Abstract
Background
Hyalomma marginatum and Hyalomma rufipes are two-host tick species, which are mainly distributed in southern Europe, Africa and middle-eastern Asia. They are well-known vectors of Crimean Congo hemorrhagic fever (CCHF) virus and other viruses as well as Rickettsia aeschlimannii. In recent years, these tick species have been found sporadically in Germany, but they do not belong to the autochthonous tick fauna in Germany.
Methods
Ticks with unusual morphology were collected and sent from private persons or public health offices to involve institutions for morphological identification and further testing. All ticks identified as Hyalomma spp. were tested using molecular detection methods for CCHF virus, Rickettsia spp., Coxiella burnetii and Coxiella-like organisms, Babesia spp. and Theileria spp.
Results
Thirty-five ticks with an unusual appearance or behaviour were reported to us during summer-autumn 2018. For 17 of them, the description or photos implied that they belong to the hard tick genus Hyalomma. The remaining 18 ticks were sent to us and were identified as adult Hyalomma marginatum (10 specimens) or adult Hyalomma rufipes (8 specimens). All ticks tested negative for CCHF virus, Coxiella burnetii, Coxiella-like organisms, Babesia spp. and Theileria spp. The screening for rickettsiae gave positive results in 9 specimens . The Rickettsia species in all cases was identified as R. aeschlimannii.
Conclusions
These results show that exotic tick species imported into Germany were able to develop from the nymphal to the adult stage under appropriate weather conditions. Fifty percent of the ticks carried R. aeschlimannii, a human pathogen, while CCHF virus or other pathogens were not detected. Imported Hyalomma ticks may be the source of exotic diseases acquired in Germany.
Keywords: Hyalomma marginatum, Hyalomma rufipes, Sheep, Horse, Human, Rickettsia aeschlimannii, Germany
Background
The genus Hyalomma is a small genus, with 27 species that are mainly present in the Afrotropical Region and parts of the Palaearctic Region [1]. A considerable amount of work on the genus Hyalomma, with an important input on classification, morphology, hosts and distribution has been done by Apanaskevich and colleagues [2–6].
Hyalomma (Euhyalomma) marginatum Koch, 1844 is the type-species of the H. marginatum complex, formed by Hyalomma isaaci, Hyalomma marginatum (sensu stricto), Hyalomma rufipes, Hyalomma turanicum and Hyalomma glabrum [7]. Hyalomma marginatum is known as the “Mediterranean” Hyalomma [8] (the synonym Hyalomma plumbeum has been used in some Russian and eastern European literature [2, 9, 10]). Hyalomma marginatum has a large geographical distribution, ranging from southern Europe and North Africa to the Ukraine and southern Russia and the Middle East [2]. Like some other Hyalomma species, especially of the H. marginatum complex, H. marginatum is known to be a vector of a wide variety of pathogens of medical and veterinary importance, including Crimean Congo hemorrhagic fever (CCHF) virus [8, 10], West Nile, Thogoto, Dhori and other viruses [10], as well as Rickettsia aeschlimannii [11, 12], Babesia caballi and Theileria annulata [8, 13]. Petney et al. [14] reviewed the tick species in Germany and found a few previous reports of H. marginatum, but in the majority of these cases the identification remained uncertain. A more recent study georeferenced ixodid ticks in Germany and reported one location where H. marginatum was identified [15, 16]. In 2017, one H. marginatum specimen was detected on a human in Tübingen, Federal State of Baden-Württemberg [17].
Hyalomma rufipes Koch, 1844 known as “the hairy Hyalomma” or “the coarse bont-legged Hyalomma” [8, 18], was considered a subspecies of H. marginatum [19, 20], but is currently accepted as a valid species [2]. Hyalomma rufipes is the most widespread Hyalomma species in Africa, but is also present in Greece, Turkey, Russia, Iraq, Syria, Pakistan, Egypt (Nile Valley), Yemen, Oman and northern China [8, 21–24]. Both larvae and nymphs of H. marginatum and H. rufipes use small mammals and birds as hosts, while adults are mainly found on cattle, sheep, goats, wild ungulates and horses [8, 23]. As some other Hyalomma species, H. rufipes is known to be a vector of CCHF virus [8, 18, 25] as well as of Rickettsia conorii [8, 18], R. aeschlimannii [26–28], Anaplasma marginale and Babesia occultans [8, 18]. Some authors implicated Hyalomma species in tick facial paralysis in humans [29, 30]. Larvae and nymphs of H. rufipes have been occasionally found on migratory birds in some European countries (e.g. the Netherlands and Norway) [31]. One H. rufipes specimen was described recently in Germany near Frankfurt, Federal State of Hesse [32]. Hoffman et al. [33] detected Alkhurma hemorrhagic fever virus RNA in immature H. rufipes ticks infesting northward migratory birds caught in the North Mediterranean Basin.
However, probably due to the current climatic conditions, no permanent Hyalomma populations have been recognized in northern or central Europe so far. Here, we report 18 imported specimens of H. marginatum and H. rufipes in Germany in 2018. The individual ticks were tested for various pathogens known to be carried by these two Hyalomma species.
Methods
Tick collection and identification
Ticks were collected from sheep, horses, a human, a house, and from one unknown site, in different locations and districts in Germany, from June to October 2018 (Table 1, Fig. 1). Ticks were shipped as individual specimens by the collecting persons directly or via public health offices to our laboratories. These ticks were further analysed in the present study. They were identified by morphological characters according to Apanaskevich & Horak [2]. In addition, some other collected ticks, not available for shipment, were included in this study and their identification as Hyalomma were based on photos sent by the animal owner.
Table 1.
Hyalomma spp. collection samples and detected pathogens in Germany, 2018
| Collection date | Locality and district | Hyalomma spp. | Stage | Host | Pathogen | ||||
|---|---|---|---|---|---|---|---|---|---|
| CCHF virus | Rickettsia aeschlimannii | Coxiella burnetii | Coxiella-like | Babesia/Theileria spp. | |||||
| 26 June | Wächtersbach, Hesse | H. marginatum | Female | Sheep | − | + | − | − | − |
| 30 July | Wardenburg, Lower Saxony | H. marginatum | Male | Horse | − | − | − | − | − |
| 30 July | Hannover, Lower Saxony | H. rufipes | Female | Horse | − | − | − | − | − |
| 05 August | Wächtersbach, Hesse | H. marginatum | Male | Horse | − | + | − | − | − |
| 20 August | Hannover, Lower Saxony | H. rufipes | Female | Horse | − | + | − | − | − |
| 20 August | Lützelhausen, Hesse | H. rufipes | Male | Horse | − | − | − | − | − |
| 24 August | Hannover, Hesse | H. marginatum | Male | Car | − | + | − | − | − |
| 13 September | Heiligenberg, Baden-Württemberg | H. marginatum | Male | Horse | − | − | − | − | − |
| 22 August | Neuenhaus, Lower Saxony | H. marginatum | Female | Horse | − | + | − | − | − |
| 21 August | Koblenz, Rhineland-Palatinate | H. marginatum | Female | Horse | − | − | − | − | − |
| 10 September | Borgdorf-Seedorf, Schleswig-Holstein | H. rufipes | Male | Horse | − | − | − | − | − |
| 23 August | Hohenaspe, Schleswig-Holstein | H. marginatum | Male | Horse | − | − | − | − | − |
| 04 September | Fechenheimer Aue, Hesse | H. marginatum | Female | House | − | + | − | − | − |
| 11 September | Winsen/Aller, Lower Saxony | H. rufipes | Female | Unknown | − | − | − | − | − |
| 18 August | Saulheim, Rhineland-Palatinate | H. rufipes | Female | Horse pasture | − | + | − | − | − |
| 13 October | Neuenkirchen, Lower Saxony | H. rufipes | Male | Horse | − | + | − | − | − |
| 20 October | Wessel, North Rhine Westphalia | H. marginatum | Female | Horse | − | − | − | − | − |
| 13 October | Mörsdorf, Rhineland-Palatinate | H. rufipes | Male | Horse | − | + | − | − | − |
Key: +, present; −, absent
Fig. 1.
Distribution of introduced Hyalomma spp. in Germany, 2018
Nucleic acid extraction and PCR
Total nucleic acid was extracted using MagNA Pure LC RNA/DNA Kit (Roche, Mannheim, Germany) in a MagNA Pure LC instrument (Roche) according to the manufacturer’s instructions. The extracted total nucleic acid was stored at -80 °C until use.
Ticks were tested for CCHF virus using a previously published real-time RT-PCR [34], Rickettsia spp. DNA using a pan-Rickettsia real-time PCR to amplify part of the gltA gene [35], followed by a 23S-5S intergenic spacer region PCR [36] to identify the Rickettsia species and an ompA PCR [37] and ompB PCR [38] for further molecular characterization. Furthermore, the ticks were tested for Babesia spp. and Theileria spp. using a conventional PCR amplifying part of the 18S rRNA gene [39]. Additionally, by real-time PCR and conventional PCR, respectively, ticks were tested for the occurrence of Coxiella burnetii and Coxiella-like organisms as described earlier [40, 41].
Sequence analysis of rickettsial ompA, ompB and 23S intergenic spacer region
The 23S intergenic spacer region amplicon sequences (334 bp) and the partial ompA sequences were compared to sequences from GenBank using the nucleotide blast algorithm. A phylogenetic tree based on the partial ompB sequences was generated using the maximum-likelihood (ML) method of Mega v.5.0 [42]. Best fitting substitution models were determined with the Akaike information criterion using the ML model test implemented in MEGA v.5.0. Support for the topologies was tested by bootstrapping over 1000 replicates and gaps were excluded from the comparisons. The substitution model was GTR + I. Sequences from R. aeschlimannii available on GenBank (HM050278.1, AF123705.1, KU961544.1, KU723521.1, MF002557.1, KT318745.1) were included to compare the newly generated sequences. Two sequences of R. helvetica (AF 123725.1, GU 324465.1) were used as an outgroup.
Results
A total of 18 tick specimens were received in our laboratories and identified as H. marginatum (5 females and 5 males) and H. rufipes (4 females and 4 males) (Table 1). Ticks were found in locations in western Germany, from the northern part of the Federal State of Baden-Württemberg along the Federal States of Hesse, Rhineland-Palatine to Lower Saxony and Schleswig-Holstein (Fig. 1).
The molecular testing of the ticks for potential pathogens of both species for CCHF virus, C. burnetii, Coxiella-like organisms, Babesia spp. and Theileria spp. were negative. The pan-Rick PCR tested positive for rickettsiae in 5 out of the 10 H. marginatum and 4 out of 8 H. rufipes. The amplification of the 23S-5S intergenic spacer region, ompA (ompA1 and ompA4) and ompB fragments with specific PCRs identified R. aeschlimannii.
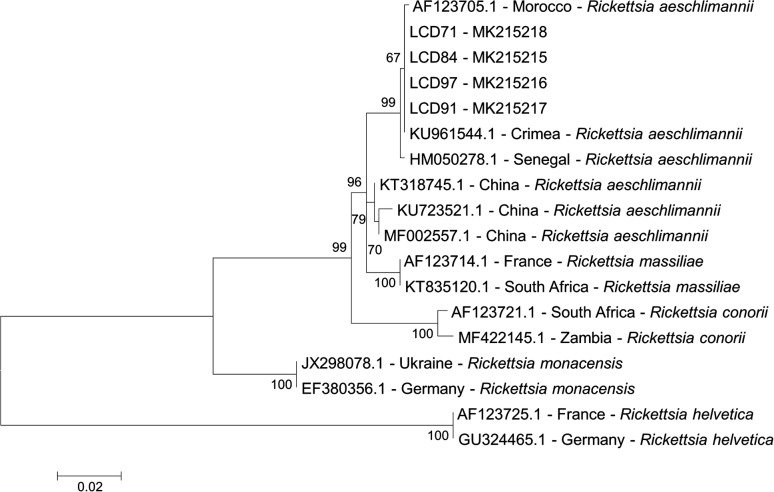
All nine Rickettsia spp. positive panRick PCR samples were further studied by amplifying and sequencing different gene fragments. 23S gene fragments were obtained and sequenced for all nine samples, ompA fragments for six samples and ompB fragments for four samples. The obtained sequences for the 23S-5S intergenic spacer region amplicon showed 100% identity with R. aeschlimannii sequences (GenBank: AY125016.1 and MG450333.1) on GenBank. The six ompA4 sequences (861 bp) were 100% identical to the R. aeschlimannii sequence from the strain MC16 (GenBank: U83446.1). Six out of seven ompA1 sequences obtained from the German samples showed a 100% identity to strains from different areas in the world (Russia, Israel, Spain, Portugal and Turkey), while one R. aeschlimannii sequence from a H. marginatum tick had a single nucleotide polymorphism at position 264 in the alignment (273 bp), which is identical to a sequence from Senegal (GenBank: HM050290.1). The four sequences obtained for the ompB gene (MK215215-MK215218) were 100% identical and cluster with strains from Morocco and Senegal (GenBank: HM050278.1, AF123705.1) (Fig. 2).
Fig. 2.
Maximum likelihood based on partial ompB sequences (776 nucleotides)
Discussion
Here we report an unusually high introduction of Hyalomma spp. into Germany. From the 35 recorded Hyalomma ticks, 18 specimens were received and identified as H. marginatum (10 specimens) and H. rufipes (8 specimens). The others (17 ticks) were identified based on photos. Detection of Hyalomma ticks in central Europe and also northern Europe, i.e. outside of the known areas of distribution of these tick species, is not totally new. Hyalomma marginatum was described for the first time in northern Europe in 1939 on the Island of Bornholm [43]. Later they were described on several occasions in Finland, Sweden and Norway [44–46]. In Poland, four specimens of unfed H. marginatum males were found in Bytom, Upper Silesia, in June 1935 (1 specimen) and June 1943 (3 specimens), which are archived in Bytom’s museum collection, Upper Silesia [47]. In Germany, four reports of Hyalomma ticks are known to the best of our knowledge. Two cases of adults, one H. rufipes male collected from a horse [32] and one H. marginatum female collected from a human [17] in the Frankfurt area and Tübingen, respectively, were reported in Germany, and two other reports date from 2007 and 2011 [15, 48]. Therefore, in 2018, the reporting of 35 putative and identified ticks of the genus Hyalomma and the final confirmation of identification and analysis of 18 specimens in Germany are exceptional.
All reported and confirmed tick findings were located in western Germany. Ticks were found along the Rhine River and continuing up to Schleswig-Holstein in northern Germany. This implies that the main route of introduction was most likely via the western migratory route of birds from West Africa via Spain and France to Scandinavia.
While in Scandinavia nymphal ticks were collected from migrating birds, all Hyalomma specimens described and tested in 2018 in Germany were adult ticks sampled from large animals or humans. The immature stages of H. marginatum are commonly found on migratory passerine birds [10], which may transport these ticks over long distances [49–52]. Up to 21% of birds migrating from Africa to the United Kingdom were infested with H. marginatum nymphs [53]. Therefore, it can be estimated that every year hundreds of thousands of immature Hyalomma ticks are transported via migratory birds into or over central Europe during the spring migration of birds from southern Europe and Africa. Hyalomma marginatum also attacks humans [54]. In a report, Hyalomma species were transported from one continent to the other by humans [55].
Usually, only few of these imported ticks seem to develop into the mature stage and, so far, no established populations of Hyalomma ticks in central Europe are known. However, the weather conditions in 2018 in Germany allowed the molting into adult ticks, and these adult stages were subsequently found on animals, humans or as questing ticks as described above. According to the German National Weather Service, 2018 was the warmest year ever recorded since the beginning of weather recording in 1881. In addition, 2018 was the second driest year since 1881 [56]. Only the year 1911 was drier than 2018 [56]. The combination of dry and hot conditions probably favored the development and molting of imported nymphs of Hyalomma ticks into adults.
An accurate modelling has hypothesized that the current northern distribution limit for this tick species should be 47°N [57]. Interestingly, the same authors have hypothesized the expansion of the geographical areas, where H. marginatum could complete the life-cycle up to some areas in Germany and the Netherlands by the 2050s, if not before [58, 59]. Despite these forecasts, adult Hyalomma ticks attached to mammalian hosts in areas further north of the forecasted hypothetical geographical limit were recently reported [17, 32]. These findings confirm and even anticipate the forecasts of the models mentioned above [58, 59].
Ticks belonging to the H. marginatum complex are known to transmit viral and bacterial agents with the potential to cause diseases of variable severity in humans. Among the viruses, CCHF virus is of greatest medical importance. Hyalomma marginatum is the most important vector of this virus in the Mediterranean area [8, 10]. Besides CCHF virus, a number of other viruses have been detected in Hyalomma ticks, among them Wad Medani virus, Bahig virus, Matruh virus and Wanowrie virus [60]. The pathogenicity of these arboviruses is unknown. In the Ukraine, the European subtype and the Siberian subtype of tick-borne encephalitis (TBE) virus were isolated in several instances from H. marginatum [61]. However, the biological role of H. marginatum to support the natural transmission cycle under the ecological conditions of the Ukraine and the medical importance of this tick species for the transmission of TBE virus to humans and animals (with the potential alimentary infection by milk and cheese) are unknown. In several instances West Nile virus was isolated from H. marginatum [62, 63]. However, similar to TBE virus, the role of ticks in the natural transmission cycle and in the transmission to humans and animals needs to be further elucidated. In presumably H. rufipes nymphs collected from migratory birds on the Island of Capri, Italy, and in Andikithira, Greece, Alkhumra virus, a flavivirus of the tick-borne flavivirus group, was detected [33]. This virus causes a severe form of hemorrhagic fever which occurs mainly at the Arabian Peninsula but was also detected in travellers returning from Egypt [64].
Another pathogen associated with ticks of the genus Hyalomma is R. aeschlimannii [11, 12, 65, 66], a member of the spotted fever group (SFG). Rickettsia aeschlimannii was first described in H. marginatum ticks in Morocco [67]. Later it was detected in the same tick species in Europe [66, 68] and in several African countries, such as Niger, Mali and Senegal [26]. Rickettsia aeschlimannii was also identified by molecular means in ticks of the H. marginatum complex collected from birds in Pakendorf and Zerbst, Saxony-Anhalt, Germany, in May 2007 [48]. However, no identification of the tick to species level was done. In a recent study on SFG rickettsiae in ticks from migratory birds, almost 50% of ticks of the genus Hyalomma found as immature stages on birds in Italy and Greece were infected with rickettsiae. Among 657 collected ticks of the genus Hyalomma, 230 ticks (35%), exclusively larvae and nymphs, were found positive for R. aeschlimannii. Our data are comparable with these data. However, our ticks were exclusively adult stages. Here, 5/10 (50%) H. marginatum were found positive and 4/8 (50%) H. rufipes (Table 1) contained R. aeschlimannii DNA. Rickettsia aeschlimannii was detected in non-engorged adult ticks. These results confirm transstadial transmission of R. aeschlimannii from the nymphal to adult stage and show the potential risk of transmission of this rickettsial species to humans and animals by the imported ticks. It is also unclear whether large animals may play a role in the transmission cycle of this rickettsial species and whether other tick species, mainly of the Ixodes ricinus complex, may become infected and establish a transmission cycle under central European ecological conditions. Raoult et al. [69] detected R. aeschlimannii for the first time in a patient, who developed symptoms after returning from Morocco.
Nine of the introduced specimens were positive for R. aeschlimannii showing a 100% identity with R. aeschlimannii sequences from GenBank for the 23S intergenic spacer region (GenBank: AY125016.1 and MG450333.1), two ompA fragments (GenBank: U83446.1, HM050290.1, DQ459390.1) as well as an ompB fragment (GenBank: AF123705.1, HM050278.1). Due to the high homology of the analyzed sequences of the rickettsial gene fragments, a phylogenetic analysis of the R. aeschlimannii sequences and the ticks is difficult. However, the occurrence mainly in the western part of Germany and the closest phylogenetic relationship of ompB R. aeschlimannii sequences (Fig. 2) let us speculate that the main direction of introduction was along the southwestern route of bird migration.
For C. burnetii, the agent of Q fever, the main method of transmission is inhalation or ingestion, rather than an infective tick bite [70], although this pathogen occurs in different tick species including Hyalomma. In addition, tick endosymbionts (as Coxiella-like organisms) have been identified regularly in blood-feeding ticks [71]. However, in our study all tested specimens were negative for C. burnetii and Coxiella-like agents.
All ticks tested were found negative for Babesia spp. and Theileria spp. So far, there is only little information available on the importance of H. marginatum and H. rufipes as vectors for these two pathogen groups. Theileria equi was found in 9.2% and Babesia (B.) caballi in 1.6% of Hyalomma ticks in Tunisia [72]. In another study from Tunisia only 3/120 ticks tested were found positive for B. occultans and Babesia sp. Kayseri I [73]. In Somalia, none of the three Hyalomma species tested were found positive for Theileria spp. [74]. In Turkey, only one of 30 H. marginatum ticks was found positive for B. occultans [75]. These limited data show that Hyalomma ticks seem not to exhibit a high prevalence of piroplasms, which is in concordance with our results. However, no data on the occurrence and prevalence of Babesia and Theileria species in ticks are available for the assumed areas of origin in southwestern Europe and western Africa.
Conclusions
As Hyalomma larvae and nymphs are regularly found on migratory birds, there is good reason to assume that these ticks are regularly imported as feeding nymphs by migratory birds coming from endemic areas in southern Europe and Africa to central Europe. This is an example of a tropical or sub-tropical tick species molting from the nymphal stage to the adult under favorable weather conditions outside the usual distribution area. The detection of R. aeschlimannii in the imported H. marginatum and H. rufipes to Germany is of importance, as it is a human pathogen.
Acknowledgements
We thank Dr Dmitry Apanaskevich (Georgia Southern University, Statesboro, Georgia, USA) for confirming our morphological identification. All people are especially acknowledged for providing important details on the cases and sending us photos or ticks. Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th CVBD World Forum Symposium.
Funding
The work was funded in part by a grant of the German Ministry of Health (grant no. 1369-492 to GD).
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The newly generated ompB sequences for R. aeschlimannii were deposited to the GenBank database under the accession numbers MK215215-MK215218.
Authors’ contributions
GD, CS, UM, MD and AL received the samples. LCD performed or confirmed the morphological identification of the ticks made by MD and AL. LCD, SS and RR performed nucleic acid extraction of the ticks and SS and RR performed the PCR for CCHF virus and Rickettsia species. DF performed the Coxiella burnetii and Coxiella-like PCRs, while AS performed the Babesia/Theileria spp. PCR. MB performed the genetic analyses for Rickettsia aeschlimannii sequences. LCD and GD analyzed and assembled the data, and wrote the manuscript draft. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CCHF
Crimean Congo hemorrhagic fever
- PCR
reverse transcription-polymerase chain reaction
- ML
maximum likelihood
- TBE
tick-borne encephalitis
Contributor Information
Lidia Chitimia-Dobler, Email: lydiachitimia@gmail.com.
Sabine Schaper, Email: SabineSchaper@bundeswehr.org.
Ramona Rieß, Email: ramonariess@bundeswehr.org.
Karin Bitterwolf, Email: Karin.Bitterwolf@mkk.de.
Dimitrios Frangoulidis, Email: DimitriosFrangoulidis@Bundeswehr.org.
Malena Bestehorn, Email: Malena1Bestehorn@bundeswehr.org.
Andrea Springer, Email: andrea.springer@tiho-hannover.de.
Rainer Oehme, Email: rainer.oehme@rps.bwl.de.
Marco Drehmann, Email: Marco.Drehmann@uni-hohenheim.de.
Alexander Lindau, Email: Alexander.Lindau@uni-hohenheim.de.
Ute Mackenstedt, Email: mackenst@uni-hohenheim.de.
Christina Strube, Email: Christina.Strube@tiho-hannover.de.
Gerhard Dobler, Email: gerharddobler@bundeswehr.org.
References
- 1.Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG. The hard ticks of the world (Acari: Ixodida: Ixodidae) Berlin: Springer; 2014. [Google Scholar]
- 2.Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844: V. Re-evaluation of the taxonomic rank of taxa comprising the H. (Euhyalomma) marginatum Koch complex of species (Acari: Ixodidae) with re-description of all parasitic stages and notes on biology. Int J Acarol. 2008;34:13–42. doi: 10.1080/01647950808683704. [DOI] [Google Scholar]
- 3.Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844: IX. Redescription of all parasitic stages of H. (Euhyalomma) impeltatum Schulze & Schlottke, 1930 and H. (E.) somalicum Tonelli Rondelli, 1935 (Acari: Ixodidae) Syst Parasitol. 1935;2009(73):199–218. doi: 10.1007/s11230-009-9190-x. [DOI] [PubMed] [Google Scholar]
- 4.Apanaskevich DA, Santos-Silva MM, Horak IG. The genus Hyalomma Koch, 1844: IV. Redescription of all parasitic stages of H. (Euhyalomma) lusitanicum Koch, 1844 and the adults of H. (E.) franchinii Tonelli Rondelli, 1932 (Acari: Ixodidae) with a first description of its immature stages. Folia Parasitol. 2008;55:61–74. doi: 10.14411/fp.2008.009. [DOI] [PubMed] [Google Scholar]
- 5.Apanaskevich DA, Schuster AL, Horak IG. The genus Hyalomma: VII. Redescription of all parasitic stages of H. (Euhyalomma) dromedarii and H. (E.) schulzei (Acari: Ixodidae) Morphol Syst Evol. 2008;45:817–831. doi: 10.1603/0022-2585(2008)45[817:tghvro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Apanaskevich DA, Filippova NA, Horak IG. The genus Hyalomma Koch, 1844: X. Redescription of all parasitic stages of H. (Euhyalomma) scupense Schulze, 1919 (= H. detritum Schulze) (Acari: Ixodidae) and notes on its biology. Folia Parasitol. 2010;57:69–78. doi: 10.14411/fp.2010.009. [DOI] [PubMed] [Google Scholar]
- 7.Capek M, Literak I, Kocianova E, Sychra O, Najer T, Trnka A, et al. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick Borne Dis. 2014;5:489–493. doi: 10.1016/j.ttbdis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Walker AR, Bouattour A, Camicas JL, Estrada-Peña A, Horak IG, Latif AA, et al. Ticks of domestic animals in Africa: guide to identification of species. Zaragoza: ICTTD; 2003. [Google Scholar]
- 9.Feider Z. Fauna of the Peoples Republic of Romania. Suprafamily Ixodoidea. Ticks. Bucharest: Academiei Republicii Populare Romane; 1965. [Google Scholar]
- 10.Kaiser MN, Hoogstraal H. Ticks (Ixodoidea) on migrating birds in Cyprus, fall 1967 and spring 1968, and epidemiological considerations. Bull Entomol Res. 1974;64:97–110. doi: 10.1017/S0007485300027024. [DOI] [Google Scholar]
- 11.Sentausa E, El Karkouri K, Michelle C, Raoult D, Fournier PE. Draft genome sequence of Rickettsia aeschlimannii, associated with Hyalomma marginatum ticks. Genome Announc. 2014;2:e00666-14. doi: 10.1128/genomeA.00666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallménius K, Barboutis C, Fransson T, Jaenson TGT, Lindgren P-E, Nyström F, et al. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit Vectors. 2014;7:318. doi: 10.1186/1756-3305-7-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Kok JB, d’Oliveira C, Jongejan F. Detection of the protozoan parasite Theileria annulata in Hyalomma ticks by the polymerase chain reaction. Exp Appl Acarol. 1993;17:379–381. doi: 10.1007/BF00225857. [DOI] [PubMed] [Google Scholar]
- 14.Petney TN, Pfäffle MP, Skuballa JD. An annotated checklist of the ticks (Acari: Ixodida) of Germany. Syst Appl Acarol. 2012;17:115–170. [Google Scholar]
- 15.Kampen H, Poltz W, Hartelt K, Wölfel R, Faulde M. Detection of a questing Hyalomma marginatum adult female (Acari, Ixodidae) in southern Germany. Exp Appl Acarol. 2007;43:227–231. doi: 10.1007/s10493-007-9113-y. [DOI] [PubMed] [Google Scholar]
- 16.Rubel F, Bugger K, Monazahian M, Habedank B, Dautel H, Leverenz S, Kahl O. The first German map of georeferenced ixodid ticks locations. Parasit Vectors. 2014;7:477. doi: 10.1186/s13071-014-0477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oehme R, Bestehorn M, Wölfel S, Chitimia-Dobler L. Hyalomma marginatum in Tübingen, Germany. Syst Appl Acarol. 2017;22:1–6. doi: 10.11158/saa.22.1.1. [DOI] [Google Scholar]
- 18.Latif AA. Ixodid ticks of major economic importance and their distribution in South Africa. Ticks and tick-borne diseases: Monograph 1. Johannesburg: AGRI Connect (Pty) Ltd.; 2013.
- 19.Camicas JL, Hervy JP, Adam F, Morel PC. The ticks of the world nomenclature, described stages, hosts, distribution (Acarida, Ixodida) Paris: Orston Editions; 1998. [Google Scholar]
- 20.Horak IG, Camicas JL, Keirans JE. The Argasidae, Ixodidae and Nutalliellidae (Acari: Ixodida): a world list of valid tick names. Exp Appl Acarol. 2002;28:27–54. doi: 10.1023/A:1025381712339. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Yang X, Bu F, Yang X, Yang X, Liu J. Ticks (Acari Ixodoidea: Argasidae, Ixodidae) of China. Exp Appl Acarol. 2010;51:393–404. doi: 10.1007/s10493-010-9335-2. [DOI] [PubMed] [Google Scholar]
- 22.Bakirci S, Sarali H, Aydin L, Latif A, Eren H, Karagenc T. Hylomma rufipes (Koch, 1844) infesting cattle in the West Aegean region of Turkey. Turk J Anim Sci. 2011;35:359–363. [Google Scholar]
- 23.Latif AA. Illustrated guide to identification of African tick species. Ticks and tick-borne diseases: Monograph 2. Johannesburg: AGRI Connect (Pty) Ltd.; 2013.
- 24.Chaligiannis I, Papa A, Sotiraki S. Ticks feeding on ruminants and humans in Greece. Parasit Vectors. 2014;7(Suppl. 1):O1. doi: 10.1186/1756-3305-7-S1-O1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayedi MH, Chinikar S, Mostafavi E, Khakifirouz S, Jalali T, Hosseini-Chegeni A, et al. Crimean-Congo hemorrhagic fever virus clade IV (Asia 1) in ticks of western Iran. J Med Entomol. 2015;52:1144–1149. doi: 10.1093/jme/tjv081. [DOI] [PubMed] [Google Scholar]
- 26.Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4:e82. doi: 10.1371/journal.pntd.0000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djerbouh A, Kernif T, Beneldjouzi A, Socolovschi C, Kechemir N, Parola R, et al. The first molecular detection of Rickettsia aeschlimannii in the ticks of camels from southern Algeria. Ticks Tick Borne Dis. 2012;3:374–376. doi: 10.1016/j.ttbdis.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Bitam I. Vectors of rickettsiae in Africa. Ticks Tick Borne Dis. 2012;3:381–385. doi: 10.1016/j.ttbdis.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Gürbüz MK, Erdogan M, Dogan N, Birdane L, Cingi E. Case report: isolated facial paralysis with a tick. Turkiye Parazitol Derg. 2010;34:61–64. [PubMed] [Google Scholar]
- 30.Dogan M, Devge C, Tanriöver Pata YS, Sönmezoglu M. Case report: facial nerve paralysis due to intra-aural Hyalomma tick infestation. Turkiye Parazitol Derg. 2012;36:254–257. doi: 10.5152/tpd.2012.60. [DOI] [PubMed] [Google Scholar]
- 31.Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Fransses L, et al. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007;7:585–595. doi: 10.1089/vbz.2007.0130. [DOI] [PubMed] [Google Scholar]
- 32.Chitimia-Dobler L, Nava S, Bestehorn M, Dobler G, Wölfel S. First detection of Hyalomma rufipes in Germany. Ticks Tick Borne Dis. 2016;7:1135–1138. doi: 10.1016/j.ttbdis.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman T, Lindeborg M, Barboutis C, Erciyas-Yavuz K, Evander M, Fransson T, et al. Alkhurma hemorrhagic fever virus RNA in Hyalomma rufipes ticks infesting migratory birds, Europe and Asia Minor. Emerg Infect Dis. 2018;24:879–882. doi: 10.3201/eid2405.171369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson B, Chamberlain J, Logue CH, Cook N, Bruce C, Dowall SD, et al. Development of a real-time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector Borne Zoonotic Dis. 2012;12:786–793. doi: 10.1089/vbz.2011.0770. [DOI] [PubMed] [Google Scholar]
- 35.Wölfel R, Essbauer S, Dobler G. Diagnostics of tick-borne rickettsioses in Germany: a modern concept for a neglected disease. Int J Med Microbiol. 2008;298(Suppl 1):368–374. doi: 10.1016/j.ijmm.2007.11.009. [DOI] [Google Scholar]
- 36.Chitimia-Dobler L, Rieß R, Kahl O, Wölfel S, Dobler G, Nava S, et al. Ixodes inopinatus—Occurring also outside the Mediterranean region. Ticks Tick Borne Dis. 2018;9:196–200. doi: 10.1016/j.ttbdis.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Fournier PE, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48:839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- 38.Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int J Syst Evol Microbiol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- 39.Casati S, Sager H, Gern L, Piffaretti J. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65. [PubMed] [Google Scholar]
- 40.Frangoulidis D, Meyer H, Kahlhofer C, Splettstoesser WD. ‘Real-time’ PCR-based detection of Coxiella burnetii using conventional techniques. FEMS Immunol Med Microbiol. 2012;64:134–136. doi: 10.1111/j.1574-695X.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- 41.Al-Deeb MA, Frangoulidis D, Walter MC, Kömpf D, Fischer SF, Petney T, et al. Coxiella-like endosymbiont in argasid ticks (Ornithodoros muesebecki) from a Socotra Cormorant colony in Umm Al Quwain, United Arab Emirates. Ticks Tick Borne Dis. 2016;7:166–171. doi: 10.1016/j.ttbdis.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnsen P. Hyalomma marginatum Koch, a tick new to Denmark. Entomol Med. 1943;22:381–383. [Google Scholar]
- 44.Nuorteva P, Hoogstraal H. The incidence of ticks (Ixodoidea, Ixodidae) on migratory birds arriving in Finland during the spring of 1962. Ann Med Exp Biol Fenn. 1963;27:197–204. [PubMed] [Google Scholar]
- 45.Saikku P, Ulmanen I, Brummer-Korvenkontio M. Ticks (Ixodidae) on migratory birds in Finland. Acta Entomol Fenn. 1971;28:46–51. [Google Scholar]
- 46.Mehl R, Michaelsen J, Lid G. Ticks (Acari: Ixodides) on migratory birds in Norway. Fauna Norv Ser B. 1984;31:46–58. [Google Scholar]
- 47.Cuber P. Ticks (Ixodida) from the collection of the Natural History Department, Museum of Upper Silesia in Bytom, Poland—a contribution to knowledge on tick fauna and the first record of Hyalomma marginatum presence in Poland. Ann Agric Environ Med. 2016;23:379–381. doi: 10.5604/12321966.1203910. [DOI] [PubMed] [Google Scholar]
- 48.Rumer L, Graser E, Hillebrand T, Talaska T, Dautel H, Mediannikov O, et al. Rickettsia aeschlimannii in Hyalomma marginatum ticks, Germany. Emerg Infect Dis. 2011;17:325–326. doi: 10.3201/eid1702.100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoogstraal H, Kaiser MN, Traylor MA, Gaber S, Guindy E. Ticks (Ixodidae) on birds migrating from Africa to Europe and Asia. Bull World Health Organ. 1961;24:197–212. [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen B, Jaenson TGT, Bergström S. Prevalence of Borrelia burgdorferi sensu lato infected ticks on migrating birds. Appl Environ Microbiol. 1995;61:3082–3087. doi: 10.1128/aem.61.8.3082-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishiguro F, Takada N, Masuzawa T, Fukui T. Prevalence of Lyme disease Borrelia spp. in ticks from migratory birds on the Japanese mainland. Appl Environ Microbiol. 2000;66:982–986. doi: 10.1128/AEM.66.3.982-986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alekseev A, Dubinina HV, Semenkov AV, Bolshakov CV. Evidence of ehrlichiosis agents found in ticks (Acari: Ixodidae) collected from migratory birds. J Med Entomol. 2001;38:471–474. doi: 10.1603/0022-2585-38.4.471. [DOI] [PubMed] [Google Scholar]
- 53.Jameson LJ, Morgan PJ, Medlock JM, Watola G, Vaux AGC. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into United Kingdom by migratory birds. Ticks Tick-borne Dis. 2012;3:95–99. doi: 10.1016/j.ttbdis.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Estrada-Peña A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. doi: 10.1023/A:1006241108739. [DOI] [PubMed] [Google Scholar]
- 55.Mathison BA, Gerth WJ, Pritt BS, Baugh S. Introduction of the exotic Hyalomma truncatum on a human with travel to Ethiopia: a case report. Ticks Tick-Borne Dis. 2015;6:152–154. doi: 10.1016/j.ttbdis.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deutscher Wetterdienst. http://www.dwd.de/DE/Home/home_node.html. Accessed 2 Nov 2018.
- 57.Estrada-Peña A, Martínez Avilés M, Muñoz Reoyo MJ. A population model to describe the distribution and seasonal dynamics of the tick Hyalomma marginatum in the Mediterranean Basin. Transbound Emerg Dis. 2011;58:213–223. doi: 10.1111/j.1865-1682.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 58.Estrada-Peña A, Sánchez N, Estrada-Sánchez A. An assessment of the distribution and spread of the tick Hyalomma marginatum in the western Palearctic under different climate scenarios. Vector Borne Zoonotic Dis. 2012;12:758–768. doi: 10.1089/vbz.2011.0771. [DOI] [PubMed] [Google Scholar]
- 59.Estrada Peña A, de la Fuente J, Latapia T, Ortega C. The impact of climate trends on a tick affecting public health: a retrospective modeling approach for Hyalomma marginatum (Ixodidae) PLoS ONE. 2015;10:e0125760. doi: 10.1371/journal.pone.0125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karabatsos N. International Catalogue of Arboviruses, including certain other viruses of vertebrates. San Antonio: American Society of Tropical Medicine and Hygiene; 1985. [DOI] [PubMed] [Google Scholar]
- 61.Yurchenko OO, Dubina O, Vynograd NO, Gonzalez J-P. Partial characterization of tick-borne encephalitis virus isolates from ticks of Southern Ukraine. Vector Borne Zoonotic Dis. 2017;17:550–557. doi: 10.1089/vbz.2016.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubalek Z, Halouzka J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolodziejek J, Marinov M, Kiss BJ, Alexe V, Nowotny N. The complete sequence of a West Nile virus lineage 2 strain detected in a Hyalomma marginatum marginatum tick collected from a song thrush (Turdus philomelos) in Eastern Romania in 2013 revealed closest genetic relationship to strain Volgograd 2017. PLoS ONE. 2014;9:e109905. doi: 10.1371/journal.pone.0109905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carletti F, Castilletti C, Di Caro A, Capobianchi MR, Nisii C, Suter F, et al. Alkhurma hemorrhagic fever in travelers returning from Egypt, 2010. Emerg Infect Dis. 2010;16:1979–1982. doi: 10.3201/eid1612.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–700. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson MO, Tolf C, Tamba P, Stefanache M, Radbea G, Frangoulidis D, et al. Molecular survey of neglected bacterial pathogens reveals an abundant diversity of species and genotypes in ticks collected from animal hosts across Romania. Parasit Vectors. 2018;11:144. doi: 10.1186/s13071-018-2756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beati L, Meskini M, Thiers B, Raoult D. Rickettsia aesclimannii sp. nov., new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int J Syst Bacteriol. 1997;47:548–554. doi: 10.1099/00207713-47-2-548. [DOI] [PubMed] [Google Scholar]
- 68.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raoult D, Fournier PE, Abboud P, Caron F. First documented human Rickettsia aeschlimannii infection. Emerg Infect Dis. 2002;8:748–749. doi: 10.3201/eid0807.010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong J. Coxiella-like endosymbionts. Adv Exp Med Biol. 2012;984:365–379. doi: 10.1007/978-94-007-4315-1_18. [DOI] [PubMed] [Google Scholar]
- 72.Ros-Garcia A, M’Ghirbi Y, Hurtado A, Bouattour A. Prevalence and genetic diversity of piroplasm species in horses and ticks from Tunisia. Infect Genet Evol. 2013;17:33–37. doi: 10.1016/j.meegid.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 73.Ros-Garcia A, M’Ghirbi Y, Bouattour A, Hurtado A. First detection of Babesia occultans in Hyalomma ticks from Tunisia. Parasitology. 2011;138:578–582. doi: 10.1017/S0031182011000060. [DOI] [PubMed] [Google Scholar]
- 74.Tomassone L, Grego E, Callà G, Rodighiero P, Pressi G, Gebre S, et al. Ticks and tick-borne pathogens in livestock from nomadic herds in the Somali Region, Ethiopia. Exp Appl Acarol. 2012;56:391–401. doi: 10.1007/s10493-012-9528-y. [DOI] [PubMed] [Google Scholar]
- 75.Orkun Ö, Karaer Z, Cakmak A, Nalbantogluo S. Identification of tick-borne pathogens in ticks feeding on humans in Turkey. PLoS Negl Trop Dis. 2014;8:e3067. doi: 10.1371/journal.pntd.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this article are included within the article. The newly generated ompB sequences for R. aeschlimannii were deposited to the GenBank database under the accession numbers MK215215-MK215218.