Abstract
It is unclear how long-term medical utilization and costs from diverse care settings and their age-related patterns may differ by cardiovascular health (CVH) status earlier in adulthood. We followed 17,195 participants of the Chicago Heart Association Detection Project Industry (1967–1973) with linked Medicare claims (1992 to 2010). Baseline CVH is a composite measure of blood pressure, body mass index, diabetes, cholesterol, and smoking and includes four mutually exclusive strata: all factors were favorable (5.5%), one or more factors were elevated but none high (20.3%), one factor was high (40.9%), and two or more factors were high (33.2%). We assessed differences in the quantities (using negative binomial models) of and costs (using quantile regressions) for inpatient admissions, ambulatory care, home health care, and others between less favorable and all favorable CVH. All analyses adjusted for baseline age, race, sex, education, age at follow-up, year, state of residence, and death. We found that all favorable CVH in earlier adulthood was associated with lower long-term utilization and costs in all settings and the gap widened with age. Compared to all favorable CVH, the annual number of acute inpatient admissions per person was 79% greater (p-value < 0.001) for poor CVH, the median annual Medicare payment per person was $640 greater (41%, p-value < 0.001), and the mean was $4,628 greater (67%, p-value < 0.001). The cost differences were greatest for acute inpatient, followed by ambulatory, post-acute inpatient, home health, and other. Early prevention efforts may potentially result in compressed all-cause morbidity in later years of age, along with reductions in resource use and health care costs for associated conditions.
Keywords: cardiovascular health, healthcare utilization, healthcare costs
Introduction
U.S. healthcare spending is projected to grow from 17.8% of national gross domestic product (GDP) in 2015 to 20.1% of national GDP by 2025.1,2 Although the rise in healthcare spending is attributable to myriad factors, growing Medicare costs due to an aging population with a greater incidence of chronic diseases could likely further increase spending.3,4,5,6 According to a recent report published by the American Heart Association, by 2035 approximately 45% (131.2 million) of the U.S. adult population will have some form of cardiovascular diseases (CVD), up from 41.5% (102.7 million) in 2015 and CVD-related medical costs will more than double during this period.7
Past research indicates that better cardiovascular health (CVH) is associated with compressed all-cause morbidity and lower medical care costs at later ages.8–19 However, the evidence to date is mostly limited by short follow-ups or small cohorts and a particular focus on overall medical care costs, failing to distinguish the distribution of costs across health resources. Specifically, it is unclear how long-term medical utilization and costs from diverse care settings (e.g., acute inpatient and home health care) and their age-related patterns may differ by baseline CVH status. Answers to these questions provide a unique lens to understand how and where favorable CVH earlier in life may be resulting in reduced costs in later ages.
Using linked, complete Medicare Part A and Part B claims from 1992 to 2010 for participants of the Chicago Heart Association Detection Project Industry (CHA, 1967 – 1973), we examined the associations between their baseline cardiovascular health (CVH) status and annual medical utilization and costs from various care settings at older ages. The study is novel given its long follow-up period (up to 40 years) and the fact that it uses all Part A and Part B claims. Specifically, we examined total costs and the quantities of and costs for acute inpatient admissions, post-acute inpatient admissions, ambulatory care visits, home health visits, and others. The answers to these questions will provide unique evidence on the later-life healthcare experiences of individuals with various CVH status earlier in adulthood and generate policy implications regarding to the potential health and financial benefits of investments in health early in life.
Methods
Sample
The CHA Study assessed cardiovascular health for 39,665 participants (baseline aged between 18 and 77 years) between 1967 and 1972.15 The study collected information on participants’ demographic characteristics at baseline including age, sex, race, education, and biometrics data including height, weight, systolic blood pressure, diastolic blood pressure, serum cholesterol (total cholesterol), serum uric acid, 1-hour plasma glucose, cigarette smoking, diabetes, and medical history such as history of diagnosed high blood pressure, high cholesterol, and hyperuricemia.
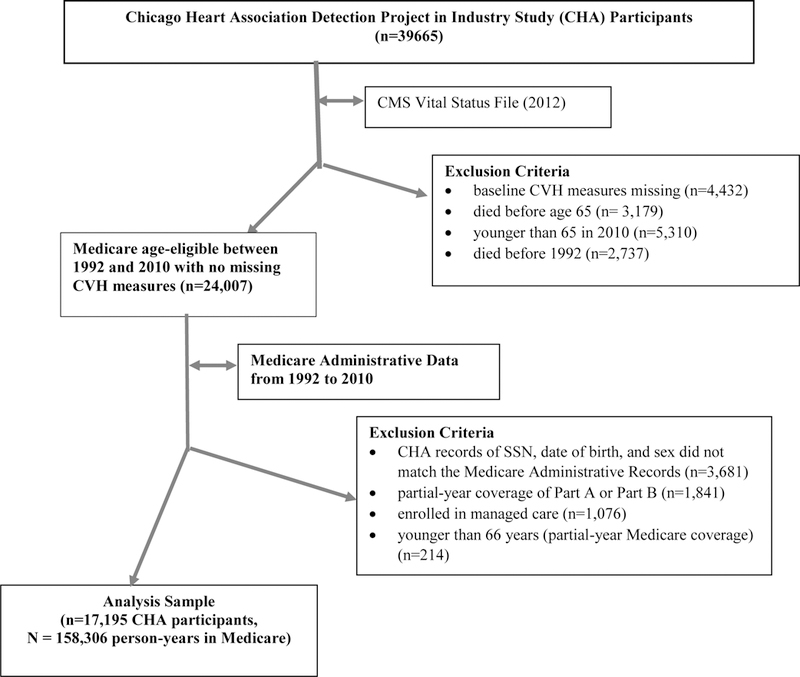
Among the CHA participants (see Appendix A for the diagram for sample selection), we excluded those whose baseline CVH measures were missing (n=4,432). To focus on participants who were age-eligible for Medicare during follow-up (and therefore likely enrolled in Medicare) between 1992 and 2010 (when linked data were available), we excluded those who died before age 65 (n=3,179), were younger than 65 years in 2010 (n=5,310), or died before 1992 (n=2,737). These exclusions resulted in 24,007 age-eligible participants between 1992 and 2010. Of them, 20,326 participants were successfully matched to the Administrative Medicare enrollment file by SSN, year of birth, and sex. We then included only participants who had both Medicare Part A and Part B benefits for the full year (number excluded =1,841). Claims for managed care beneficiaries were not available, therefore, we excluded individuals who were enrolled in managed care for any month during a year (n=1,076). Finally, we excluded individuals who were younger than 66 years because they may have only partial-year Medicare coverage (n=214). This study has been continuously approved by the Institutional Review Board and has a waiver of consent.
Our final longitudinal analysis sample included 17,195 unique CHA participants aged between 22 and 76 years (mean age =43) at baseline followed for 158,306 person-years during Medicare eligibility (i.e., after age 66 years). The unit of observation is person-year. On average, a participant was followed in Medicare for 9.2 years.
Baseline Cardiovascular Health (CVH)
Baseline CVH risk factors measured in the CHA study in 1967–1972 included blood pressure, body mass index (BMI), diabetes, total cholesterol, and cigarette smoking. As in prior studies,11 we divided the sample into four mutually exclusive CVH strata: all factors were favorable (hereafter, all favorable), one or more factors were elevated but none was high (hereafter, 1+ elevated, none high), one factor was high (thereafter, 1 high), and two or more factors were high (thereafter, 2+ high). Table 1 provides details on the definitions of favorable, elevated, and high levels of CVH factors, which reflect standard clinical practice guidelines and have been used in prior publications.11 Table 2 provides the percent distribution of each of the five risk factors for each CVH stratum.
Table 1.
Definitions of Favorable, Borderline, and High Levels of Cardiovascular Risk Factors
| Blood Pressure | Cholesterol | Diabetes | BMI | Smoking | |
|---|---|---|---|---|---|
| Favorable | Untreated SBP ≤ 120 mm Hg and DBP ≤ 80 mm Hg | Untreated serum cholesterol < 200 mg/dL (<5.17 mmol/L) | No diabetes | BMI < 25 | Non-smoker |
| Elevated but not High | Untreated SBP 121–139 or DBP 81–89 | Untreated serum cholesterol 200–239 mg/dL (5.17 – 6.18 mmol/L) | No diabetes | BMI 25.0–29.9 | Non-smoker |
| High | SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg or taking antihypertensive medications | Serum cholesterol level ≥ 240 mg/dL (≥6.21 mmol/L) or taking cholesterol-lowering medication | Diabetes | BMI ≥ 30 | Current Smoker |
Note: we followed the definition used in previous study by Allen NB, Zhao L, Lei L, et al. Favorable Cardiovascular Health, Compression of Morbidity and Healthcare Costs: 40-Year Follow-up of the Chicago Heart Association Detection Project in Industry. Circulation. 2017;135(18):1693–1701. doi: 10.1161/CIRCULATIONAHA.116.026252.
Table 2.
Percent Distribution of Cardiovascular Risk Factors at Baseline by CVH Strata
| Blood Pressure | Cholesterol | Diabetes | BMI | Smoking | |
|---|---|---|---|---|---|
| All Favorable | Untreated SBP ≤ 120 mm Hg and DBP ≤ 80 mm Hg | Untreated serum cholesterol < 200 mg/dL (<5.17 mmol/L) | No diabetes | BMI < 25 | Non-smoker |
| (% of stratum) | 100% | 100% | 100% | 100% | 100% |
| 1+ Elevated, None High | Untreated SBP 121–139 or DBP 81–89 | Untreated serum cholesterol 200–239 mg/dL (5.17 – 6.18 mmol/L) | No diabetes | BMI 25.0–29.9 | Non-smoker |
| (% of Stratum) | 60.7% | 51.5% | 100% | 54.3% | 100% |
| 1 High | SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg or taking antihypertensive medications | Serum cholesterol level ≥ 240 mg/dL (≥6.21 mmol/L) or taking cholesterol-lowering medication |
Diabetes | BMI ≥ 30 | Current Smoker |
| (% of stratum) | 46.3% | 9.8% | 0.9% | 5.0% | 38.1% |
| 2+ High | SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg or taking antihypertensive medications | Serum cholesterol level ≥ 240 mg/dL (≥6.21 mmol/L) or taking cholesterol-lowering medication | Diabetes | BMI ≥ 30 | Current Smoker |
| (% of stratum) | 84.8% | 42.2% | 4.4% | 32.7% | 62.0% |
Medical Care Utilization and Costs
Medical care utilization and costs were ascertained from linked Medicare Part A and Part B claims from 1992 to 2010. These claims files included Medicare Provider Analysis and Review (inpatient and skilled nursing facilities), outpatient (hospital outpatient), carrier (physician/supplier Part B claims), durable medical equipment (DME), home health agency, and hospice files.
We calculated the medical care utilization and costs for each beneficiary by year (therefore by age). Medical care utilization included the number and total length (in days) of acute inpatient admissions, the number and total length of post-acute inpatient admissions, the number of ambulatory care visits, and the number of home health visits. Acute and post-acute inpatient cost/services were defined using the algorithms recommended in the Chronic Condition Data Warehouse (CCW) Technical Guidance for using CMS Medicare Administrative Research Files (July 2016, Version 2.3) published by CMS. Specifically, post-acute inpatient admissions included admissions to long-term care hospitals (the 3rd and 4th digits of the provider number are 20, 21, or 22), inpatient rehabilitation facilities (the last 4 digits of the provider number are between 3025 and 3099 or the 3rd digit being R or T), and skilled nursing facilities (the SNF indicator). Acute inpatient admissions include admissions to inpatient prospective payment system reimbursed hospitals (the 3rd digit of provider number is zero), critical access hospitals (3rd and 4th digits equal to 13), and other hospitals not counted as post-acute care hospitals.20 Ambulatory care visits include visits to physician offices and clinics, hospital outpatient departments, emergency rooms without inpatient admission, and other non-institutional ambulatory care providers. Because claims for ambulatory care are located in both the outpatient and carrier files, we combined the two files and defined a visit by beneficiary ID, and claim from and through date to avoid double counting a visit with multiple claims.
We also analyzed these utilization categories according to the primary reason for admission or visit, defined as a cardiovascular disease (CVD) or non-cardiovascular disease (non-CVD). CVD visits and admissions were identified using primary diagnosis codes (ICD-9-CM) and included ischemic heart diseases, heart failure, peripheral vascular diseases, and stroke (ICD-9 codes included 410 through 414, 402.01, 402.11, 402.91, 429.3, 425, 428, 440, 4412, 441.4, 441.7, 441.9, 443.1 through 443.9, 447.1, 557.1, 557.9, v434, 434, and 436).21
We then examined differences in medical care costs for those with less favorable CVH at baseline compared to those with all favorable CVH. Because charges can vary across care facilities for reasons unrelated to patients’ medical conditions,22–24 we measured costs using Medicare payment amount, which was the amount Medicare paid for covered services and therefore a good representation of the financial burden to the Medicare program. In a small number of cases where the Medicare payment amount was negative because a beneficiary’s deductible or coinsurance amount exceeded the amount Medicare paid, we set the Medicare payment amount to zero.25 This was required in only 0.03% of all inpatient claims, 0.1% of all outpatient and carrier claims, and there were no changes for DME, home health, and hospice claims. We excluded denied claims before calculating utilization and costs. All costs were adjusted to 2016 dollars using the medical care services component of the consumer price indexes published by the U.S. Bureau of Labor Statistics.
We calculated total costs as total Medicare payment amount incurred in all care settings for each individual by year. To calculate costs by types of care, we divided the total costs into mutually exclusive categories, including costs for acute inpatient admissions, post-acute inpatient admissions, ambulatory care visits, home health care visits, and others. All costs vary by person and year. Acute inpatient costs is the total amount that Medicare paid hospitals and physicians for acute inpatient services; post-acute inpatient costs is the total amount that Medicare paid facilities and physicians for post-acute inpatient services; ambulatory care costs is the total amount Medicare paid for ambulatory care; home health costs is the total amount Medicare paid for home health visits; and other costs include costs incurred in hospice care and retail DME.20 Similar to utilization, we also categorized costs related to CVD and costs related to non-CVD.
Statistical Analysis
Sample characteristics were summarized by CVH strata and included average age at follow-up and at baseline, percent female, white, black, with a high school degree, and with a more than high school degree. We also calculated the mean and median of medical utilization and costs for each stratum. F test was used to compare differences across CVH stratum. To assess the age pattern in medical costs, we calculated average costs by age for each stratum and plotted them using a local polynomial smoothing method.
We used a multivariate regression model where an individual’s medical care utilization (or costs) is a function of baseline CVH, age at follow-up (entered as linear and quadratic terms), year effects, state of residence effects, and other covariates including baseline age, race, sex, education, and an indicator for whether an individual died during the year. We entered baseline CVH as three dummy variables representing the three less favorable CVH strata: 1+ elevated, none high; 1 high; and 2+ high, using all favorable as the reference stratum.
We controlled for the year effect to account for aggregate temporal differences (such as new medical technology/treatment) that could lead to differences in utilization and costs across periods and, in particular, across birth cohorts at the same age. We also controlled for state effects to account for potential geographic differences in healthcare utilization and costs because the Medicare enrollment files show that over time CHA participants have moved to diverse regions of the country and because both medical care utilization and reimbursement vary by locality.26–28 We found that because baseline CVH only weakly correlated with later state of residence, the inclusion of state effects had little impact on the estimates. Finally, because end-of-life health costs may follow a different pattern from average annual costs for an individual,29 we included an indicator for whether a person died during the year. In sensitivity analyses we stratified by whether an individual died in sample to confirm that findings were consistent.
We used a negative binomial model to examine differences in utilization between less favorable CVH and all favorable CVH. Negative binomial models are commonly used in modeling count data (e.g. number of admissions/visit) and have less restrictive assumptions than do Poisson models.30 Marginal effects associated with less favorable CVH were calculated at the mean of covariates. We used cluster-robust standard errors clustered by individual to account for within individual correlation in healthcare utilization across periods.30 These standard errors are larger than the Huber-White standard errors which suggests that our estimates of statistical significance are conservative. In order to assess age patterns in the differences in utilization, marginal effects were calculated for each age with all other covariates fixed at their mean. We conducted the analyses on the full sample and also stratified by sex.
We used quantile regressions to examine differences in costs between poor CVH and all favorable CVH. Quantile regression methods estimate the conditional quantile functions instead of conditional mean functions and are therefore less likely to be influenced by outliers than conditional mean comparisons.30,31 An additional advantage of quantile regressions is that it allows us to examine cost differences at different points of the cost distribution.30 To examine whether the differences in cost vary across the cost distribution, we estimated quantile regressions at various percentiles of the costs: the 25th percentile, 50th percentile, 75th percentile, 90th percentile, and 95th percentile where applicable due to the highly skewed nature of the cost data and the mass of true zeros. We also supplemented the quantile estimates with GLM estimates which represent mean differences in costs conditional on covariates. We used a gamma distribution and a log-link function which provided the best fit to the cost data compared with alternative distributions. Combined, quantile regressions and GLM provide a fuller understanding of the cost differences than either approach alone. Again, we used cluster-robust standard errors at the individual level to adjust for intra-individual correlation of healthcare costs across periods.30 We performed the analyses on the full sample and also stratified by sex. All statistical analyses were performed using Stata statistical software (version 14, StataCorp, TX).
Results
Sample Characteristics and Summary Statistics
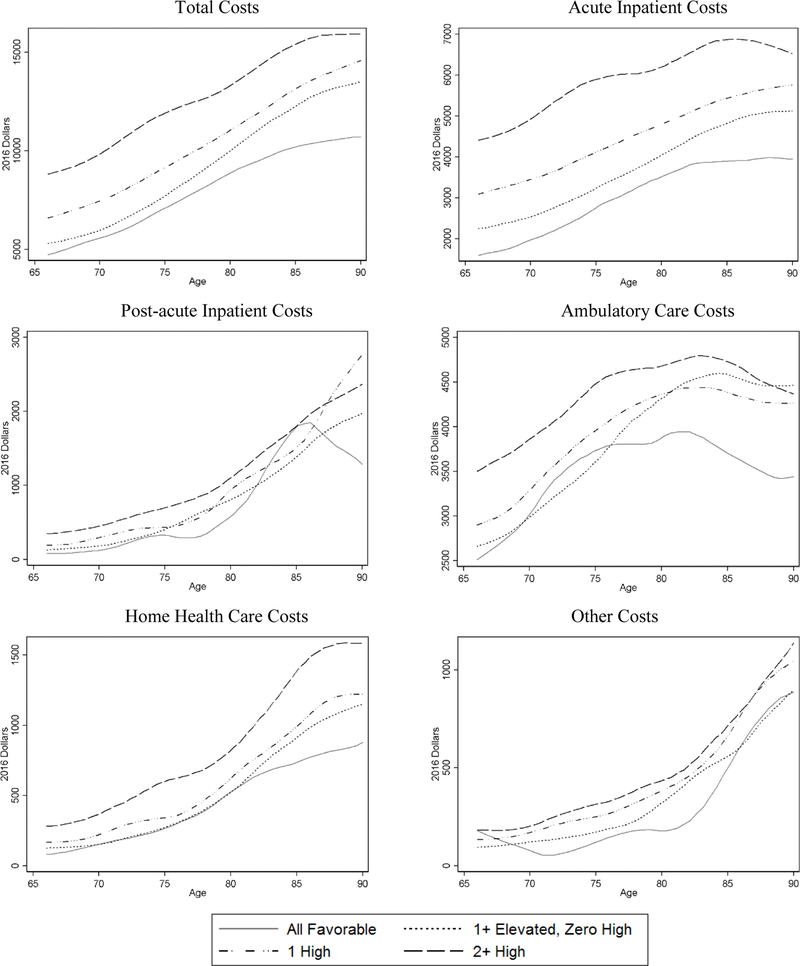
Among the 17,195 CHA participants, 5.5% (951) had all favorable CVH, 20.3% (3,496) had one or more risk factors elevated but none high, 40.9% (7,040) had 1 risk factor high, and 33.2% (5,708) had 2+ high risk factors at baseline (Table 3). Table 2 shows the percent distribution of each risk factor by CVH strata. For example, nearly 33% of the participants in the 2+ high group were obese and 62% were current smokers, compared to 5% and 38% respectively in the 1 High group. On average, less favorable CVH strata were slightly older, had fewer women, and had lower educational attainment than the all favorable stratum. Overall, medical care utilization and costs (unadjusted) were substantially lower for individuals with all favorable CVH than those with less favorable CVH across all care settings (Table 3). In addition, the average costs by age (unadjusted) were generally lower for individuals with all favorable CVH for all types of care (Figure 1). Across the cost distribution for each type of care, costs at various percentiles were also generally lower for individuals with all favorable CVH (Supplemental Figure 1).
Table 3.
Summary Statistics by CVH Strata
| CVH Strata |
|||||||
|---|---|---|---|---|---|---|---|
| All Favorable | 1+ Elevated, None High | 1 High | 2 + High | P-value | |||
| Sample Characteristics: | |||||||
| Avg. Age at Follow-up (years) | 74.5 | 75.8 | 76.0 | 76.3 | <0.001 | ||
| Female, % | 61.9 | 41.1 | 41.6 | 36.6 | <0.001 | ||
| White, % | 88.2 | 91.5 | 90.6 | 90.7 | 0.194 | ||
| Black, % | 6.3 | 5.2 | 6.6 | 6.9 | 0.005 | ||
| Avg. Age at CV Risk Measurement (years) | 37.9 | 41.2 | 42.5 | 44.3 | <0.001 | ||
| With a High School Degree, % | 34.6 | 34.6 | 38.5 | 39.9 | <0.001 | ||
| With a More than High School Degree, % | 55.6 | 52.0 | 43.6 | 35.7 | <0.001 | ||
| Utilization and Costs: | |||||||
| Avg. No. of Acute Inpatient Admissions | 0.19 | 0.24 | 0.29 | 0.38 | <0.001 | ||
| Avg. Acute Inpatient Length of Stay (days) | 0.91 | 1.2 | 1.6 | 2.1 | <0.001 | ||
| Avg. No. of Post-acute inpatient admissions | 0.04 | 0.04 | 0.05 | 0.07 | <0.001 | ||
| Avg. Post-acute inpatient Length of Stay (days) | 0.97 | 1.3 | 1.5 | 1.9 | <0.001 | ||
| Avg. No. of Ambulatory Care Visits | 14.6 | 15.4 | 15.8 | 17.1 | <0.001 | ||
| Avg. No. of Home Health Visits | 1.4 | 2.0 | 2.5 | 3.8 | <0.001 | ||
| Total Costs, $ | Median | 1,548 | 1,819 | 1,854 | 2,241 | <0.001 | |
| Mean | 6,903 | 8,312 | 9,613 | 12,111 | <0.001 | ||
| Acute Inpatient Costs, $ | Median | 0 | 0 | 0 | 0 | − | |
| Mean | 2,601 | 3,378 | 4,202 | 5,742 | <0.001 | ||
| Post-acute Inpatient Costs, $ | Median | 0 | 0 | 0 | 0 | − | |
| Mean | 454 | 628 | 746 | 948 | <0.001 | ||
| Ambulatory Care Costs, $ | Median | 1,464 | 1,679 | 1,665 | 1,906 | <0.001 | |
| Mean | 3,343 | 3,631 | 3,806 | 4,298 | <0.001 | ||
| Home Health Care Costs, $ | Median | 0 | 0 | 0 | 0 | − | |
| Mean | 317 | 405 | 498 | 723 | <0.001 | ||
| Other Costs*, $ | Median | 0 | 0 | 0 | 0 | − | |
| Mean | 187 | 270 | 361 | 401 | <0.001 | ||
| Sample Size (person-year) | 8,226 | 34,945 | 64,980 | 50,155 | <0.001 | ||
| Number of CHA Participants | 951 | 3,496 | 7,040 | 5,708 | <0.001 | ||
Notes: Total sample size is 158,306 person-years. Costs are measured in 2016 dollars. P-values are obtained from Wald tests for the equality of the sample characteristics among the four risk strata.
*Other costs include costs for hospice care and durable medical equipment.
Figure 1.
Average Annual Medical Care Costs by Age and CVH Strata
Notes: Plots are local polynomial smoothed lines of average annual medical cost by age (unadjusted).
Medical Care Utilization: Estimates from Negative Binomial Regressions
After adjusting for differences in observed characteristics, individuals with less favorable baseline CVH used significantly more medical care services later in life than individuals with favorable CVH; and the poorer the baseline CVH, the greater the utilization (Table 4). For example, while approximately 19% of the all favorable stratum had acute inpatient admissions per year, roughly 34% of the 2+ high risk stratum did (79% greater, p-value < 0.001). Additionally, the number of home health visits per person per year for the 2+ high risk stratum was 1.76 (125%, p-value < 0.001) greater than that of the all favorable stratum. We observed few significant differences in the association by sex (Panel B of Table 4). Across all categories examined, the gaps in medical care utilization between individuals with less favorable CVH and those with all favorable CVH widened with age (Figure 2).
Table 4.
Differences in Annual Medicare Care Utilization Per Person between Less Favorable CVH and All Favorable (N = 158,306)
| Acute Inpatient | Post-acute Inpatient | Ambulatory Care | Home health | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Admissions | Length of Stay | No. of Admissions | Length of Stay | No. of Visits | No. of Visits | |||
| Panel A: Overall | ||||||||
| All Favorable (ref. mean) | 0.19 | 0.91 | 0.04 | 0.97 | 14.57 | 1.41 | ||
| Marginal effect from ref.; Mean [SE] | ||||||||
| 1+ Elevated, None High | 0.04*** | 0.29*** | 0.006** | 0.15 | 0.80 | 0.56** | ||
| (0.01) | (0.09) | (0.003) | (0.12) | (0.46) | (0.24) | |||
| 1 High | 0.09*** | 0.63*** | 0.01*** | 0.30** | 1.40*** | 0.93*** | ||
| (0.01) | (0.09) | (0.003) | (0.12) | (0.44) | (0.22) | |||
| 2 + High | 0.15*** | 1.03*** | 0.02*** | 0.59*** | 3.04*** | 1.76*** | ||
| (0.01) | (0.09) | (0.003) | (0.12) | (0.45) | (0.22) | |||
|
Panel B: P-values on the Interaction Term between CVH and Female Indicator | ||||||||
| All Favorable | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 1+ Elevated, None High | 0.94 | 0.47 | 0.95 | 0.47 | 0.46 | 0.78 | ||
| 1 High | 0.95 | 0.59 | 0.93 | 0.56 | 0.37 | 0.93 | ||
| 2 + High | 0.91 | 0.78 | 0.82 | 0.60 | 0.06 | 0.73 | ||
Notes: Each column within a panel is from a separate regression. The sample size for each regression is 158,306. Panel A shows coefficients (marginal effects calculated at the mean of covariates) and cluster-robust standard errors (in parenthesis) obtained from negative binomial models. The coefficients in Panel A represent the per person difference in the dependent variable between less favorable CVH strata and all favorable CVH strata. In Panel B, we estimated separate models that also included the interaction term between CVH and the female indicator and the values in cells are the p-values on the interaction terms. All regressions also control for age at follow-up, state of residence, year, baseline age, race, sex, baseline education, and whether the participant died during the year.
p-value<0.05
p-value<0.01
Figure 2.
Estimated Differences in Utilization between Less Favorable CVH and All Favorable by Age
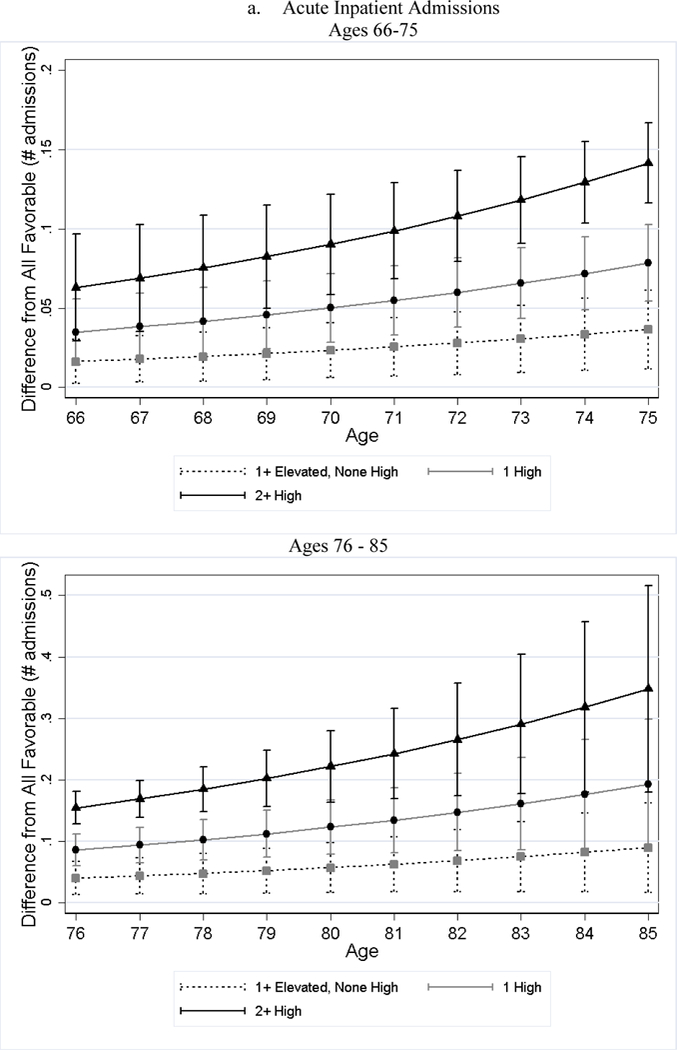
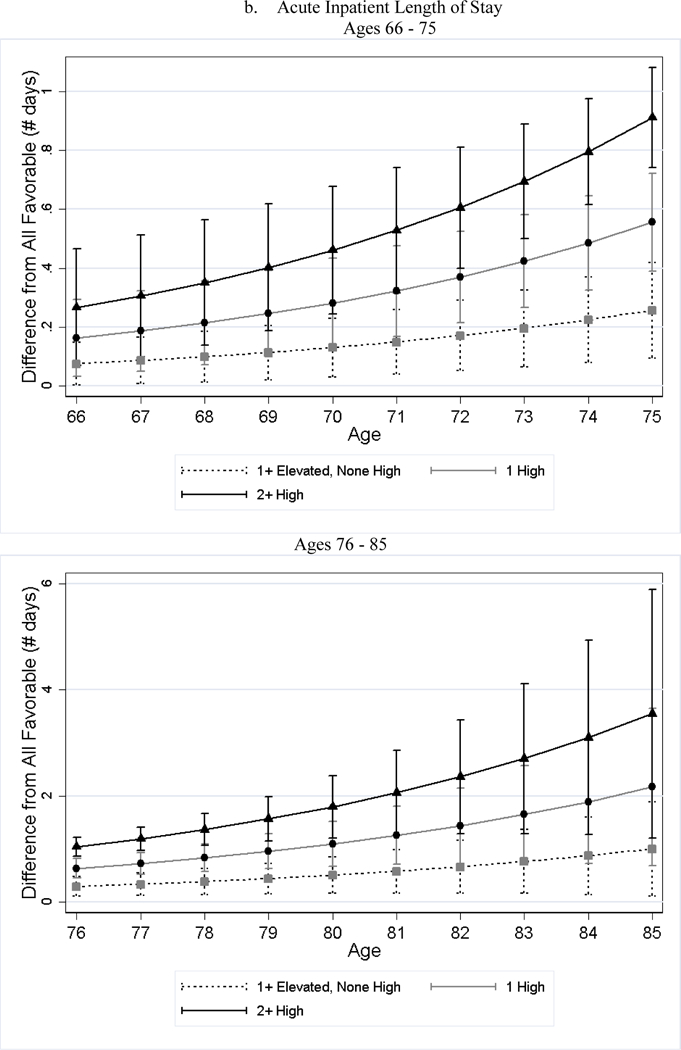
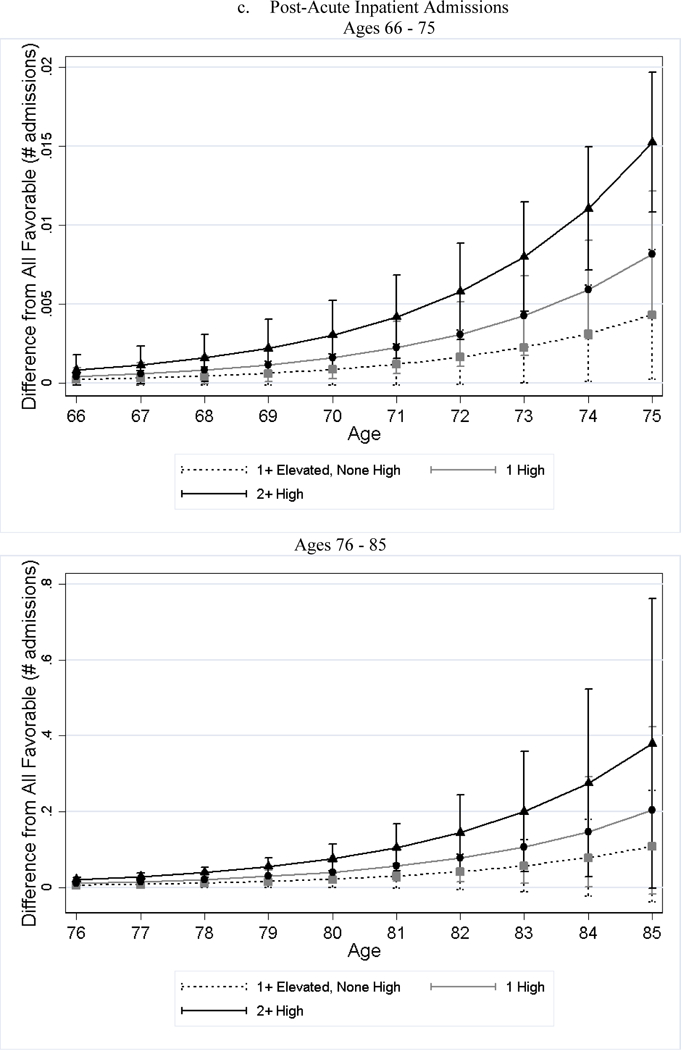
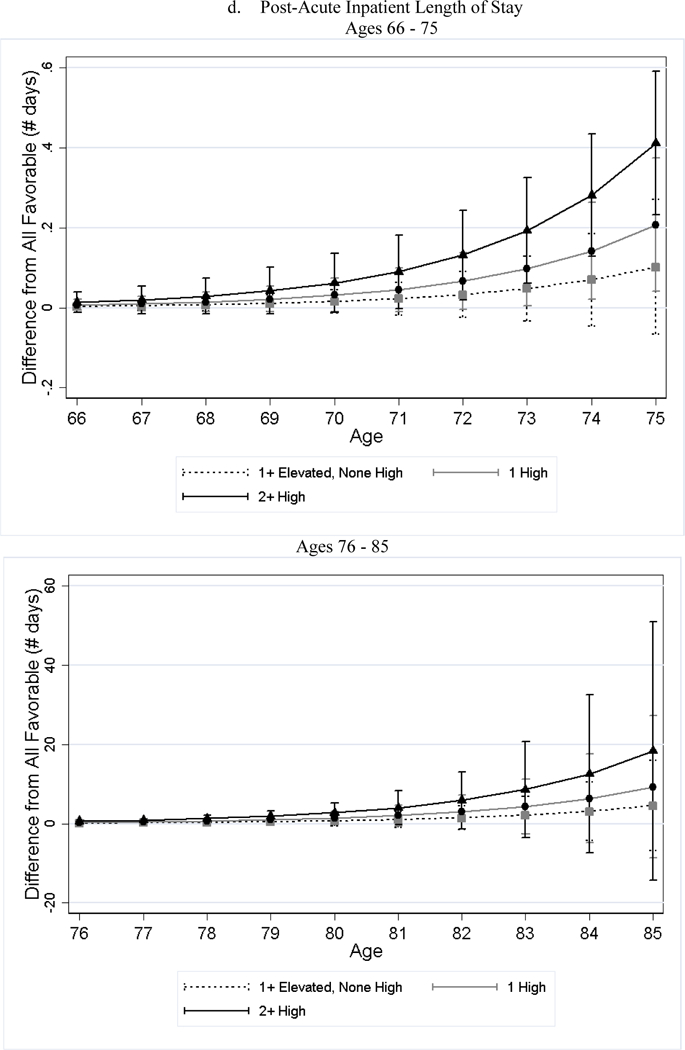
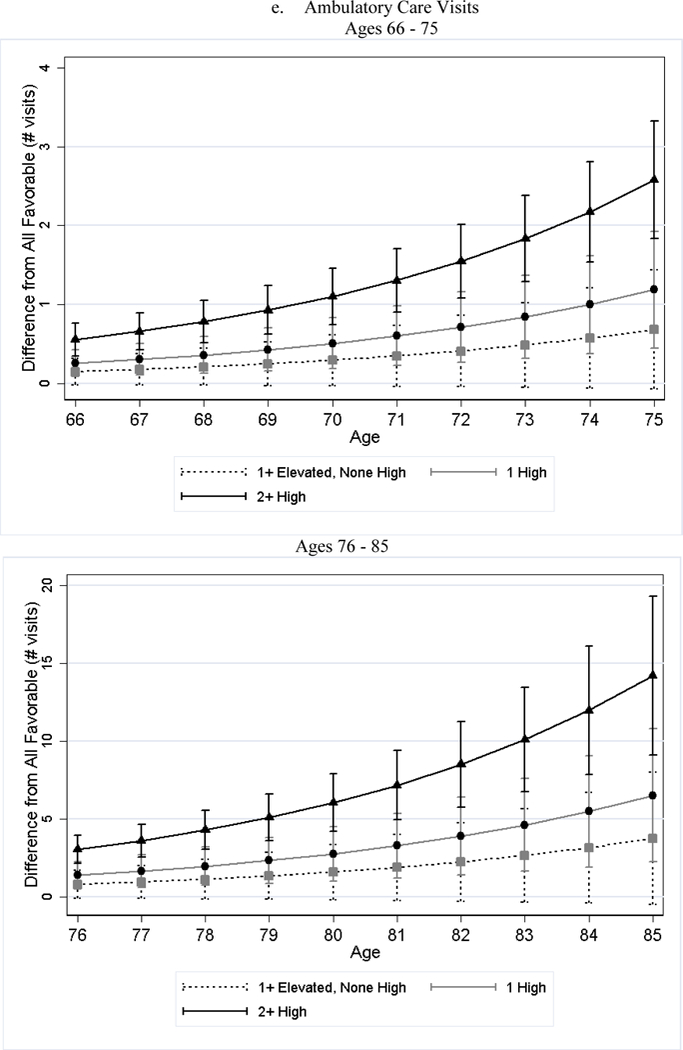
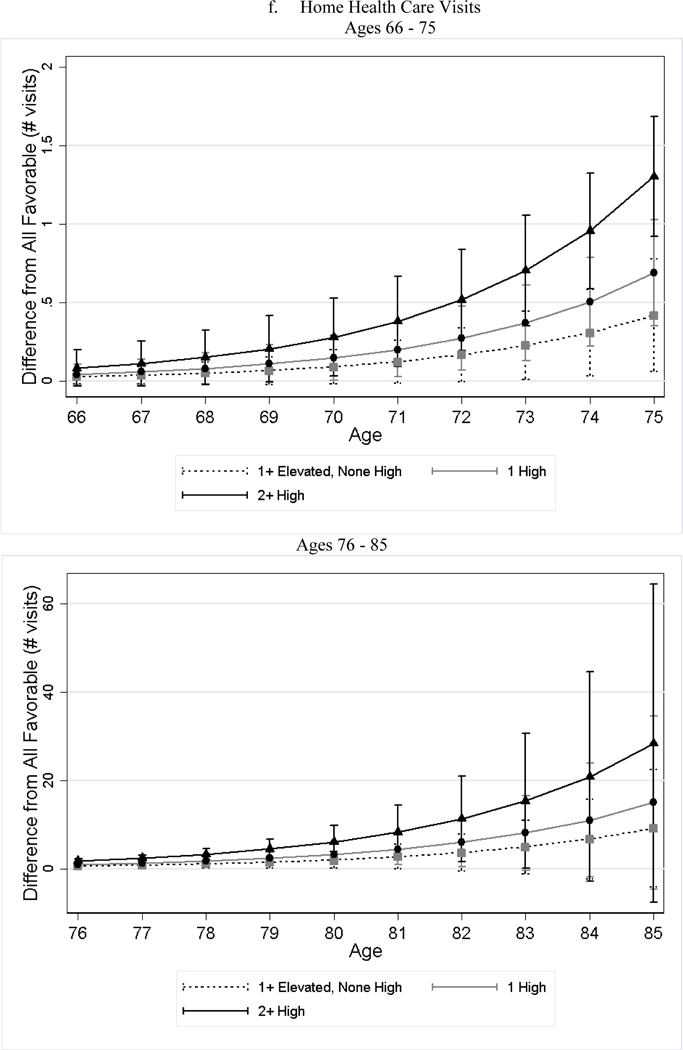
Notes: All favorable CVH stratum is the reference stratum. Estimated difference in utilization between less favorable CVH strata and all favorable CVH stratum and their 95% CI are obtained from negative binomial regressions. We plotted the differences in two figures because the scale is too large that the lines are overlapped if all ages are plotted in one figure.
Additionally, both CVD and non-CVD related utilizations were substantially higher for individuals in less favorable baseline CVH (Supplemental Table 1). For example, compared to the all favorable stratum, the number of CVD related acute inpatient admissions per person per year was 0.05 greater (250%, p-value < 0.001) for the 2+ high risk stratum, and the number of non-CVD related admissions was 0.10 (59%, p-value < 0.001) greater. While the absolute differences were greater for non-CVD related utilization, the relative (%) differences were greater for CVD related utilizations.
Medical Care Costs: Estimates from Quantile Regressions and GLM
Across all quantiles, less favorable CVH was associated with higher costs including total costs and costs by primary reason for care and care setting (Table 5). For example, compared to the all favorable stratum, the median of annual Medicare payment amount per person was $640 higher (p-value < 0.001) for the 2+ high risk stratum (41% higher than the median annual costs of $1,548 for the all favorable group) and the mean of annual Medicare payment amount per person was $4,628 (67%, p-value < 0.001) higher. The cost differences (in dollars) per person per year between less favorable CVH strata and all favorable stratum were greatest for acute inpatient care, followed (in descending order) by ambulatory care, post-acute inpatient care, home health care, and other care (hospice and durable medical equipment).
Table 5.
Differences in Annual Medical Care Costs between Less Favorable CVH and All Favorable (N = 158,306)
| Quantiles Regressions | GLM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q25 | Q50 | Q75 | Q90 | Q95 | |||||||||
| Total Costs | |||||||||||||
| All Favorable (ref., $) | 416 | 1,548 | 4,844 | 16,962 | 35,084 | 6,903 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | 89*** | 207*** | 368 | 1,266 | 4,001*** | 1,157*** | |||||||
| (30) | (66) | (189) | (809) | (1,334) | (440) | ||||||||
| 1 High | 82*** | 282*** | 892*** | 4,438*** | 10,219*** | 2,429*** | |||||||
| (28) | (64) | (187) | (839) | (1,226) | (425) | ||||||||
| 2 + High | 164*** | 640*** | 3,019*** | 13,087*** | 21,572*** | 4,628*** | |||||||
| (30) | (72) | (240) | (989) | (1,403) | (428) | ||||||||
| CVD-related Costs | |||||||||||||
| All Favorable (ref., $) | 0 | 0 | 0 | 380 | 1,116 | 616 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | − | − | 1 | 34 | 27 | 743*** | |||||||
| (2) | (17) | (52) | (197) | ||||||||||
| 1 High | − | − | 7*** | 145*** | 424*** | 1,140*** | |||||||
| (2) | (32) | (98) | (190) | ||||||||||
| 2 + High | − | − | 175*** | 1,058*** | 7,319*** | 1,758*** | |||||||
| (13) | (78) | (633) | (186) | ||||||||||
| Non-CVD-related Costs | |||||||||||||
| All Favorable (ref., $) | 391 | 1,448 | 4,464 | 14,810 | 31,784 | 6,287 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | 83*** | 188*** | 274 | 512 | 3,136** | 665 | |||||||
| (26) | (62) | (168) | (671) | (1,268) | (371) | ||||||||
| 1 High | 67*** | 226*** | 636*** | 2,825*** | 7,260*** | 1,507*** | |||||||
| (25) | (59) | (164) | (692) | (1,324) | (359) | ||||||||
| 2 + High | 121*** | 479*** | 1,886*** | 8,239*** | 15,677*** | 3,013*** | |||||||
| (26) | (64) | (200) | (827) | (1,435) | (363) | ||||||||
| Acute Inpatient Costs | |||||||||||||
| All Favorable (ref., $) | 0 | 0 | 0 | 6,386 | 17,137 | 2,601 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | − | − | − | 322 | 2,146** | 716*** | |||||||
| (305) | (956) | (268) | |||||||||||
| 1 High | − | − | − | 2,467*** | 6,152*** | 1,496*** | |||||||
| (438) | (983) | (258) | |||||||||||
| 2 + High | − | − | − | 7,676*** | 13,254*** | 2,673*** | |||||||
| (493) | (1,053) | (257) | |||||||||||
| Post-acute Inpatient Costs | |||||||||||||
| All Favorable (ref., $) | 0 | 0 | 0 | 0 | 0 | 454 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | − | − | − | − | 9 | 217*** | |||||||
| (6) | (69) | ||||||||||||
| 1 High | − | − | − | − | 11 | 313*** | |||||||
| (6) | (66) | ||||||||||||
| 2 + High | − | − | − | − | 21*** | 494*** | |||||||
| (7) | (66) | ||||||||||||
| Ambulatory Care Costs | |||||||||||||
| All Favorable (ref., $) | 405 | 1,464 | 3,943 | 7,746 | 11,766 | 3,343 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | 83*** | 171*** | 242 | 593 | 882** | 201 | |||||||
| (28) | (61) | (142) | (311) | (379) | (160) | ||||||||
| 1 High | 70*** | 200*** | 399*** | 1,182*** | 1,704*** | 419*** | |||||||
| (27) | (58) | (137) | (309) | (385) | (156) | ||||||||
| 2 + High | 137*** | 452*** | 1,070*** | 2,502*** | 3,511*** | 921*** | |||||||
| (28) | (63) | (145) | (413) | (157) | |||||||||
| Home Health Costs | |||||||||||||
| All Favorable (ref., $) | 0 | 0 | 0 | 0 | 144 | 317 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1 Elevated, None High | − | − | − | 4** | 21 | 99** | |||||||
| (1) | (12) | (50) | |||||||||||
| 1 High | − | − | − | 5*** | 37*** | 200*** | |||||||
| (1) | (13) | (50) | |||||||||||
| 2 + High | − | − | − | 12*** | 1,310*** | 356*** | |||||||
| (2) | (173) | (47) | |||||||||||
| Other Costs | |||||||||||||
| All Favorable (ref., $) | 0 | 0 | 0 | 85 | 282 | 187 | |||||||
| Marginal effect from ref.; Mean [SE] | |||||||||||||
| 1+ Elevated, None High | − | − | − | 8*** | 26*** | 62 | |||||||
| (2) | (7) | (48) | |||||||||||
| 1 High | − | − | − | 15*** | 82*** | 136*** | |||||||
| (2) | (18) | (45) | |||||||||||
| 2 + High | − | − | − | 116*** | 415*** | 175*** | |||||||
| (18) | (50) | (44) | |||||||||||
Notes: Coefficients (cluster-robust standard errors in parenthesis) are obtained from quantile regressions. Each column by outcome is one regression. The sample size for each regression is 158,306. All regressions also control for age at follow-up, state of residence, year, baseline age, race, sex, baseline education, and whether the participant died during the year.
p<0.05
p<0.01s
There were some statistically significant sex differences in the association between CVH and CVD related costs (Supplemental Table 2 and Supplemental Table 3). Specifically, the cost differences at the 75th, 90th, and 95th percentile between 2+ High and all favorable and between 1 High and all favorable were smaller for women than men. However, the differences in mean costs were statistically indistinguishable.
Consistent with greater absolute differences in non-CVD related utilization (Supplemental Table 1), the cost differences (in dollars) associated with less favorable CVH were greater for non-CVD related costs (Table 5). For example, compared to the all favorable stratum, the 90th percentile costs for the 2+ high stratum was $8,239 greater (p-value < 0.001) per person per year for non-CVD related cost, compared to $1,058 greater (p-value < 0.001) for CVD related cost.
Sensitivity Analysis
Our estimates of utilization and cost differences between less favorable CVH and all favorable CVH represent annual differences per person. Among the 17,195 participants, approximately 49% had died by year 2010, including 26% of the all favorable stratum, 38% of the 1+ elevated, none high stratum, 48% of the 1 high stratum, and 61% of the 2+ high stratum. Because greater cardiovascular risk burden earlier in life is associated with shorter life expectancy,15,32 and to gauge how the end of life care may affect our results, we conducted stratified analyses on those were still alive in 2010 (hereafter, survivors) and those who died in sample by 2010 (hereafter, decedents). Compared to the stratified estimates (Supplemental Table 4a and 4b), our main estimates on utilization and costs (Table 4 and Table 5) are closer to those from the decedent sample than from the survivor sample.
Additionally, those with less favorable CVH were followed slightly longer, specifically, the average length of follow-up was 8.65 years, 9.99 years, 9.23 years, and 8.79 years for those with favorable CVH, with 1+ Elevated, None High, with 1 High, and with 2+ High (p-value < 0.001). However, our results are robust (almost identical) when the length of follow-up in Medicare was included as an additional regressor, suggesting that our results were not driven by the differences in the lengths of follow-up between the CVH groups (Supplemental Table 5).
Discussion
In this paper, we used a composite measure of cardiovascular health earlier in adulthood that incorporated major risk factors including blood pressure, total cholesterol, diabetes, BMI, and smoking and assessed its association with medical care utilization and costs at older ages, up to 40 years later. We found that favorable cardiovascular health in young and middle adulthood was associated with significantly lower medical care utilization and costs at older ages (66 years and older) across all care settings.
Individuals with all favorable baseline CVH had approximately half as many acute inpatient admissions (which were also half the length), post-acute inpatient admissions, home health care visits, and three fewer ambulatory care visits per person per year than those with poor baseline CVH at older ages. Moreover, the differences in utilization persist through life and become more pronounced with age. These findings suggest that individuals with favorable CVH earlier in adulthood had lower morbidity burden by age at old ages, indicating compressed morbidity.11 Consistent with patterns of utilization, individuals with favorable baseline CVH had significantly lower costs across all settings. The median amount that Medicare paid for medical care per person per year was $640 smaller for individuals with all favorable CVH and the mean amount was $4,628 smaller. Because we used the amount Medicare paid for medical care goods and services as our measure of costs, our costs do not include patient cost-sharing and, therefore, our cost estimates may understate the actual cost-savings associated with favorable CVH.
Past research indicates that better cardiovascular health (CVH) is associated with lower risks of diseases and medical care costs at later ages.8–19 Other studies of the CHA cohort, all but one with shorter follow-ups, found that favorable CVH in middle age was associated with lower all-cause morbidity and lower medical costs later in life.11–15 Studies of other cohorts, albeit with smaller cohorts or shorter follow-ups, found that better CVH was associated with lower CVD risks and lower CVD and non-CVD costs.16, 18 Additionally, cross-sectional comparisons of individuals with different CVH status also suggest that favorable CVH was associated with lower healthcare expenditures and utilization.17,19
Additional to the longer follow-up (up to 40 years), to our knowledge this study is the first to dissect the overall healthcare utilization and costs by all settings of care. Because we used all Medicare Part A and Part B claims for fee-for-service Medicare enrollees, our estimates represent closely the full resource and cost savings. This approach provides a unique lens to understand how and where favorable CVH earlier in life may be resulting in reduced costs in the long term. Importantly, our results suggest that investing in health early in life may have substantial health and financial benefits later in life.
This study has several limitations. One limitation of this study is that we cannot assess changes in CVH status between baseline and Medicare claims. However, prior studies have found baseline CVH to be strongly predictive of later life CVH status.11,14,32,33 Moreover, using Medicare claims we examined the prevalence of hypertension (ICD-9 codes: 401), diabetes (ICD-9 codes: 250), obesity (ICD-9 codes: 278), and hyperlipidemia (ICD-9 codes: 272.4) which corresponded to the CVH risk factors assessed as baseline. The results (Supplemental Table 6) show that participants with less favorable baseline CVH continued to have poorer CVH at baseline than those with more favorable CVH (p-value <0.01). If, however, individuals with less favorable CVH at baseline were more likely to improve their subsequent health behaviors than individuals with favorable CVH, then our estimates would likely be an underestimate and therefore conservative. An additional limitation is that there were no data on diet, physical activity, or family history of CVD. Information on subsequent lifestyle factors (e.g., diet and physical activity), various medical care after baseline, and family history of CVD would provide valuable insights into these factors’ potential mediating roles between baseline CVH and later-life health care costs and would generate relevant policy implications. To this end, our results reflect the average differences in later-life medical care utilization and costs associated with baseline CVH. While we are unable to separately estimate the mediating impact of subsequent lifestyle factors and medical care, our estimates suggest that baseline CVH remain a strong predictor of medical care utilization and costs up to 40 years later and are therefore policy relevant.
If individuals with less favorable baseline CVH were to receive more medical care and more likely to adjust lifestyle factors following the baseline (because they were sicker), then our results may be an underestimate and therefore conservative. If individuals with more favorable CVH were to receive more medical care and more likely to adjust lifestyle factors following the baseline, then our estimates may be an overestimate. However, the second situation seems less likely especially because we also controlled for education (which is also a good proxy for income) – comparisons are made between individuals with the same levels of education.
A third limitation is that claims data were not available for managed care enrollees. If individuals with different baseline CVH non-randomly switched between fee-for-service (FFS) and managed care between periods, the estimates may potentially be biased. To assess such a possibility, we included in the model three additional covariates that measure potential shifts in sample composition by CVH strata and year: fraction switched from FFS to managed care, fraction switched from managed care to FFS, and fraction stayed in managed care. The inclusion of these additional covariates had limited impact on our estimates (Supplemental Table 7). Additionally, the estimates on costs by care setting and primary reason for admission/visit are also close to the main estimates shown in Table 4 and 5. Therefore, this does not seem to have affected our results.
Additionally, our analysis sample did not include CHA participants who died before the age 65 due to the lack of Medicare claims data. Using CMS Vital Status File (2012), we found that 4.9% of CHA participants with all favorable CVH died before the age 65, while 4.7% of the 1+ elevated, none high group, 8.3% of the 1 high group, and 13.1% of the 2+ high group died before the age 65. This evidence may suggest potential selective mortality, which if true, would suggest that our results excluding these individuals may be conservative (biased downward). A fifth limitation is the lack of Medicare Part D drug claims, which, however, were not available before 2006 – the year when Part D was implemented. As a result, our measure of total costs does not include the costs for prescription drugs filled under Medicare Part D benefits. Assume that the medication usage follows a similar pattern with other medical utilizations we examined in the paper, the differences in total costs and therefore cost-savings associated with favorable CVH are likely underestimated. Finally, our results may not generalize to populations with different demographic characteristics from the CHA sample, for example, the CHA sample is predominantly White. Due to the small sample sizes for non-White races, we are unable to identify whether there is heterogeneity in the association between baseline CVH and later-life costs by race.
Our findings are relevant to policy makers, public health, and the general audience. Up to 40 years later, cardiovascular health status in young and middle adulthood remains a strong predictor of medical care utilization and costs (therefore, overall health). Favorable CVH in young and middle adulthood was associated with significantly lower Medicare spending during Medicare eligibility, raising the possibility that primordial and primary prevention of cardiovascular risks may provide substantial savings to Medicare and taxpayers in the long term. In fact, many of the major cardiovascular risk factors are modifiable and preventable through changes in life style such as diet, physical activity and use of cost effective lipid or blood pressure lowering medication.34–37 Although cost analysis of early prevention efforts is not the focus of this study, our estimates suggest potentially substantial long-term benefits from having a favorable CVH at young and middle age.
Supplementary Material
Acknowledgments
Funding: This work was supported by a grant from NHLBI to Dr. Allen (R01–60036640).
Appendix A. Diagram for Sample Selection
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Centers for Medicare and Medicaid Services (CMS). [December 20, 2016] National Health Expenditures 2015 Highlights https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf
- 2.Centers for Medicare and Medicaid Services. [December 19, 2016] National Health Expenditure (NHE) Fact Sheet https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nhe-fact-sheet.html
- 3.Moses H, Matheson DHM, Dorsey ER, George BP, Sadoff D, Yoshimura S. The Anatomy of Health Care in the United States. JAMA 2013;310(18):1947–1964. doi: 10.1001/jama.2013.281425 [DOI] [PubMed] [Google Scholar]
- 4.Kaiser Family Foundation. [December 20, 2016] The Rising Cost of Living Longer: Analysis of Medicare Spending by Age for Beneficiaries in Traditional Medicare http://files.kff.org/attachment/report-the-rising-cost-of-living-longer-analysis-of-medicare-spending-by-age-for-beneficiaries-in-traditional-medicare
- 5.World Health Organization. [December 20, 2016] Global Health and Aging October 2011. http://www.who.int/ageing/publications/global_health.pdf
- 6.Office of Disease Prevention and Health Promotion. [December 20, 2016] Healthy People 2020: Older Adults https://www.healthypeople.gov/2020/topics-objectives/topic/older-adults#2
- 7.The American Heart Association. [April 03, 2017] Cardiovascular Disease: A Costly Burden For America - Projections Through 2035 http://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_491543.pdf
- 8.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. The New England journal of medicine 2012;366(4):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM. Cardiovascular health and protection against CVD: more than the sum of the parts? Circulation 2014;130(19):1671–3. [DOI] [PubMed] [Google Scholar]
- 10.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Feinglass J, Guralnik JM, Greenland P, Stamler J. Favorable cardiovascular risk profile in middle age and health-related quality of life in older age. Archives of internal medicine 2003;163(20):2460–8. [DOI] [PubMed] [Google Scholar]
- 11.Allen NB, Zhao L, Lei L, Daviglus M, Liu K, Fries J, Shih T, Garside DB,Vu TH, Stamler J, Lloyd-Jones DM. Favorable Cardiovascular Health, Compression of Morbidity and Healthcare Costs: 40-Year Follow-up of the Chicago Heart Association Detection Project in Industry. Circulation 2017;135(18):1693–1701. doi: 10.1161/CIRCULATIONAHA.116.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daviglus ML, Liu K, Greenland P, Dyer AR, Garside DB, Manheim L, Lowe LP, Rodin M, Lubitz J, Stamler J. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. The New England journal of medicine 1998;339(16):1122–9. [DOI] [PubMed] [Google Scholar]
- 13.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Greenland P, Manheim LM, Dyer AR, Wang R, Lubitz J, Manning WG, Fries JF, Stamler J. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Archives of internal medicine 2005;165(9):1028–34. [DOI] [PubMed] [Google Scholar]
- 14.Daviglus ML, Stamler J, Pirzada A, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA 2004;292:1588–1592. [DOI] [PubMed] [Google Scholar]
- 15.Stamler J, Dyer AR, Shekelle RB, Neaton J, Stamler R. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology 1993;82(2–3):191–222. [DOI] [PubMed] [Google Scholar]
- 16.Willis BL, DeFina LF, Bachmann JM, Franzini L, Shay CM, Gao A, et al. Association of ideal cardiovascular health and long-term healthcare costs. American journal of preventive medicine 2015;49(5):678–85. [DOI] [PubMed] [Google Scholar]
- 17.Valero-Elizondo J, Salami JA, Ogunmoroti O, Osondu CU, Aneni EC, Malik R, et al. Favorable cardiovascular risk profile is associated with lower healthcare costs and resource utilization. Circulation: Cardiovascular Quality and Outcomes 2016;9(2):143–53. [DOI] [PubMed] [Google Scholar]
- 18.Aaron KJ, Colantonio LD, Deng L, Judd SE, Locher JL, Safford MM, et al. Cardiovascular Health and Healthcare Utilization and Expenditures Among Medicare Beneficiaries: The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc 2017;6(2). 10.1161/JAHA.116.005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osondu CU, Aneni EC, Valero-Elizondo J, Salami JA, Rouseff M, Das S, et al. , editors. Favorable Cardiovascular Health Is Associated With Lower Health Care Expenditures and Resource Utilization in a Large US Employee Population: The Baptist Health South Florida Employee Study. Mayo Clinic Proceedings; 2017: Elsevier. [DOI] [PubMed] [Google Scholar]
- 20.Chronic Condition Data Warehouse. [January 20, 2017] CCW Technical Guidance: Getting Started with CMS Medicare Administrative Research Files.Version 2.3 https://www.ccwdata.org/web/guest/technical-guidance-documentation.
- 21.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017. doi: 10.1161/cir.0000000000000485. [DOI] [PMC free article] [PubMed]
- 22.Hsia RY, Akosa Antwi Y, Weber E. Analysis of variation in charges and prices paid for vaginal and caesarean section births: a cross-sectional study. BMJ Open 2014;4:e004017. doi: 10.1136/bmjopen-2013-004017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaca Z, Moore B. Geographic Variation in Hospital Inpatient List Prices in the United States, 2013. HCUP Statistical Brief #209 August 2016. Agency for Healthcare Research and Quality, Rockville, MD: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb209-GeographicVariation-Hosptial-Inpatient-Prices.pdf. [PubMed] [Google Scholar]
- 24.Cooper Z, Craig SV, Gaynor M, Reenen JV. The Price Ain’t Right? Hospital Prices and Health Spending on the Privately Insured NBER Working Paper #21815. 2015. doi: http://www.nber.org/papers/w21815 [DOI] [PMC free article] [PubMed]
- 25.Research Data Assistance Center. [December 20, 2016] Claim Payment Amount https://www.resdac.org/cms-data/variables/Claim-Payment-Amount-0
- 26.MaCurdy T, Shafrin J, DeLeire T, DeVaro J, Bounds M, Pham D, Chai A. Geographic Adjustment of Medicare Payments to Physicians: Evaluation of IOM Recommendations Centers for Medicare and Medicaid Services Web site; 2012. https://www.cms.gov/medicare/medicare-fee-for-service-payment/physicianfeesched/downloads/geographic_adjustment_of_medicare_physician_payments_july2012.pdf [Google Scholar]
- 27.MaCurdy T, Bhattacharya J, Shafrin J, Au-Yeung A, Bashour H, Chicklis C, Cronen K, Lipton B, Saneinejad S, Shrestha E, Zaidi S. Geographic Variation in Spending, Utilization and Quality: Medicare and Medicaid Beneficiaries Committee on Geographic Variation in Health Care Spending and Promotion of High Value Care; 2013. doi: http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2013/Geographic-Variation/Sub-Contractor/Acumen-Medicare-Medicaid.pdf [Google Scholar]
- 28.Skinner J, Fisher ES, Reflections on Geographic Variation in U.S. Health Care The Dartmouth Institute For Health Policy and Clinical Practice. doi: http://www.dartmouthatlas.org/downloads/press/Skinner_Fisher_DA_05_10.pdf Published March 31, 2010. Updated May 12, 2010. Accessed December 18, 2016. [Google Scholar]
- 29.Kaiser Family Foundation. [December 18, 2016] 10 FAQs: Medicare’s Role in End-of-life Care http://files.kff.org/attachment/10-FAQs-Medicares-Role-in-End-of-Life-Care
- 30.Cameron AC,Trivedi PK, Microeconometrics Using Stata 2nd ed. College Station, TX: Stata Press; 2010. [Google Scholar]
- 31.Parente PMDC and Santos Silva JMC Quantile Regression with Clustered Data. Journal of Econometric Methods 2016; 5(1):1–15 [Google Scholar]
- 32.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA : the journal of the American Medical Association 1999;282(21):2012–8. [DOI] [PubMed] [Google Scholar]
- 33.Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd-Jones DM. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 2012; 125(8): 996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000; 343: 16–22. DOI: 10.1056/NEJM200007063430103 [DOI] [PubMed] [Google Scholar]
- 35.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men. Circulation 2006; 114: 160–167. 10.1161/CIRCULATIONAHA.106.621417 [DOI] [PubMed] [Google Scholar]
- 36.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation 2011;124(8):967–90. [DOI] [PubMed] [Google Scholar]
- 37.Kahn R, Robertson RM, Smith R, Eddy D. The Impact of Prevention on Reducing the Burden of Cardiovascular Disease. Circulation 2008;118:576–585. DOI: 10.1161/circulationaha.108.190186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.