Abstract
Background
Human melanoma is a malignant tumor originated from melanocytes with high invasion, metastasis, and poor prognosis. In this study, the effects of naphthalimides UNBS5162 and amonafide on the properties of proliferation and apoptosis in human melanoma cells were confirmed.
Methods
Cell proliferation was determined by CCK8 and clone formation assay. Transwell assay was performed to detect the migration and invasion of M14 and A375 cells. Cell apoptosis was estimated using flow cytometry.
Results
In a drug sensitivity assay, cell viability decreased with increasing concentrations of UNBS5162 or amonafide. Likewise, proliferation of M14 or A375 cells treated with 10 μM UNBS5162 or 8 μM amonafide decreased significantly when compared with negative control (NC) cells, their inhibition effect verified by means of a clone formation assay. After the treatment with UNBS5162 or amonafide, the migration of melanoma cells was inhibited in a dosede-pendent manner. The number of invaded cells treated with UNBS5162 was also significantly reduced when compared with those of the NC cells. The apoptotic cell numbers treated with UNBS5162 or amonafide decreased significantly when compared with the M14 and A375 cells in the NC group. According to Western blot results, phosphorylation of AKT and expressions of mesenchymal marker factors were inhibited in cells treated with UNBS5162 or amonafide.
Conclusion
These results reveal that UNBS5162 inhibits the cell activity of melanoma cells through the AKT/mTOR signaling pathway, and reverses epithelial–mesenchymal transition conversion in human melanoma cells. This study on UNBS5162 and amonafide in melanomas provides an experimental basis of their uses and potential value on human melanoma treatment.
Keywords: cell apoptosis, proliferation, M14, A375, epithelial-mesenchymal transition
Introduction
Human melanomas are malignant tumors that originated from melanocytes with high invasion, metastasis, and poor prognosis. Melanomas accounts for about 1%–2% of human malignant tumors, with less than a 90% 5-year survival rate.1 At initial diagnosis, about 20% of patients with melanomas have tumor metastasis. Unfortunately, there is still a deficiency of effective drugs for melanoma, thus new anti-neoplastic drugs that have fewer side effects are needed.2,3 Therefore, our study has aimed to investigate the inhibitory effect of UNBS5162 and amonafide on human melanoma.
As DNA intercalating agents, naphthalimides have high potential as anti-neoplastic agents. Currently, naphthalimides have been evaluated clinically; this includes mitonafide, Elinafide, amonafide, bisnafide, and UNBS5162.4,5 According to present research, UNBS5162 and amonafide are promising antitumor agents endowed with fewer side effects and a higher efficiency of cancer suppression.6–8 However, the clinical efficacy of UNBS5162 and amonafide for human melanoma remains unclear. In this study, we investigated the effects of UNBS5162 and amonafide on the properties of proliferation and apoptosis in humans. Our purpose is to determine the functionary mechanism of UNBS5162 and amonafide in human melanomas in vitro.
Materials and methods
Cell lines
The human melanoma cell lines M14 (BRAFV600E positive) and A375 (BRAFV600E positive) were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Cells were cultured in DMEM containing 10% FBS, streptomycin (100 units/mL), and penicillin (100 units/mL; Invitrogen Corp., Waltham, MA, USA).
Compounds and antibodies
UNBS5162, amonafide, and 5FU were purchased from the American MedChemExpress, Monmouth Junction, NJ, USA. Further, reference antibodies Bcl-2 (proteintech, Rosemont, IL, USA; Cat# 12789–1-AP), active-Caspase3 (Abcam, Cam-bridge, UK; Cat# ab32042), Bax (proteintech, Cat# 50599–2-Ig), P70/S6K (Abcam, Cat# ab109393), AKT (Abcam, Cat# ab8805), p-AKT (Abcam, Cat# ab38449), mTOR (Abcam, Cat# ab32028), p-mTOR (Abcam, Cat# ab109268), Cyclin D1 (proteintech, Cat# 12363–1-AP), E-cad (Abcam, Cat# ab40772), Snail (proteintech, Cat# 13099–1-AP), N-cad (Abcam, Cat# ab6528), Twist (proteintech, Cat# 11752–1-AP), Slug (proteintech, Cat#) and GAPDH (proteintech, Cat# 60004–1-Ig) were used in this study. Our electrochemiluminescence kit was obtained from proteintech.
Drug sensitivity assay
The cells were seeded in 96-well plates at 1,000 cells/well. Nineteen gradients of UNBS5162 were prepared, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 60, 80, 100, 150, and 200 μM. Nineteen gradients of amonafide were likewise prepared: 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 100, and 150 μM. After cells were treated with UNBS5162 or amonafide for 24 hours, they were cultured with cell-counting kit-8 (CCK8) reagent (Solarbio Science & Technology Company, Beijing, China) for 2 hours, and the OD value of each well was read at 450 nm. Three duplicates were used for each determination.
Cell proliferation assay
A CCK8 assay was performed to detect the proliferation of melanoma cells. Cells of UNBS5162 group were treated with 10 μM UNBS5162, amonafide cells were treated with 8 μM amonafide, and negative control (NC) cells were treated with 0.1% DMSO. A total of 1,000 cells per well were seeded in a 96-well plate. Cellular activity was measured every 24 hours, adding 10 μL CCK8 reagent per well and reading the absorbance at a wavelength of 450 nm. Cell proliferation was assessed relative to the NC group, and SD was assessed from triplicates.
Clone formation assay
Melanoma cells were treated with UNBS5162 or amonafide for 24 hours. Cells were then collected and seeded on a 6-cm dish at a total number of 200–300. Cells were continually cultured until clones could be observed by the naked eye. After being stained with 0.1% crystal violet for 20 minutes and washed with PBS twice, HCC cells were imaged and counted.
Transwell assay
Cell migration and invasion were confirmed using transwell chambers (Millipore, Billerica, MA, USA), which was performed according to the manufacturer’s protocol. Cells were seeded into the upper chamber of a 24-well invasion assay containing matrigel (BD Biosciences, San Jose, CA, USA). Then, DMEM medium with 10% FBS was added into the lower chamber. After 48 hours incubation, cells that migrated to the lower surface of the chamber were stained with 0.1% crystal violet. Experiments were performed in triplicate.
Western blotting
Total proteins were extracted from M14 or A375 cells by a RIPA buffer containing 0.1% proteinase inhibitor. Proteins were separated on 7% or 10% SDS-PAGE and were transferred to a PVDF membrane. Primary antibodies were used at 1:1,000 dilutions, and secondary antibodies were used at 1:5,000 dilutions at 1 hour incubation. Enhanced chemiluminescent (ECL) developer was ordered from proteintech. Experiments were performed in triplicate.
Flow cytometry analysis
Cells were seeded into a 6 cm plate and treated with 10 μM UNBS5162 or 8 μM amonafide, being incubated for 48 hours. Annexin V-FITC/PI was added into the cell suspension, and incubated for 5 minutes in the dark. After staining with 10 μl dye liquor, the samples were analyzed by a flow cytometer (BD FACSC anto II, BD Biosciences). Apoptosis was analyzed using Flowjo software.
Statistical analyses
Results were expressed as the means ± SDs. Relative densitometry values were calculated considering GAPDH as an internal control using ImageJ software and were plotted in GraphPad Prism 7. A t-test was performed in order to analyze the changes between each group. All results were tested in three independent experiments. P<0.05 was considered as significant.
Results
UNBS5162 and amonafide inhibits the proliferation of human melanoma cells
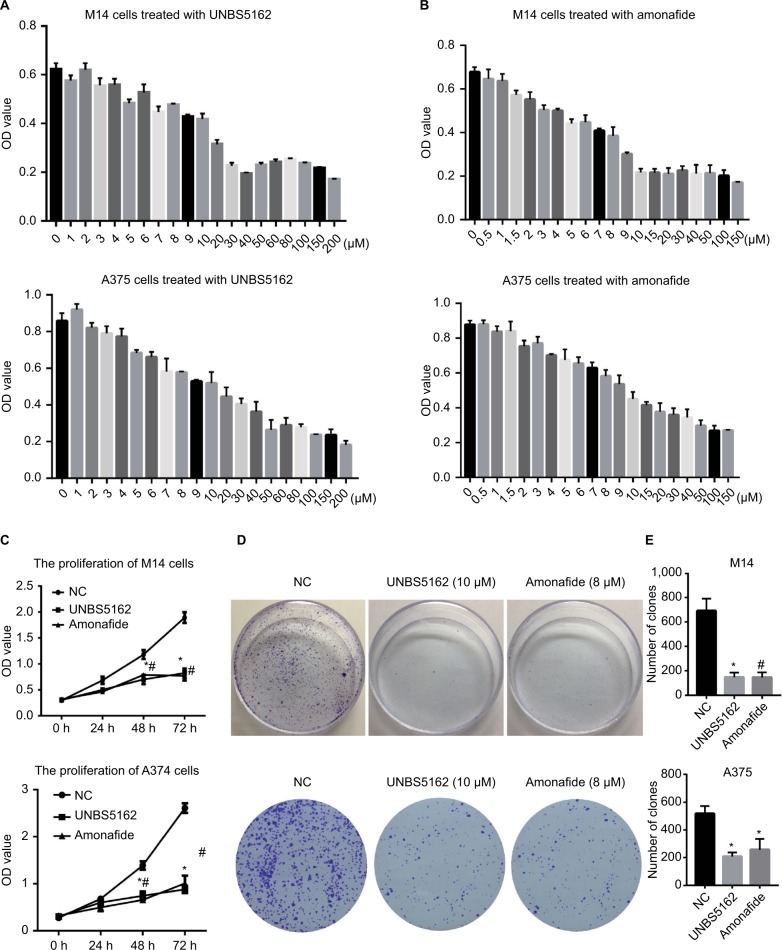
Based on previous findings, we predicted that UNBS5162 and amonafide would inhibit melanoma cell proliferation.9,10 Drug sensitivity assay was performed in order to confirm our prediction. Human melanoma cells were treated with various concentrations of UNBS5162 or amonafide and cultured with CCK8 reagent in order to determine OD values. As shown in Figures 1(A and B), cell viability decreased with increasing concentrations of UNBS5162 and amonafide, with 10 μM UNBS5162 and 8 μM amonafide being chosen as experimental concentration. We then examined the proliferation of the UNBS5162 group (10 μM UNBS5162), the amonafide group (8 μM amonafide), and the NC group (0.1% DMSO) in M14 and A375 cells by means of CCK8 assay. We observed that the OD value of M14 and A375 cells treated with UNBS5162 or amonafide was significantly reduced when compared to the NC group (Figure 1C). Clone formation assay was then performed to further verify the effects of UNBS5162 and amonafide on cell proliferation (Figure 1D). Likewise, the clone numbers of M14 or A375 cells treated with UNBS5162 and amonafide both decreased significantly when compared with that of NC cells (Figure 1E). These results proved that UNBS5162 and amonafide prevented the melanoma cells from proliferating in vitro.
Figure 1.
UNBS5162 and amonafide inhibits the proliferation of melanoma cells. (A and B) The proliferation of M14 or A375 cells treated with various concentrations of UNBS5162 and amonafide, respectively. (C) Cell-counting kit-8 assay was performed in order to estimate the proliferation of melanoma cells treated with 10 µM UNBS5162 or 8 µM amonafide; the OD value of the UNBS5162 and amonafide groups decreased significantly compared with the negative control (NC) group both in M14 and A375 cells. (D and E) Clone numbers of melanoma cells treated with 10 µM UNBS5162 or 8 µM amonafide decreased significantly compared with the NC group.
Notes: *P<0.05, the UNBS5162 group vs the NC group; #P<0.05, the amonafide group vs the NC group.
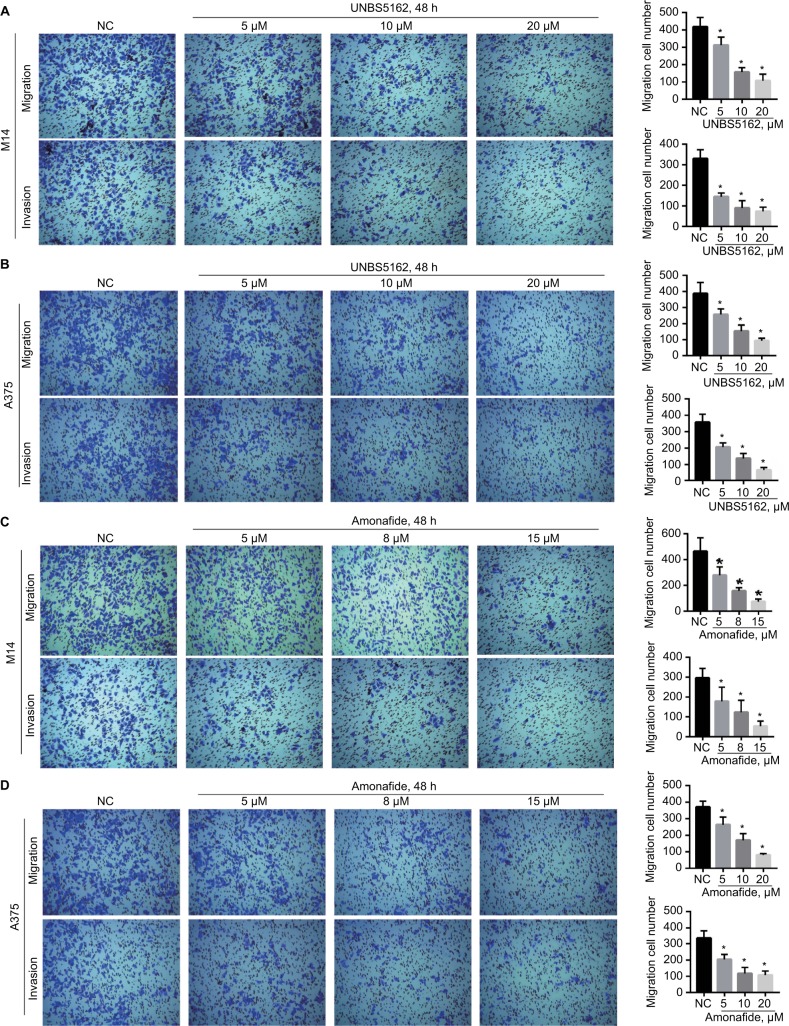
UNBS5162 and amonafide inhibits the migration and invasion of human melanoma cells
Transwell assay was performed to investigate the effect of UNBS5162 and amonafide on the migration and invasion of melanoma cells. After treatment for 48 hours, UNBS5162 dose dependently decreased the migration and invasion of M14 and A375 cells (Figures 2A and B). Additionally, amonafide (5–15 μM) decreased the migration and invasion of M14 and A375 cells when compared with NC cells (Figures 2C and D). These results prove that UNBS5162 and amonafide prevent the migration and invasion of melanoma cells in vitro.
Figure 2.
UNBS5162 and amonafide inhibits the migration and invasion of melanoma cells. (A and B) Representative images of the transwell assay for migration and invasion in melanoma cells with treatment of UNBS5162. (C and D) Representative images of transwell assay for migration and invasion in melanoma cells with treatment of amonafide. Invasion or migration cell numbers decreased significantly in UNBS5162 or amonafide treated cells when compared with negative control (NC) group both in M14 and A375 cells.
Note: *P<0.05.
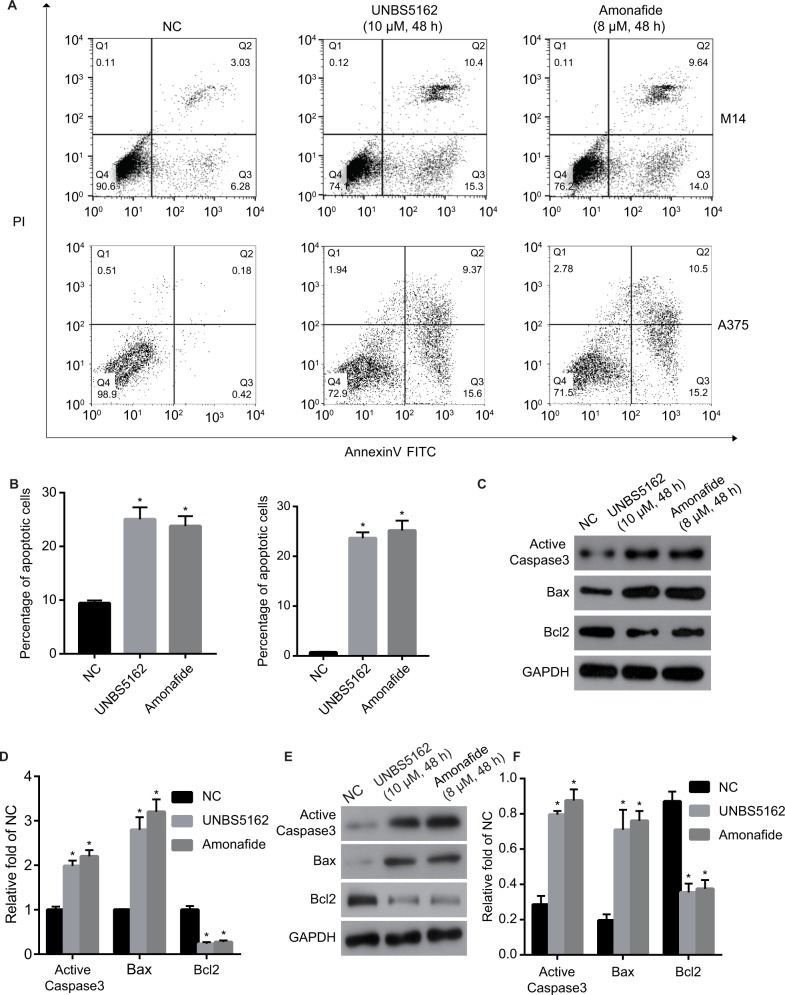
UNBS5162 and amonafide promote apoptosis of melanoma cells in vitro
The role of UNBS5162 and amonafide on apoptosis was confirmed by means of flow cytometry. As shown in Figures 3A and B, the apoptosis cell numbers of melanoma cells treated with UNBS5162 (M14, 25.8%; A375 24.97%) and amonafide (M14, 23.64%; A375, 25.7%) increased significantly when compared with NC cells (M14, 9.31%; A375, 0.6%). Western blot was then performed to investigate the change of expression in apoptotic-related genes in UNBS5162, amonafide, and NC groups (Figures 3C and E). Results demonstrate that Active Caspase3 and Bax expression increased in UNBS5162 and amonafide group cells, while Bcl-2 was inhibited (Figures 3D and F).
Figure 3.
UNBS5162 and amonafide promote the apoptosis of melanoma cells. (A) Cell apoptosis was detected by flow cytometry. (B) Apoptosis cell number of M14 and A375 cells treated with 10 µM UNBS5162 or 8 µM amonafide decreased significantly when compared with negative control (NC) cells. (C and E) The expressions of apoptotic-related genes were detected by Western blot. (D and F) Active Caspase3 and Bax expression were inhibited in UNBS5162 and amonafide groups, while Bcl-2 was promoted when compared with NC cells.
Note: *P<0.05.
Abbreviations: PI, propidium iodide; FITC, fluorescein isothiocyanate.
UNBS5162 and amonafide inhibits growth of melanoma cells by the AKT/mTOR pathway
According to our knowledge, AKT/mTOR is a crucial signaling pathway in tumorigenesis; this also includes melanomas.11,12 However, the role of UNBS5162 and amonafide on the AKT/mTOR pathway in melanoma progression remains unknown. Therefore, we hypothesized that UNBS5162 and amonafide would regulate the signaling pathway and, thus, inhibit tumor progression. To investigate UNBS5162 and amonafide’s effect on the AKT/mTOR pathway, we measured the expression of related genes in M14 and A375 cells. As shown in Figures 4A–D, phosphorylation of AKT and mTOR were inhibited in M14 and A375 cells when compared with NC cells. The cells were treated with 10 μM UNBS5162 or 8 μM amonafide for 24 hours. Further, we detected expressions of P70/S6K and Cyclin D1, which in the UNBS5162 group and amonafide groups were reduced significantly. These results reveal that UNBS5162 and amonafide influences cell viability of melanoma cells through the AKT/mTOR signaling pathway.
Figure 4.
UNBS5162 and amonafide inhibit the activation of the AKT/mTOR pathway and reverse the epithelial–mesenchymal transition process in human melanoma cells. (A–D) Western blot was performed to confirm the pathway by which UNBS5162 and amonafide inhibited tumor progression; phosphorylation of AKT and mTOR were inhibited in M14 and A375 cells treated with 10 µM UNBS5162 or 8 µM amonafide for 48 hours, as well as P70/S6K and Cyclin D1. (C–H) The expressions of N-cad, Snail, Vimentin, and slug in UNBS5162 and amonafide groups decreased significantly, whereas E-cad increased significantly.
Note: *P<0.05.
Abbreviation: NC, negative control.
UNBS5162 and amonafide inhibit epithelial–mesenchymal transition (EMT) conversion in human melanoma cells
In outside studies, it was found that UNBS5162 and amonafide inhibited the invasion of melanoma cells. Therefore, we further explore the role of UNBS5162 and amonafide in the EMT process of human melanoma cells by means of Western blot. Our results demonstrated that N-cad, Snail, Vimentin, and slug expression in the UNBS5162 and amonafide groups decreased significantly and that E-cad increased significantly in both groups, whereas Twist in the amonafide group decreased significantly (Figures 4E–H). Thus, our results suggest that UNBS5162 and amonafide reverse EMT conversion in human melanoma cells.
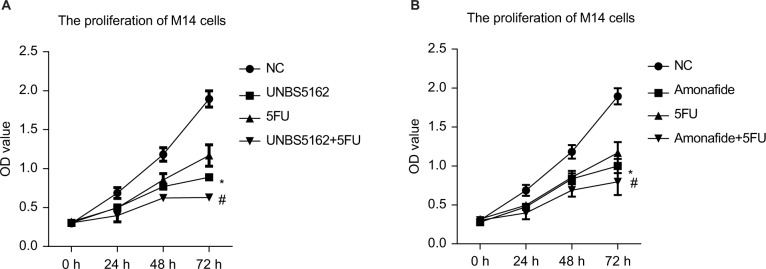
5FU combined with UNBS5162 or amonafide inhibits the proliferation of human melanoma cells
5FU is currently one of the most widely used anticancer drugs in clinical practice,13 so we chose it for comparative study. The effects of 5FU combined independently with UNBS5162 or amonafide were tested using a CCK8 assay. These effects were tested in order to measure the proliferation of M14 cells. We found that the CCK8 OD of cells treated with 5 μM 5FU combined with 10 μM UNBS5162 was significantly decreased when compared with cells only treated with 5FU (Figure 5A). Furthermore, similar results were observed in the amonafide-treated cells. The CCK8 OD of M14 and A375 cells decreased when treated with 5FU combined with 8 μM amonafide. This was measured in comparison to 5FU treated cells (Figure 5B).
Figure 5.
5FU combined with UNBS5162 or amonafide inhibits the proliferation of human melanoma cells. (A) CCK8 assay was performed to confirm the proliferation of cells when treated with 5FU combined with 10 µM UNBS5162. (B) CCK8 assay was performed to confirm the proliferation of cells with treatment of combined 5FU and 8 µM amonafide.
Notes: *P<0.05 vs the NC group; #P<0.05 vs the 5FU group.
Abbreviations: CCK8, cell-counting kit-8; NC, negative control
Discussion
Naphthalimides plays an important role in tumor chemotherapy, and its clinical application has continued to attract researchers.14–16 Brana et al17 first synthesized naphthalimide and its derivatives in 1979, and it has proved effective in being embedded into DNA, increasing the length of DNA, and unwinding superhelix DNA. UNBS5162 and amonafide are two important members of the naphthalimide family, and have recently entered clinical trials.18,19 The National Cancer Institute has already evaluated UNBS5162 anti-tumor ability, and has confirmed that it is effective for multiple tumor models. UNBS5162 inhibits tumor progression with less-toxic side effects.20 It was already known that amonafide is also an inhibitor of topoisomerase II (TopoII).
In our present study, we have investigated the role of UNBS5162 and amonafide in melanoma cell growth. A drug sensitivity assay was performed to estimate the change in proliferation of cells treated with UNBS5162 or amonafide. Our results showed that UNBS5162 and amonafide inhibited melanoma cell proliferation, which was consistent with previous results.20,21 Next, UNBS5162 and amonafide’s effect on cell proliferation was verified by CCK8 and clone formation assay. We then estimated the UNBS5162 and amonafide ability of invasion in melanoma cells by means of a transwell. The cells treated with UNBS5162 or amonafide ability of invasion were reduced significantly when compared with NC cells. Flow cytometry was then performed to confirm the role of UNBS5162 and amonafide on apoptosis. The apoptosis cell numbers of the UNBS5162 group and the amonafide group increased significantly when compared with the NC group. Finally, we confirmed the mechanism by which UNBS5162 inhibits progression of melanoma cells. The expression of genes related to the AKT/mTOR signaling pathway decreased significantly when compared with the NC group. Moreover, UNBS5162 and amonafide significantly reverses the EMT conversion in human melanoma cells.
As a naphthalimide, UNBS5162 can reduce the expression of CXCL chemokine in tumors. Chemokines are a class of chemically inducible cytokines that can promote inflammation and can further cause chemotaxis, cell survival and proliferation, intracellular calcium production, and gene transcription.22,23 It has also been verified that chemokines are closely related to the occurrence and development of tumors.24–30 Furthermore, according to recent findings, CXCLs such as CXCL1, CXCL8, CXCL12, CXCL10, CXCL6, CXCL4, and CXCL2 are all associated with the progression of melanomas.31–36 Therefore, we predict that UNBS5162 may play a tumor suppressive role in melanomas by means of regulating CXCL. It has been demonstrated that UNBS5162 can induce cancer in PC3 prostate cancer cells. It does this by decreasing expression of Hsp70 by means of the lysosomal membrane permeabilization. Notably, UNBS5162 also decreases the expression of cancer cell’s chemoresistance gene HERP in many human tumors.20 Research has confirmed that UNBS5162 is a pan-antagonist of CXCL chemokine expression. When administered alone, UNBS5162 displays antitumor effects in experimental models of refractory prostate cancer; when coadministered with the taxoid, UNBS5162 enhances taxol activity.37 In sum, our data suggested that UNBS5162 and amonafide could inhibit the growth of human melanoma cells by the AKT/mTOR signaling pathway, and thus might have clinical potential in treating melanomas. Further studies of UNBS5162 and amonafide’s efficacy and mechanism in vivo and in vitro are needed, however, and will thus be the direction of our future research. They can cause single-strand breaks, double-strand breaks and DNA-protein cross-links in human leukemic cells. Studies have shown that amonafide has a therapeutic effect on advanced breast cancer and leukemia.8 Clinical trials of amonafide in a variety of tumors are also in progress.21,38
Furthermore, we proved that combined 5FU and UNBS5162 or amonafide could significantly inhibit the proliferation of melanoma cells in comparison to only 5FU treated cells. The combined effect of two drugs on cell proliferation was significantly stronger than that of each drug alone, which had a synergistic effect. The low-dose combination of two drugs played a synergistic role in anti-proliferation, and also avoided the side effects caused by high-dose drugs on the body, thereby improving the quality of life of patients. However, the specific mechanism of the above effects remains to be further studied.
In conclusion, we demonstrated that UNBS5162 and amonafide could inhibit the growth of human melanoma cells by AKT/mTOR signaling pathway. The present study on the clinical efficacy of UNBS5162 and amonafide in melanoma provides an experimental basis of its use and expands the potential value on human melanoma treatment.
Acknowledgments
The present study was supported by the Key Discipline foundation of Hwa Mei Hospital, university of Chinese Academy of Science (grant no. 2016013), China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150(2):179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 2.Michielin O, Hoeller C. Gaining momentum: new options and opportunities for the treatment of advanced melanoma. Canc Treat Rev. 2015;41(8):660–670. doi: 10.1016/j.ctrv.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seregard S, Singh AD. Retinoblastoma: direct chemotherapeutic drug delivery into the vitreous cavity. Br J Ophthalmol. 2012;96(4):473–474. doi: 10.1136/bjophthalmol-2012-301528. [DOI] [PubMed] [Google Scholar]
- 5.Kumari G, Singh RK. Green synthesis, antibacterial activity, and SAR of some novel naphthalimides and allylidenes. Med Chem Res. 2015;24(1):171–181. [Google Scholar]
- 6.Mijatovic T, Mahieu T, Bruyère C, et al. UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers. Neoplasia. 2008;10(6):573–586. doi: 10.1593/neo.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruyère C, Mijatovic T, Denève N, et al. 47 poster UNBS5162: a new naphthalimide derivative with radiosensitizing and anti-angiogenic activity entering phase I. EJC Suppl. 2008;6:18. [Google Scholar]
- 8.Costanza ME, Berry D, Henderson IC, et al. Amonafide: an active agent in the treatment of previously untreated advanced breast cancer—a cancer and leukemia group B study (CALGB 8642) Clin Canc Res. 1995;1(7):699–704. [PubMed] [Google Scholar]
- 9.Wang B, Shen J, Wang J. UNBS5162 inhibits proliferation of human retinoblastoma cells by promoting cell apoptosis. Onco Targets Ther. 2017;10:5303–5309. doi: 10.2147/OTT.S145518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornek G, Raderer M, Depisch D, et al. Amonafide as first-line chemotherapy for metastatic breast cancer. Eur J Canc. 1994;30(3):398–400. doi: 10.1016/0959-8049(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 11.Jazirehi AR, Wenn PB, Damavand M. Therapeutic implications of targeting the PI3Kinase/AKT/mTOR signaling module in melanoma therapy. Am J Canc Res. 2012;2(2):178–191. [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Yu J, Yan J, et al. PI3K/Akt/mTOR pathway inhibitors inhibit the growth of melanoma cells with mTOR H2189Y mutations in vitro. Canc Biol Ther. 2018;19(7):584–589. doi: 10.1080/15384047.2018.1435221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zatta K, Frank L, Reolon L, et al. An inhalable powder formulation based on micro- and nanoparticles containing 5-fluorouracil for the treatment of metastatic melanoma. Nanomaterials. 2018;8(2):75. doi: 10.3390/nano8020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Lin Y, Wang Q, Yuan Y, Zhang H, Qian X. The novel anti-tumor agents of 4-triazol-1,8-naphthalimides: synthesis, cytotoxicity, DNA inter-calation and photocleavage. Eur J Med Chem. 2011;46(4):1274–1279. doi: 10.1016/j.ejmech.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Cholody W, Kosalowska-Cholody T, Michejda C, inventors. HEALTH AND HUMAN SERVICES GOVERNMENT OF United States, DEARTMENT OF, Secretary of, assignee 1,8-naphthalimide imidazo{4,5,1-de}acridones with anti-tumor activity. United States patent US20030203916A1. 2003 Aug 1;
- 16.Ji L, Yang S, Li S, et al. A novel triazolonaphthalimide induces apoptosis and inhibits tumor growth by targeting DNA and DNA-associated processes. Oncotarget. 2017;8(23):37394–37408. doi: 10.18632/oncotarget.16962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braña MF, Castellano JM, Roldán CM, Santos A, Vázquez D, Jiménez A. Synthesis and mode(s) of action of a new series of imide derivatives of 3-nitro-1,8 naphthalic acid. Canc Chemother Pharmacol. 1980;4(1):61–66. doi: 10.1007/BF00255461. [DOI] [PubMed] [Google Scholar]
- 18.Mahadevan D, Northfelt DW, Chalasani P, et al. Phase I trial of UNBS5162, a novel naphthalimide in patients with advanced solid tumors or lymphoma. Int J Clin Oncol. 2013;18(5):934–941. doi: 10.1007/s10147-012-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen SL, Kolitz JE, Lundberg A, et al. Phase I study of amonafide + cytosine arabinoside (araC) in patients with poor risk acute myeloid leukemia (AML) J Clin Oncol. 2005;23(16_suppl):6602. [Google Scholar]
- 20.Mahieu T, Mijatovic T, Dumont P, et al. UNBS5162: a novel naphthalimide derivative with potent pro-autophagic effects in human cancer cells. Canc Res. 2007;67:4831. [Google Scholar]
- 21.Gellerman G. Recent developments in the synthesis and applications of anticancer amonafide derivatives. A mini review. Let Drug Des Discov. 2016;13(1) [Google Scholar]
- 22.Lira SA, Furtado GC. The biology of chemokines and their receptors. Annu Rev Immunol. 2012;54:111–120. doi: 10.1007/s12026-012-8313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48(1):171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 24.Akram IG, Georges R, Hielscher T, Adwan H, Berger MR. The chemokines CCR1 and CCRL2 have a role in colorectal cancer liver metastasis. Tumour Biol. 2016;37(2):2461–2471. doi: 10.1007/s13277-015-4089-4. [DOI] [PubMed] [Google Scholar]
- 25.Verbeke H, Geboes K, van DJ, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta: Rev Canc. 1825;2012:117–129. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Schrader AJ, Lechner O, Templin M, et al. CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Canc. 2002;86(8):1250–1256. doi: 10.1038/sj.bjc.6600221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan JT, Sun W, Hussain SF, Deangulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by gliomasecreted chemokines can be blocked with chemotherapy. Canc Immunol Immunother. 2008;57(1):123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng ZH, Shi YX, Yuan M, Xiong D, Zheng JH, Zhang ZY. Chemokines and their receptors in lung cancer progression and metastasis. J Zhejiang Univ Sci B. 2016;17(5):342–351. doi: 10.1631/jzus.B1500258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagarsheth N, Peng D, Kryczek I, et al. PRC2 epigenetically silences Th1-type chemokines to suppress effector T-cell trafficking in colon cancer. Canc Res. 2016;76(2):275–282. doi: 10.1158/0008-5472.CAN-15-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Canc Lett. 2008;267(2):271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Nobeyama Y, Nakagawa H. Tumor-suppressive effects of natural-type interferon-β through CXCL10 in melanoma. Biochem Biophys Res Commun. 2015;464(2):416–421. doi: 10.1016/j.bbrc.2015.06.122. [DOI] [PubMed] [Google Scholar]
- 32.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72(1):9. [PMC free article] [PubMed] [Google Scholar]
- 33.O’Boyle G, Swidenbank I, Marshall H, et al. Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070. Br J Canc. 2013;108(8):1634–1640. doi: 10.1038/bjc.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S, Singh S, Varney ML, Kindle S, Singh RK. Modulation of CXCL-8 expression in human melanoma cells regulates tumor growth, angiogenesis, invasion, and metastasis. Canc Med. 2012;1(3):306–317. doi: 10.1002/cam4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, S A, Varney ML, Nannuru KC, Singh RK. CXCR1, CXCR2, melanoma, CXCL-8. Int J Canc J Int Du Canc. 2010;126:328. doi: 10.1002/ijc.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Arcangelo D, Facchiano F, Nassa G, et al. PDGFR-alpha inhibits melanoma growth via CXCL10/IP-10: a multi-omics approach. Oncotarget. 2016;7(47):77257. doi: 10.18632/oncotarget.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mijatovic T, Mahieu T, Bruyère C, et al. UNBS5162, CXCL, prostate cancer. Neoplasia. 2008;10:573–586. doi: 10.1593/neo.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone RM, Mazzola E, Neuberg D, et al. Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol. 2015;33(11):1252–1257. doi: 10.1200/JCO.2014.57.0952. [DOI] [PubMed] [Google Scholar]