Abstract
Objective
Frailty indices are important predictors of major health outcomes, but mostly designed by and for researchers and specialists. Three of the most commonly used theory-based indices are composite measures that are subject to arbitrary assumptions and biases introduced due to data processing. A complicated index can be simplified with fewer items. The theory-based frailty indices are not optimal and neglect patients’ perspectives. This study aims to compare different definitions of frailty and propose a self-rated measure of frailty index and status.
Results
Frailty was defined differently by laypeople and researchers/clinicians. Patients’ and laypeople’s perspectives seemed neglected. Existing frailty indices had shortcomings related to the use of composite measures, assumptions of frailty theories, and the lack of novel information. To avoid these shortcomings, we suggested asking individuals “on a scale of 0 to 10, how frail do you think you are?” and “by answering yes or no, do you consider yourself to be frail?” to determine frailty on continuous and dichotomous scales respectively. However, there will be other issues emerging with these new measures, such as the need for feasibility and validity studies, as well as acceptability by researchers.
Keywords: Frailty, Frailty index, Frailty status, Self-rated frailty measure, Phenotype Model, Accumulation of Deficits Model
Introduction
Frailty is often considered a geriatric syndrome by researchers [1, 2], characterized by weakness, slow gait, and other aging-related symptoms or diseases [1–3]. In a 2018 review, the mostly commonly adopted theory was the Phenotype Model by Fried et al., followed by the Accumulation of Deficits Model by Rockwood et al. [4]. According to the Phenotype Model, frailty can be defined if at least three of the following present: weight loss, low handgrip strength, slow walking speed, exhaustion, and reduced physical activity [5]. The frailty measured by the Accumulation of Deficits Model is defined with 70 variables that characterize the deficits in cognition, cardiovascular system, metabolism, and others [6, 7]. Despite their popularity in the research field, these two models have been recognized for problems in the assumptions they rely on and the biases introduced by data processing [1].
The assumptions made in the creation of the two frailty indices by the two models include assigning input variables with equal weights, arbitrary age criteria, and the lack of evidence for formulating the thresholds for frailty statuses based on frailty indices [1]. The input variables have been conventionally recognized as equally important and assigned equal weight to derive frailty indices [1]. Recent studies suggest that this assumption needs to be tested [1]. The age criteria for the diagnosis of frailty proposed by the two models differ, a minimum age of 65 and 70 years respectively [1, 2]. Lastly, the proposed thresholds that define frailty statuses and are used to determine the prevalence estimates differ, at 0.6 and 0.2 respectively [1]. These thresholds may also influence the magnitudes of the biases that can be introduced to the indices [1].
Furthermore, these frailty indices defined by the two models are subject to interpretability problems for different reasons [1]. The Phenotype Model relates to biases generated purely by data processing [1]. Bias alone can explain more than 71% of the variance of the frailty index defined by the Phenotype Model [1]. The 70-item frailty index defined by the Accumulation of Deficits Model can be further simplified with fewer input variables [1]. This suggests that many input variables may in fact be similar or highly correlated [1]. As a result, these two models fail to reflect the theories they are based on [1].
In addition, patients’ perspectives are increasingly emphasized [8]. The two frailty models are generated mostly for research purposes using administrative or survey data [2]. Research-oriented frailty indices have been criticized for focusing purely on the clinical aspects of this aging phenomenon [3]. The frailty indices defined by the two models may not be closely linked to patients’ perception. To improve the research on frailty, this study aims to (1) highlight the differences in frailty definitions adopted by laypeople and clinicians/researchers, and (2) propose self-rated frailty measures that avoid the shortcomings existing in many research-oriented indices.
Main texts
A search for definitions of frailty was performed and the results were listed in Table 1. Frailty could be defined from at least two perspectives: layperson and researcher/clinician. From a layperson’s perspective, frailty was often referred to as weakness, delicacy, and being fragile [9, 10]. For statisticians, frailty models were those that took random effects into consideration and frailty was used to describe the unobserved heterogeneity of a deterioration of health [11]. Since the 1990s, statisticians have used frailty to refer to random effects in statistical models, especially survival analysis [11]. For aging researchers and clinicians, frailty became a popular research topic in medical research since the 2000s; however a variety of frailty definitions have been used [3]. According to an expert consensus, frailty could be defined as “a clinical syndrome characterized by declining reserve and diminished resistance to stressors” [3, 12]. Compared to the layperson’s perspective, which primarily links frailty to physical weakness, researchers and clinicians often consider frailty in terms of aging and interaction with stressors or physical environments according to the three definitions used by frailty researchers [3]. Although the Accumulation of Deficits Model did not explicitly link frailty to aging or interaction with external factors, this model also set up age threshold for the diagnosis of frailty [2, 3].
Table 1.
Frailty defined by the public, statisticians and aging researchers
| Perspectives | Definitions |
|---|---|
| Laypeople | “The quality or state of being frail” (frail: easily broken or destroyed; physically weak) [9] |
| “The condition of being weak and delicate” [10] | |
| Researchers/clinicians | The random effects that “account for association and unobserved heterogeneity” in statistical models [11] |
| “A state of vulnerability that becomes more prevalent with age and affects an individual’s resilience and ability to deal with minor and major stressors, which can include illnesses or infections” (National Institute on Ageing definition) [3] | |
| “A clinical syndrome characterized by declining reserve and diminished resistance to stressors” (expert consensus) [12] | |
| “A phenotype, which is defined as an individual’s observable traits that result from the interaction of their genetic information with their physical environment.” (Phenotype Model) [3] | |
| “An accumulation of deficits, which can be physical, cognitive, and clinical challenges an individual may be facing, including falls, changes in the ability to carry out everyday activities, depression, restlessness, memory changes, and congestive heart failure—the more deficits an individual has, the greater their level of frailty” (Accumulation of Deficits Model) [3] |
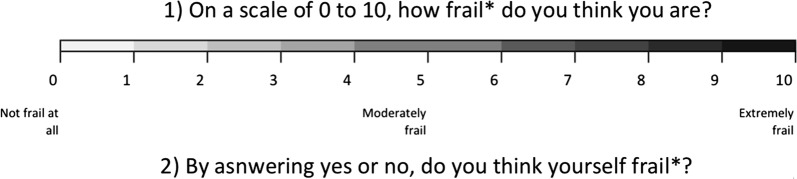
To address the discrepancy between the frailty defined by laypeople and researchers and avoid the problematic assumptions, we developed frailty measures that directly adopted the individual inputs presented in Fig. 1. Before measuring frailty, a definition of frailty should be given and well explained to the participants. The definitions could be adopted from the commonly-used models aforementioned. To determine frailty ranking on a continuous scale, individuals would be asked directly, “on a scale of zero to ten, how frail do you think you are?” In the other question, individuals would be questioned, “by answering yes or no, do you consider yourself to be frail?” to understand whether they consider themselves frail.
Fig. 1.
Proposed patient-oriented frailty scales. Asterisk: the definition of frailty needs to be clarified and should be understood by the interviewees
Moreover, the new assessment scales were also designed to avoid the problematic assumptions aforementioned and the shortcomings that had been identified in existing frailty indices, listed in Table 2 (a full list published elsewhere [1]). These shortcomings were related to the use of index, the characteristics of the frailty theories, or information collection [1]. Without adopting composite measures for frailty measurement, issues, including the assumptions of equal weights, relatively poorer predictive power than input variables, data processing biases, and excessive complexity of indices [1, 13], could be completely avoided. The theory-based assumptions, such as the thresholds of frailty indices for the diagnosis of frailty status, age criteria, and important components of frailty [13], could be directly assessed with patient perception. The self-rated frailty measures directly introduced patient input and could potentially lead to public engagement by surveying a large population. Also, most frailty indices used existing variables from administrative or survey data [1] and lacked new information that could have the potential to improve our understanding in frailty.
Table 2.
The issues that a patient-oriented frailty scale might address and those that might emerge
| Classifications of the shortcomings | Issues that can be avoided by a patient-oriented frailty scale | Issues merging if patient-oriented frailty scales in use |
|---|---|---|
| Index related | 1. Unclear rationales for equal weighting of domain variables that leads to unequal weighting of input variables and inclusion of duplicate information | |
| 2. Biases introduced by data processing that is not based on evidence | ||
| 3. Reproducibility limited by measurement devices and data processing | 1. Subjective measurement | |
| 4. Disconnection between frailty theories and produced indices because of excessive numbers of input variables and biases introduced due to data processing | ||
| 5. Complex indices that can be simplified | ||
| 6. Constraints on the regression coefficients of input or domain variables | ||
| 7. Relatively poorer predictive power regarding mortality than input variables | 2. Predictive power to be tested | |
| Frailty theory-related | 8. Arbitrary thresholds of frailty indices for the diagnosis of frailty statuses | |
| 9. Arbitrary assumptions about frailty distribution, age correlation, and input variable eligibility of input variables | ||
| 10. Potential disconnection between biology of frailty and the measurement | ||
| 11. Patients’ and the public’ perspectives ignored | 3. Deviation from researchers’ definitions | |
| 12. Disconnection to socio-economic determinants | 4. Questions on socioeconomic status may deter some to respond | |
| Information generation | 13. Old information shuffled, if frailty estimated based on available research or administrative data | 5. Reliability and validity to be tested |
There were other successful precedents that adopted subjective measures to understand individual health status. One of the most prominent examples was self-rated health status that had been proven important for the prediction of mortality [14], depression [15], cardiovascular disease, diabetes, and other adverse events [16]. This subjective measure was examined and proven valid in the population [17]. Self-rating was also applied in mental health research [18]. For aging research, the subjective measures of frailty and the important components had not been sufficiently studied. Ideally, even the components in the frailty models could be investigated for the usefulness of the subjective measurement. For example, the five items in the Phenotype Model could be measured by subjective judgements. In fact, four of the five items in the Phenotype Model could be measured subjectively with validated questionnaires: weight loss [19], exhaustion [20], walking speed [21], and physical activity [22]. It might be the time to understand the usefulness of subjective frail measures and the relationships between subjective and objective frailty measures.
Limitations
However, it would be possible that the proposed scales might be subject to certain limitations. There were several potential obstacles for the wide adoption of the subjective measures of frailty. Although there was some consensus on the definition of frailty [12, 23], it remained uncertain whether this consensus would be understood and accepted by most laypeople or researchers. It had been reported that some seniors might not prefer the term, “frail” [24]. Interviewees might be unwilling to consider and rate themselves frail. The acceptability of the term, “frail”, for self-assessment remained unanswered. The proposed subjective measure of frailty, similar to other patient reported outcomes, such as quality of life [25], needed to be tested in different populations to understand it validity and reliability [26]. In general, indices predicted mortality worse than input variables [13]. However, it remained unclear whether this new measure would predict mortality or other outcomes better than existing frailty indices. Currently, no resources were available for us to conduct feasibility test for the proposed frailty measure. Lastly, we did a limited literature search for the definitions of frailty. A systematic search for all frailty definitions might be useful.
Authors’ contributions
YSC conceptualized the self-rated frailty measures, drafted and reviewed the manuscript. DM, CJW, HCW, and WCC reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent to publish
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi-Sheng Chao, Email: chaoyisheng@post.harvard.edu.
Danielle McGolrick, Email: danielle.mcgolrick@gmail.com.
Chao-Jung Wu, Email: chao-jung.wu@mail.mcgill.ca.
Hsing-Chien Wu, Email: s881023@gmail.com.
Wei-Chih Chen, Email: wiji.chen@gmail.com.
References
- 1.Chao Y-S, Wu H-C, Wu C-J, Chen W-C. Index or illusion: the case of frailty indices in the Health and Retirement Study. PLoS ONE. 2018;13(7):e0197859. doi: 10.1371/journal.pone.0197859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57(5):830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Ageing. We can’t address what we don’t measure consistently. Building consensus on frailty in Canada. Toronto: National Institute on Ageing; 2018. https://www.ryerson.ca/nia/white-papers/frailty-paper.pdf.
- 4.Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A. 2018 doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Biol Sci Med Sci. 2007 doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 8.Clark DA, Khan U, Kiberd BA, Turner CC, Dixon A, Landry D, et al. Frailty in end-stage renal disease: comparing patient, caregiver, and clinician perspectives. BMC Nephrol. 2017;18:148. doi: 10.1186/s12882-017-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merriam-Webster I. Merriam-Webster’s collegiate dictionary. 11. Springfield: Merriam-Webster, Incorporated; 2004. [Google Scholar]
- 10.Stevenson A. Oxford Dictionary of English. Oxford: OUP Oxford; 2010. [Google Scholar]
- 11.Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1(3):255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol Series A. 2013;68(1):62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao Y-S, Wu C-J. Principal component-based weighted indices and a framework to evaluate indices: results from the Medical Expenditure Panel Survey 1996 to 2011. PLoS ONE. 2017;12(9):e0183997. doi: 10.1371/journal.pone.0183997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800–808. doi: 10.2105/AJPH.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambresin G, Chondros P, Dowrick C, Herrman H, Gunn JM. Self-rated health and long-term prognosis of depression. Ann Fam Med. 2014;12(1):57–65. doi: 10.1370/afm.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waller G. Self-rated health in general practice: a plea for subjectivity. Br J Gen Pract. 2015;65(632):110. doi: 10.3399/bjgp15X683833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnittker J, Bacak V. The increasing predictive validity of self-rated health. PLoS ONE. 2014;9(1):e84933. doi: 10.1371/journal.pone.0084933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mawani FN, Gilmour H. Validation of self-rated mental health. Health Rep. 2010;21(3):61–75. [PubMed] [Google Scholar]
- 19.Zentenius E, Andersson-Assarsson JC, Carlsson LMS, Svensson PA, Larsson I. Self-reported weight-loss methods and weight change: ten-year analysis in the Swedish Obese Subjects Study Control Group. Obesity (Silver Spring, Md) 2018;26(7):1137–1143. doi: 10.1002/oby.22200. [DOI] [PubMed] [Google Scholar]
- 20.Glise K, Hadzibajramovic E, Jonsdottir IH, Ahlborg G., Jr Self-reported exhaustion: a possible indicator of reduced work ability and increased risk of sickness absence among human service workers. Int Arch Occup Environ Health. 2010;83(5):511–520. doi: 10.1007/s00420-009-0490-x. [DOI] [PubMed] [Google Scholar]
- 21.Stamatakis E, Kelly P, Strain T, Murtagh EM, Ding D, Murphy MH. Self-rated walking pace and all-cause, cardiovascular disease and cancer mortality: individual participant pooled analysis of 50 225 walkers from 11 population British cohorts. Br J Sports Med. 2018;52(12):761. doi: 10.1136/bjsports-2017-098677. [DOI] [PubMed] [Google Scholar]
- 22.Holen MS, Een R, Mildestvedt T, Eide GE, Meland E. Two valid measures of self-rated physical activity and capacity. Open Cardiovasc Med J. 2012;6:156–162. doi: 10.2174/1874192401206010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Direct Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson S. Going grey: the mediation of politics in an ageing society. London: Taylor & Francis; 2016. [Google Scholar]
- 25.Carr AJ, Higginson IJ. Are quality of life measures patient centred? BMJ. 2001;322(7298):1357–1360. doi: 10.1136/bmj.322.7298.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use. Oxford: Oxford University Press; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.