Abstract
Background
Haemonchosis affects sheep husbandry and its treatment is often compromised due to the development of anthelminthic resistance. Plant-derived bioactive compounds have been studied as alternative to control Haemonchus contortus. The objective of this study was to evaluate the effect of Senecio brasiliensis extracts on H. contortus egg hatching and infective larvae migration.
Results
The aqueous extract from dried and fresh plant and alkaloid-enriched fraction of the previously dried leaves of S. brasiliensis inhibited H. contortus egg hatching. The main plant compound in alkaloid fraction was integerrimine, a pyrrolizidine alkaloid (PA). However, the aqueous extract from dried plant displayed higher efficacy when compared to their alkaloid enriched or non-polar fractions, meaning that, although PAs contributed to the ovicidal effect, other compounds in the plant can also contribute to their effect. Furthermore, the aqueous extract from dried plant also had higher efficacy than aqueous extract from fresh plant in larvae migration inhibition. Finally, extract from dried plant presented low in vitro cytotoxic effect.
Conclusion
Taken together our results suggest a good anthelmintic effect of S. brasiliensis, especially when aqueous extract is prepared from dried plant. Further in vivo studies should be performed focused on forms of administration of this extract in rearing sheep.
Keywords: Anthelmintic, Egg hatching, Infective larvae migration, Plant extract, Pyrrolizidine alkaloids, Small ruminants
Background
Endoparasitic diseases are a high concern in sustainable sheep production [1]. Haemonchus contortus [2] (barber’s pole worm), a blood-sucking nematode and member of the order Strongylida and family Trichostrongylidae, can cause anemia, anorexia, diarrhea, gastritis, and even death in animals [3]. This parasitic infection results in direct economic losses related to decreased animal performance and/or death as well as indirect economic losses linked to the high cost of anthelmintic drugs and the labor and equipment required for the control of parasitosis [4, 5].
H. contortus control is mainly based on the use of commercial anthelmintics; however, these drugs have lost their effectiveness due to the development of drug-resistant parasite strains [6, 7]. To aid the control of nematodes, in conjunction with the use of anthelmintics, alternative strategies have been developed, such as pasture management, nutritional adjustments, genetic selection, use of nematophagous fungi, and the development of plant-derived anthelmintic compounds [8, 9].
The genus Senecio (Asteraceae) comprises approximately 1500 species [10]. Plants belonging to this genus are known for the production of compounds such as alkaloids, sesquiterpenes, and flavonoids [11] and for their anti-inflammatory, vasodilator, antiemetic, antimicrobial, and parasiticide activities [12–14].
Senecio brasiliensis is a native species found in south and southeast of Brazil [15]. It is toxic to the livestock [16, 17] due to the presence of pyrrolizidine alkaloids (PAs), which are widely found in the Asteraceae, Boraginaceae, and Fabaceae families [18, 19]. However, the leaves and inflorescence of S. brasiliensis are used in traditional medicine for the treatment of stomach pain [20], a practice justified by the antiulcerogenic and cytoprotective effect of PAs [21, 22].
Based on reports of the pharmacological effects of the genus Senecio and the species S. brasiliensis, we aimed to evaluate the effect of aqueous extract of this plant and its non-polar and alkaloid fractions on H. contortus egg hatching and infective larvae migration, besides evaluating the cytotoxic effect of this plant.
Results
The GC-MS analysis showed the presence of four constituents for the alkaloid fraction (AF) from dried plant (DP) and eight constituents for AF from fresh plant (FP) (Table 1). In AF from DP, all peaks were attributed to PAs. On the other hand, in AF from FP, in addition to the alkaloids, carboxylic acids and ketones were found. For both extracts, the major compound was integerrimine (~ 91% in AF from DP and ~ 79% in AF from FP).
Table 1.
GC/MS analysis of Senecio brasiliensis alkaloid fraction from dried and fresh plant
| Peak | tR | IK | Name of the compound | Peak area (%) |
|---|---|---|---|---|
| Alkaloid Fraction from Dried Plant | ||||
| 1 | 17.020 | 1464 | Senecionine | 3.34 |
| 2 | 18.114 | 1436 | Integerrimine | 91.14 |
| 3 | 18.400 | 1456 | Platyphylline | 1.46 |
| 4 | 19.704 | 1444 | 3(2H)-isoquinolinone, octahydro-, (4ar-trans) | 4.06 |
| Alkaloid Fraction from Fresh plant | ||||
| 1 | 4.830 | 1381 | Cyclohexanone, 5-methyl-2-(1-methylethylidene) | 4.15 |
| 2 | 5.721 | 1491 | 4-amino-2-nitro-benzaldehyde oxime | 0.84 |
| 3 | 7.178 | 1648 | 1,2-cyclopropanedicarboxylic acid, 3-(1-methylethenyl)-, diethyl ester | 4.12 |
| 4 | 8.959 | 1406 | 2-(4-nitrobutyryl) cyclo-octanone | 1.19 |
| 5 | 17.071 | 1363 | Senecionine | 4.45 |
| 6 | 18.254 | 1446 | Integerrimine | 79.26 |
| 7 | 19.068 | 1500 | 2-anilino-4-methylquinoline | 0.82 |
| 8 | 19.73 | 1445 | Neo-triangularine | 1.5 |
GC/MS Gas Chromatography-Mass Spectrometry, tR retention time, IK Kovats Index
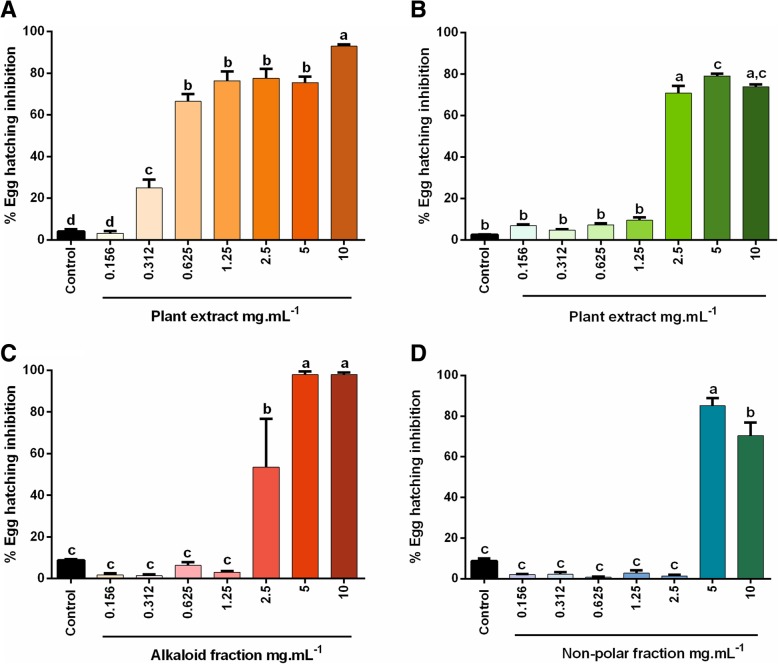
The effective concentrations to inhibit H. contortus egg hatching (Fig. 1) by 50% (EC50) were 0.660 mg.mL− 1 for DP and 2.596 mg.mL− 1 for FP, respectively (Table 2). DP showed high inhibitory activity at the highest concentration tested (94%) (Fig. 2a), whereas FP showed moderate inhibitory activity at the highest concentration tested (73%) (Fig. 2b). The percentage of inhibition of egg hatching ranged from 1 to 94% for DP and from 4 to 73% for FP. For DP aqueous extract, inhibition rates above 50% were observed for concentrations higher than 0.625 mg.mL− 1 (Fig. 2a). On the other hand, for FP, significant egg hatching inhibition was observed only for concentrations above 2.5 mg.mL− 1 (Fig. 2b).
Fig. 1.

Optical microscopy of eggs and hatched larvae: representative image of H. contortus eggs with larva failing eclosion (white arrow) and larva (black arrow). Eggs were treated with dried plant extract during 24 h. 100x magnification
Table 2.
Mean (SE) of effective concentration to inhibit 50% (EC50) of egg hatching or larval migration and to kill 50% of the cells
| Extract | In vitro testb | EC50 (mg.mL−1) |
|---|---|---|
| Dried plant | EHT | 0.660 (0.506 to 0.859) |
| LMIT | 10.150 (8.022 to 12.840) | |
| CT | 45.430 (37.580 to 54.920) | |
| Alkaloids from dried plant | EHT | 2.435 (2.143 to 2.765) |
| Non-polar fraction from dried plant | EHT | 4.356 (0.434 to 43.760) |
| Fresh plant | EHT | 2.596 (2.139 to 3.086) |
| LMIT | 1712 (44.020 to 66.560) | |
| Alkaloids from fresh plant | EHT | a |
| Non-polar fraction from fresh plant | EHT | a |
aIt was not possible to estimate
bEHT Egg Hatching Test, LMIT Larval Migration Inhibition Test, CT Cytotoxicity Test
Fig. 2.
Effect of Senecio brasiliensis extracts on Haemonchus contortus egg hatching inhibition: a Dried plant extract (DP); b Fresh plant extract; c Alkaloid fraction of DP extract; d Non-polar fraction of DP extract. Different letters mean difference between groups (p < 0.05; one-way ANOVA followed by Tukey’s post-hoc test)
The estimated EC50 was 2.435 mg.mL− 1 for the AF from DP. Non-polar enriched fraction (NPF) from DP showed EC50 of 4.356 mg.mL− 1. The EC50 values for AF and NPF from FP could not be determined, due to low effect, since the eclosion inhibition was less than 10% in all tested concentrations. At the highest evaluated concentrations, AF (Fig. 2c) and NPF (Fig. 2d) from DP exhibited egg hatching inhibition of 98 and 70%, respectively. The average inhibition for AF from DP ranged from 1.7 to 98% and started at 2.5 mg.mL− 1, while the average inhibition for NPF from DP ranged from 0.5 to 70% and started at 5 mg.mL− 1. The egg hatching inhibition in the positive control (thiabendazole 5 mg.mL− 1) was 98%.
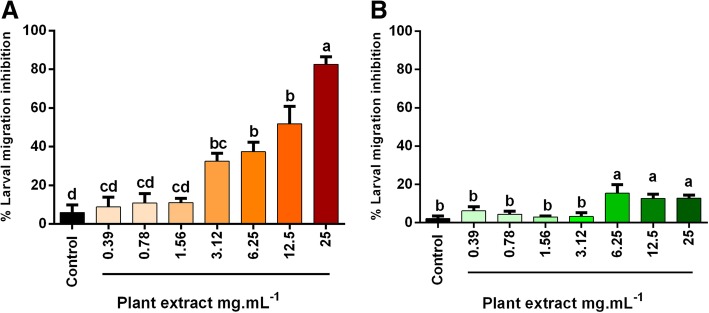
Concerning larval migration inhibition, the EC50 were 10.15 mg.mL− 1 for DP and 1712 mg.mL− 1 for FP (Table 2). The maximal larval migration inhibition, observed when high concentrations of plant extract were used, was 15% for FP and 82% for DP. The percentage of inhibition migration ranged from 6 to 82% for DP and from 2 to 15% for FP. For DP, migration inhibition rates above 50% were observed only in concentrations higher than 12.5 mg.mL− 1 (Fig. 3a). On the other hand, no migration inhibition above 50% for FP was observed (Fig. 3b). The larval migration inhibition of the positive control (levamisole 6.25 μg.mL− 1) was 96%.
Fig. 3.
Effect of Senecio brasiliensis extracts on Haemonchus contortus larval migration inhibition: a Dried plant extract; b Fresh plant extract. Different letters mean difference between groups (p < 0.05; one-way ANOVA followed by Tukey’s post-hoc test)
A dose-dependent pattern is observed for cytotoxic activity of DP aqueous extract (Fig. 4). However, the effect of this extract in mammal cell lethality was pronouncedly less intense than for egg hatching or larval migration inhibition, as demonstrated by the SI that was 68.63 and 4.48, respectively. The cytotoxic EC50 of DP was estimated in 45.43 mg.mL− 1 (Table 2). Maximal cell lethality was 54.94% when 50 mg.mL− 1 of DP extract was used.
Fig. 4.
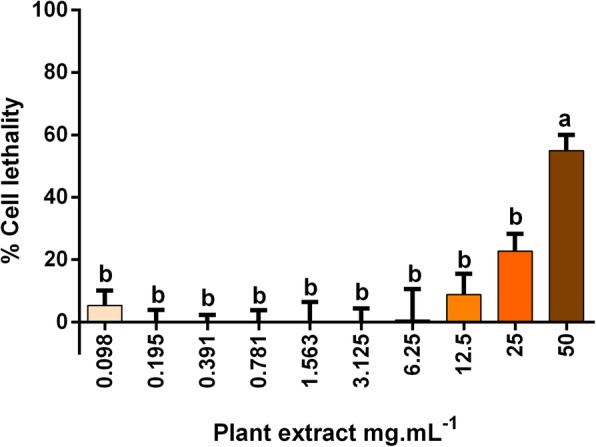
Cytotoxic effect of dried Senecio brasiliensis extract on Vero cells. Different letters mean difference between groups (p < 0.05; one-way ANOVA followed by Tukey’s post-hoc test)
Discussion
Integerrimine, found in plant extracts, is among the main PAs in Senecio [22–24]. Although integerrimine was the most abundant component in both AFs, its concentration was higher in AF from DP as compared to AF from FP, possibly because the drying procedure at 40 °C increased the concentration of some compounds while caused the loss of others, what could also be a plausible explanation for the reduction of compounds diversity in AF from DP when compared to AF from FP. Previous studies have shown that hot-drying can affect the chemical composition of plant extracts when compared with those obtained by freeze-drying (lyophilization) [25, 26].
An anthelmintic is considered effective if it exhibits at least 90% of inhibitory activity [27]. Considering this cut-off, DP and AF from DP from S. brasiliensis showed efficacy against egg hatching of H. contortus isolated from sheep feces. In our experiment we observed the phenomenon ‘failing eclosion’, described by Vargas-Magaña et al., [28] and Chan-Pérez et al., [29] in studies with plant extracts. These authors used this term to differentiate larval eggs from morulated eggs, which were classified as dead. In our work we observed the presence of morulated eggs only in the test with the positive ovicidal control Thiabendazole. We believe that the extract of S. brasiliensis prevents the larva exit of the egg, by mechanisms still unknown. Regarding larval migration inhibition, it is not possible to conclude an effectiveness of S. brasiliensis extracts, since at the tested concentrations; the highest inhibitory activity observed was 82.6%, observed for DP aqueous extract. Taking into consideration the effect of DP on egg hatchability, we believe that, in further studies, S. brasiliensis extract should be considered to be used in reduction of pastures contamination, acting as an environmental control, as observed by Niezen et al., [30].
Among the set of alkaloids identified in AF from DP, PAs were the most abundant, suggesting their participation in the observed effect. In addition, the efficacy of those substances in combating plant nematodes has already been reported [31]. Surprisingly, the effect of DP (EC50 = 0.660 mg.mL− 1) was higher than AF from DP (EC50 = 2.435 mg.mL− 1), suggesting that PAs are not the only responsible components for anthelmintic effects observed, which has already been reported for other plant extracts. In the study conducted by Brandão et al. [32], the total extract of Bidens sp. (Asteraceae) was more effective in reducing Plasmodium sp. parasitemia than its fractions. Gomes et al. [33] also recorded a similar event in their in vitro study with Zizyphus joazeiro against H. contortus; the saponins found in a plant fraction acted together with other substances present in the total extract. Saponins, tannins, phlobatannins, phenols, anthraquinones, flavonoids, glycosides, steroids, terpenes, cardenolides, and chalcones [34, 35] have been identified in other Senecio species and some of these compounds have been associated with anthelmintic effects [36–38].
Even according to the traditional medicine, whole plants are used rather than isolated compounds. This phenomenon can be attributed to the synergic effect, what occurs when the combination of components of a plant extract or mixture of plant extracts effect is greater than the sum of individual effects [39]. Although synergy is a well-documented effect, there is a lack in acknowledgment concerning its mechanism of action [40]. An example of synergy can be found in the work of Klongsiriwet et al., [41], who detected a greater inhibition of the exsheathment of H. contortus infective larvae when tannins and flavonoids were mixed, in relation to the effect of these compounds in the isolated form. However, these authors only speculated how the synergy occurred, as Williamson [40] and Wagner and Ulrich Merzenich [42] cite in their works. Although we have not performed experiments with isolated components and their possible mixtures, we suppose that DP is more potent than its fractions due to some degree of synergy between the alkaloids and the other components present in aqueous extract of the plant.
Despite S. brasiliensis having ovicidal effect, this plant may be toxic to animals [43], which may be attributed to liver metabolism of pyrrolizidine alkaloids. Three metabolic ways may occur with PA in liver: dehydrogenation, hydrolysis and n-oxidation [44, 45]. According to these authors, dehydrogenation forms pyrroles that are toxic, whereas hydrolysis and n-oxidation leads to non-toxic metabolites. The pyrroles originating from dehydrogenation of PAs can bind with the hepatocyte DNA, inhibiting cellular mitosis and thereby causing hepatic dysfunction [46, 47]. However, it is known that sheep, the targets of our study, are more resistant to Senecio intoxication than cattle due to their ruminal microbiota [48] and liver metabolization system [49].
It worth mentioning that in vitro anthelmintic and cytotoxic effects of S. brasiliensis were conducted with plant extracts in a non-metabolized form. The hypothesis of animal toxicity after liver metabolization of the compounds corroborate our results, in which in vitro cytotoxicity was low [50, 51] and which confirms previous researches that claim that alkaloids are not toxic in the non-metabolized form [44, 52]. Thus, future studies involving new formulations in order to avoid liver metabolization or to protect that compounds against organism degradation need to be carried out.
Conclusions
DP of S. brasiliensis displays good anti-H. contortus egg hatchability and, less pronouncedly, anti-larvae activity, presenting low in vitro cytotoxicity to Vero cells. Future studies are required to clarify the mechanism of action on H. contortus eggs and the pharmacological mechanisms of the plant extract in animals to validate the DP uses in integrated nematodes control programs.
Methods
Reagents
Sodium hydroxide (NaOH), sulfuric acid (H2SO4), dichloromethane (CH2Cl2), sodium sulfate (Na2SO4), and methanol (MeOH) (High Performance Liquid Chromatography grade) were purchased from Vetec® (Brazil). For the parasitological tests, thiabendazole, tween® 80, levamisole, sodium hypoclorite (NaOCl) and sodium chloride (NaCl) were purchased from Sigma-Aldrich (USA). Rezasurin used in cytotoxicity evaluation was also purchased from Sigma-Aldrich (USA).
Plant material
S. brasiliensis was collected from fields that belong to Embrapa Pecuária Sul, located in the city of Bagé, Rio Grande do Sul, Brazil (31°21′13.3″S 54°00′36.2″W). A voucher specimen (103517) was stored in the herbarium of the Embrapa, Brasília, Distrito Federal, Brazil.
Extraction and fractionation
Aqueous extract from fresh plant (FP)
Leaves of S. brasiliensis were collected, immediately macerated and mixed with water (100 mg.mL− 1) at room temperature. The material was filtered through cotton, concentrated by freeze-drying using a lyophilization apparatus (LP510, Liotop®, Brazil), and stored at − 20 °C.
Aqueous extract from dried plant (DP)
Collected leaves of S. brasiliensis were dried at 40 °C for 2 days. Subsequently, the material was macerated, powdered, and mixed with distilled water (40 °C for 30 min) to make a 100 mg.mL− 1 stock solution. The solution was filtered through cotton, concentrated by lyophilization, and stored at − 20 °C.
Alkaloid and non-polar fractions
The alkaloid and non-polar organic fractions (AF and NPF, respectively) were prepared according to the procedure described by Torras-Claveria et al. [53] and Andrade et al. [54] with some modifications. Lyophilized samples were dissolved in 40 mL of MeOH by sonication (Sanders, Brazil). Next, the sample was acidified to pH 2 with 2% H2SO4, and the organic fraction was removed by mixing with CH2Cl2 followed by decantation. This procedure was repeated three times. The lower fraction was enriched with non-polar compounds and was called NPF. The upper fraction (polar) was basified up to pH 11 with 10% NaOH, and the alkaloids were extracted by liquid-liquid partition with CH2Cl2 three times. Afterwards, the lower fractions were enriched with alkaloids compounds and called AF. Finally, the NPF and AF were concentrated in a rotary evaporator (Tecnal, Brazil).
GC-MS analysis of alkaloid extracts
The dried alkaloid-enriched extracts were dissolved in CHCl3 and directly injected into the GC-MS (Gas Chromatography-Mass Spectrometry) apparatus (injection volume: 1 μL) consisting of a Hewlett Packard 6890 coupled with a mass spectroscopy device (5975 GC/MS, Hewlett Packard, USA) operating in EI (Ionization by Electron) mode at 70 eV. An HP-5 MS column (30 m × 0.25 mm i.d., film thickness 0.25 m) was used. The temperature gradients were as follows: 100–180 °C at 15 °C/min, 180–300 °C at 5 °C/min, 10 min hold at 300 °C and 2 min at 100 °C. The injection temperature was 250 °C, and the flow rate of the carrier gas (helium) was 1 μL/min. A split ratio of 1:5 was applied. Furthermore, the arithmetic retention indexes were calculated by linear interpolation relative to the retention times (tR) of a series of n-alkanes (C7–C30). The obtained values were compared with the published retention index (Kovats index) values [55, 56]. Mass spectra were deconvoluted using AMDIS® 2.64 software (NIST) [54].
Parasitological tests
Animals
Eggs were obtained from the feces of sheep infected and maintained with a monospecific culture of H. contortus. These animals belong to the Embrapa Pecuária Sul, which has own sheep farming, and where the research was carried out. The recovery of eggs was performed according to the method described by Coles et al. [57]. Feces were homogenized in water and filtered through sieves of diminishing pore diameter (180, 90, 68, 38 μm), and the eggs were separated by centrifugation (3000 rpm for 5 min) in a saturated NaCl solution. Immediately, the eggs were washed with distilled water and used in experiments. The experimental protocol was in accordance with the directives of Brazilian National Experiment Control Council (Ethics Committee on Use of Animals – Embrapa Pecuária Sul, protocol under registration number 01/2017). To obtain third stage larvae, the eggs obtained in the same manner as described above were incubated under aerobic conditions in fecal cultures for 7 days at 28 °C and 80–85% humidity. Larvae obtained from these cultures were isolated using a Baermann funnel system and larval cultures were stored at 4–8 °C. After experiments, the animals were treated with anthelmintics and returned to the Embrapa Pecuária Sul fields.
Egg hatching test
The in vitro egg hatching test (EHT) was based on the method described by Coles et al. [58] and standardized by von Samson-Himmelstjerna et al. [59] with modifications. DP and FP were diluted in distilled water and AF and NPF were diluted in 5% tween® 80 aqueous solution. Diluents alone were used as negative controls. Thiabendazole (5 mg.mL− 1) was used as positive control, diluted in 5% tween® 80. Approximately 120 H. contortus eggs diluted in water or 5% tween® 80 were incubated with final concentration of 10, 5, 2.5; 1.25, 0.625, 0.312, and 0.156 mg.mL− 1 of DP, FP, AF or NPF in a BOD incubator, for 24 h at 28 °C and 80% humidity. Next, 1% lugol’s iodine was added to each well, and the eggs and larvae at the first stage (L1) were microscopically quantified. The test was only validated when hatching in the negative control was greater than 90%. To calculate the percentage of inhibition of larval hatching [58], the formula [(A)/(A + B)] × 100 was used, where A = number of eggs and B = number of larvae. The data were corrected using the Abbott formula [60]. Tests were performed in quadruplicate.
Larval migration inhibition test
Larval migration inhibition test (LMIT) was performed according to Demeler et al., [61, 62] with some modifications. DP and FP were diluted in distilled water, which alone was used as negative control. Levamisole (6.25 μg.mL− 1) was used as positive control, diluted in distilled water. About 120 third stage larvae, previously exsheathed with a solution of sodium hypochlorite (0.3%), were incubated in seven different concentrations of plant extract (25; 12.5; 6.25; 3.12; 1.56; 0.78 and 0.39 mg.mL− 1) in 24 well plates in a final volume of 1000 μL per well. After 24 h at 28 °C, the total volume (extract + larvae) was deposed into and 25-μm sieves mounted on wells and incubated for more 24 h at 28 °C. After the second incubation, sieves were carefully removed and the remaining non-migrated larvae were washed with distilled water into another well. 1% lugol’s iodine was added to each well and migrated and non-migrated larvae were quantified at 100x magnification. The percentage of non-migrated larvae to the total amount of larvae was calculated for the controls and every concentration tested, as mentioned in EHT, with A = number of non-migrated larvae and B = number of migrated larvae and Abbott formula [60] also was used. Tests were performed in quadruplicate.
Cell citotoxicity assay
Vero cells were maintained in RPMI media (Cultilab, Brazil) supplemented with penicillin (100 UI.mL− 1) (Sigma-Aldrich), streptomycin (0.01 mg.mL− 1) (Sigma-Aldrich), gentamycin (10 μg.mL-1 (Sigma-Aldrich), and 10% bovine calf serum (Cultilab). Confluent cells were trypsinized and seed on 96 well plates, totalizing 1.5 × 104 cells/well. Four hours later, when cells were completely adhered, test solutions were added. Two fold serial dilutions were carried out with DP diluted in distillated water to obtain final concentrations ranging from 50 to 0.098 mg.mL− 1. The microplates were sealed and incubated at 37 °C in normal atmosphere during 24 h. After this period, resazurin solution (Sigma-Aldrich) was added to each well, to a final concentration of 0.3 mM and the plates were incubated at 37 °C for additional 18 h. The absorbance values were read by dual wavelength using a microplate spectrophotometer (Versa-Max, Molecular Devices) at 570 and 600 nm. All experiments were performed three times each in triplicate. Percentage of viable cells (VC) was calculated as described by Rolón et al., [63]. Percentage of citotoxicity was calculated as 100-VC and was used to calculate EC50. Selectivity index (SI), the ratio between cytotoxicity EC50 and egg hatching or larval migration inhibition EC50, was calculated to evaluate the safety of the extract tested [50].
Statistical analysis
An analysis of variance (ANOVA) followed by Tukey’s test was performed on the test results. A p-value ≤0.05 indicated statistical significance. A logistic equation with a variable slope was used to fit the dose-response data by non-linear regression and find the EC50. All analyses were performed after transforming the concentrations into their logarithms (X = log X) and constraining the bottom value to 0% and top value to 100%. All statistical analyses were performed using GraphPrism® version 6.07 (GraphPad Software, USA).
Acknowledgments
The authors would like to thank to Vice-Reitoria de Pós-Graduação of Universidade de Passo Fundo (VRPPG-UPF) for the writing support, CAPES for the scholarship and Rossana Leitzke Granada (Embrapa Pecuária Sul) and Ana Sheila de Queiroz Souza (Universidade Federal do Ceará) for the technical support.
Funding
Embrapa (Empresa Brasileira de Pesquisa Agropecuária) funding all that involved in study design, collection, analysis and interpretation of data. CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) also funded with scholarship.
Availability of data and materials
The data generated or analyzed during this study are included in this published article (and its supplementary information files). Data that are not in this article are available from corresponding author on reasonable request.
Abbreviations
- °C
Celsius degree
- AF
Alkaloid fraction
- DNA
Deoxyribonucleic acid
- DP
Dry plant
- EC
Effective concentration
- EHT
Egg hatching test
- FP
Fresh plant
- GC-MS
Gas Chromatography-Mass Spectrometry
- H. contortus
Haemonchus contortus
- LMIT
Larval migration inhibition test
- mg.mL−1
Milligram per mL
- mM
Millimolar
- NaCl
Sodium chloride
- nm
Nanometers
- NPF
Non-polar fraction
- PA
Pyrrolizidine alkaloid
- S. brasiliensis
Senecio brasiliensis
- SI
Selectivity index
Authors’ contributions
APM, RD, SMS and MIBV conceived the studies. The laboratory experiments and analyses were performed by SMS, RD, PAdS, EBG and KMC. The statistical analyses were performed by RD, SMS and EBG. APM and MIBV were responsible for funding acquisition. This work were under supervision of APM and MIBV. The original draft and review of writing were performed by SMS, EBG, APM and MIBV. All authors read and approved the manuscript.
Ethics approval
The experimental protocol was in accordance with the directives of Brazilian National Council for the Control of Animal Experimentation (CONCEA) (Ethics Committee on Use of Animals – Embrapa Pecuária Sul, protocol under registration number 01/2017).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suelen Mendonça Soares, Phone: +55 54 996840111, Email: suelensoares.vet@gmail.com.
Robert Domingues, Email: domingues@embrapa.br.
Emanuelle Baldo Gaspar, Email: emanuelle.gaspar@embrapa.br.
Patrício Azevedo dos Santos, Email: patricio.azevedo@hotmail.com.
Kirley Marques Canuto, Email: kirley.canuto@embrapa.br.
Alessandro Pelegrine Minho, Email: alessandro.minho@embrapa.br.
Maria Isabel Botelho Vieira, Email: marisabel@upf.br.
References
- 1.Mavrot F, Hertzberg H, Torgerson P. Effect of gastro-intestinal nematode infection on sheep performance: a systematic review and meta-analysis. Parasit Vectors. 2015;8(1):557. doi: 10.1186/s13071-015-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolphi KA. Neue beobachtungen über die eingeweidewtirmer. In: Archiv für Zoologie und Zootomie. Braunschweig: Boffifchen Buchhandlung; 1803.
- 3.Gasser R, von Samson-Himmelstjerna G. Haemonchus contortus and Haemonchosis–Past, Present and Future Trends, vol. 93. San Diego: Academic Press; 2016.
- 4.Qamar MF, Maqbool A, Ahmad N. Economic losses due to haemonchosis in sheep and goats. Sci Int. 2011;23(4):321–324. doi: 10.13140/2.1.3987.8084. [DOI] [Google Scholar]
- 5.Charlier J, van der Voort M, Kenyon F, Skuce P, Vercruysse J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30(7):361–367. doi: 10.1016/j.pt.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Sangster N. Anthelmintic resistance: past, present and future. Int J Parasitol. 1999;29(1):115–124. doi: 10.1016/S0020-7519(98)00188-X. [DOI] [PubMed] [Google Scholar]
- 7.Besier RB, Kahn LP, Sargison ND, Van Wyk JA. Diagnosis, treatment and management of Haemonchus contortus in small ruminants. Adv Parasitol. 2016;93:181–238. doi: 10.1016/bs.apar.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Katiki LM, Chagas AC, Takahira RK, Juliani HR, Ferreira JF, Amarante AF. Evaluation of Cymbopogon schoenanthus essential oil in lambs experimentally infected with Haemonchus contortus. Vet Parasitol. 2012;186(3–4):312–318. doi: 10.1016/j.vetpar.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Hoste H, Torres-Acosta JF, Alonso-diaz MA, Brunet S, Sandoval-Castro C, Adote SH. Identification and validation of bioactive plants for the control of gastrointestinal nematodes in small ruminants. Trop Biomed. 2008;25(1 Suppl):56–72. [PubMed] [Google Scholar]
- 10.Romo-Asunción D, Ávila-Calderón MA, Ramos-López MA, Barranco-Florido JE, Rodríguez-Navarro S, Romero-Gomez S, Aldeco-Pérez EJ, Pacheco-Aguilar JR, Rico-Rodríguez MA. Juvenomimetic and insecticidal activities of Senecio salignus (Asteraceae) and Salvia microphylla (Lamiaceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae) Fla Entomol. 2016;99(3):345–351. doi: 10.1653/024.099.0301. [DOI] [Google Scholar]
- 11.Romo de Vivar A, Pérez-Castorena A-L, Arciniegas A, Villaseñor JL. Secondary metabolites from Mexican species of the tribe Senecioneae (Asteraceae) J Mex Chem Soc. 2007;51(3):160–172. [Google Scholar]
- 12.Loizzo MR, Statti GA, Tundis R, Conforti F, Bonesi M, Autelitano G, Houghton PJ, Miljkovic-Brake A, Menichini F. Antibacterial and antifungal activity of Senecio inaequidens DC. and Senecio vulgaris L. Phytother Res. 2004;18(9):777–779. doi: 10.1002/ptr.1562. [DOI] [PubMed] [Google Scholar]
- 13.Hernández R, C López Pérez E. Actividad insecticida e insectistática de la chilca (Senecio salignus) sobre Zabrotes subfasciatus Manejo Integrado de Plagas (CATIE)(no 59) 2001. pp. 19–26. [Google Scholar]
- 14.Nibret E, Sporer F, Asres K, Wink M. Antitrypanosomal and cytotoxic activities of pyrrolizidine alkaloid-producing plants of Ethiopia. J Pharm Pharmacol. 2009;61(6):801–808. doi: 10.1211/jpp.61.06.0014. [DOI] [PubMed] [Google Scholar]
- 15.Karam FSC, Schild AL, Mello JRB. Intoxicação por Senecio spp. em bovinos no Rio Grande do Sul: condições ambientais favoráveis e medidas de controle. Pesqui Vet Bras. 2011;31(7):603–609. doi: 10.1211/jpp.61.06.0014. [DOI] [Google Scholar]
- 16.Gava A, Barros CS. Senecio spp. poisoning of horses in southern Brazil. Pesqui Vet Bras. 1997;17(1):36–40. doi: 10.1590/S0100-736X1997000100006. [DOI] [Google Scholar]
- 17.Panziera W, Gonçalves MA, Oliveira LG, Lorenzett MP, Reis M, Hammerschmitt ME, Pavarini SP, Driemeier D. Senecio brasiliensis poisoning in calves: pattern and evolution of hepatic lesions. Pesqui Vet Bras. 2017;37(1):8–16. doi: 10.1590/S0100-736X2017000100002. [DOI] [Google Scholar]
- 18.Artiles LRV, Reina M, Coloma AG, Pérez RC, Mesia LR. Pyrrolizidine alkaloids of Senecio sp from Peru. Quím Nova. 2011;34(6):992–995. doi: 10.1590/S0100-40422011000600015. [DOI] [Google Scholar]
- 19.Bull LB, Culvenor C, Dick A. The pyrrolizidine alkaloids: their chemistry, pathogenicity and other biological properties. Front Biol. 1968;(9). 10.1086/406383.
- 20.Vendruscolo GS, Mentz LA. Levantamento etnobotânico das plantas utilizadas como medicinais por moradores do bairro Ponta Grossa, Porto Alegre, Rio Grande do Sul, Brasil. Iheringia Série Botânica. 2006;61(1/2):83–103. [Google Scholar]
- 21.Toma W, Trigo JR, Bensuaski de Paula AC, Monteiro Souza Brito AR. Modulation of gastrin and epidermal growth factor by pyrrolizidine alkaloids obtained from Senecio brasiliensis in acute and chronic induced gastric ulcers. Can J Physiol Pharmacol. 2004;82(5):319–325. doi: 10.1016/j.jep.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Toma W, Trigo JR, de Paula ACB, Brito ARMS. Preventive activity of pyrrolizidine alkaloids from Senecio brasiliensis (Asteraceae) on gastric and duodenal induced ulcer on mice and rats. J Ethnopharmacol. 2004;95(2):345–351. doi: 10.1016/j.jep.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Suau R, Cabezudo B, Rico R, Nájera F, López-Romero JM, García AI. Pyrrolizidine alkaloids from three Spanish Senecio species. Biochem Syst Ecol. 2002;30(10):981–984. doi: 10.1016/S0305-1978(02)00031-5. [DOI] [Google Scholar]
- 24.Klitzke CF, Trigo JR. New records of pyrrolizidine alkaloid-feeding insects Hemiptera and Coleoptera on Senecio brasiliensis. Biochem Syst Ecol. 2000;28(4):313–318. doi: 10.1016/S0305-1978(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 25.Fan L, Li J, Deng K, Ai L. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr Polym. 2012;87(2):1849–1854. doi: 10.1016/j.carbpol.2011.10.018. [DOI] [Google Scholar]
- 26.Chan E, Lim Y, Wong S, Lim K, Tan S, Lianto F, Yong M. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113(1):166–172. doi: 10.1016/j.foodchem.2008.07.090. [DOI] [Google Scholar]
- 27.Vercruysse J, Holdsworth P, Letonja T, Barth D, Conder G, Hamamoto K, Okano K. International harmonisation of anthelmintic efficacy guidelines. Vet Parasitol. 2001;96(3):171–193. doi: 10.1016/S0304-4017(00)00443-X. [DOI] [PubMed] [Google Scholar]
- 28.Vargas-Magaña J, Torres-Acosta J, Aguilar-Caballero A, Sandoval-Castro C, Hoste H, Chan-Pérez J. Anthelmintic activity of acetone–water extracts against Haemonchus contortus eggs: interactions between tannins and other plant secondary compounds. Vet Parasitol. 2014;206(3):322–327. doi: 10.1016/j.vetpar.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Chan-Pérez J, Torres-Acosta J, Sandoval-Castro C, Hoste H, Castañeda-Ramírez G, Vilarem G, Mathieu C. In vitro susceptibility of ten Haemonchus contortus isolates from different geographical origins towards acetone: water extracts of two tannin rich plants. Vet Parasitol. 2016;217:53–60. doi: 10.1016/j.vetpar.2015.11.00. [DOI] [PubMed] [Google Scholar]
- 30.Niezen J, Waghorn G, Graham T, Carter J, Leathwick D. The effect of diet fed to lambs on subsequent development of Trichostrongylus colubriformis larvae in vitro and on pasture. Vet Parasitol. 2002;105(4):269–283. doi: 10.1016/S0304-4017(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 31.Thoden TC, Boppré M. Plants producing pyrrolizidine alkaloids: sustainable tools for nematode management? Nematology. 2010;12(1):1–24. doi: 10.1163/138855409X12549869072248. [DOI] [Google Scholar]
- 32.Brandao MG, Krettli AU, Soares LS, Nery CG, Marinuzzi HC. Antimalarial activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. J Ethnopharmacol. 1997;57(2):131–138. doi: 10.1016/S0378-8741(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 33.Gomes DC, de Lima HG, Vaz AV, Santos NS, Santos FO, Dias ÊR, Botura MB, Branco A, Batatinha MJM. In vitro anthelmintic activity of the Zizyphus joazeiro bark against gastrointestinal nematodes of goats and its cytotoxicity on Vero cells. Vet Parasitol. 2016;226:10–16. doi: 10.1016/j.vetpar.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Ajiboye B, Ibukun E, Edobor G, Ojo A, Onikanni S. Qualitative and quantitative analysis of phytochemicals in Senecio biafrae leaf. Int J Invent Pharm Sci. 2013;1(5):428–432. [Google Scholar]
- 35.Zellagui A, Tijani S, Gherraf N, Rhouati S. Phytochemical screening and evaluation of antibacterial activity of alkaloids extract of Senecio delphinifolius Vahl. Der Pharma Chemica. 2012;4(5):2080–2084. [Google Scholar]
- 36.Mukherjee N, Mukherjee S, Saini P, Roy P, P Sinha Babu S. Phenolics and terpenoids; the promising new search for anthelmintics: a critical review. Mini Rev Med Chem. 2016;16(17):1415–1441. doi: 10.2174/1389557516666151120121036. [DOI] [PubMed] [Google Scholar]
- 37.Minho AP, Bueno ICS, Louvandini H, Jackson F, Gennari SM, Abdalla AL. Effect of Acacia molissima tannin extract on the control of gastrointestinal parasites in sheep. Anim Feed Sci Technol. 2008;147(1–3):172–181. doi: 10.1016/j.anifeedsci.2007.09.016. [DOI] [Google Scholar]
- 38.Ouattara M, Sissouma D, Koné MW, Menan HE, Touré SA, Ouattara L. Synthesis and anthelmintic activity of some hybrid benzimidazolyl-chalcone derivatives. Trop J Pharm Res. 2011;10(6):767–775. doi: 10.4314/tjpr.v10i6.10. [DOI] [Google Scholar]
- 39.Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar J. 2011;10(Suppl 1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- 41.Klongsiriwet C, Quijada J, Williams AR, Mueller-Harvey I, Williamson EM, Hoste H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int J Parasitol Drugs Drug Resist. 2015;5(3):127–134. doi: 10.1016/j.ijpddr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2–3):97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Wiedenfeld H, Edgar J. Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem Rev. 2011;10(1):137–151. doi: 10.1007/s11101-010-9174-0. [DOI] [Google Scholar]
- 44.Segall HJ, Wilson DW, Lamé MW, Morin D, Winter CK. Metabolism of Pyrrolizidine Alkaloids. In: Handbook of Natural Toxins: Toxicology of Plant and Fungal Compounds. Volume 6, edn. Edited by Keeler RF and Tu AT. New York: Marcel Dekker; 1991.
- 45.Mattocks A. Chemistry and toxicology of pyrrolizidine alkaloids. London: Academic Press; 1986.
- 46.Fu PP, Xia Q, Lin G, Chou MW. Pyrrolizidine alkaloids—genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 2004;36(1):1–55. doi: 10.1081/DMR-120028426. [DOI] [PubMed] [Google Scholar]
- 47.Santos JCA, Riet-Correa F, Simões SV, Barros CS. Patogênese, sinais clínicos e patologia das doenças causadas por plantas hepatotóxicas em ruminantes e eqüinos no Brasil. Pesqui Vet Bras. 2008;28(1):1–14. doi: 10.1590/S0100-736X2008000100001. [DOI] [Google Scholar]
- 48.Craig AM, Latham CJ, Blythe LL, Schmotzer WB, O’Connor OA. Metabolism of toxic pyrrolizidine alkaloids from tansy ragwort (Senecio jacobaea) in ovine ruminal fluid under anaerobic conditions. Appl Environ Microbiol. 1992;58(9):2730–2736. doi: 10.1128/aem.58.9.2730-2736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huan JY, Miranda CL, Buhler DR, Cheeke PR. Species differences in the hepatic microsomal enzyme metabolism of the pyrrolizidine alkaloids. Toxicol Lett. 1998;99(2):127–137. doi: 10.1016/S0378-4274(98)00152-0. [DOI] [PubMed] [Google Scholar]
- 50.Adamu M, Naidoo V, Eloff JN. Efficacy and toxicity of thirteen plant leaf acetone extracts used in ethnoveterinary medicine in South Africa on egg hatching and larval development of Haemonchus contortus. BMC Vet Res. 2013;9(1):38. doi: 10.1186/1746-6148-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Likhitwitayawuid K, Angerhofer CK, Cordell GA, Pezzuto JM, Ruangrungsi N. Cytotoxic and antimalarial bisbenzylisoquinolme alkaloids from Stephania erecta. J Nat Prod. 1993;56(1):30–38. doi: 10.1021/np50091a005. [DOI] [PubMed] [Google Scholar]
- 52.Prakash AS, Pereira TN, Reilly PE, Seawright AA. Pyrrolizidine alkaloids in human diet. Mutat Res. 1999;443(1–2):53–67. doi: 10.1016/S1383-5742(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 53.Torras-Claveria L, Berkov S, Codina C, Viladomat F, Bastida J. Daffodils as potential crops of galanthamine. Assessment of more than 100 ornamental varieties for their alkaloid content and acetylcholinesterase inhibitory activity. Ind Crop Prod. 2013;43:237–244. doi: 10.1016/j.indcrop.2012.07.034. [DOI] [Google Scholar]
- 54.de Andrade JP, Giordani RB, Torras-Claveria L, Pigni NB, Berkov S, Font-Bardia M, Calvet T, Konrath E, Bueno K, Sachett LG. The Brazilian Amaryllidaceae as a source of acetylcholinesterase inhibitory alkaloids. Phytochem Rev. 2016;15(1):147–160. doi: 10.1007/s11101-015-9411-7. [DOI] [Google Scholar]
- 55.Van den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 56.Adams R. Identification of essential oil components by gas chromatography/mass spectroscopy. J Am Soc Mass Spectrom. 1997;6(8):671–672. [Google Scholar]
- 57.Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, Waller PJ. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44(1–2):35–44. doi: 10.1016/0304-4017(92)90141-U. [DOI] [PubMed] [Google Scholar]
- 58.Coles G, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136(3–4):167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 59.von Samson-Himmelstjerna G, Coles GC, Jackson F, Bauer C, Borgsteede F, Cirak VY, Demeler J, Donnan A, Dorny P, Epe C, et al. Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol Res. 2009;105(3):825–834. doi: 10.1007/s00436-009-1466-1. [DOI] [PubMed] [Google Scholar]
- 60.Abbott W. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18(2):265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 61.Demeler J, Kuttler U, El-Abdellati A, Stafford K, Rydzik A, Varady M, Kenyon F, Coles G, Hoglund J, Jackson F, et al. Standardization of the larval migration inhibition test for the detection of resistance to ivermectin in gastro intestinal nematodes of ruminants. Vet Parasitol. 2010;174(1–2):58–64. doi: 10.1016/j.vetpar.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Demeler J, Kuttler U, von Samson-Himmelstjerna G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Vet Parasitol. 2010;170(1–2):61–70. doi: 10.1016/j.vetpar.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 63.Rolón M, Vega C, Escario JA, Gómez-Barrio A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol Res. 2006;99(2):103–107. doi: 10.1007/s00436-006-0126-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analyzed during this study are included in this published article (and its supplementary information files). Data that are not in this article are available from corresponding author on reasonable request.