Abstract
Background
Lactic acidosis with an elevated lactate–pyruvate ratio suggesting anoxia is a common feature of severe falciparum malaria. High lactate levels are associated with parasitized erythrocyte sequestration in the microcirculation. To assess if there is an additional contribution to hyperlactataemia from relatively inadequate total oxygen delivery, oxygen consumption and delivery were investigated in patients with malaria.
Methods
Adult Bangladeshi and Indian patients with uncomplicated (N = 50) or severe (N = 46) falciparum malaria or suspected bacterial sepsis (N = 27) and healthy participants as controls (N = 26) were recruited at Chittagong Medical College Hospital, Chittagong, Bangladesh and Ispat General Hospital, Rourkela, India. Oxygen delivery (DO2I) was estimated from pulse oximetry, echocardiographic estimates of cardiac index and haematocrit. Oxygen consumption (VO2I) was estimated by expired gas collection.
Results
VO2I was elevated in uncomplicated median (IQR) 185.1 ml/min/m2 (135–215.9) and severe malaria 192 ml/min/m2 (140.7–227.9) relative to healthy persons 107.9 ml/min/m2 (69.9–138.1) (both p < 0.001). Median DO2I was similar in uncomplicated 515 ml/min/m2 (432–612) and severe 487 ml/min/m2 (382–601) malaria and healthy persons 503 ml/min/m2 (447–517) (p = 0.27 and 0.89, respectively). The VO2/DO2 ratio was, therefore, increased by similar amounts in both uncomplicated 0.35 (0.28–0.44) and severe malaria 0.38 (0.29–0.48) relative to healthy participants 0.23 (0.17–0.28) (both p < 0.001). VO2I, DO2I and VO2/DO2 did not correlate with plasma lactate concentrations in severe malaria.
Conclusions
Reduced total oxygen delivery is not a major contributor to lactic acidosis in severe falciparum malaria.
Electronic supplementary material
The online version of this article (10.1186/s12936-019-2733-y) contains supplementary material, which is available to authorized users.
Keywords: Malaria; Acidosis, lactic; Microcirculation; Oxygen consumption; Haemodynamics; Cardiac output
Background
Severe falciparum malaria is a life-threatening infection requiring prompt treatment with intravenous artesunate [1]. Patients present with a range of syndromes reflecting vital organ dysfunction. The depth of coma, degree of metabolic/lactic acidosis and severity of renal impairment are the major prognosticators for a fatal outcome. During blood stage infection with Plasmodium falciparum, 48-h cycles of asexual replication in red cells result in exponential growth of the infecting parasite biomass and the corresponding release of haemoglobin and haemozoin pigment-containing digestive vacuoles at schizont rupture. As the P. falciparum parasites mature the infected red cells sequester heterogeneously in the microvasculature causing microvascular obstruction and impairment of tissue perfusion [1]. Plasma lactate is elevated in relation to the proportion of non-perfused capillaries [2]. Hyperlactataemia is associated with increased glucose turnover [3] and increased lactate-pyruvate ratios [4], consistent with hypoxia rather than hypermetabolism as the cause. Increased plasma lactate also correlates inversely with functional liver blood flow suggesting impaired hepatic clearance contributes to hyperlactataemia [5].
Previous studies have investigated the haemodynamics of severe malaria using invasive techniques [2, 6–8] and reported lack of association between total oxygen delivery and plasma lactate [2]. In this article the hypothesis that inadequate oxygen delivery contributes to the metabolic acidosis of severe malaria is evaluated. Oxygen consumption was measured to assess whether inhibition of oxidative metabolism contributes to lactic acidosis, and determined the ratio of oxygen consumption to delivery (VO2/DO2) to provide a better estimate of the adequacy of oxygen delivery.
Methods
Patients
Adult patients with severe or uncomplicated falciparum malaria, patients with suspected bacterial sepsis of any severity meeting the systemic inflammatory response (SIRS) criteria, and healthy adult volunteers were recruited in Chittagong Medical College Hospital (CMCH), Chittagong, Bangladesh and Ispat General Hospital (IGH), Rourkela, India. Patients with malaria had asexual stages visible on a thick or thin blood film. Severe malaria was defined as being present when one or more of the following features were present: cerebral malaria (Glasgow coma score < 11); parasites > 100,000/mm3 with either severe anaemia (haematocrit < 20%) or bilirubin level of > 2.5 mg/dl; renal failure (creatinine > 265 μmol/l or anuria); hypoglycaemia < 2.2 mmol/l; systolic blood pressure < 80 mmHg with cold extremities; pulmonary oedema; spontaneous bleeding; generalized convulsions (> 1 in 24 h); venous bicarbonate < 15 mmol/l, hyperparasitaemia > 10%, venous lactate > 4 mmol/l. Participants with paired oxygen consumption and oxygen delivery estimates were included in this analysis. All participants (or legally acceptable representatives in cases where patients lacked capacity during the acute illness) gave informed, written consent. The study was approved by the Oxford Tropical Research, Chittagong Medical College and IGH ethical committees. Patients with severe malaria received intravenous artesunate, and those with uncomplicated malaria received either oral artemether–lumefantrine (Chittagong) or artesunate–sulfadoxine/pyrimethamine (Rourkela) according to national guidelines.
On enrolment a history and examination were recorded, and blood for biochemistry and haematology was collected. Plasma lactate and base excess were measured by iSTAT (Abbott laboratories, Illinois, USA). Plasma Plasmodium falciparum histidine rich protein 2 (PfHRP2) was measured by ELISA (Cellabs, Brookvale, Australia). Plasma cell free haemoglobin was measured by ELISA (Bethyl laboratories, Texas, USA). Plasma asymmetric dimethyl arginine (ADMA) and arginine were estimated by HPLC. Acute kidney injury (AKI) was staged based on enrolment plasma creatinine and baseline creatinine estimated by back-calculation using the Modification of Diet in Renal Disease equation [9]. This assumed a baseline glomerular filtration rate of 75 ml/min. Stage 1 AKI was defined as creatinine 1.5–1.9× baseline, stage 2 as creatinine 2–2.9× baseline, and stage 3 as creatinine ≥ 3× baseline or creatinine 353.6 µmol/l. The coma acidosis malaria (CAM) score was calculated using Glasgow coma score and base excess as previously [10].
Oxygen consumption and delivery
A timed collection of expired air (typically 45 s) was collected from participants into a Douglas bag using a mask and three way tap with one-way valves [11]. When conscious, participants were asked to breathe normally. The measurement was not possible in patients requiring supplementary oxygen or nasogastric tubes. The volume of gas (RSS 100HR Research Pneumotach, Hans Rudolph, Shawnee, Kansas, USA) and partial pressures of oxygen (Model MO-200, Apogee instruments, Logan, Utah, USA) and carbon dioxide (Tidal Wave S Capnograph, Novametrix Medical Systems, Wallingford, CT, USA) in the bag were measured and used to calculate oxygen consumption (VO2) and carbon dioxide production (VCO2) [11]. The respiratory quotient (RQ) was calculated as the ratio of VCO2/VO2. VO2 and VCO2 were indexed to body surface area (Haycock) (VO2I, VCO2I, respectively). The within participant standard deviations for VO2I, VCO2I and RQ estimated in 11 patients with malaria were 36 ml/m2, 30 ml/m2 and 0.15, respectively. Cardiac output was estimated by transthoracic echocardiography using heart rate, aortic velocity time integral and left ventricular outflow tract diameter [12]. Arterial blood oxygen content was calculated from haemoglobin and oxygen saturation (by pulse oximetry) ignoring dissolved oxygen [13]. Oxygen delivery (DO2) was calculated as the product of arterial blood oxygen content and cardiac output and indexed to body surface area (DO2I) [13]. The ratio of VO2/DO2 was then calculated.
Statistics
Data were analysed using Stata version 14 (StataCorp, Texas, USA). Correlations were assessed using Spearman’s rank. Kruskal–Wallis tests or chi2 tests were used to compare continuous and binary data across groups.
Results
Patients and outcomes
Baseline characteristics and outcome in the different groups are shown in Table 1. A total of 97 patients were studied in Bangladesh and 26 in India. Mortality was 11/46 (24%) in severe malaria and 5/27 (19%) in the sepsis groups. In severe malaria the median CAM score was 2 (IQR 2 to 3).
Table 1.
Participant characteristics
| Variable | Healthy (N = 26) | Uncomplicated malaria (N = 50) | Severe malaria (N = 46) | Sepsis (N = 27) | p value |
|---|---|---|---|---|---|
| Sex (% male) | 81 | 78 | 67 | 56 | 0.12 |
| Coma (% GCS < 11) | 0 | 0 | 57 | 15 | < 0.001 |
| Lactate > 4 mmol/l (%) | 0 | 0 | 46 | 4 | < 0.001 |
| Creatinine > 3 mg/dl (%) | 0 | 0 | 26 | 7 | < 0.001 |
| Died (%) | 0 | 0 | 24 | 19 | 0.001 |
| Age (years) | 28 (26 to 35) | 25 (20 to 40) | 35 (26 to 42) | 32 (23 to 55) | 0.196 |
| Temperature (°C) | 36.8 (36.6 to 37) | 37.5 (36.9 to 38.7) | 37.6 (37.1 to 38.7) | 38.8 (38.2 to 39.3) | < 0.001 |
| Heart rate (/min) | 67 (60 to 74) | 95 (85 to 112) | 105 (87 to 123) | 102 (88 to 114) | < 0.001 |
| Base excess (mM) | 2 (− 1 to 2) | − 1 (− 3 to 1) | − 7 (− 11 to − 2) | 1 (− 3 to 3) | < 0.001 |
| Plasma lactate (mM) | 1.1 (0.9 to 1.4) | 1.5 (1.1 to 2) | 3.7 (2.5 to 6.5) | 1.7 (1.1 to 2.2) | < 0.001 |
| Creatinine (mg/dl) | 0.9 (0.8 to 1) | 1 (0.8 to 1.2) | 1.4 (1 to 3.3) | 1 (0.9 to 1.3) | < 0.001 |
| Parasitaemia (/μl) | NA | 18,903 (3768 to 56,796) | 74,920 (19,091 to 282,751) | NA | 0.002 |
| Plasma PfHRP2 (ng/ml) | NA | 312 (172 to 763) | 2492 (1664 to 3950) | NA | < 0.001 |
| Plasma arginine (μM) | 101 (85 to 107) | 52 (37 to 64) | 53 (37 to 75) | 49 (39 to 54) | < 0.001 |
| Plasma ADMA (μM) | 0.53 (0.48 to 0.56) | 0.58 (0.48 to 0.75) | 0.58 (0.47 to 0.89) | 0.5 (0.39 to 0.61) | 0.032 |
| Plasma arginine/ADMA | 186 (160 to 227) | 87 (70 to 112) | 77 (66 to 98) | 92 (68 to 132) | < 0.001 |
| Plasma CFH (μM) | 2.7 (1.3 to 4.8) | 3.6 (1.9 to 5.1) | 8 (3.8 to 14.5) | 2.3 (0.6 to 5.6) | < 0.001 |
| VO2I (ml/min/m2) | 107.9 (69.9 to 138.1) | 185.1 (135 to 215.9) | 192 (140.7 to 227.9) | 155.3 (132.1 to 196.4) | < 0.001 |
| VCO2I (ml/min/m2) | 86.3 (56.5 to 105.6) | 118.3 (89.3 to 155) | 132.6 (98.4 to 145.6) | 105.2 (73.9 to 131.3) | 0.003 |
| RQ | 0.77 (0.7 to 0.86) | 0.63 (0.58 to 0.76) | 0.67 (0.59 to 0.74) | 0.64 (0.59 to 0.83) | 0.004 |
| CI (ml/min/m2) | 2575 (2340 to 3111) | 3792 (3404 to 4439) | 4167 (3564 to 4876) | 4142 (2988 to 4916) | < 0.001 |
| Haematocrit (%) | 43 (38 to 46) | 32 (25 to 37) | 26 (20 to 35) | 37 (30 to 42) | < 0.001 |
| O2 saturation (%) | 97 (96 to 98) | 97 (96 to 99) | 96 (95 to 97) | 95 (92 to 97) | < 0.001 |
| DO2I (ml/min/m2) | 503 (447 to 517) | 515 (432 to 612) | 487 (382 to 601) | 575 (513 to 694) | 0.02 |
| VO2/DO2 | 0.23 (0.17 to 0.28) | 0.35 (0.28 to 0.44) | 0.38 (0.29 to 0.48) | 0.26 (0.21 to 0.34) | < 0.001 |
For non-binary variables statistics shown are median (interquartile range)
VO2I oxygen consumption index, VCO2I carbon dioxide production index, RQ respiratory quotient, CI cardiac index, DO2I oxygen delivery index, NA not applicable. CFH cell free haemoglobin
P-value is for Kruskal–Wallis test across the four groups or chi2 test
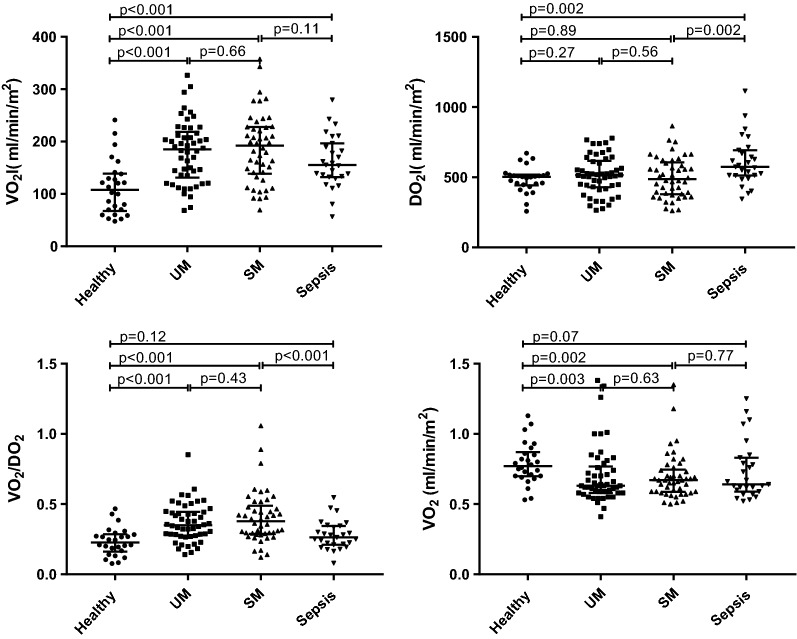
VO2I, VCO2I, DO2I and their ratios in the different patient groups
Compared to healthy participants, oxygen consumption (Fig. 1, Table 1) was higher in uncomplicated and severe malaria and sepsis (all p < 0.001). Oxygen consumption was not different between patients with uncomplicated versus severe falciparum malaria (p = 0.655) or sepsis versus severe falciparum malaria (p = 0.105). VCO2I was significantly higher in uncomplicated and severe malaria and sepsis than in healthy participants (p = 0.002, p < 0.001, p = 0.022 respectively). Despite the increased VO2I in malaria, oxygen delivery (Table 1) was similar in both uncomplicated and severe malaria to values in healthy persons (p = 0.27 and 0.89, respectively), but was significantly higher in sepsis than health (p = 0.002). Consequently, VO2/DO2 values were higher in both uncomplicated and severe malaria than in healthy persons (both p < 0.001) or sepsis (p = 0.005 and p < 0.001, respectively), but were similar between patients with sepsis and healthy participants (p = 0.117). There was no difference in the VO2/DO2 ratio between uncomplicated and severe malaria patients (p = 0.433). Compared to healthy subjects the respiratory quotient was reduced in uncomplicated and severe malaria but not sepsis (p = 0.003, 0.002, 0.072, respectively). Similar results were found for VO2, DO2 and VO2/DO2 when the subset of severe malaria patients with hyperlactataemia were compared to the control groups (Additional file 1).
Fig. 1.
Oxygen consumption and delivery indices in different groups of participant. UM, uncomplicated falciparum malaria; SM, severe falciparum malaria. Bars indicate medians and interquartile ranges. VO2I, oxygen consumption index; DO2I, oxygen delivery index; VO2I/DO2I, ratio of oxygen consumption to delivery; RQ, respiratory quotient
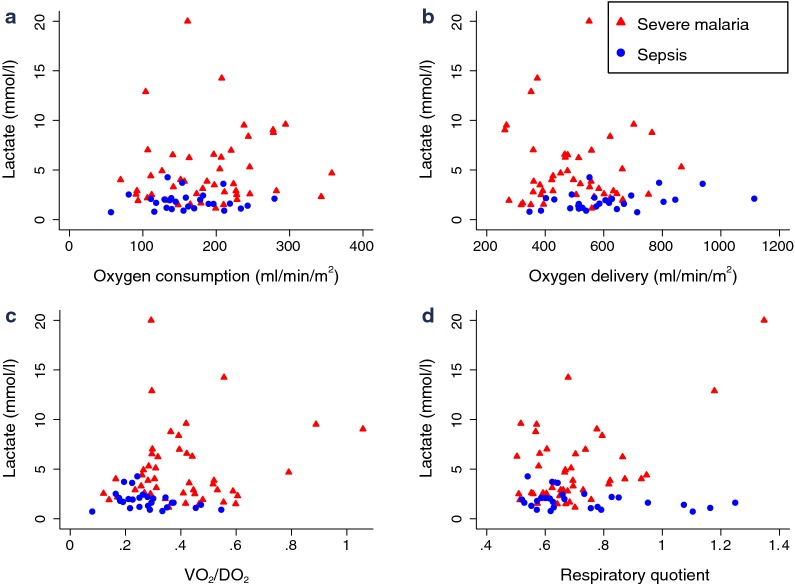
VO2I, VCO2I, DO2I and their ratios in severe malaria and sepsis
In severe malaria VO2I, DO2I and VO2I/DO2I were not significantly different in patients with or without hyperlactataemia (all p > 0.05). There was no correlation between VO2I, DO2I, RQ or the ratio of VO2/DO2 and plasma lactate in severe malaria or sepsis (all p > 0.05, Fig. 2). There was however a positive correlation between VCO2 and plasma lactate in severe malaria (ρ = 0.34, p = 0.02, N = 46). Plasma lactate correlated with parasitaemia (ρ = 0.40, p = 0.006, N = 46) and plasma PfHRP2 (ρ = 0.30, p = 0.04, N = 46) as markers of parasite biomass. In severe malaria, base excess correlated positively with DO2I (ρ = 0.29, p = 0.049, N = 46), negatively with VO2/DO2 (ρ = − 0.30, N = 46, p = 0.04) but not RQ, VO22I or VCO2I. In a multivariate linear regression model for admission base excess in severe malaria patients using AKI (categorical) and VO2/DO2 or DO2I as independent variables, AKI but not VO2I/DO2 or DO2I remained a significant predictor of base excess in the model.
Fig. 2.
Oxygen consumption and delivery indices and plasma lactate in patients with severe malaria or sepsis. a: Lactate and oxygen consumption. b: Lactate and oxygen delivery. c: Lactate and VO2/DO2. d: Lactate and respiratory quotient. VO2/DO2, ratio of oxygen consumption to delivery
Oxygen consumption did not correlate with markers of nitric oxide bioavailability in severe malaria (plasma arginine, asymmetric dimethylarginine (ADMA), the arginine/ADMA ratio, plasma cell free haemoglobin) or with temperature (p > 0.05). There was no correlation between oxygen consumption and measures of parasite biomass (plasma PfHRP2 and parasitaemia) (p > 0.05).
Discussion
Severe and uncomplicated falciparum malaria were both accompanied by an approximate 75% increase in VO2I compared to healthy individuals. The increase in VCO2I was approximately 50%. In patients admitted with suspected bacterial sepsis these values were also higher than in healthy subjects but the increments were approximately half those observed in patients with malaria. Cardiac index was increased in malaria, but unlike sepsis this did not result in a rise in DO2I due to anaemia. Consequently, the ratio of oxygen consumption to delivery rose in both severe and uncomplicated malaria but not sepsis. VO2/DO2 and DO2I did not correlate with lactate in severe malaria or in sepsis consistent with tissue hypoxia not being determined by overall oxygen delivery.
The increase in VO2I and VCO2I observed in both severe and uncomplicated malaria and sepsis is consistent with an increase in metabolic rate. The increase observed in severe malaria is consistent with a previous small study using invasive techniques [14]. A previous study in sepsis found elevated VO2 in sepsis, which decreased as disease severity increased [15]. This inverse relationship with severity was not found in malaria. The lack of an inverse relationship with plasma lactate indicates that widespread mitochondrial dysfunction, caused by hypoxia or other factors, is not a major contributor to acidosis in severe malaria. In conditions with mitochondrial dysfunction, such as biguanide intoxication, a low VO2 and inverse relationship with lactate is observed [16]. From the perspective of increases in VO2 on exercise, the increase in severe malaria of about 1.8-fold appears modest relative to the tenfold increase from rest expected when running at 6 miles per hour [17].
Tissue oxygen uptake may be limited by diffusion or convection [18]. In severe falciparum malaria, microvascular obstruction resulting from sequestration of erythrocytes containing mature parasites has been directly visualized [19] and results in focal tissue hypoxia [1]. The VO2/DO2 ratio, which is numerically equivalent to the oxygen extraction ratio [13] was elevated in both severe and uncomplicated malaria. Despite an increase in VO2/DO2 in malaria, which would be expected to result in a fall in end-capillary oxygen tension, no relationship between VO2/DO2 and plasma lactate was observed. Consistent with previous findings, no relationship between DO2 and plasma lactate was found [2]. The lack of relationship between VO2I/DO2 or DO2 and plasma lactate suggests that DO2 is adequate and that variation in the oxygen content of perfused capillaries does not increase oxygen tension significantly around non-perfused capillaries. This could be because non-perfused capillaries occur in small patches as seen in malaria retinopathy observed by fluorescein angiography [20] or because of microvascular dysfunction in perfused capillaries with failure of compensatory responses [21].
The reason for the elevated VO2I in both severe and uncomplicated malaria is likely multifactorial. Hypermetabolism from the host inflammatory response and increased cardiac work and work of breathing may contribute to the elevation in VO2I. Elevated catecholamines in malaria and sepsis may increase the VO2 of many organs including skeletal muscle [22], possibly due in part to mitochondrial uncoupling. Nitric oxide can inhibit mitochondrial respiration and reduced NO bioavailability in malaria could therefore result in an increased VO2I [21]; however, no association between markers of NO bioavailability and VO2I was observed. While whole body oxygen consumption is increased in malaria, this increase is probably heterogeneous across different tissues. Previous studies have investigated oxygen consumption in different organs. In cerebral malaria, despite normal blood flow cerebral oxygen consumption was reduced and correlated inversely with lactate production, consistent with hypoxia driving anaerobic glycolysis [7]. Skeletal muscle oxygen consumption assessed with near infrared spectroscopy (NIRS) was found to be higher in malaria than healthy individuals, but similar in severe and uncomplicated malaria [21]. In severe malaria, an inverse relationship between muscle oxygen consumption and plasma lactate was noted, consistent with inadequate oxygen availability resulting in anaerobic glycolysis [21]. Whilst anaerobic glycolysis does not consume oxygen directly, clearance of the resulting lactate via gluconeogenesis (Cori cycle) or oxidation does. As such, certain organs (the brain) or small hypoxic patches of tissue may have a reduced VO2 and produce lactate which may be cleared in other areas which have an increased VO2. Patients with severe malaria have increased metabolic requirements as evidenced by their increased VO2.
This study had several shortcomings: the number of patients with the different syndromes of severe malaria and sepsis was relatively small. There were too few septic patients to determine the relationship between VO2 and severity in this category. The assessment of oxygen consumption and CO2 production from expired gas assumes steady state conditions; application of the mask may have caused hyperventilation, increasing the respiratory minute volume and hence CO2 elimination in particular.
Inadequate DO2I is unlikely to contribute to tissue hypoxia in severe malaria. Future studies should examine the potential role of blood transfusion and fluid therapy in severe malaria in preserving haemodynamic stability and renal function as opposed to improving lactic acidosis. Whilst blood transfusion is unlikely, except in extreme anaemia, to improve outcome due to reducing tissue hypoxia, it might lessen the chance of cardiovascular decompensation developing by increasing the cardiac index reserve. The optimal blood transfusion threshold in adult severe malaria has not been well established. A large randomized controlled trial of blood transfusion in paediatric severe malaria is ongoing (ISRCTN84086586).
Conclusion
Falciparum malaria was associated with increased oxygen consumption but this was not related to disease severity. Lactic acidosis did not result from inadequate overall macrocirculatory tissue oxygen delivery, but more likely from patchy microvascular perfusion abnormalities combined with impaired hepatic clearance. How the haemodynamic status can be optimized to avoid decompensation in the period after antimalarial treatment needs further investigation.
Additional file
Additional file 1: Table S1. Comparison of hyperlactatemic severe malaria patients with control groups.
Authors’ contributions
Designed study: HWK, AMD, RJM, NMA. Collected data: HWK, MTH, KP, HI, SJL, RJM, BI. Analyzed data: HWK, VR. Interpreted data, revised manuscript: HWK, AG, VR, RJM, SM, NPJD, NJW, MAH, NMA, AMD. Drafted manuscript: HWK, AMD, NJW. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the patients and their relatives for agreeing to participate in these studies and the assistance of colleagues in patient recruitment. We thank the doctors and staff of Chittagong Medical College Hospital and ISPAT General Hospital for their support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All participants (or legally acceptable representatives in cases where patients lacked capacity during the acute illness) gave informed, written consent. The study was approved by the Oxford Tropical Research, CMC and IGH ethical committees.
Funding
This work was supported by an Australian Government UPRS & PIRTS scholarship (HWK), the Wellcome Trust as part of the Wellcome Trust Major Overseas Programme funding and the National Health and Medical Research Council (Grant Number 605807 and Practitioner Fellowship to NMA).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ADMA
asymmetric dimethyl arginine
- AKI
acute kidney injury
- CAM
coma acidosis malaria
- CI
cardiac index
- CFH
cell free haemoglobin
- CMCH
Chittagong Medical College Hospital
- DO2
oxygen delivery
- DO2I
oxygen delivery index
- IGH
Ispat General Hospital
- IQR
interquartile range
- PfHRP2
Plasmodium falciparum histidine rich protein 2
- RQ
respiratory quotient
- SM
severe falciparum malaria
- UM
uncomplicated falciparum malaria
- VCO2
carbon dioxide production
- VCO2I
carbon dioxide production index
- VO2
oxygen consumption
- VO2I
oxygen consumption index
- VO2/DO2
ratio of oxygen consumption to delivery
References
- 1.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 2.Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, et al. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis. 2012;206:571–579. doi: 10.1093/infdis/jis400. [DOI] [PubMed] [Google Scholar]
- 3.Davis TM, Binh TQ, le Thu TA, Long TT, Johnston W, Robertson K, et al. Glucose and lactate turnover in adults with falciparum malaria: effect of complications and antimalarial therapy. Trans R Soc Trop Med Hyg. 2002;96:411–417. doi: 10.1016/S0035-9203(02)90377-9. [DOI] [PubMed] [Google Scholar]
- 4.Day NP, Phu NH, Mai NT, Chau TT, Loc PP, Chuong LV, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000;28:1833–1840. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Pukrittayakamee S, White NJ, Davis TM, Looareesuwan S, Supanaranond W, Desakorn V, et al. Hepatic blood flow and metabolism in severe falciparum malaria: clearance of intravenously administered galactose. Clin Sci (Lond). 1992;82:63–70. doi: 10.1042/cs0820063. [DOI] [PubMed] [Google Scholar]
- 6.Day NP, Phu NH, Mai NT, Bethell DB, Chau TT, Loc PP, et al. Effects of dopamine and epinephrine infusions on renal hemodynamics in severe malaria and severe sepsis. Crit Care Med. 2000;28:1353–1362. doi: 10.1097/00003246-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Warrell DA, White NJ, Veall N, Looareesuwan S, Chanthavanich P, Phillips RE, et al. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet. 1988;2:534–538. doi: 10.1016/S0140-6736(88)92658-X. [DOI] [PubMed] [Google Scholar]
- 8.Charoenpan P, Indraprasit S, Kiatboonsri S, Suvachittanont O, Tanomsup S. Pulmonary edema in severe falciparum malaria. Hemodynamic study and clinicophysiologic correlation. Chest. 1990;97:1190–1197. doi: 10.1378/chest.97.5.1190. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 10.Hanson J, Lee SJ, Mohanty S, Faiz MA, Anstey NM, Charunwatthana P, et al. A simple score to predict the outcome of severe malaria in adults. Clin Infect Dis. 2010;50:679–685. doi: 10.1086/649928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plowman A, Smith D, Appendix B. In exercise physiology for health, fitness, and performance. 2. San Francisco: Benjamin Cummings; 2003. [Google Scholar]
- 12.Huntsman LL, Stewart DK, Barnes SR, Franklin SB, Colocousis JS, Hessel EA. Noninvasive Doppler determination of cardiac output in man. Clinical validation. Circulation. 1983;67:593–602. doi: 10.1161/01.CIR.67.3.593. [DOI] [PubMed] [Google Scholar]
- 13.Varon J, Fromm RE. Cardiovascular facts and formulas. Acute and critical care formulas and laboratory values. New York: Springer; 2014. pp. 1–23. [Google Scholar]
- 14.Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hien TT, et al. The effects of dopamine and adrenaline infusions on acid–base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219–223. doi: 10.1016/S0140-6736(96)09096-4. [DOI] [PubMed] [Google Scholar]
- 15.Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med. 1993;21:1012–1019. doi: 10.1097/00003246-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Protti A, Russo R, Tagliabue P, Vecchio S, Singer M, Rudiger A, et al. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14:R22. doi: 10.1186/cc8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 18.McClatchey PM, Frisbee JC, Reusch JEB. A conceptual framework for predicting and addressing the consequences of disease-related microvascular dysfunction. Microcirculation. 2017;24:6. doi: 10.1111/micc.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, Faiz MA, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 20.Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis. 2009;199:263–271. doi: 10.1086/595735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis. 2013;207:528–536. doi: 10.1093/infdis/jis692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonsen L, Bulow J, Madsen J, Christensen NJ. Thermogenic response to epinephrine in the forearm and abdominal subcutaneous adipose tissue. Am J Physiol. 1992;263:E850–E855. doi: 10.1152/ajpendo.1992.263.5.E850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparison of hyperlactatemic severe malaria patients with control groups.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.