Abstract
Ovarian cancer is one of the most common causes of morbidity related to gynecologic malignancies. Possible risk factors are including hereditary ovarian cancer, obesity, diabetes mellitus, alcohol consumption, aging, and smoking. Various molecular signaling pathways including inflammation, oxidative stress, apoptosis and angiogenesis are involved in this progression of ovarian cancer. Standard treatments for recently diagnosed patients are Surgery and chemotherapy such as co-treatment with other drugs such that the exploitation of neoadjuvant chemotherapy is expanding. Melatonin (N-acetyl-5-methoxy-tryptamine), an endogenous agent secreted from the pineal gland, has anti-carcinogenic features, such as regulation of estradiol production, cell cycle modulation, stimulation of apoptosis as well as anti-angiogenetic properties, anti-inflammatory activities, significant antioxidant effects and modulation of various immune system cells and cytokines. Multiple studies have shown the significant beneficial roles of melatonin in various types of cancers including ovarian cancer. This paper aims to shed light on the roles of melatonin in ovarian cancer treatment from the standpoint of the molecular aspects.
Keywords: Melatonin, Ovarian cancer, Signaling pathways, Anti-inflammatory activities, Anti-angiogenetic properties
Introduction
Ovarian cancer is one of the most common causes of morbidity related to gynecologic malignancies [1]. This illness is frequently diagnosed in advanced stages [2] and can be divided into at least five histological types that are characterized by recognizable risk factors, molecular manifestations and clinical properties [3]. Ovarian tumors are graded into 3 types including benign, borderline malignant or malignant [4]. Possible risk factors are including hereditary [5], obesity [6], diabetes mellitus [7], alcohol consumption [8], aging [9], and smoking [10]. Due to absence of noticeable early symptoms in this cancer such as lack of special pelvic or gastrointestinal symptoms [11] its morbidity is high [12]. Standard treatments for recently diagnosed patients are surgery and chemotherapy such as co-treatment with carboplatin and paclitaxel. Although the exploitation of neoadjuvant chemotherapy is expanding [13], treatment in high-grade serous ovarian carcinoma remains a clinical challenge [14].
Melatonin (N-acetyl-5-methoxy-tryptamine), an endogenous agent secreted from the pineal gland [15], transfers information about period of darkness to all cells [16] which its peak is at highest level among 2AM and 5AM [17]. Melatonin has a critical role in determining homeostasis, neurohumoral stableness and circadian rhythms through synergetic activities with other hormones and neuropeptides [18]. Melatonin is classified as an autocoid, a chronobiotic, a sleep-inducing agent, an immune modulator and a biological adjusting agent. In addition, melatonin has anti-carcinogenic features, such as regulation of estradiol production, cell cycle modulation, upgrading apoptosis [19] as well as anti-angiogenetic properties [20], significant antioxidant effects [21] and modulation of various immune system cells and cytokines [22]. There is considerable evidence of its ability in prevention and treatment of cancers [23]. Multiple studies have shown significant beneficial roles of melatonin in various cancers types such as breast cancer [24] pancreatic cancer [25] lung cancer [26] and ovarian cancer [27]. A retrospective study showed that melanin levels are lower in women with ovarian cancer compared with healthy ones (41.8 versus 82.4 pg/mL) [28]. Even though there is no significant relationship between urinary melatonin levels and ovarian cancer risk in women, several evidence shows that melatonin have potential therapeutic properties against this cancer. A recent in vivo study reported that melatonin treatment for 60 days potentially decreased ovarian cancer mass without any peritoneal adhesions and tumor incidence including high-grade serous papillary, sarcoma and undifferentiated carcinoma [29]. In addition, an in vitro study observed that response to melatonin in various types of ovarian cancer cells was different. In fact, there was a non-heterogeneous response to melatonin as one of the cell lines inhibited by 90% while another one inhibited by 30% [30]. This report sheds the new light on the roles of melatonin in prevention, treatment and chemotherapetic interplay in ovarian carcinoma as identified in recent studies and clinical trials.
Ovarian cancer pathogenesis
Although some causes of ovarian cancer remain unclear, several hypothesizes have been advanced. One is the incessant ovulation hypothesis which proposes the formation and progression of ovarian cancer throughout cyclical ovulatory processes. This hypothesis suggests that replicative DNA mistakes are increased in ovarian epithelial cells during follicular growth and ovulation [31]. Another hypothesis is the gonadotrophin theory suggesting that gonadotrophins lead to excessive proliferation of ovarian epithelial cells which result in tumor formation [32]. Hormonal influences are the third hypothesis which suggests the effects of hormones such as androgen and progesterone on proliferation of the ovarian epithelial cells and therefore ovarian cancer formation [33]. The surface of the ovarian epithelium is part of the peritoneal lining and, therefore, is exposed to substances which exist in the peritoneal cavity. Most of these substances have inflammatory features. A primary physiological role of the ovary is ovulation, which has pro-inflammatory properties [34]. During the ovulatory processes followed by immediate ovum release, a large number of molecules are generated including cytokines and chemokines, prostaglandins, plasminogen activators, bioactive eicosanoids, interleukins, collagenases, tumor necrosis factors, several growth factors and also various immune cells which all activate a pro-inflammatory cascade. Some of these pro-inflammatory molecules including CCL2/MCP-1, CCL5/RANTES and IL-8 are activated during cyclical ovulation; thus, the incessant ovulation theory suggests that inflammation along with other physiological conditions enhances the progression of ovarian cancer [35]. Conversely, as suggested by the gonadotrophin and hormonal hypothesizes, increased estrogens and androgens recruit several pro-inflammatory cells and molecular stimulators leading to immune activation [36]. Collectively, these hypothesizes indicate the influences of ovulation, gonadotrophin and hormonal changes on formation and progression of ovarian cancer which are all related to activation of inflammatory mediators as well as persistent creation of genomic damages.
Melatonin and ovarian cancer; molecular mechanisms
Treatment by melatonin leads to a reduction in various proteins involved in ovarian cancer signaling pathways including oxidative stress, inflammation, apoptosis, cell cycle and proliferation. One of these molecules is E-cadherin which is a tumor suppressor and a key molecule for sustaining adherent junctions in cell surface [37]. Up-regulation of E-cadherin expression in ovarian cancer tissues has prognostic value to distinguish tumors in late and early stages [38]. Melatonin increases E-cadherin in ovarian cancer cells [39]. Another factor modulated by melatonin is the estrogen receptor α (ERα). This molecule is one a member of nuclear receptor super family and modulates cell multiplication, homeostasis, and differentiation in multiple tissues. Sustained exposure to estrogen/estradiol (E2) up-regulates the growth of ovarian cancers [40]. Melatonin is a special ERα suppressor [41] and plays an anti-carcinogenic role through the estrogen receptor (ER) pathway in tumor cells [42].
Antioxidant effects of melatonin
There is agreement that melatonin is an important endogenous free-radical scavenger [43] and possesses several antioxidant roles by influencing the electron transfer chain, preventing peroxynitrite levels via regulation of nitric oxide synthases (iNOS, nNOS) and thereby reducing NO levels. Melatonin enhances the intra-mitochondrial anti-oxidative potential by improving glutathione levels and promoting glutathione peroxidase, manganese-superoxide dismutase (Mn-SOD) in the matrix and copper, zinc (Cu, Zn-SOD) in the inter-membrane space [44]. Melatonin treatment leads to ROS reduction and activation of several anti-oxidant enzymes such as superoxide dismutase, catalase and glutathione [45]; melatonin also acts as a pro-oxidant in different cancers [46]. In pre-ovulatory follicular fluid, melatonin alleviates the carcinogenic effect of ROS in follicular fluid [47]. Some studies revealed that cyclooxygenase-2 (COX2) is over-expressed in tumor cells. Melatonin suppresses COX2 activity [48] leading to prevention of DNA damage [49].
Melatonin and apoptosis
Apoptosis, a type of programmed cell death, has both specific morphological features and biochemical mechanisms. Melatonin regulates apoptosis in several types of cancers by multiple mechanisms. Caspases are interleukin-1beta-converting enzyme family members which are also aspartate-specific cysteine proteases [50] and have pivotal roles in regulation of the initiation, transduction and promotion of apoptotic signals. In ovarian cancer the expression of cleaved caspase-3 is increased [51] while melatonin reduces the over-expression and activation of this molecule [52]. Thus melatonin administration enhances apoptosis in ovarian cancer cells [53].
Evidences suggest that treatment with melatonin enhances apoptosis by increasing the expression of p53, a tumor suppressor, in ovarian cancer cells [53]. P53 signaling pathway in various carcinoma cell lines acts significantly as a transcription factor, resulting in arrest cell cycle or apoptosis [54] by inhibition of the cell cycle in the G2-phase [55]. Accumulated p53 significantly interacts with proteins which are important in tumor cell maintenance and thereby neutralizes them [56]. Melatonin increases [57] and activates p53 and therefore increases apoptosis in several cancers such as the colon [58] and the uterus [59]. Two important members of apoptosis-related genes are Bcl-2 and BAX [60]. Melatonin motivates BAX gene expression, and down regulates the expression of anti-apoptotic gene BCL-2 [61, 62]; thus, melatonin regulates the Bax/Bcl-2 ratio [63]. A recent study suggested the role of melatonin in induction of apoptosis in ovarian cancer cells by increasing BAX expression and reducing in Bcl-2 levels [53].
Melatonin as an anti-inflammatory agent
In addition to its anti-oxidant effects melatonin’s anti-inflammatory and immune regulatory properties are well documented. Melatonin induces the release of interleukin-2, interleukin-10 and interferon-γ which leads to the enhancement of T-helper cells which respond to these substances. T-helper cells have a significant anti-cancer role. Nuclear factor-kappa B (NF-kappa B) enhances ROS generation leading to DNA damage [64, 65]. In the etiology of the ovarian cancer, NF-kB is a significant marker of inflammation [66]. Melatonin suppresses the NF-kB phospho-activation [67]. Furthermore, melatonin decreases H2O2-induced oxidative stress by regulation of Erk/Akt/NFkB pathway [68]. Melatonin therapy down-regulates the mRNA expression of NFκB1, NFκB2 in mice [69]. This indolamine also inhibits the expression of TNF-α which is an important member of TNF/TNFR cytokine super-family; via these mechanisms melatonin acts as an anti-inflammatory agent [70]. TNF-α is a pro-inflammatory agent which causes pathological processes such as chronic inflammation and malignancy [71]. In ovarian cancer cells the expression of TNF-α is elevated [72]. Melatonin administration importantly inhibits this increase in ovarian cancer cells. HER-2 is another factor involved in the initiation and maintenance of inflammation and tumorogenesis in cancer cells. HER2 initiates a feed-forward activation circle of IL-1α and IL-6 that induces NF-κB and STAT3 pathways for induction and preservation of cancer cells [73]. Her2 stimulates kinases and transcript agents which support cancer medicine resistance and metastasis. Melatonin critically suppresses this invasive/metastatic aspect; the mechanism involves the repression of mesenchymal-to-epithelial transition, either by helping mesenchymal-to-epithelial conversion, and/or by obstructing important signaling pathways implicated in later stages of metastasis [74]. Melatonin also regulates Her-2 system in invasive tumors by decreasing the Her-2 expression [75]. Signaling pathways of transforming growth factor-β (TGF-β) have significant roles in ovarian cancer [76]. TGF-β may enhance cell survival by positive modulation of the cell cycle as well as by preventing apoptosis [77]. The expression of TGF-ß1 and its receptors may play an important role in promotion and proliferation of tumor cells [78]. Melatonin prevents [79] and decreases the expression of TGF-β1 in epithelial ovarian cancer [80]. Collectively, the findings indicate that melatonin administration significantly regulates signaling pathways in ovarian cancer [81].
Melatonin and its ani-angigenesis effects
One of important aspects of metastatic spread and proliferation of cancer cells is presence of necessary nutrients and oxygen and also should be removed waste products [82, 83]. In this regards, vascular network and new growth of vessels are known as well players for these actions. New lymphatic and blood and vessels form via processes which are known as lymphangiogenesis and angiogenesis respectively [84]. Multiple lines evidence revealed that angiogenesis could be modulated by both inhibitor and activator molecules. Several proteins have been observed as angiogenic inhibitors and activators. Despite many efforts, antiangiogeic inhibitors have not documented useful in terms of long-term survival. Therefore, there is a serious require for finding, and developing new effect therapeutic platforms combining anti-angiogenic therapies along with conventional cytoreductive treatments in the management of different cancers [83]. Vascular endothelial growth factor (VEGF) is significantly over-expresses in cancer patients [85, 86] VEGF inhibits apoptosis, protects tumor and vascular growth, and enhances proliferation and inflammation leading to carcinogenesis [87]. Melatonin decreases VEGF secretion resulting in inhibition of angiogenesis in tumors [88]. Melatonin also was shown to inhibit angiogenesis by decreasing angiopoietins and VEGF in an animal model of ovarian cancer [89].
Besides different factors which are associated to over expression and activation of pro-angiogenic growth factors and their receptors, hypoxia has been emerged as key factor. Given that cells employed a variety of genes to adapt to hypoxia in low-oxygen positions. Among of these genes, HIF-1 is major and primary players in hypoxic conditions [90, 91]. HIF-1α and HIF-1β are well-known subunits of HIF-1. These subunits are known as a basic helix-loop-helix (bHLH) transcription factor family. It has been showed that HIF-1α/HIF-1β dimer has various targets such as VEGF. Hence, this protein is related to increasing of angiogenesis. Along to different signaling pathways involved in angiogenesis, STAT3 is another angiogenesis factor which is able to elevate the expression of VEGF and stimulate HIF-1α stability. Increasing evidence indicated, when STAT3 and HIF-1α link to CBP/p300 (is known as a co-activator in the VEGF promoter); there were normal activation of VEGF transcription [88]. The activated STAT3 is associated with initiation and progression of several malignancies such as ovarian cancer, and melanoma. It has a crucial role in different biological processes including survival, proliferation of cells, migration, invasion, and angiogenesis [92]. As mentioned above, melatonin shows anti-cancer activities such as anti-angiogenesis property. In this regards, given that melatonin enables to effect on angiogenesis thought targeting HIF-1α under hypoxic conditions [46, 93]. In a study, Park et al. [94] revealed that there is a decrease for HIF-1α and pVHL binding during hypoxic conditions in colon cancer cell lines. While the presence of melatonin is able to recover PHD activity in the treatment group and then elevated the binding of HIF-1α and pVHL. In another study, Zhang et al. [95] documented melatonin increases binding of pVHL and HIF-1α during hypoxia in glioblastoma cells. Taken together, expression levels of angiogenic factors are associated with the tumor cells aggressiveness. Thus, identification of new angiogenic inhibitors (e.g., melatonin) could contribute to decrease both mortality and morbidity from carcinomas.
Melatonin and metabolic alterations in ovarian cancer
Alteration in cancer cell metabolism is one of the important events occurred during tumorogenesis and cancer progression. Cancer cells need to be able to proliferate and growth in a hypoxic and nutrient-poor microenvironment which require a reprogramming of metabolism in these cells, especially the key metabolic substrates such as glucose, lipid and etc. in this way, mitochondria gets more functional changes among other organelles. Despite of the high glycolytic rate in cancer cells, the produced pyruvate is not used in Krebs cycle and it is transformed into lactate independently from oxygen availability in the so-called Warburg effect [96]. In addition, many clinical studies reported that plasma glucose levels in cancer patients is highly elevated and may be a significant prognostic indicator for cancer. Besides, some recent studies demonstrated that the expression of glucose transporter protein 1 (GLUT1) increased in ovarian cancer cells leading to elevation of glucose uptake in these cells [97]. Recently, new beneficial therapeutic targets which involve in cellular pathways responsible for energy generation required to control cancer cell growth are emerging. Several studies showed that melatonin, at both physiological and pharmacological concentrations, is able to regulate cellular metabolism through different mechanisms [98]. It has been proved that melatonin crosses cell membranes via glucose transporters which may lead to reduction of glucose uptake in cancer cells [99]. In addition, melatonin is able to decrease the production of lactate, but the certain mechanism which in how melatonin affects glycolysis is not clear yet [100]. Moreover, melatonin influences insulin secretion from pancreas by its MT1 and MT2 receptors leading to reduction in blood glucose and elevation in fatty acids [101]. Recent findings showed that melatonin reduced proteins related to metabolic systems including production of several metabolites and energy, endoplasmic reticulum stress related pathways, cancer-associated proteoglycan, HIF-1 signaling and antigen processing in ovarian cancer. Indeed, melatonin down-regulates several proteins related to metabolism including glyceraldehydes-3- phosphate dehydrogenase, pyruvate kinase isozymes M1/M2, fructose-bisphosphate, aldolase A, lactate dehydrogenase A chain, creatine kinase B, protein disulfide isomerase A3 and A6, subunit α of ATP synthase, 78-kDa glucose-regulated protein and peptidyl-prolyl cis-trans isomerase A. these alterations in metabolism may significantly influence aerobic glycolysis leading to reduction in proliferation and metastasis in ovarian cancer cells. Besides, melatonin overexpressed some molecules including subunit β of ATP synthase, fatty acid binding protein and 10-kDa heat chock protein in ovarian cancer cells [81].
Melatonin as an adjuvant therapy in ovarian cancer treatment
Recent investigations have described the role of melatonin in combination with radio- or chemotherapy in several cancers including ovarian cancer [102]. These studies also showed that the optimal dose of melatonin is safe and effective in enhancing radiotherapy ratio therapeutic effects as well as providing radioprotection [103]. Melatonin up regulates the tolerance of normal tissues to toxic effects of ionizing radiation in patients who undergo radiotherapy by enhancing DNA damage responses and reducing the risk of instability. Melatonin plays synergistic roles in radiotherapy and chemotherapy as an antioxidant which relieving the side effects of these destructive treatments [104].
In ovarian cancer this powerful antioxidant potentially supports the ovaries against damage induced by cisplatin; this is the main chemotherapeutic agent for this cancer [105]. Recently it has been shown that melatonin improved fertility in ovarian cancer [106] via the promotion of ERK/p90RSK/HSP27 cascade in SK-OV-3 cells. Moreover, melatonin and cisplatin co-administration promoted apoptosis induced by cisplatin. It was also demonstrated that melatonin improved cis-diamminedichloroplatinum sensitivity in HTOA and OVCAR-3 cells; both these lines are ovarian cancer cells [107]. Other investigations also showed that melatonin co-administration potentially boosted laser effectiveness by induction of apoptosis in ovarian tumor cells leading to significant improvement of apoptosis/necrosis ratio, and also increasing the expression of heat shock protein 70 compared to administration each factor alone [108]. Thus, melatonin acts as a powerful synergistic agent with cisplatin therapy [109] and can be applied as an adjuvant in ovarian cancer treatment.
Conclusions
Ovarian cancer is a gynecologic malignancy with a high morbidity rate. The pathogenesis of this cancer is complex from its molecular aspects. Several factors and various molecular signaling pathways such as inflammation, oxidative stress, apoptosis and angiogenesis are involved in its progression. There is a large amount of evidence that documents the potential beneficial effects of melatonin in inhibition of development and progression of ovarian cancer via its multiple potential features including antioxidant, anti-inflammatory, metabolic effects and apoptosis induction activities in these tumor cells (Fig. 1). Also melatonin has beneficial effects with several anti-cancer drugs such as cisplatin. Thus, it is suggested that melatonin can be co-administrated as a potent adjuvant agent in combination with other chemotherapeutic drugs in ovarian cancer treatment. Current therapeutic strategies for ovarian cancer are often limited and evidence has shown that chemotherapy alone is not completely efficient to reduce tumor cells. Thus, finding new therapeutic options with low adverse effects is considerable. Melatonin is endogenously produced and its pharmacological doses are available with no toxicity. Although these potential roles have long been known, it has not been fully exploited in clinical trials. Melatonin as a natural molecule with its potential anti-cancer properties and its efficiency in decreasing side effects of current treatments may be an appropriate option in the treatment of ovarian cancer.
Fig. 1.
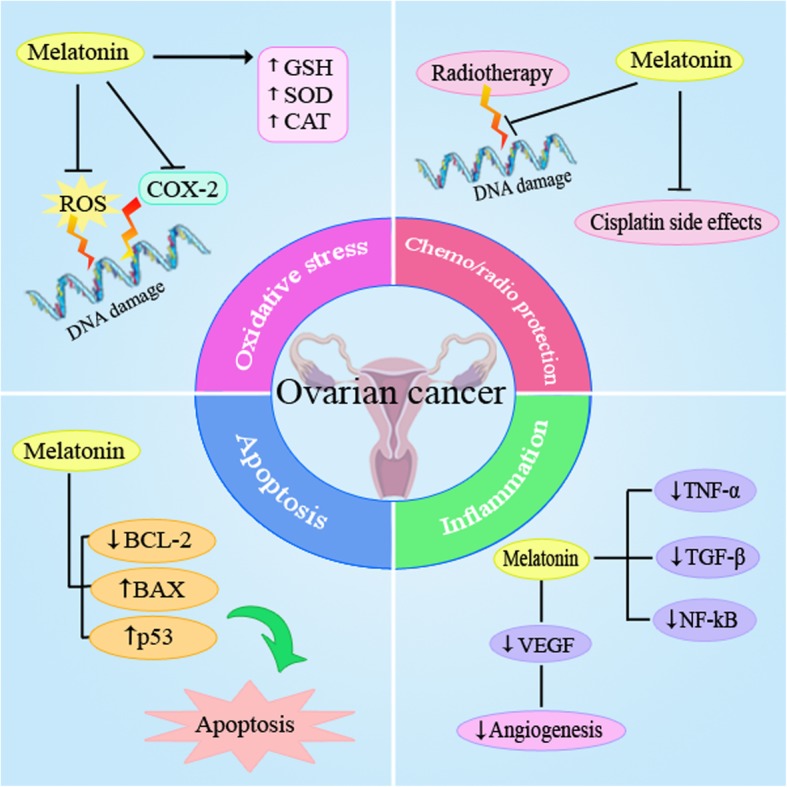
Schematic representation in targeting different signaling pathways using melatonin as a novel therapeutic strategy in the treatment of ovarian cancer
Acknowledgements
Not applicable.
Funding
The present study was founded by a grant from the Vice Chancellor for Research, Kashan University of Medical Sciences, in Iran.
Availability of data and materials
The primary data for this study is available from the authors on direct request.
Abbreviations
- ERα
estrogen receptor α
- TGF-β
transforming growth factor-β
Authors’ contributions
ZA contributed in conception, design, and drafting of the manuscript. HZ, RS and RJ.R contributed in data collection and manuscript drafting. All authors approved the final version for submission. ZA oversaw the study.
Ethics approval and consent to participate
This study was considered exempt by the KAUMS Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hadis Zare, Email: Hadis.Zare11@gmail.com.
Rana Shafabakhsh, Email: r.shafabakhsh@gmail.com.
Russel J. Reiter, Email: REITER@uthscsa.edu
Zatollah Asemi, Phone: +98-31-55463378, Email: asemi_r@yahoo.com.
References
- 1.Kujawa KA, Lisowska KM. Ovarian cancer--from biology to clinic. Postepy Hig Med Dosw (Online) 2015;69:1275–1290. doi: 10.5604/17322693.1184451. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez L, Kim MK, Lyle LT, Bunch KP, House CD, Ning F, et al. Characterization of ovarian cancer cell lines as in vivo models for preclinical studies. Gynecol Oncol 2016;142:332–340. [DOI] [PMC free article] [PubMed]
- 3.Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okumura T, Muronosono E, Tsubuku M, Terao Y, Takeda S, Maruyama M. Anaplastic carcinoma in ovarian seromucinous cystic tumor of borderline malignancy. J Ovarian Res. 2018;11:77. doi: 10.1186/s13048-018-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Vecchia C. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev. 2017;26:55–62. doi: 10.1097/CEJ.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Metzinger MN, Lewellen KA, Cripps SN, Carey KD, Harper EI, et al. Obesity contributes to ovarian cancer metastatic success through increased lipogenesis, enhanced vascularity, and decreased infiltration of M1 macrophages. Cancer Res. 2015;75:5046–5057. doi: 10.1158/0008-5472.CAN-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D, Li N, Xi Y, Zhao Y, Wang T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res Clin Pract. 2017;130:43–52. doi: 10.1016/j.diabres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Goodman MT, Tung KH. Alcohol consumption and the risk of borderline and invasive ovarian cancer. Obstet Gynecol. 2003;101:1221–1228. doi: 10.1016/s0029-7844(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 9.Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Physician. 2016;93:937–944. [PubMed] [Google Scholar]
- 10.Licaj I, Jacobsen BK, Selmer RM, Maskarinec G, Weiderpass E, Gram IT. Smoking and risk of ovarian cancer by histological subtypes: an analysis among 300 000 Norwegian women. Br J Cancer. 2017;116:270–276. doi: 10.1038/bjc.2016.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erondu CO, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy M, Cote ML, et al. The association between body mass index and presenting symptoms in African American women with ovarian cancer. J Women's Health (Larchmt) 2016;25:571–578. doi: 10.1089/jwh.2015.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51(Suppl 3):e72–e76. doi: 10.4103/0019-509X.154049. [DOI] [PubMed] [Google Scholar]
- 13.Matulonis UA. Management of newly diagnosed or recurrent ovarian cancer. Clin Adv Hematol Oncol. 2018;16:426–437. [PubMed] [Google Scholar]
- 14.Mendiola M, Redondo A, Heredia-Soto V, Herranz J, Berjon A, Hernandez A, et al. Predicting response to standard first-line treatment in high-grade serous ovarian carcinoma by angiogenesis-related genes. Anticancer Res. 2018;38:5393–5400. doi: 10.21873/anticanres.12869. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Campa C, Menendez-Menendez J, Alonso-Gonzalez C, Gonzalez A, Alvarez-Garcia V, Cos S. What is known about melatonin, chemotherapy and altered gene expression in breast cancer. Oncol Lett. 2017;13:2003–2014. doi: 10.3892/ol.2017.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erren TC, Reiter RJ. A generalized theory of carcinogenesis due to chronodisruption. Neuro Endocrinol Lett. 2008;29:815–821. [PubMed] [Google Scholar]
- 17.Nogueira LM, Sampson JN, Chu LW, Yu K, Andriole G, Church T, et al. Individual variations in serum melatonin levels through time: implications for epidemiologic studies. PLoS One. 2013;8:e83208. doi: 10.1371/journal.pone.0083208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dragojevic Dikic S, Jovanovic AM, Dikic S, Jovanovic T, Jurisic A, Dobrosavljevic A. Melatonin: a "Higgs boson" in human reproduction. Gynecol Endocrinol 2015;31:92–101. [DOI] [PubMed]
- 19.Poole EM, Schernhammer E, Mills L, Hankinson SE, Tworoger SS. Urinary melatonin and risk of ovarian cancer. Cancer Causes Control. 2015;26:1501–1506. doi: 10.1007/s10552-015-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl):S224–Ss43. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinther AG, Claesson MH. [The influence of melatonin on the immune system and cancer]. Ugeskr Laeger. 2015;177:V10140568. [PubMed]
- 23.Bondy SC, Campbell A. Mechanisms underlying tumor suppressive properties of melatonin. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 24.Vriend J, Reiter RJ. Breast cancer cells: modulation by melatonin and the ubiquitin-proteasome system--a review. Mol Cell Endocrinol. 2015;417:1–9. doi: 10.1016/j.mce.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z. Melatonin and pancreatic cancer: current knowledge and future perspectives. J Cell Physiol. 2018. [DOI] [PubMed]
- 26.Ma Z, Yang Y, Fan C, Han J, Wang D, Di S, et al. Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget. 2016;7:46768–46784. doi: 10.18632/oncotarget.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed]
- 28.Zhao M, Wan J, Zeng K, Tong M, Lee AC, Ding J, et al. The reduction in circulating melatonin level may contribute to the pathogenesis of ovarian cancer: a retrospective study. J Cancer. 2016;7:831–836. doi: 10.7150/jca.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuffa LG, Fioruci-Fontanelli BA, Mendes LO, Fávaro WJ, Pinheiro PF, Martinez M, et al. Characterization of chemically induced ovarian carcinomas in an ethanol-preferring rat model: influence of long-term melatonin treatment. PLoS One. 2013;8:e81676. doi: 10.1371/journal.pone.0081676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartsch H, Buchberger A, Franz H, Bartsch C, Maidonis I, Mecke D, et al. Effect of melatonin and pineal extracts on human ovarian and mammary tumor cells in a chemosensitivity assay. Life Sci. 2000;67:2953–2960. doi: 10.1016/s0024-3205(00)00882-1. [DOI] [PubMed] [Google Scholar]
- 31.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 32.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–721. [PubMed] [Google Scholar]
- 33.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 34.Bonello N, McKie K, Jasper M, Andrew L, Ross N, Braybon E, et al. Inhibition of nitric oxide: effects on interleukin-1 beta-enhanced ovulation rate, steroid hormones, and ovarian leukocyte distribution at ovulation in the rat. Biol Reprod. 1996;54:436–445. doi: 10.1095/biolreprod54.2.436. [DOI] [PubMed] [Google Scholar]
- 35.Freedman RS, Deavers M, Liu J, Wang E. Peritoneal inflammation - a microenvironment for epithelial ovarian cancer (EOC) J Transl Med. 2004;2:23. doi: 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison L. Health and disease among women: Biological and environmental influences. BMJ (Clinical research ed). 2000;320:193a. [PMC free article] [PubMed]
- 37.Pecina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17. doi: 10.1186/1475-2867-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosso M, Majem B, Devis L, Lapyckyj L, Besso MJ, Llaurado M, et al. E-cadherin: a determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS One. 2017;12:e0184439. doi: 10.1371/journal.pone.0184439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbarzadeh M, Rahbarghazi R, Nabat E, Movassaghpour AA, Shanehbandi D, Faramarzian Azimi Maragheh B, et al. The impact of different extracellular matrices on melatonin effect in proliferation and stemness properties of ovarian cancer cells. Biomed Pharmacother. 2017;87:288–295. doi: 10.1016/j.biopha.2016.12.119. [DOI] [PubMed] [Google Scholar]
- 40.Anbalagan M, Rowan BG. Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer. Mol Cell Endocrinol. 2015;418(Pt 3):264–272. doi: 10.1016/j.mce.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Pai P, Velmurugan BK, Kuo CH, Yen CY, Ho TJ, Lin YM, et al. 17beta-estradiol and/or estrogen receptor alpha blocks isoproterenol-induced calcium accumulation and hypertrophy via GSK3beta/PP2A/NFAT3/ANP pathway. Mol Cell Biochem. 2017;434:181–195. doi: 10.1007/s11010-017-3048-3. [DOI] [PubMed] [Google Scholar]
- 42.Lopes J, Arnosti D, Trosko JE, Tai MH, Zuccari D. Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer. 2016;7:209–217. doi: 10.18632/genesandcancer.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galano A, Tan DX, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018;23. 10.3390/molecules23030530. [DOI] [PMC free article] [PubMed]
- 44.Hardeland R. Melatonin and the electron transport chain. Cell Mol Life Sci. 2017;74:3883–3896. doi: 10.1007/s00018-017-2615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehrzadi S, Safa M, Kamrava SK, Darabi R, Hayat P, Motevalian M. Protective mechanisms of melatonin against hydrogen-peroxide-induced toxicity in human bone-marrow-derived mesenchymal stem cells. Can J Physiol Pharmacol. 2017;95:773–786. doi: 10.1139/cjpp-2016-0409. [DOI] [PubMed] [Google Scholar]
- 46.Chuffa LGA, Reiter RJ, Lupi LA. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38:945–952. doi: 10.1093/carcin/bgx054. [DOI] [PubMed] [Google Scholar]
- 47.Huang HS, Chu SC, Hsu CF, Chen PC, Ding DC, Chang MY, et al. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: initiation of fimbria carcinogenesis. Carcinogenesis. 2015;36:1419–1428. doi: 10.1093/carcin/bgv132. [DOI] [PubMed] [Google Scholar]
- 48.Deng WG, Tang ST, Tseng HP, Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. 2006;108:518–524. doi: 10.1182/blood-2005-09-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortiz-Franco M, Planells E, Quintero B, Acuna-Castroviejo D, Rusanova I, Escames G, et al. Effect of melatonin supplementation on antioxidant status and DNA damage in high intensity trained athletes. Int J Sports Med. 2017;38:1117–1125. doi: 10.1055/s-0043-119881. [DOI] [PubMed] [Google Scholar]
- 50.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin. 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 51.Hu Q, Peng J, Liu W, He X, Cui L, Chen X, et al. Elevated cleaved caspase-3 is associated with shortened overall survival in several cancer types. Int J Clin Exp Pathol. 2014;7:5057–5070. [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai CC, Lin YJ, Yu HR, Sheen JM, Tain YL, Huang LT, et al. Melatonin alleviates liver steatosis induced by prenatal dexamethasone exposure and postnatal high-fat diet. Exp Ther Med. 2018;16:917–924. doi: 10.3892/etm.2018.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chuffa LG, Alves MS, Martinez M, Camargo IC, Pinheiro PF, Domeniconi RF, et al. Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr Relat Cancer. 2016;23:65–76. doi: 10.1530/ERC-15-0463. [DOI] [PubMed] [Google Scholar]
- 54.Fischer M, Quaas M, Steiner L, Engeland K. The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 2016;44:164–174. doi: 10.1093/nar/gkv927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, Selth LA, Callen DF. MiR-766 induces p53 accumulation and G2/M arrest by directly targeting MDM4. Oncotarget. 2017;8:29914–29924. doi: 10.18632/oncotarget.15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang-Hartwich Y, Soteras MG, Lin ZP, Holmberg J, Sumi N, Craveiro V, et al. p53 protein aggregation promotes platinum resistance in ovarian cancer. Oncogene. 2015;34:3605–3616. doi: 10.1038/onc.2014.296. [DOI] [PubMed] [Google Scholar]
- 57.Song J, Ma SJ, Luo JH, Zhang H, Wang RX, Liu H, et al. Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol Rep. 2018;39:1975–1983. doi: 10.3892/or.2018.6282. [DOI] [PubMed] [Google Scholar]
- 58.Casado J, Inigo-Chaves A, Jimenez-Ruiz SM, Rios-Arrabal S, Carazo-Gallego A, Gonzalez-Puga C, et al. AA-NAT, MT1 and MT2 correlates with cancer stem-like cell markers in colorectal cancer: study of the influence of stage and p53 status of tumors. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed]
- 59.Zhang L, Zhang Z, Wang F, Tian X, Ji P, Liu G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod Biol Endocrinol. 2017;15:78. doi: 10.1186/s12958-017-0297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan LL, Wang AY, Huang YQ, Luo Y, Ling M. Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac J Cancer Prev. 2014;15:7065–7068. doi: 10.7314/apjcp.2014.15.17.7065. [DOI] [PubMed] [Google Scholar]
- 61.Alonso-Gonzalez C, Menendez-Menendez J, Gonzalez-Gonzalez A, Gonzalez A, Cos S, Martinez-Campa C. Melatonin enhances the apoptotic effects and modulates the changes in gene expression induced by docetaxel in MCF7 human breast cancer cells. Int J Oncol. 2018;52:560–570. doi: 10.3892/ijo.2017.4213. [DOI] [PubMed] [Google Scholar]
- 62.Xu L, Jin QD, Gong X, Liu H, Zhou RX. Anti-gastric cancer effect of melatonin and Bcl-2, Bax, p21 and p53 expression changes. Sheng Li Xue Bao. 2014;66:723–729. [PubMed] [Google Scholar]
- 63.Sanchez DI, Gonzalez-Fernandez B, Crespo I, San-Miguel B, Alvarez M, Gonzalez-Gallego J, et al. Melatonin modulates dysregulated circadian clocks in mice with diethylnitrosamine-induced hepatocellular carcinoma. J Pineal Res. 2018;65:e12506. doi: 10.1111/jpi.12506. [DOI] [PubMed] [Google Scholar]
- 64.Nichols TC, Fischer TH, Deliargyris EN, Baldwin AS., Jr Role of nuclear factor-kappa B (NF-kappa B) in inflammation, periodontitis, and atherogenesis. Ann Periodontol. 2001;6:20–29. doi: 10.1902/annals.2001.6.1.20. [DOI] [PubMed] [Google Scholar]
- 65.Ask TF, Lugo RG, Sutterlin S. The neuro-immuno-senescence integrative model (NISIM) on the negative association between parasympathetic activity and cellular senescence. Front Neurosci. 2018;12:726. doi: 10.3389/fnins.2018.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yilmaz E, Gul M, Melekoglu R, Koleli I. Immunhistochemical analysis of nuclear factor kappa Beta expression in etiopathogenesis of ovarian tumors. Acta Cir Bras. 2018;33:641–650. doi: 10.1590/s0102-865020180070000009. [DOI] [PubMed] [Google Scholar]
- 67.Mao L, Dauchy RT, Blask DE, Dauchy EM, Slakey LM, Brimer S, et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J Pineal Res. 2016;60:167–177. doi: 10.1111/jpi.12298. [DOI] [PubMed] [Google Scholar]
- 68.Moniruzzaman M, Ghosal I, Das D, Chakraborty SB. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol Res. 2018;51:17. doi: 10.1186/s40659-018-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuesta S, Kireev R, Forman K, Garcia C, Escames G, Ariznavarreta C, et al. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8) Exp Gerontol. 2010;45:950–956. doi: 10.1016/j.exger.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 70.Konturek PC, Burnat G, Brzozowski T, Zopf Y, Konturek SJ. Tryptophan free diet delays healing of chronic gastric ulcers in rat. J Physiol Pharmacol. 2008;59(Suppl 2):53–65. [PubMed] [Google Scholar]
- 71.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 72.Hong L, Wang S, Li W, Wu D, Chen W. Tumor-associated macrophages promote the metastasis of ovarian carcinoma cells by enhancing CXCL16/CXCR6 expression. Pathol Res Pract. 2018;214:1345–1351. doi: 10.1016/j.prp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Lee JS, Jie C, Park MH, Iwakura Y, Patel Y, et al. HER2 overexpression triggers an IL1alpha proinflammatory circuit to drive tumorigenesis and promote chemotherapy resistance. Cancer Res. 2018;78:2040–2051. doi: 10.1158/0008-5472.CAN-17-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao L, Summers W, Xiang S, Yuan L, Dauchy RT, Reynolds A, et al. Melatonin represses metastasis in HER2-postive human breast cancer cells by suppressing RSK2 expression. Mol Cancer Res : MCR. 2016;14:1159–1169. doi: 10.1158/1541-7786.MCR-16-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreira GM, Martinez M, Camargo IC, Domeniconi RF, Martinez FE, Chuffa LG. Melatonin attenuates her-2, p38 MAPK, p-AKT, and mTOR levels in ovarian carcinoma of ethanol-preferring rats. J Cancer. 2014;5:728–735. doi: 10.7150/jca.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian X, Guan W, Zhang L, Sun W, Zhou D, Lin Q, et al. Physical interaction of STAT1 isoforms with TGF-beta receptors leads to functional crosstalk between two signaling pathways in epithelial ovarian cancer. J Exp Clin Cancer Res. 2018;37:103. doi: 10.1186/s13046-018-0773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mansouri-Attia N, Tripurani SK, Gokul N, Piard H, Anderson ML, Eldin K, et al. TGFbeta signaling promotes juvenile granulosa cell tumorigenesis by suppressing apoptosis. Mol Endocrinol. 2014;28:1887–1898. doi: 10.1210/me.2014-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Komiyama S, Kurahashi T, Ishikawa M, Tanaka K, Komiyama M, Mikami M, et al. Expression of TGFss1 and its receptors is associated with biological features of ovarian cancer and sensitivity to paclitaxel/carboplatin. Oncol Rep. 2011;25:1131–1138. doi: 10.3892/or.2011.1151. [DOI] [PubMed] [Google Scholar]
- 79.Wang YR, Hong RT, Xie YY, Xu JM. Melatonin ameliorates liver fibrosis induced by carbon tetrachloride in rats via inhibiting TGF-beta1/Smad signaling pathway. Curr Med Sci. 2018;38:236–244. doi: 10.1007/s11596-018-1871-8. [DOI] [PubMed] [Google Scholar]
- 80.Shin NR, Park JW, Lee IC, Ko JW, Park SH, Kim JS, et al. Melatonin suppresses fibrotic responses induced by cigarette smoke via downregulation of TGF-beta1. Oncotarget. 2017;8:95692–95703. doi: 10.18632/oncotarget.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chuffa LG, Lupi Junior LA, Seiva FR, Martinez M, Domeniconi RF, Pinheiro PF, et al. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J Proteome Res. 2016;15:3872–3882. doi: 10.1021/acs.jproteome.6b00713. [DOI] [PubMed] [Google Scholar]
- 82.Mashreghi M, Azarpara H, Bazaz MR, Jafari A, Masoudifar A, Mirzaei H. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol. 2018;233:2949–2965. doi: 10.1002/jcp.26049. [DOI] [PubMed] [Google Scholar]
- 83.Goradel NH, Asghari MH, Moloudizargari M, Negahdari B, Haghi-Aminjan H, Abdollahi M. Melatonin as an angiogenesis inhibitor to combat cancer: mechanistic evidence. Toxicol Appl Pharmacol. 2017;335:56–63. doi: 10.1016/j.taap.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 84.Hashemi Goradel N, Ghiyami-Hour F, Jahangiri S, Negahdari B, Sahebkar A, Masoudifar A, et al. Nanoparticles as new tools for inhibition of cancer angiogenesis. J Cell Physiol. 2018;233:2902–2910. doi: 10.1002/jcp.26029. [DOI] [PubMed] [Google Scholar]
- 85.Zhu G, Lin Y, Liu H, Jiang D, Singh S, Li X, et al. Dll4-Notch1 signaling but not VEGF-a is essential for hyperoxia induced vessel regression in retina. Biochem Biophys Res Commun. 2018;507:400–406. doi: 10.1016/j.bbrc.2018.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barreta A, Sarian LO, Ferracini AC, Costa LBE, Mazzola PG, de Angelo AL, et al. Immunohistochemistry expression of targeted therapies biomarkers in ovarian clear cell and endometrioid carcinomas (type I) and endometriosis. Hum Pathol. 2018. 10.1016/j.humpath.2018.10.028. [DOI] [PubMed]
- 87.Xu J, Zheng T, Hong W, Ye H, Hu C, Zheng Y. Mechanism for the decision of ovarian surface epithelial stem cells to undergo neo-oogenesis or ovarian tumorigenesis. Cell Physiol Biochem. 2018;50:214–232. doi: 10.1159/000494001. [DOI] [PubMed] [Google Scholar]
- 88.Carbajo-Pescador S, Ordonez R, Benet M, Jover R, Garcia-Palomo A, Mauriz JL, et al. Inhibition of VEGF expression through blockade of Hif1alpha and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 2013;109:83–91. doi: 10.1038/bjc.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonzalez-Gonzalez A, Gonzalez A, Alonso-Gonzalez C, Menendez-Menendez J, Martinez-Campa C, Cos S. Complementary actions of melatonin on angiogenic factors, the angiopoietin/Tie2 axis and VEGF, in cocultures of human endothelial and breast cancer cells. Oncol Rep. 2018;39:433–441. doi: 10.3892/or.2017.6070. [DOI] [PubMed] [Google Scholar]
- 90.Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 92.Momtaz S, Niaz K, Maqbool F, Abdollahi M, Rastrelli L, Nabavi SM. STAT3 targeting by polyphenols: novel therapeutic strategy for melanoma. Biofactors. 2017;43:347–370. doi: 10.1002/biof.1345. [DOI] [PubMed] [Google Scholar]
- 93.Colombo J, Maciel JM, Ferreira LC, RF DAS, Zuccari DA. Effects of melatonin on HIF-1alpha and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol Lett 2016;12:231–237. [DOI] [PMC free article] [PubMed]
- 94.Park SY, Jang WJ, Yi EY, Jang JY, Jung Y, Jeong JW, et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res. 2010;48:178–184. doi: 10.1111/j.1600-079x.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Liu Q, Wang F, Ling EA, Liu S, Wang L, et al. Melatonin antagonizes hypoxia-mediated glioblastoma cell migration and invasion via inhibition of HIF-1alpha. J Pineal Res. 2013;55:121–130. doi: 10.1111/jpi.12052. [DOI] [PubMed] [Google Scholar]
- 96.Cutruzzola F, Giardina G, Marani M, Macone A, Paiardini A, Rinaldo S, et al. Glucose metabolism in the progression of prostate cancer. Front Physiol. 2017;8:97. doi: 10.3389/fphys.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rudlowski C, Moser M, Becker AJ, Rath W, Buttner R, Schroder W, et al. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology. 2004;66:404–410. doi: 10.1159/000079489. [DOI] [PubMed] [Google Scholar]
- 98.Lamkin DM, Spitz DR, Shahzad MM, Zimmerman B, Lenihan DJ, Degeest K, et al. Glucose as a prognostic factor in ovarian carcinoma. Cancer. 2009;115:1021–1027. doi: 10.1002/cncr.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, Miar A, Rodriguez-Garcia A, Tan DX, et al. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res. 2015;58:234–250. doi: 10.1111/jpi.12210. [DOI] [PubMed] [Google Scholar]
- 100.Dauchy RT, Hoffman AE, Wren-Dail MA, Hanifin JP, Warfield B, Brainard GC, et al. Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth. Comparative medicine. Comp Med. 2015;65:473–485. [PMC free article] [PubMed] [Google Scholar]
- 101.Bazwinsky-Wutschke I, Bieseke L, Muhlbauer E, Peschke E. Influence of melatonin receptor signalling on parameters involved in blood glucose regulation. J Pineal Res. 2014;56:82–96. doi: 10.1111/jpi.12100. [DOI] [PubMed] [Google Scholar]
- 102.Menendez-Menendez J, Martinez-Campa C. Melatonin: an anti-tumor agent in hormone-dependent cancers. Int J Endocrinol. 2018;2018:3271948. doi: 10.1155/2018/3271948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shabeeb D, Najafi M, Musa AE, Keshavarz M, Shirazi A, Hassanzadeh G, et al. Biochemical and histopathological evaluation of the radioprotective effects of melatonin against gamma ray-induced skin damage in rats. Curr Radiopharm. 2018. 10.2174/1874471012666181120163250. [DOI] [PubMed]
- 104.Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Najafi M, Sahebkar A. Melatonin and cancer: from the promotion of genomic stability to use in cancer treatment. J Cell Physiol. 2018. 10.1002/jcp.27391. [DOI] [PubMed]
- 105.Barberino RS, Menezes VG, Ribeiro A, Palheta RC, Jr, Jiang X, Smitz JEJ, et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod. 2017;96:1244–1255. doi: 10.1093/biolre/iox053. [DOI] [PubMed] [Google Scholar]
- 106.Jang H, Hong K, Choi Y. Melatonin and fertoprotective adjuvants: prevention against premature ovarian failure during chemotherapy. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed]
- 107.Futagami M, Sato S, Sakamoto T, Yokoyama Y, Saito Y. Effects of melatonin on the proliferation and cis-diamminedichloroplatinum (CDDP) sensitivity of cultured human ovarian cancer cells. Gynecol Oncol. 2001;82:544–549. doi: 10.1006/gyno.2001.6330. [DOI] [PubMed] [Google Scholar]
- 108.Akbarzadeh M, Nouri M, Banekohal MV, Cheraghi O, Tajalli H, Movassaghpour A, et al. Effects of combination of melatonin and laser irradiation on ovarian cancer cells and endothelial lineage viability. Lasers Med Sci. 2016;31:1565–1572. doi: 10.1007/s10103-016-2016-6. [DOI] [PubMed] [Google Scholar]
- 109.Kim JH, Jeong SJ, Kim B, Yun SM, Choi DY, Kim SH. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J Pineal Res. 2012;52:244–252. doi: 10.1111/j.1600-079X.2011.00935.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on direct request.


