Abstract
Background
The interplay of speed of activity of acaricidal products and tick-borne pathogen transmission time is the major driver for disease prevention. This study aimed to investigate the time required for transmission of Anaplasma phagocytophilum by adult Ixodes ricinus ticks in vivo on dogs, and to confirm the time required for transmission observed in vivo, in vitro.
Methods
Nymphs of I. ricinus were experimentally infected with an A. phagocytophilum strain of canine origin. Dogs were allocated to 6 groups of 3 dogs each. Groups 1–5 were infested with 50 A. phagocytophilum-infected female adult ticks on Day 0. Ticks were removed post-infestation at 3, 6, 12, 24 and 48 h. Dogs in Group 6 were infested with 60 A. phagocytophilum-infected female adult ticks (left on dogs until engorged). Dogs were observed daily for general health and clinically examined on Day 0, and weekly from Day 14. Blood was collected for qPCR and serological analysis on Day 0 (pre-challenge) and weekly thereafter. In the in vitro study each artificial feeding chamber was seeded with 10 adult ticks (5 male/5 female), attachment assessed, and blood pools sampled for qPCR at 6 h intervals up to 72 h after first tick attachment.
Results
Anaplasma phagocytophilum specific antibodies and DNA were detected in all 3 dogs in Group 6. No A. phagocytophilum-specific antibodies or DNA were detected in any dogs in Groups 1–5. All dogs remained healthy. Female tick attachment in 60 artificial feeding chambers over 72 h ranged between 20–60%. Anaplasma phagocytophilum DNA was detected in the blood collected from 5% of chambers sampled at 6 h, with the highest number of positive samples (16.3%) observed at 36 h.
Conclusions
Transmission of A. phagocytophilum by I. ricinus ticks starts within a few hours after attachment but establishment of infections in dogs is apparently dependent on a minimum inoculation dose that was only observed when ticks attached for greater than 48 h. These findings highlight the need for acaricidal products to exert a repellent and/or rapid killing effect on ticks to forestall transmission and subsequent disease.
Keywords: Anaplasma phagocytophilum, Ixodes ricinus, Transmission, In vivo, In vitro, Dogs
Background
Anaplasma phagocytophilum, transmitted by ixodid ticks, is regarded as an emerging pathogen of humans, horses and dogs worldwide. In dogs, this pathogen is the causative agent of canine granulocytic anaplasmosis, a disease with nonspecific clinical signs like lethargy and reduced activity, fever and inappetence most frequently observed [1–5]. Studies suggest that multiple strains of A. phagocytophilum may be circulating in wild and domestic animal populations, with differential host tropisms and pathogenicity and that co-infections with other tick-borne pathogens may occur, especially Borrelia burgdorferi [6]. Due to the health risks posed to dogs by tick-borne diseases, the importance of acaricidal products to protect against tick infestations and the pathogens they transmit is a growing concern worldwide. As such, various studies have been conducted evaluating the ability of acaricidal products to forestall transmission of tick-borne pathogens like Babesia spp., Ehrlichia canis, B. burgdorferi and A. phagocytophilum [7–12].
The protective ability of an acaricidal product to prevent transmission of a tick-borne pathogen can be explained by several properties of the acaricidal molecule: a repellent/irritant effect inhibiting tick infestation and attachment, a neuro-hormonal disruption of tick attachment and intake of the blood meal, and/or a quick speed of kill before transmission can occur [13]. Moreover, the relevance of these properties in preventing transmission is dependent on the speed of transmission of the specific pathogen by its tick vector. These transmission times are highly variable and can be slow as is the case for Babesia spp. because of the 36–48 h minimum duration of attachment and initial feeding required for sporogony, or fast as is the case for tick-transmitted bacteria like E. canis (within 3 h) or viruses like Powassan virus (within 15 min) [14–16]. Moreover, transmission times can also be shortened once a tick has taken a blood meal and feeding is interrupted, as demonstrated for male Dermacentor reticulatus infected with B. canis, shortening the attachment time needed for transmission from a minimum of 36–48 h to less than 8 h [17].
Knowledge of the speed at which a specific pathogen is transmitted by its tick vector is therefore imperative to determine the “grace” period within which a specific acaricidal product could be able to prevent transmission. Although the speed of transmission has been investigated for various tick-transmitted pathogens like B. canis and E. canis in dogs, very little information is available on the speed of transmission of A. phagocytophilum by Ixodes ricinus ticks [16, 17]. Various authors have cited that ticks must attach for 36–48 h for transmission of A. phagocytophilum to occur based on research done by Hodzic et al. [18] and Katavolos et al. [19]. Although both these studies provided valuable insights into the transmission dynamics of A. phagocytophilum, both were conducted using nymphal Ixodes scapularis ticks on mice and with a human Ehrlichia phagocytophila isolate, later reclassified as A. phagocytophilum [20]. Considering that A. phagocytophilum is regarded as an emerging pathogen of dogs worldwide, it is important to understand the transmission dynamics of this pathogen in dogs in more detail [6].
The aim of this study was to determine the time required for transmission of A. phagocytophilum by adult I. ricinus ticks in vivo on dogs, and to confirm the time required for transmission observed in vivo in an in vitro experiment using artificial feeding membranes.
Methods
Anaplasma phagocytophilum strain
The A. phagocytophilum strain used (“TIBA strain”) was isolated in June 2015 from a clinical case (dog) in Terschelling, the Netherlands. Amplification of the ank gene was performed as described by Massung et al. [21], followed by Sanger sequencing of the PCR product on both strands. The assembled sequence was subjected to BLAST analysis and 142 sequences from GenBank (having a > 99% coverage of the query sequence) were used in a multiple alignment using MAFFT, followed by Bayesian inference analysis (HKY85 substitution model; 2 heated chains with a chain length of 4,000,000; sampling frequency of 1000; 25% ‛burn-inʼ) using GU236882 as the outgroup.
Infection of Ixodes ricinus ticks with Anaplasma phagocytophilum
Ixodes ricinus nymphs were fed on a sheep infected with the “TIBA strain” of A. phagocytophilum described above. Sheep were confirmed infected using qPCR analysis of blood. Nymphs were left to feed until engorged, after which the detached fully engorged nymphs were collected and allowed to moult at 20 °C, 90% relative humidity (RH) and 16 h:8 h Light:Dark photoperiod.
The methodology described above was used to breed 3 infected tick batches; 2 tick batches were used for the in vivo study and 1 tick batch for the in vitro study. Successful infection of adult I. ricinus ticks was confirmed by qPCR on a sample of 50 ticks (25 male/25 female) taken from the each tick batch collected from the donor sheep.
Dog study design
The in vivo component of the study was conducted at Clinvet Morocco with 6 groups of 3 dogs each. At the time of enrolment all dogs were between 2–6 years of age, and weighed between 12–21 kg. All dogs were healthy based on clinical examination by a veterinarian and seronegative for A. phagocytophilum antibodies prior to inclusion in the study. The study dogs had not been treated with any acaricidal product for 12 weeks prior to the first tick challenge. Dogs were individually housed in indoor cages fitted with a sleeping bench, were fed a commercial dog food once daily and provided water ad libitum.
Tick infestation, attachment observations, counts and removal
To allow for an accurate assessment of tick attachment and removal, ticks were infested in chambers fitted to the skin of the dogs in Groups 1–5. In these groups, each dog was fitted with 2 feeding chambers (10 cm in diameter) on the lateral shoulder. The chambers were joined to the shaved skin of dogs using cyanoacrylate adhesive applied to the chambers immediately before placement. Pressure was applied for at least 30 s after joining the feeding chambers to the skin. Dogs were fitted with Elizabethan collars from the time of chamber attachment to the time of removal to minimize the risk of damaging or dislodging the chambers containing the ticks. At each assessment period, the chambers fitted to each dog and the site of fitment were examined for any abnormality. All chambers were removed from the dogs after completion of assessments using DMSO to dissolve the cyanoacrylate adhesive.
Each dog in Groups 1–5 was infested with 50 female ticks (25 ticks per chamber) with a confirmed infectivity of 37%, whilst dogs in Group 6 received a full body infestation with 60 female I. ricinus ticks with a confirmed infectivity of 21%.
At 3 h after tick infestation, all non-attached ticks were removed from each feeding chamber and counted. At 3, 6, 12, 24 and 48 h, all remaining ticks were removed, sexed, counted, categorized based on attachments status and viability for Groups 1–5, respectively. All attached female ticks were assessed by qPCR for A. phagocytophilum DNA to confirm infectivity. Male ticks were discarded. Ticks infested on dogs in Group 6 were allowed to feed until engorged and all engorged detached ticks were collected from the cage environment.
Monitoring of dogs for general health and Anaplasma phagocytophilum infection
All dogs were observed daily for general health and clinically examined by a veterinarian on Day 0, and weekly from Day 14 up to study completion. Clinical examinations included general appearance by body system, respiration rate, heart rate and body temperature. Particular attention was given to the most common clinical manifestations of anaplasmosis, which included lethargy and reduced activity, fever and inappetence. Rectal body temperatures were recorded daily from Day 5 to study completion (Day 63 for Groups 1–5 and Day 42 for Group 6). Group 6 terminated on Day 42 as all dogs had already presented with 2 positive serology results by this day. At least 3.5 ml of blood was collected in EDTA tubes for qPCR and serological analysis on Day 0 (prior to tick challenge) and weekly thereafter until study completion.
Laboratory assays
Blood collected from dogs (200 µl) was directly subjected to genomic DNA isolation using the NucleoMag Vet kit (Macherey-Nagel, Dűren, Germany) using a KingFisher Flex 96 instrument (Thermo Fisher Scientific, Waltham, USA). The DNA isolation procedure was modified to include a post-lysis RNase A treatment (10 µl of 20 mg/ml RNase A per sample) for 30 min at room temperature. DNA was recovered using 100 µl of elution buffer and quantified spectrophotometrically and assessed using agarose gel electrophoresis. A total of 2 µl of DNA served as template for subsequence qPCR detection. Anaplasma phagocytophilum-specific qPCR primers and probe targeting the MSP2 region were used to detect the presence of A. phagocytophilum DNA in the extract [22]. Anaplasma phagocytophilum MSP2 quantification was performed for dogs in Group 6 (A. phagocytophilum infected female adult ticks were left on the dogs until engorged). SsoAdvancedTM Universal Probes Supermix (Bio-Rad, Hercules, USA) was used in a 20 µl reaction volume containing 300 nM each primer and 200 nM probe, followed by thermal cycling at 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Control reactions included positive, negative, extraction and no template controls, as well as an internal amplification control to limit false negative results.
Tick infectivity was determined by homogenizing individual ticks using high density zirconium oxide beads, followed by genomic DNA isolation and qPCR detection as described above.
For serology, 3 drops of whole blood were transferred to a micro tube for the detection of antibodies to A. phagocytophilum using a SNAP® 4Dx® Plus test (IDEXX Laboratories Inc., Westbrook, ME, USA). The samples were processed according to the manufacturer’s instructions.
In vitro study
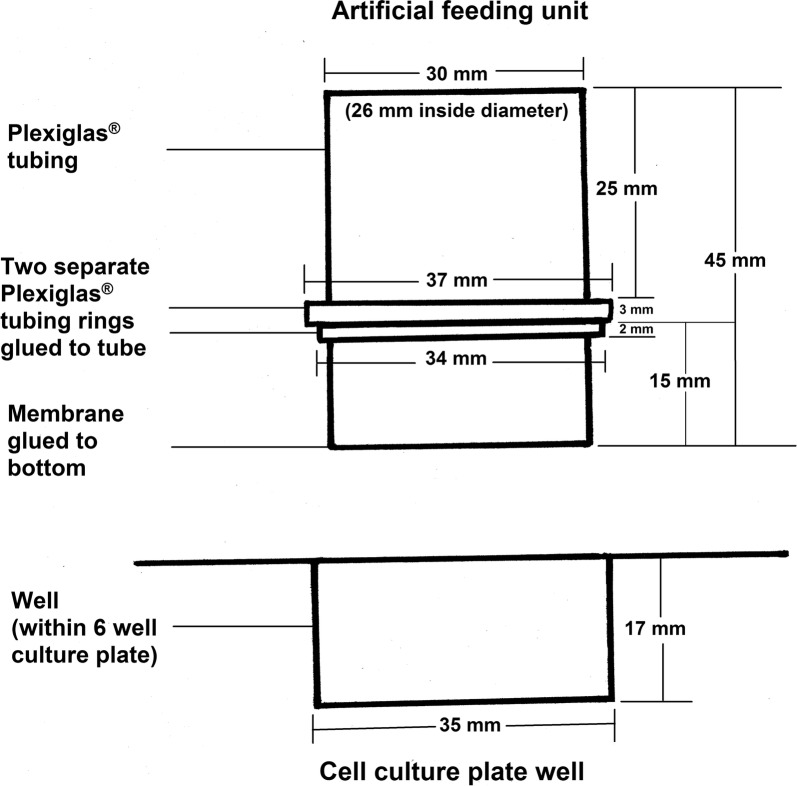
A total of 60 membrane feeding units in 6-well culture plates (35 mm diameter) containing bovine blood were used. The feeding chamber units, prepared according to Kröber & Guerin [23] were made of Plexiglas® tubing (26 mm inside diameter, 2 mm wall thickness, 45 mm high; see Fig. 1).
Fig. 1.
Schematic diagram of the feeding chambers used (as per Kröber & Guerin [23])
These units were designed to fit into the wells of the 6-well culture plates so that the bottom of the feeding unit, which was covered by an artificial feeding membrane, was slightly elevated above the bottom of the plate. This allowed for the entire area of the feeding membrane to be covered with blood upon insertion into the well (Fig. 2). The artificial membrane was prepared as described in Fourie et al. [16].
Fig. 2.

Example of feeding units in 6-well plates containing bovine blood in an incubator. Note the net covered stopper to preclude tick escape
Cattle blood (from 3 donor cattle) was collected into Fenwal Blood Collection Bags [containing 2.45 g dextrose (monohydrate), 2.2 g sodium citrate (dihydrate) and 730 mg citric acid (anhydrous) per 100 ml of blood] on the day of tick seeding. Blood was stored at 4 °C until used for replacement of blood pools. Commercial gentamycin (5 µg/ml) and ATP (10 µm in the blood) were added to the blood just before it was filled into the wells. Approximately 3 ml of blood was required for each well. Prior to blood replacement, the required volume of blood, as well as saline used during the exchange process, was heated to approximately 37 °C. The chambers were kept in an incubator with a light/dark cycle of 18 h light:6 h dark. A thin layer of bovine hair, cut into pieces of approximately 4–7 mm was used to cover the membrane. A laboratory-bred strain of I. ricinus infected with A. phagocytophilum (predetermined A. phagocytophilum infectivity of 60%) was used to seed the chambers (see Fig. 3).
Fig. 3.
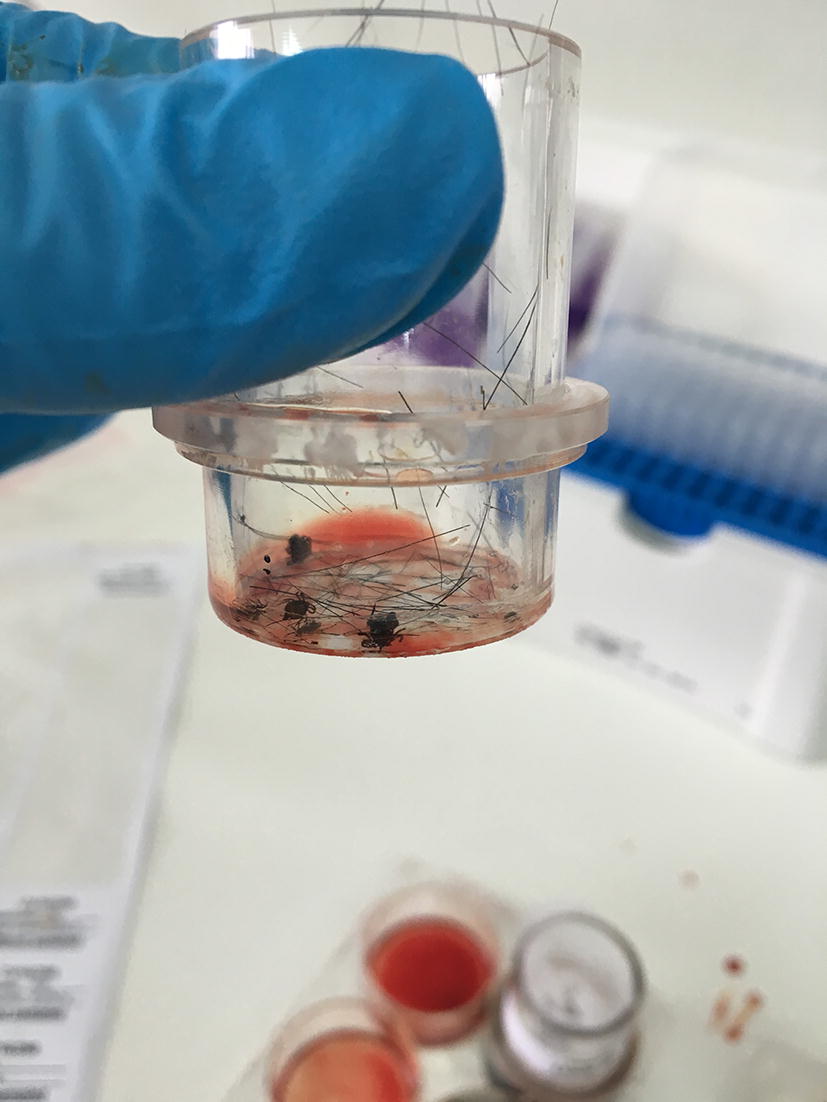
Ticks on the artificial membrane within the feeding unit after removal from the 6-well plate containing bovine blood (visible in the background). Also note the bovine hair clippings in the feeding unit
Feeding units were seeded with 10 ticks (5 males/5 females) and a stopper covered with the netting was placed above the ticks to prevent escape. Once ticks had been added to the feeding units, each unit was placed into a well containing blood (warmed to 37 °C), ensuring no air bubbles were present. Blood was replaced at least every 18 h or every 6 h once tick attachment was observed. This was done by adding fresh blood to a clean culture plate and by moving the feeding units to the clean plate. The membrane surface facing the blood was rinsed with warm sterile saline (37 °C) before placing the feeding unit into the fresh well. An image of the membrane, with a tick attached, is shown in Fig. 4.
Fig. 4.

A tick hypostome as viewed from beneath the artificial membrane of the feeding unit after removal from a culture plate well containing cattle blood warmed to 37 °C
Tick attachment observations were conducted on all chambers every 6 h. At the first time point where attachment was observed, all unattached female ticks were removed, the blood was sampled, the chamber placed into a fresh blood pool and the time recorded. The time at which the first tick attachment was observed, was considered the 6 h time-point.
At every subsequent assessment, the number of attached female ticks was recorded and detached female ticks were collected and stored in 70% ethanol. The blood pool was sampled (to allow analysis by qPCR for the presence of A. phagocytophilum DNA) and the tick chamber transferred to fresh blood pool. Assessments continued for a period of up to 72 h after first attachment, or until no further ticks were attached. After the final assessments, all ticks were removed and stored in 70% ethanol.
qPCR analysis of the blood from the feeding chambers
Assessment of transmission of A. phagocytophilum to the blood in the feeding chambers required an alternative approach to ensure that each qPCR assessment contained significantly more of the target when compared to the traditional approach. For qPCR analysis on the blood collected from the artificial feeding chambers, frozen whole blood (up to 3 ml) was thawed and subjected to centrifugation for 10 min at 20,000 rcf at room temperature and the supernatant discarded. The pellet was resuspended in 1 ml 5% (w/v) ox bile ([24]; Sigma-Aldrich, St. Louis, USA) and incubated at room temperature for 10 min followed by centrifugation at 20,000 rcf for 10 min at room temperature. The supernatant was discarded, and the pellet again resuspended in 1 ml 5% (w/v) ox bile (Sigma-Aldrich) and incubated at room temperature for 10 min followed by centrifugation at 20,000 rcf for 10 min at room temperature. The supernatant was discarded and the pellet was resuspended in 200 µl PBS (Invitrogen, Carlsbad, USA) and subjected to genomic DNA isolation and qPCR detection as described above.
Statistical analyses
Successful transmission of A. phagocytophilum by ticks to dogs was based on the detection of A. phagocytophilum-specific antibodies or DNA in dogs. Successful transmission of A. phagocytophilum by ticks feeding on artificial membranes was based on the detection of A. phagocytophilum DNA in the blood pools used for feeding. The first time point at which A. phagocytophilum was successfully detected was considered the minimum time required for the transmission of this bacterium by infected I. ricinus ticks in vivo and in vitro. No formal statistical analysis was conducted and the results are given descriptively.
Results
Anaplasma phagocytophilum strain
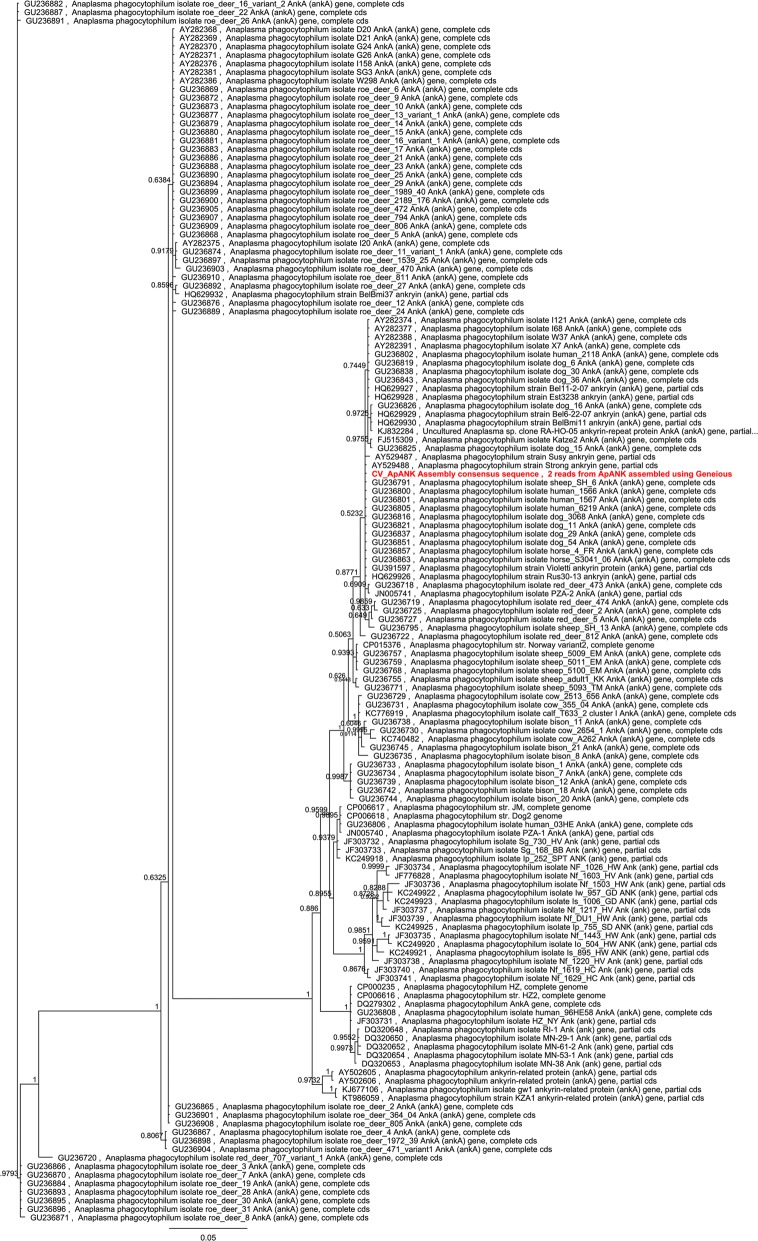
Amplification of the ank gene and sequencing of the PCR product revealed that this specific strain is closely related to other strains isolated from humans (USA and Slovenia), dogs, sheep and horses (Europe) based on the phylogenetic tree (Fig. 5). All these strains belong to the ank gene cluster I group [25].
Fig. 5.
Phylogenetic tree based on amplification of the ank gene and sequencing of the PCR product
Dog study
All dogs included in the study were judged as clinically healthy by a veterinarian and were seronegative for A. phagocytophilum-specific antibodies. The mean number of attached female ticks in the infestation chambers (2 chambers per dog in Groups 1–5) ranged between 28.7–37.7 per dog. The arithmetic mean number of attached female ticks for Group 6 was 47.7 (see Table 1).
Table 1.
Arithmetic mean number of female Ixodes ricinus ticks collected from the 6 study groups at the specific target times after infestation (Groups 1–5) or when fed until engorged (Group 6)
| Hours post-infestation | Group | Mean no. of ticks |
|---|---|---|
| 3 | 1 | 37.7 |
| 6 | 2 | 32.0 |
| 12 | 3 | 31.7 |
| 24 | 4 | 28.7 |
| 48 | 5 | 32.3 |
| Females allowed to feed until engorged | 6 | 47.7 |
Exposure of dogs to infected ticks was confirmed by conducting qPCR on pools of DNA extracted from attached female ticks removed from each dog. Each pool consisted of up to 5 ticks. The percentage of pooled DNA testing positive for A. phagocytophilum DNA ranged between 44.44–100%, confirming all dogs were exposed to infected ticks. No A. phagocytophilum-specific antibodies or DNA could be detected in any of the dogs in Groups 1–5. Anaplasma phagocytophilum-specific antibodies and DNA were detected in all 3 dogs in Group 6. In these dogs, A. phagocytophilum DNA was first detected in blood samples collected 7 days post-tick infestation in the first dog, 14 days post-infestation in the second dog and 21 days post-infestation in the third dog. All subsequent blood samples tested for these 3 dogs remained positive for A. phagocytophilum DNA. Seroconversion was first observed in 1 dog in Group 6, 28 days post-tick infestation and in the other two dogs 35 days post-infestation (see Table 2).
Table 2.
Detection of Anaplasma phagocytophilum DNA and antibodies in blood samples taken from dogs in Groups 1–6 prior to tick infestation (Day 0) and weekly thereafter up to 63 days post-tick infestation
| Group | Dog ID | Day (PCR/ SNAP® 4Dx® Plus) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | 63 | ||
| 1 | 216, 83, 184 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 2 | 121, 230, 513 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 3 | 863, 222, 075 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 4 | 833, 376, 851 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 5 | 895, 206, 226 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 6 | 495 | −/− | −/− | −/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ |
| 835 | −/− | +/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| 859 | −/− | −/− | +/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ | |
Key: −, no DNA or antibodies detected; +, DNA or antibodies detected
In Group 6, where A. phagocytophilum infected female adult ticks were left on the dogs until engorged, A. phagocytophilum MSP2 copy numbers detected by qPCR increased over time (Days 0 to 28 for 1 dog and Days 0 to 28 for the remaining 2 dogs; see Table 3).
Table 3.
Relative copy number of the MSP2 target in blood collected from dogs in Group 6 (ticks fed on dogs until engorged)
| Group 6 Dog ID | Relative copy number of the MSP2 target on: | ||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
| 495 | 0 | 8 | 0 | 304 | 719 | 274 | 130 |
| 835 | 0 | 15 | 5588 | 5135 | 1263 | 431 | 2108 |
| 859 | 0 | 0 | 804 | 3222 | 234 | 74 | 115 |
The body temperatures for all dogs ranged between 36.5–39.4 °C, which was within the range considered normal for dogs. No clinical symptoms associated with acute canine granulocytic anaplasmosis were observed in any of the dogs.
In vitro study
Tick attachment in the 60 chambers over the 72 h ranged between 20–60% (i.e. 1–3 female ticks attached in each chamber) with all 60 chambers having at least 1 attached female tick. The speed at which at least one tick attached in each chamber ranged from 6–18 h post-seeding with attachment observed in 56.7% of chambers at 6 h. By 72 h after observing the first attachment, 66.7% of the chambers still contained at least 1 attached tick. Anaplasma phagocytophilum DNA was detected in the blood collected from 3 (5%) out of 60 chambers at 6 h (defined as the time point where the first attached tick was observed) with the highest number of positive samples (8 out of 49; 16.3%) in chambers with ticks still attached at 36 h (Fig. 6).
Fig. 6.
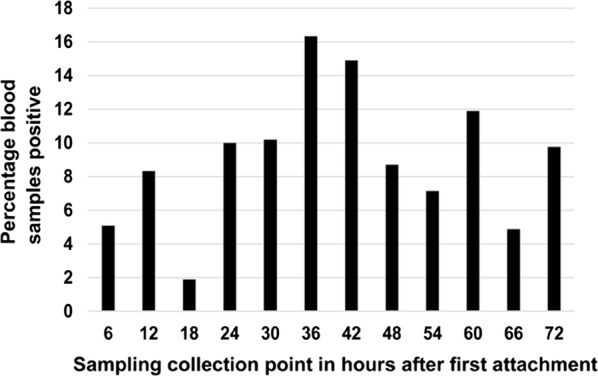
Percentage of blood samples in which Anaplasma phagocytophilum DNA was detected (out of total number tested) at each of the respective time points during the in vitro experiment
Detection of A. phagocytophilum in blood samples collected and replaced every 6 h from individual chambers were intermittent, with no consistent detection of DNA being observed from feeding units with ticks attached for longer than 18 h after first attachment.
Discussion
The experimental infection of sheep with an A. phagocytophilum strain (“TIBA strain”) isolated from a clinical case (dog) enabled the successful infection of multiple tick batches by feeding I. ricinus nymphs until repletion on a bacteremic host. The experimental infection of ticks yielded an infectivity between 21–60% in different batches and was sufficient to demonstrate transmission of A. phagocytophilum bacterium in vivo in dogs and in vitro using an artificial feeding system. Furthermore, the temporal increase in copy number of the A. phagocytophilum MSP2 target in DNA isolated from whole blood obtained from dogs in Group 6 demonstrates that A. phagocytophilum was able to multiply in blood over time and that the bacteria were thus alive. This further validates the success of the model employed. Transmission of A. phagocytophilum bacterium was only detected based on qPCR and specific antibody assay (SNAP® 4Dx® Plus test) in dogs that infected ticks had fed on until engorged. No infection was detected in dogs when ticks were removed from 3 up to 48 h post-infestation. In contrast, A. phagocytophilum DNA was observed as early as 6 h post-feeding in the blood pools that infected ticks had fed on in vitro. Using an ox bile pretreatment of the blood from the feeding chambers, resulted in the reduction host DNA contamination of the isolated DNA yielding a 15-fold increase in effective blood volume that could be assessed during qPCR and an increased detection sensitivity compared to the untreated methodology recommended by the nucleic acid isolation kit manufacturer. Taking this, as well as the > 100 copies of the MSP2 qPCR targets present per A. phagocytophilum genome into account, this extremely sensitive approach enabled detection of A. phagocytophilum DNA present in the blood pools [26]. The seemingly contradictory results based on qPCR observed in vivo in dogs and in vitro using artificial feeding units could potentially be attributed to the increased sensitivity of the assay used to assess the blood pools from the in vitro test. Moreover, transmission of A. phagocytophilum by ticks has been demonstrated in vitro to take place soon after attachment. As a result, establishment and detection of an infection in dogs might be dependent on a minimum A. phagocytophilum bacteria dose inoculated to achieve infection and consequent detection on the multiplication of A. phagocytophilum bacteria in the host, until the detection threshold for qPCR is reached. In the present study, no seroconversion was observed in dogs challenged with infected ticks when removed within 48 h after infestation, although transmission of A. phagocytophilum bacteria should have occurred. Hodzic et al. [18] demonstrated that, although they could not accurately determine the tick-borne infectious dose, infection with A. phagocytophilum is dose-dependent and that relatively high doses of organisms appear to be needed to infect a mouse. This is also the case for other related organisms such as Ehrlichia risticii, E. canis, Rickettsia australis and Rickettsia conorii where dosage studies have demonstrated that the innate defense mechanisms of hosts can protect against or eliminate low dose inoculation and it is only at higher doses that infection and disease is established [27, 28]. Moreover, it has also been shown that replication of A. phagocytophilum bacteria occurred within feeding ticks, also increasing the effectiveness of transmission and ultimately the speed at which the minimum inoculation dose needed for infection in the host is reached [18]. Considering the results of the present study, as well as the dosage studies done on other hosts like mice, infection with A. phagocytophilum in dogs seems also to be dose-dependent and relatively high doses of organisms appear to be needed for the establishment of infection. More research is, however, needed to determine the minimum infective dose for A. phagocytophilum in dogs.
Conclusions
Transmission of A. phagocytophilum by I. ricinus ticks starts within a few hours after attachment, but establishment of infections in dogs is apparently dependent on a minimum inoculation dose that was only observed in the present study when ticks attached for greater than 48 h. These findings highlight the need for acaricidal products to exert a repellent and/or rapid killing effect on ticks to forestall or interrupt transmission of A. phagocytophilum and ultimately prevent clinical infection and disease in dogs.
Acknowledgements
The authors would like to thank the research team (research and technical personnel) at Clinvet Morocco for their assistance in conducting this study. Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th CVBD Word Forum Symposium.
Funding
The study was funded by Bayer Animal Health (sponsor company). The study itself was performed by Clinvet.
Availability of data and materials
Due to confidentiality agreements study documentation is not freely available, but will be stored in the archives of the sponsor (Bayer Animal Health) and contract research organization (CRO, Clinvet) as per respective standard operating procedures (SOPs) in place.
Authors’ contributions
JF and DC compiled the first draft manuscript. JF also acted as technical advisor for this study. AE performed the study and reviewed the manuscript, whilst BS and MP acted as sponsor representatives and reviewed the manuscript. ML was responsible for the design and conduct of the PCR assays. All authors provided input on draft versions to produce the final manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Approval from the Institutional Animal Care and Use Committee (IACUC) was received for conduct of the study. Upon approval, a certificate of approval was issued. Members of the IACUC had the authority to inspect the study site and the animals at will. The animals were purpose-bred and belonged to Clinvet International (Pty) Ltd. The husbandry and accommodation of the dogs was in compliance with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Consent for publication
Not applicable.
Competing interests
JF and AE are employed by Clinvet and DC, ML and MM by Clinglobal, the organizations that performed the study for Bayer Animal Health (sponsor company who funded the study). MP and BS are employed by Bayer Animal Health GmbH.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- DMSO
dimethyl sulfoxide
- DNA
deoxyribonucleic acid
- EDTA
ethylenediamine tetraacetic acid
- ID
identification
- PCR
polymerase chain reaction
Contributor Information
Josephus J. Fourie, Email: josephus.fourie@clinvet.com
Alec Evans, Email: alec.evans@clinvet.com.
Michel Labuschagne, Email: michel.labuschagne@clinglobal.com.
Dionne Crafford, Email: dionne.crafford@clinglobal.com.
Maxime Madder, Email: maxime.madder@clinglobal.com.
Matthias Pollmeier, Email: matthias.pollmeier@bayer.com.
Bettina Schunack, Email: bettina.schunack@bayer.com.
References
- 1.Greig B, Asanovich KM, Armstrong PJ, Dumler JS. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla SK. Genetic variants of Anaplasma phagocytophilum infecting dogs in western Washington State. J Clin Microbiol. 2005;43:796–801. doi: 10.1128/JCM.43.2.796-801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic ehrlichiosis in 18 naturally infected dogs. J Vet Intern Med. 2008;22:1289–1295. doi: 10.1111/j.1939-1676.2008.0180.x. [DOI] [PubMed] [Google Scholar]
- 4.Johansson KE, Pettersson B, Uhlén M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horse by direct solid phase sequencing of PCR products. Res Vet Sci. 1995;58:109–112. doi: 10.1016/0034-5288(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 5.Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59:112–120. doi: 10.1111/jsap.12787. [DOI] [PubMed] [Google Scholar]
- 6.Carrade DD, Foley JE, Borjesson DL, Sykes JE. Canine granulocytic anaplasmosis: a review. J Vet Intern Med. 2009;23:1129–1141. doi: 10.1111/j.1939-1676.2009.0384.x. [DOI] [PubMed] [Google Scholar]
- 7.McCall JW, Baker CF, Mather TN, Chester ST, McCall SD, Irwin JP, et al. The ability of a topical novel combination of fipronil, amitraz and (S)-methoprene to protect dogs from Borrelia burgdorferi and Anaplasma phagocytophilum infections transmitted by Ixodes scapularis. Vet Parasitol. 2011;179:335–342. doi: 10.1016/j.vetpar.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Honsberger NA, Six RH, Heinz TJ, Weber A, Mahabir SP, Berg TC. Efficacy of sarolaner in the prevention of Borrelia burgdorferi and Anaplasma phagocytophilum transmission from infected Ixodes scapularis to dogs. Vet Parasitol. 2016;222:67–72. doi: 10.1016/j.vetpar.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Taenzler J, Liebenberg J, Roepke RK, Heckeroth AR. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs after topical administration of fluralaner spot-on solution. Parasit Vectors. 2016;9:234. doi: 10.1186/s13071-016-1481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro C, Reymond N, Fourie J, Hellmann K, Bonneau S. Prevention of Babesia canis in dogs: efficacy of a fixed combination of permethrin and fipronil (Effitix®) using an experimental transmission blocking model with infected Dermacentor reticulatus ticks. Parasit Vectors. 2015;8:32. doi: 10.1186/s13071-015-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jongejan F, de Vos C, Fourie JJ, Beugnet F. A novel combination of fipronil and permethrin (Frontline Tri-Act®/Frontect®) reduces risk of transmission of Babesia canis by Dermacentor reticulatus and of Ehrlichia canis by Rhipicephalus sanguineus ticks to dogs. Parasit Vectors. 2015;8:602. doi: 10.1186/s13071-015-1207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jongejan F, Crafford D, Erasmus H, Fourie JJ, Schunack B. Comparative efficacy of oral administrated afoxolaner (NexGard™) and fluralaner (Bravecto™) with topically applied permethrin/imidacloprid (Advantix®) against transmission of Ehrlichia canis by infected Rhipicephalus sanguineus ticks to dogs. Parasit Vectors. 2016;9:348. doi: 10.1186/s13071-016-1636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halos L, Baneth G, Beugnet F, Bowman AS, Chomel B, Farkas R, et al. Defining the concept of “tick repellency” in veterinary medicine. Parasitology. 2012;139:419–423. doi: 10.1017/S0031182011002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piesman J, Spielman A. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29:742–746. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- 15.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71:268–271. doi: 10.4269/ajtmh.2004.71.3.0700268. [DOI] [PubMed] [Google Scholar]
- 16.Fourie JJ, Stanneck D, Luus HG, Beugnet F, Wijnveld M, Jongejan F. Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes. Vet Parasitol. 2013;197:595–603. doi: 10.1016/j.vetpar.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Varloud M, Liebenberg J, Fourie J. Early Babesia canis transmission in dogs within 24 h and 8 h of infestation with infected pre-activated male Dermacentor reticulatus ticks. Parasit Vectors. 2018;11:41. doi: 10.1186/s13071-018-2637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodzic E, Fish D, Maretzki CM, De Silva AM, Feng S, Barthold SW. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol. 1998;36:3574–3578. doi: 10.1128/jcm.36.12.3574-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katavolos P, Armstrong PM, Dawson JE, Telford SR., 3rd Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis. 1998;177:1422–1425. doi: 10.1086/517829. [DOI] [PubMed] [Google Scholar]
- 20.Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 21.Massung RF, Levin ML, Munderloh UG, Silverman DJ, Lynch MJ, Gaywee JK, et al. Isolation and propagation of the Ap-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J Clin Microbiol. 2007;45:2138–2143. doi: 10.1128/JCM.00478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex Real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–3168. doi: 10.1128/JCM.42.7.3164-3168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kröber T, Guerin PM. In vitro feeding assays for hard ticks. Trends Parasitol. 2007;23:445–449. doi: 10.1016/j.pt.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Pollard AJ. A novel method of selective removal of human DNA improves PCR sensitivity for detection of Salmonella typhi in blood samples. Inf Dis. 2012;12:164. doi: 10.1186/1471-2334-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharf W, Schauer S, Freyburger F, Petrovec M, Schaarschmidt-Kiener D, Liebisch G, et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J Clin Microbiol. 2011;49:790–796. doi: 10.1128/JCM.02051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dugat T, Lagrée A, Maillard R, Boulouis H-J, Haddad N. Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives. Front Cell Infect Microbiol. 2015;5:61. doi: 10.3389/fcimb.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng HM, Wen J, Walker DH. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol. 1993;142:1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 28.Gaunt SD, Corstvet RE, Berry CM, Brennan B. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J Clin Microbiol. 1996;34:1429–1432. doi: 10.1128/jcm.34.6.1429-1432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to confidentiality agreements study documentation is not freely available, but will be stored in the archives of the sponsor (Bayer Animal Health) and contract research organization (CRO, Clinvet) as per respective standard operating procedures (SOPs) in place.