Abstract
Reporting recently in Nature Cell Biology, Lu et al. (2015) identify two Eps15-homology-domain-containing proteins as critical effectors of ciliary vesicle formation, an early event in ciliogenesis. Functional dissection reveals that one of them works to convert small vesicles associated with mother centriole distal appendages into a larger ciliary vesicle.
The seminal work of Sergei Sorokin half a century ago continues to inspire studies on the early steps of ciliogenesis. Cilia are microtubule-based cellular projections on many types of cells that can be motile and can participate in sensing extracellular cues. Cells produce cilia in tight coordination with the cell cycle. Sorokin used electron microscopy to identify different steps in ciliogenesis and then reasoned, largely from parsimony, how these steps might be ordered (Sorokin, 1962). One important early step is the attachment of the mother centriole—the oldest centriole in the cell and the centriole that possesses distal appendages—to a vesicle, called the ciliary vesicle. Small ‘‘secondary’’ vesicles then fuse with the ciliary vesicle, enlarging it, before the microtubules of the centriole extend to invaginate the vesicle. Eventually, the distended ciliary vesicle fuses with the plasma membrane, externalizing the cilium. These early studies provide the framework for an elegant new study from Lu et al. (2015) examining the mechanisms underlying ciliary vesicle formation and early ciliogenesis.
Lu et al. found that knockdown of EHD1 and EHD3 inhibits ciliogenesis. Eps15homology-domain (EHD)-containing proteins have been previously linked to membrane remodeling, particularly in endocytosis and endosome recycling (Zhang et al., 2012). EHD1 and EHD3 are associated with membranes marked by Rab11 and Rab8, small GTP-binding regulators of membrane trafficking. Indeed, some Rab11 and Rab8 effectors also bind EHD1 and EHD3, suggesting shared functions (Zhang et al., 2012).
How might EHD1 function relative to Rab11 and Rab8? During ciliogenesis, Rab11 transports Rabin8, a guanine nucleotide exchange factor for Rab8, to the centrosome (Kno¨ dler et al., 2010; Westlake et al., 2011). Rabin8-mediated activation of Rab8 then stimulates ciliary membrane elongation and ciliogenesis. The authors demonstrate that Rabin8 colocalizes with EHD1 on preciliary vesicles but does not require EHD1 for accumulation near the mother centriole.
Using beautiful live-cell imaging, Lu et al. also examine the order in which these proteins appear at the ciliary vesicle. To visualize the ciliary vesicle and its precursors, they make creative use of fluorescently tagged Smoothened, which localizes to ciliary membranes in a regulated way but constitutively marks ciliary membranes when overexpressed. EHD1 localizes to the developing cilium before Rab8. Together with the finding that Rabin8 localizes independently of EHD1, these results suggest that EHD1 functions upstream of or in parallel to Rab11 and Rab8.
The distinct timing of EHD1 and Rab8 localization to vesicles near the mother centriole raises the possibility that they function differently in ciliogenesis. Knockdown of Rab8a and Rab8b blocked ciliogenesis at a stage when the mother centriole has docked to a large ciliary vesicle. Knockdown of EHD1 blocked ciliogenesis in a different way: the distal appendages of the mother centriole were associated with several smaller vesicles. These vesicles are similar in diameter to what Sorokin described as small secondary vesicles but are associated with the distal appendages (Sorokin, 1962). Thus, by knocking down EHD1, Lu et al. have revealed a previously unidentified step in ciliogenesis between distal appendage association with small vesicles and the coalescence of these small vesicles into a ciliary vesicle (Figure 1).
Figure 1. Stages of mother centriole landing on the ciliary vesicle.
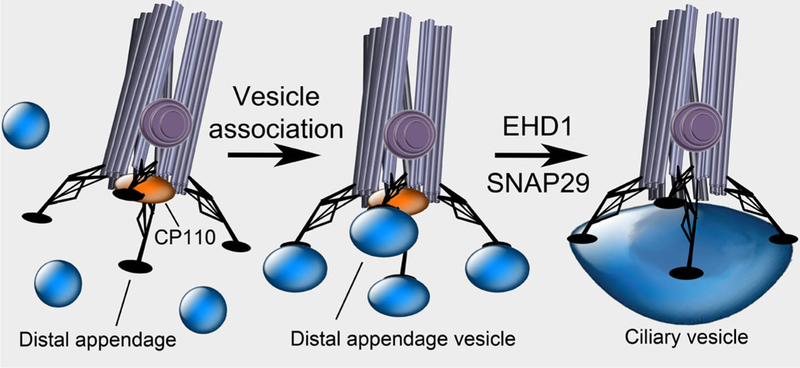
The mother centriole is distinguished from the daughter centriole by appendages including the basal foot (purple) and the distal appendages (black). Extension of ciliary microtubules is blocked by CP110 (orange). Through the distal appendages, the mother centriole docks to the membrane of vesicles. EHD1 and SNAP29 convert these distal appendage vesicles into a single ciliary vesicle. As EHD1 can tubulate membranes and SNAP29 can fuse them, membrane distortion and fusion may be essential for this step. Formation of the ciliary vesicle is associated with removal of CP110, allowing for subsequent ciliogenesis.
How might distal appendage vesicles fuse into a ciliary vesicle? Many membrane fusion events are controlled by SNARE proteins. EHD1 associates with the SNARE SNAP29 (Rotem-Yehudar et al., 2001), and Lu et al. found that SNAP29 and EHD1 colocalize at cilia. Like EHD1 knockdown, knockdown of SNAP29 inhibited ciliogenesis. Intriguingly, EHD1 does not require SNAP29 to localize to the distal centrosome, but SNAP29 localization does require EHD1, suggesting that EHD1 helps to bring SNAP29 to the centrosome to mediate distal appendage vesicle fusion. Thus, one prediction of this work is that SNAP29 will, like EHD1, be required for converting distal appendage vesicles into the ciliary vesicle.
In addition to association with a ciliary vesicle, a key early event in ciliogenesis is the removal of CP110, a distal centriolar protein, from the mother centriole (tor et al., 2007). Surprisingly, EHD1 is required to remove CP110, suggesting that, upon mother centriole association with the ciliary vesicle, the ciliary vesicle feeds back onto the mother centriole, affecting its composition to allow ciliary axoneme extension. How EHD1-dependent formation of the ciliary vesicle dislodges CP110 from the mother centriole is unclear, but one interesting candidate for mediating this effect is Tau Tubulin Kinase 2 (TTBK2). Like EHD1, this kinase is recruited early to the mother centriole and is essential for removing CP110 and promoting ciliogenesis (Goetz et al., 2012).
Following ciliogenesis, EHD1 and EHD3 localize to an interesting and poorly understood domain of cilia, the membrane of the ciliary pocket surrounding the ciliary base. The ciliary pocket is a site of active endocytosis in organisms as diverse as mammals and Trypanosomes. Following ciliogenesis, EHD1 and EHD3 could have additional roles in promoting membrane trafficking at the ciliary pocket, including the delivery of ciliary membrane proteins.
Because primary cilia interpret critical intercellular patterning cues, such as vertebrate Hedgehog signals, EHD1 and EHD3 are predicted to be required for embryonic development. Indeed, Lu et al. find that morpholino-mediated knockdown of EHD1 or EHD3 inhibited the formation of retinal cilia in zebrafish. Interestingly, the cilia of other tissues were differentially dependent on EHD1 and EHD3. For example, formation of kinocilia
in otic vesicles was dependent on EHD1 but not on EHD3. These differences suggest that different kinds of cilia may use different EHD proteins to promote ciliogenesis. In light of this work, it will also be interesting to reexamine the functions of mammalian EHD1 and EHD3 using genetic models. Knockout of mouse EHD3 by itself does not impact viability or development in clear ways, and knockout of EHD1 affects spermatogenesis (George et al., 2011; Rainey et al., 2010). Double mutants may be required to uncover clear effects on mammalian ciliogenesis.
Lu et al. have identified EHD1 and EHD3 as critical regulators of one of the earliest events of ciliogenesis, the recruitment of SNAP29 and the conversion of small distal appendage vesicles into the larger ciliary vesicle. This work builds on the foundation laid by Sorokin, helping to elucidate the molecular basis by which the ciliary vesicle forms from distal appendage-associated vesicles. The identification of distal appendage vesicles opens up the possibility of learning more about them. That they are associated with EHD1 and Smoothened suggests that they are distinct from other vesicles in the cell. Perhaps the distal appendages find these special vesicles and associate with them uniquely. Or perhaps the mother centriole indiscriminately associates with nearby vesicles and then alters the composition of these vesicles by localizing EHD1 and Smoothened to them, thus initiating a complex communication between the mother centriole and the associated vesicles that culminates in ciliogenesis.
References
- George M, Rainey MA, Naramura M, Foster KW, Holzapfel MS, Willoughby LL, Ying G, Goswami RM, Gurumurthy CB, Band V, et al. (2011). PloS One 6, e17838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Liem KF Jr., and Anderson KV (2012). Cell 151, 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knödler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, and Guo W (2010). Proceedings of the National Academy of Sciences of the United States of America 107, 6346–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, et al. (2015). Nature Cell Biology 17, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey MA, George M, Ying G, Akakura R, Burgess DJ, Siefker E, Bargar T, Doglio L, Crawford SE, Todd GL, et al. (2010). BMC Developmental Biology 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem-Yehudar R, Galperin E, and Horowitz M (2001). The Journal of Biological Chemistry 276, 33054–33060. [DOI] [PubMed] [Google Scholar]
- Sorokin S (1962). The Journal of Cell Biology 15, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor A, Tsang WY, Khoo D, and Dynlacht BD (2007). Cell 130, 678–690. [DOI] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. (2011). Proceedings of the National Academy of Sciences of the United States of America 108, 2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Naslavsky N, and Caplan S (2012). Bioscience Reports 32, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


