular B. bacilliformis strain and secondarily depended on the (genetic) predisposition of monkeys [82]. The pathologic changes in the organs of monkeys suffering from a severe course of Carrion’s disease showed high similarity to those found in human organs of fatal cases. After the death of the animals, bacteria were re-isolated from the lymphatic system, spleen, bone marrow and liver [80]. Noguchi & Battistini undertook further attempts to identify animal species susceptible to B. bacilliformis infection (dogs, donkeys, guinea pigs, java, mice, rabbits, rats, ringtails, green monkeys, chimpanzees and orangutans) but only chimpanzees and orangutans showed clinical symptoms characteristic for Carrion’s disease [83, 84]. However, compared to rhesus monkeys, the severity of symptoms was much weaker and showed less resemblance to Carrion’s disease of humans [83]. Similar results were obtained ~80 years later by infecting owl monkeys. Here, these monkeys suffered also from a microscopically-detected intraerythrocytic bacteremia upon an intravenous B. bacilliformis infection; nevertheless (and for unclear reasons), detection of B. bacilliformis via cultures and PCRs remained negative [60]. To the best of our knowledge, today the B. bacilliformis monkey-infection model is no longer applied (most likely because of animal protection reasons and economic aspects).
There have been attempts to establish a rat infection model to determine the responsible mechanism of B. bacilliformis for inducing vascular proliferations [77]. Here, polyvinyl alcohol sponge discs were subcutaneously implanted into adult Sprague-Dawley rats and were injected with B. bacilliformis culture extracts three days after implantation. Sponges were analyzed microscopically after seven days and a ~2.5-fold increase in blood vessel formation was found. It needs to be mentioned that this rat model was established for the artificial application of B. bacilliformis extracts not reflecting the natural course of infection [77]. In another experimental setting, BALB/c mice were intraperitoneally, intradermally or subcutaneously inoculated with various amounts of viable B. bacilliformis, but histopathological lesions were not detected. Moreover, no bacteremia was detected for a period of 15 days after inoculation [85], reflecting that BALB/c mice are not an appropriate B. bacilliformis animal infection model. The lack of virulence of B. bacilliformis in murine infection models can be best explained by the absence of a Trw type 4 secretion system (Trw T4SS): it was shown that a distinct Trw locus of the respective animal-pathogenic Bartonella species is crucial for facilitating host-restricted adhesion to erythrocytes [86].
A potential alternative to mimic at least the bacteremia phase of a B. bacilliformis infection in humans (Oroya fever) and to overcome the species barrier in murine infection models is the use of so-called “humanized” mice. The engraftment of NOD-scid IL2rɤ-/- mice with human hematopoietic stem cells results in de novo generation of human erythrocytes and such models have been used in analyzing e.g. the course of Plasmodium falciparum infections [87]. As B. bacilliformis is adapted to infect human erythrocytes, this promising model would probably enable to analyze some bacterial pathogenicity mechanisms. Nevertheless, in such humanized mice, endothelial cells (which represent the potential niche for B. bacilliformis) remain of murine origin and it is unknown how the murine-endothelial-cell origin affects the course of infection.
Host immune response upon B. bacilliformis infections
Only little information exists about immunity in Carrion’s disease and immune response to B. bacilliformis infections. Reasons for this are the low availability of samples from the endemic areas, a hardly existing scientific attention to the disease and the lack of suitable animal infection models. There is moderate evidence that humoral and cellular immune responses are involved during Carrion’s disease. It is known that an infection with B. bacilliformis results in a lifelong humoral immunity which confers partial immunological protection [88] and this is in-line with earlier results showing that rhesus monkeys and chimpanzee which had recovered from an infection with B. bacilliformis showed complete immunity when repetitively infected [81].
Groundbreaking findings from 1929 are still valid today [89]: to study the effects of immune sera on the course of B. bacilliformis infections, rabbit immune sera and convalescent sera from infected rhesus monkeys were tested in infections of rhesus macaques. In most cases, convalescent sera delayed the formation of verruga peruana and inhibited a proliferative blood-stream infection with B. bacilliformis when simultaneously applied with the pathogen. The injection of convalescent sera after B. bacilliformis infections resulted in negative blood cultures but showed no effect on the formation of skin lesions.
In endemic regions, seropositivity (IgM, IgG) of humans can reach ~30–35%. Recent studies reported that the number of asymptomatic B. bacilliformis carriers is ~37% in post-outbreak areas and ~52% in endemic areas [51]. These asymptomatic individuals seem to represent the main reservoir of the pathogen. In an attempt to identify serum biomarkers to detect B. bacilliformis infections it was suggested to consider IgM as a marker of a recent infection and IgG as a marker of past exposure and immunity [88]. It was also shown that IgM levels correlate with low levels of eotaxin, IL-6 and VEGF and high levels of interleukin 10 (IL-10), reflecting an immunosuppression in the acute phase of Oroya fever [88]. IL-10 is a potent anti-inflammatory cytokine that plays a crucial role in limiting the host immune response to pathogens in order to prevent host damage. It was reported that some pathogens are able to utilize the immunosuppressive properties of IL-10 to limit the host immune response [90]. A decrease of the cellular mediated immune response and increased levels of IL-10 were also observed in two pregnant patients that suffered from a severe bartonellosis [91]. It is believed that B. bacilliformis induces a long lasting immunosuppression continuing after the acute phase (Oroya fever) and during the chronic phase of Carrion’s disease [88]. Due to this, levels of TH1-related and pro-inflammatory cytokines are reduced leading to persistent infections characterized by a low level-bacteremia [88]. Furthermore, the proangiogenic cytokines VEGF and eotaxin showed a positive correlation with IgG levels and a negative correlation with IgM levels in seropositive patients [88]. It has been demonstrated that B. henselae induces VEGF production in vitro and in vivo [92, 93]. It is hypothesized that with an enhanced IgG response, B. bacilliformis evades the immune system in endothelial cells to hide and replicate in this immunoprivileged niche [88].
Conclusions
Carrion’s disease is an ancient disease. There is a worrisome lack of knowledge about vectors and possible reservoir hosts of B. bacilliformis. Insights into the dynamics of pathogen transmission by Lutzomyia species might help to gain prevention strategies. Clearly, a rigorous screening of the wildlife (animals and plants) would discard or confirm the existence of other B. bacilliformis reservoir hosts apart from human beings. Molecular mechanisms underlying host infections are also widely unknown. The use of appropriate in vitro and in vivo infection models in combination with molecular strategies using bacterial mutants (e.g. generated by random and targeted mutagenesis) and recombinant protein expression strategies (e.g. via heterologous expression libraries) could help to gain deeper insights into the infection biology of this difficult to handle pathogen and might represent a basis for the development of a potential vaccine.
Acknowledgements
The authors thank Birgit Fehrenbacher and Professor Martin Schaller (Department for Dermatology, University Hospital Tübingen, Germany), Jürgen Berger and Dr Katharina Hipp (Max Planck Institute for Developmental Biology, Tübingen, Germany), Professor Martin Aepfelbacher (Medical Microbiology and Hygiene, University Hospital Hamburg Eppendorf, Germany), and Professor Mike Minnick (College of Humanities and Science, Missoula, Montana, USA) for ongoing scientific collaborations. The authors thank especially Professor Eduardo Gotuzzo, Dr Ciro Maguiña, Ms Palmira Ventosilla and Mr Enrique Pérez from the Universidad Peruana Cayetano Heredia and the Instituto de Medicina Tropical Alexander von Humboldt, Lima, Peru for supporting with knowledge and photographs. Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th CVBD Word Forum Symposium.
Funding
This work of VAJK was supported by the LOEWE Center DRUID [(Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases (project C2)] and the Robert Koch-Institute, Berlin, Germany (Bartonella Consiliary Laboratory, 1369–354). Funding parties had no influence on data analysis, data interpretation, or writing of the manuscript.
Availability of data and materials
Not applicable.
Authors’ contributions
MGQ, AAD, HG and VAJK designed and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- DNA
deoxyribonucleic acid
- GFP
green-fluorescent protein
- HUVEC
human umbilical vein endothelial cells
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL-10
interleukin 10
- MLST
multi-locus sequence typing
- mRNA
messenger ribonucleic acid
- NF-κB
nuclear factor κB
- OMP
outer membrane protein
- PCR
polymerase chain reaction
- TAA
trimeric autotransporter adhesion
- TH1
T helper 1
- TLR5
Toll-like receptor 5
- t-PA
tissue plasminogen activator
- T4SS
type IV secretion system
- VEGF
vascular endothelial growth factor
Contributor Information
Meritxell Garcia-Quintanilla, Email: meritxelldejesus.garciaquintanilla@kgu.de.
Alexander A. Dichter, Email: alexander.dichter@kgu.de
Humberto Guerra, Email: Humberto.guerra@upch.pe.
Volkhard A. J. Kempf, Email: volkhard.kempf@kgu.de
References
- 1.Amano Y, Rumbea J, Knobloch J, Olson J, Kron M. Bartonellosis in Ecuador: serosurvey and current status of cutaneous verrucous disease. Am J Trop Med Hyg. 1997;57:174–179. doi: 10.4269/ajtmh.1997.57.174. [DOI] [PubMed] [Google Scholar]
- 2.Lydy SL, Lascano MS, Garcia-Perez JE, Williams-Newkirk AJ, Grijalva MJ. Seroprevalence and risk factors for infection with Bartonella bacilliformis in Loja Province, Ecuador. Emerg Microbes Infect. 2018;7:115. doi: 10.1038/s41426-018-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes C, Ruiz J. Carrion’s disease: the sound of silence. Clin Microbiol Rev. 2018;31:e00056. doi: 10.1128/CMR.00056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maguina C, Garcia PJ, Gotuzzo E, Cordero L, Spach DH. Bartonellosis (Carrionʼs disease) in the modern era. Clinical Infect Dis. 2001;33:772–779. doi: 10.1086/322614. [DOI] [PubMed] [Google Scholar]
- 5.Minnick MF, Anderson BE, Lima A, Battisti JM, Lawyer PG, Birtles RJ. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:e2919. doi: 10.1371/journal.pntd.0002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz MG. A history of bartonellosis (Carrionʼs disease) Am J Trop Med Hyg. 1968;17:503–515. doi: 10.4269/ajtmh.1968.17.503. [DOI] [PubMed] [Google Scholar]
- 7.Gomes C, Pons MJ, Del Valle Mendoza J, Ruiz J. Carrionʼs disease: an eradicable illness? Infect Dis Poverty. 2016;5:105. doi: 10.1186/s40249-016-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong J, Li Y, Hua X, Bai Y, Wang C, Zhu C. Lymphatic circulation disseminates Bartonella infection into bloodstream. J Infect Dis. 2017;215:303–311. doi: 10.1093/infdis/jiw526. [DOI] [PubMed] [Google Scholar]
- 9.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser PO, Riess T, O’Rourke F, Linke D, Kempf VA. Bartonella spp.: throwing light on uncommon human infections. Int J Med Microbiol. 2011;301:7–15. doi: 10.1016/j.ijmm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Pang Q, Zhao B, Vayssier-Taussat M. Molecular mechanisms of Bartonella and mammalian erythrocyte interactions: a review. Front Cell Infect Microbiol. 2018;8:431. doi: 10.3389/fcimb.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greub G, Raoult D. Bartonella: new explanations for old diseases. J Med Microbiol. 2002;51:915–923. doi: 10.1099/0022-1317-51-11-915. [DOI] [PubMed] [Google Scholar]
- 13.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 2006;14:264–270. doi: 10.1016/j.tim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Townsend CH. A Phlebotomus the practically certain carrier of verruga. Science. 1913;38:194–195. doi: 10.1126/science.38.971.194. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi H, Shannon RC, Tilden EB, Tyler JR. Etiology of Oroya fever: XIV. The insect vectors of Carrionʼs disease. J Exp Med. 1929;49:993–1008. doi: 10.1084/jem.49.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander B. A review of bartonellosis in Ecuador and Colombia. Am J Trop Med Hyg. 1995;52:354–359. doi: 10.4269/ajtmh.1995.52.354. [DOI] [PubMed] [Google Scholar]
- 18.Ulloa GM, Vasquez-Achaya F, Gomes C, Del Valle LJ, Ruiz J, Pons MJ, et al. Molecular detection of Bartonella bacilliformis in Lutzomyia maranonensis in Cajamarca, Peru: a new potential vector of Carrionʼs disease in Peru? Am J Trop Med Hyg. 2018;99:1229–1233. doi: 10.4269/ajtmh.18-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moo-Llanes DA, Arque-Chunga W, Carmona-Castro O, Yanez-Arenas C, Yanez-Trujillano HH, Cheverria-Pacheco L, et al. Shifts in the ecological niche of Lutzomyia peruensis under climate change scenarios in Peru. Med Vet Entomol. 2017;31:123–131. doi: 10.1111/mve.12219. [DOI] [PubMed] [Google Scholar]
- 20.Villaseca P, Padilla C, Ventura G, Samalvides F, Yañez H, Chevarría L, et al. Importancia de la Lutzomyia peruensis en la transmisión de la enfermedad de Carrión en el valle Sagrado de los Incas. Urubamba-Cusco, Peru. Rev Med Exp. 1999;15:28–30. [Google Scholar]
- 21.Maguina-Vargas C, Pachas P. Experiences in the prevention and control of Carrionʼs disease in Peru. Rev Peru Med Exp Salud Publica. 2014;31:348–351. [PubMed] [Google Scholar]
- 22.Romero S. Detection of Bartonella bacilliformis by real-time PCR in naturally infected sand flies. Thesis. Department of Preventive Medicine and Biometrics, University of the Health Sciences. Bethesda, MD, 2004. https://apps.dtic.mil/dtic/tr/fulltext/u2/a434804.pdf. Accessed 19 Dec 2018.
- 23.Carrazco-Montalvo AR. Detección molecular de Bartonella bacilliformis en flebótomos (Diptera: Psychodidae) en la zona fronteriza ecuatoriana-peruana. Thesis. Colegio de Ciencia Biológicas y Ambientales, University of San Francisco de Quito USFQ. Ecuador, 2017. http://repositorio.usfq.edu.ec/bitstream/23000/6480/1/131141.pdf. Accessed 30 Jan 2018.
- 24.Battisti JM, Lawyer PG, Minnick MF. Colonization of Lutzomyia verrucarum and Lutzomyia longipalpis sand flies (Diptera: Psychodidae) by Bartonella bacilliformis, the etiologic agent of Carrion’s disease. PLoS Negl Trop Dis. 2015;9:e0004128. doi: 10.1371/journal.pntd.0004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caceres AG. Geographic distribution of Lutzomyia verrucarum (Townsend, 1913) (Diptera, Psychodidae, Phlebotominae), vector of human bartonellosis in Peru. Rev Inst Med Trop Sao Paulo. 1993;35:485–490. doi: 10.1590/s0036-46651993000600002. [DOI] [PubMed] [Google Scholar]
- 26.Hambuch TM, Handley SA, Ellis B, Chamberlin J, Romero S, Regnery R. Population genetic analysis of Bartonella bacilliformis isolates from areas of Peru where Carrionʼs disease is endemic and epidemic. J Clin Microbiol. 2004;42:3675–3680. doi: 10.1128/JCM.42.8.3675-3680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorrilla V, Vásquez G, Espada L, Ramírez P. Vectores de la leishmaniasis tegumentaria y la enfermedad de Carrión en el Perú: una actualizacion. Rev Peru Med Exp Salud Publica. 2017;34:485–496. doi: 10.17843/rpmesp.2017.343.2398. [DOI] [PubMed] [Google Scholar]
- 28.Urrutia LC, Patino-Barbosa AM, Arroyave-Valencia F, Sabogal-Roman JA, Cardona-Ospina JA, Rodriguez-Morales AJ. Oroya fever, verruga peruana, and other bartonelloses incidence rates in Colombia (2009–2013) Cureus. 2018;10:e3528. doi: 10.7759/cureus.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi H. Etiology of Oroya fever: V. The experimental transmission of Bartonella bacilliformis by ticks (Dermacentor andersoni) J Exp Med. 1926;44:729–734. doi: 10.1084/jem.44.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Valle-Mendoza J, Rojas-Jaimes J, Vasquez-Achaya F, Aguilar-Luis MA, Correa-Nunez G, Silva-Caso W, et al. Molecular identification of Bartonella bacilliformis in ticks collected from two species of wild mammals in Madre de Dios: Peru. BMC Res Notes. 2018;11:405. doi: 10.1186/s13104-018-3518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laroche M, Berenger JM, Mediannikov O, Raoult D, Parola P. Detection of a potential new Bartonella species “Candidatus Bartonella rondoniensis” in human biting kissing bugs (Reduviidae; Triatominae) PLoS Negl Trop Dis. 2017;11:e0005297. doi: 10.1371/journal.pntd.0005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullins KE, Hang J, Jiang J, Leguia M, Kasper MR, Ventosilla P, et al. Description of Bartonella ancashensis sp. nov., isolated from the blood of two patients with verruga peruana. Int J Syst Evol Microbiol. 2015;65:3339–3343. doi: 10.1099/ijsem.0.000416. [DOI] [PubMed] [Google Scholar]
- 33.Regier Y, Ballhorn W, Kempf VA. Molecular detection of Bartonella henselae in 11 Ixodes ricinus ticks extracted from a single cat. Parasit Vectors. 2017;10:105. doi: 10.1186/s13071-017-2042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–438. doi: 10.1128/cmr.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birtles RJ, Canales J, Ventosilla P, Alvarez E, Guerra H, Llanos-Cuentas A, et al. Survey of Bartonella species infecting intradomicillary animals in the Huayllacallan Valley, Ancash, Peru, a region endemic for human bartonellosis. Am J Trop Med Hyg. 1999;60:799–805. doi: 10.4269/ajtmh.1999.60.799. [DOI] [PubMed] [Google Scholar]
- 36.Schikora A, Garcia AV, Hirt H. Plants as alternative hosts for Salmonella. Trends Plant Sci. 2012;17:245–249. doi: 10.1016/j.tplants.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Dehio C, Tsolis RM. Type IV effector secretion and subversion of host functions by Bartonella and Brucella species. Curr Top Microbiol Immunol. 2017;413:269–295. doi: 10.1007/978-3-319-75241-9_11. [DOI] [PubMed] [Google Scholar]
- 38.Herrer A. Carrionʼs disease. I. Studies on plants claimed to be reservoirs of Bartonella bacilliformis. Am J Trop Med Hyg. 1953;2:637–643. [PubMed] [Google Scholar]
- 39.Segers FH, Kesnerova L, Kosoy M, Engel P. Genomic changes associated with the evolutionary transition of an insect gut symbiont into a blood-borne pathogen. ISME J. 2017;11:1232–1244. doi: 10.1038/ismej.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engel P, Dehio C. Genomics of host-restricted pathogens of the genus Bartonella. Genome Dyn. 2009;6:158–169. doi: 10.1159/000235769. [DOI] [PubMed] [Google Scholar]
- 41.Pepe JC, Wachtel MR, Wagar E, Miller VL. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comanducci M, Bambini S, Brunelli B, Adu-Bobie J, Arico B, Capecchi B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–1454. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser PO, Linke D, Schwarz H, Leo JC, Kempf VA. Analysis of the BadA stalk from Bartonella henselae reveals domain-specific and domain-overlapping functions in the host cell infection process. Cell Microbiol. 2012;14:198–209. doi: 10.1111/j.1462-5822.2011.01711.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaiser PO, Riess T, Wagner CL, Linke D, Lupas AN, Schwarz H, et al. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol. 2008;10:2223–2234. doi: 10.1111/j.1462-5822.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 45.Riess T, Andersson SG, Lupas A, Schaller M, Schafer A, Kyme P, et al. Bartonella adhesin a mediates a proangiogenic host cell response. J Exp Med. 2004;200:1267–1278. doi: 10.1084/jem.20040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson LA, Kar S, McLaughlin G, Ihler GM. Entry of Bartonella bacilliformis into erythrocytes. Infect Immun. 1986;54:347–353. doi: 10.1128/iai.54.2.347-353.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mernaugh G, Ihler GM. Deformation factor: an extracellular protein synthesized by Bartonella bacilliformis that deforms erythrocyte membranes. Infect Immun. 1992;60:937–943. doi: 10.1128/iai.60.3.937-943.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scherer DC, DeBuron-Connors I, Minnick MF. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun. 1993;61:4962–4971. doi: 10.1128/iai.61.12.4962-4971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pons MJ, Silva-Caso W, Del Valle-Mendoza J, Ruiz J. Multi-locus sequence typing of Bartonella bacilliformis DNA performed directly from blood of patients with Oroyaʼs fever during a Peruvian outbreak. PLoS Negl Trop Dis. 2016;10:e0004391. doi: 10.1371/journal.pntd.0004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeaiter Z, Fournier PE, Ogata H, Raoult D. Phylogenetic classification of Bartonella species by comparing groEL sequences. Int J Syst Evol Microbiol. 2002;52:165–171. doi: 10.1099/00207713-52-1-165. [DOI] [PubMed] [Google Scholar]
- 51.Gomes C, Palma N, Pons MJ, Magallon-Tejada A, Sandoval I, Tinco-Valdez C, et al. Succinyl-CoA Synthetase: new antigen candidate of Bartonella bacilliformis. PLoS Negl Trop Dis. 2016;10:e0004989. doi: 10.1371/journal.pntd.0004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minnick MF, Smitherman LS, Samuels DS. Mitogenic effect of Bartonella bacilliformis on human vascular endothelial cells and involvement of GroEL. Infect Immun. 2003;71:6933–6942. doi: 10.1128/IAI.71.12.6933-6942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans DJ, Jr, Evans DG, Engstrand L, Graham DY. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanet A, Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect Immun. 1998;66:1023–1027. doi: 10.1128/iai.66.3.1023-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callison JA, Battisti JM, Sappington KN, Smitherman LS, Minnick MF. Characterization and expression analysis of the groESL operon of Bartonella bacilliformis. Gene. 2005;359:53–62. doi: 10.1016/j.gene.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smitherman LS, Minnick MF. Bartonella bacilliformis GroEL: effect on growth of human vascular endothelial cells in infected cocultures. Ann N Y Acad Sci. 2005;1063:286–298. doi: 10.1196/annals.1355.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taye A, Chen H, Duncan K, Zhang Z, Hendrix L, Gonzalez J, et al. Production of recombinant protein Pap31 and its application for the diagnosis of Bartonella bacilliformis infection. Ann N Y Acad Sci. 2005;1063:280–285. doi: 10.1196/annals.1355.045. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann R, Kempf VA, Schiltz E, Oberle K, Sander A. Hemin binding, functional expression, and complementation analysis of Pap 31 from Bartonella henselae. J Bacteriol. 2003;185:1739–1744. doi: 10.1128/JB.185.5.1739-1744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henriquez-Camacho C, Ventosilla P, Minnick MF, Ruiz J, Maguina C. Proteins of Bartonella bacilliformis: candidates for vaccine development. Int J Pept. 2015;2015:702784. doi: 10.1155/2015/702784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bentzel DE, Espinosa BJ, Canal E, Blazes DL, Hall ER. Susceptibility of owl monkeys (Aotus nancymaae) to experimental infection with Bartonella bacilliformis. Comp Med. 2008;58:76–80. [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell SJ, Minnick MF. Characterization of a two-gene locus from Bartonella bacilliformis associated with the ability to invade human erythrocytes. Infect Iimmun. 1995;63:1552–1562. doi: 10.1128/iai.63.4.1552-1562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller VL, Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988;56:1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller VL, Finlay BB, Falkow S. Factors essential for the penetration of mammalian cells by Yersinia. Curr Top Microbiol Immunol. 1988;138:15–39. [PubMed] [Google Scholar]
- 64.Conyers GB, Bessman MJ. The gene, ialA, associated with the invasion of human erythrocytes by Bartonella bacilliformis, designates a nudix hydrolase active on dinucleoside 5’-polyphosphates. J Biol Chem. 1999;274:1203–1206. doi: 10.1074/jbc.274.3.1203. [DOI] [PubMed] [Google Scholar]
- 65.Coleman SA, Minnick MF. Establishing a direct role for the Bartonella bacilliformis invasion-associated locus B (IalB) protein in human erythrocyte parasitism. Infect Immun. 2001;69:4373–4381. doi: 10.1128/IAI.69.7.4373-4381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coleman SA, Minnick MF. Differential expression of the invasion-associated locus B (ialB) gene of Bartonella bacilliformis in response to environmental cues. Microb Pathog. 2003;34:179–186. doi: 10.1016/s0882-4010(03)00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Derrick SC, Ihler GM. Deformin, a substance found in Bartonella bacilliformis culture supernatants, is a small, hydrophobic molecule with an affinity for albumin. Blood Cells Mol Dis. 2001;27:1013–1019. doi: 10.1006/bcmd.2001.0475. [DOI] [PubMed] [Google Scholar]
- 68.Hendrix LR. Contact-dependent hemolytic activity distinct from deforming activity of Bartonella bacilliformis. FEMS Microbiol Lett. 2000;182:119–124. doi: 10.1111/j.1574-6968.2000.tb08884.x. [DOI] [PubMed] [Google Scholar]
- 69.Iwaki-Egawa S, Ihler GM. Comparison of the abilities of proteins from Bartonella bacilliformis and Bartonella henselae to deform red cell membranes and to bind to red cell ghost proteins. FEMS Microbiol Lett. 1997;157:207–217. doi: 10.1111/j.1574-6968.1997.tb12775.x. [DOI] [PubMed] [Google Scholar]
- 70.Buckles EL, McGinnis-Hill E. Interaction of Bartonella bacilliformis with human erythrocyte membrane proteins. Microb Pathog. 2000;29:165–174. doi: 10.1006/mpat.2000.0381. [DOI] [PubMed] [Google Scholar]
- 71.Walker TS, Winkler HH. Bartonella bacilliformis: colonial types and erythrocyte adherence. Infect Immun. 1981;31:480–486. doi: 10.1128/iai.31.1.480-486.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill EM, Raji A, Valenzuela MS, Garcia F, Hoover R. Adhesion to and invasion of cultured human cells by Bartonella bacilliformis. Infect Immun. 1992;60:4051–4058. doi: 10.1128/iai.60.10.4051-4058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia FU, Wojta J, Hoover RL. Interactions between live Bartonella bacilliformis and endothelial cells. J Infect Dis. 1992;165:1138–1141. doi: 10.1093/infdis/165.6.1138. [DOI] [PubMed] [Google Scholar]
- 74.Verma A, Davis GE, Ihler GM. Infection of human endothelial cells with Bartonella bacilliformis is dependent on Rho and results in activation of Rho. Infect Immun. 2000;68:5960–5969. doi: 10.1128/iai.68.10.5960-5969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verma A, Davis GE, Ihler GM. Formation of stress fibres in human endothelial cells infected with Bartonella bacilliformis is associated with altered morphology, impaired migration and defects in cell morphogenesis. Cell Microbiol. 2001;3:169–180. doi: 10.1046/j.1462-5822.2001.00104.x. [DOI] [PubMed] [Google Scholar]
- 76.Verma A, Ihler GM. Activation of Rac, Cdc42 and other downstream signalling molecules by Bartonella bacilliformis during entry into human endothelial cells. Cellr Microbiol. 2002;4:557–569. doi: 10.1046/j.1462-5822.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 77.Garcia FU, Wojta J, Broadley KN, Davidson JM, Hoover RL. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol. 1990;136:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 78.Cerimele F, Brown LF, Bravo F, Ihler GM, Kouadio P, Arbiser JL. Infectious angiogenesis: Bartonella bacilliformis infection results in endothelial production of angiopoetin-2 and epidermal production of vascular endothelial growth factor. Am J Pathol. 2003;163:1321–1327. doi: 10.1016/S0002-9440(10)63491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noguchi H, Battistini TS. Etiology of Oroya fever: I. Cultivation of Bartonella bacilliformis. J Exp Med. 1926;43:851–64. [DOI] [PMC free article] [PubMed]
- 80.Noguchi H. Etiology of Oroya fever: III The behavior of Bartonella bacilliformis in Macacus rhesus. J Exp Med. 1926;44:697–713. doi: 10.1084/jem.44.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noguchi H. Etiology of Oroya fever: VIII. Experiments on cross-immunity between Oroya fever and verruga peruana. J Exp Med. 1927;45:781–783. doi: 10.1084/jem.45.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noguchi H. Etiology of Oroya fever: X. Comparative studies of different strains of Bartonella bacilliformis, with special reference to the relationship between the clinical types of Carrionʼs disease and the virulence of the infecting organism. J Exp Med. 1928;47:219–234. doi: 10.1084/jem.47.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noguchi H. Etiology of Oroya fever: IV. The effect of inoculation of anthropoid apes with Bartonella bacilliformis. J Exp Med. 1926;44:715–728. doi: 10.1084/jem.44.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noguchi H, Muller HR, Tilden EB, Tyler JR. Etiology of Oroya fever: XVI. Verruga in the dog and the donkey. J Exp Med. 1929;50:455–461. doi: 10.1084/jem.50.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Infante B, Villar S, Palma S, Merello J, Valencia R, Torres L, et al. BALB/c mice resist infection with Bartonella bacilliformis. BMC Res Notes. 2008;1:103. doi: 10.1186/1756-0500-1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vayssier-Taussat M, Le Rhun D, Deng HK, Biville F, Cescau S, Danchin A, et al. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog. 2010;6:e1000946. doi: 10.1371/journal.ppat.1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amaladoss A, Chen Q, Liu M, Dummler SK, Dao M, Suresh S, et al. De novo generated human red blood cells in humanized mice support Plasmodium falciparum infection. PloS One. 2015;10:e0129825. doi: 10.1371/journal.pone.0129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pons MJ, Gomes C, Aguilar R, Barrios D, Aguilar-Luis MA, Ruiz J, et al. Immunosuppressive and angiogenic cytokine profile associated with Bartonella bacilliformis infection in post-outbreak and endemic areas of Carrionʼs disease in Peru. PLoS Negl Trop Dis. 2017;11:e0005684. doi: 10.1371/journal.pntd.0005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noguchi H, Muller HR, Tilden EB, Tyler JR. Etiology of Oroya fever: XV. Effect of immune serum on the course of Bartonella bacilliformis infection in Macacus rhesus. J Exp Med. 1929;50:355–64. [DOI] [PMC free article] [PubMed]
- 90.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huarcaya E, Maguina C, Best I, Solorzano N, Leeman L. Immunological response in cases of complicated and uncomplicated bartonellosis during pregnancy. Rev Inst Med Trop Sao Paulo. 2007;49:335–337. doi: 10.1590/s0036-46652007000500012. [DOI] [PubMed] [Google Scholar]
- 92.Kempf VA, Volkmann B, Schaller M, Sander CA, Alitalo K, Riess T, et al. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 2001;3:623–632. doi: 10.1046/j.1462-5822.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 93.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 94.Sotomayor-Tribín HA. Pensamiento analógico mítico en la interpretación del arte prehispánico de interés para la arqueomedicina y la paleopatología. Repert Med Cir. 2016;25:50–71. [Google Scholar]
- 95.Riess T, Dietrich F, Schmidt KV, Kaiser PO, Schwarz H, Schafer A, et al. Analysis of a novel insect cell culture medium-based growth medium for Bartonella species. Appl Environ Microbiol. 2008;74:5224–5227. doi: 10.1128/AEM.00621-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


