Abstract
Background
Chlorhexidine as a skin surface antiseptic has been shown to be ineffective with respect to reducing Proprionibacterium acnes colonization within the dermis. The purpose of the present study was to determine whether the application of aqueous chlorhexidine solution to the dermal layer decreased P. acnes colonization during open shoulder surgery.
Methods
The present study enrolled 50 patients who were undergoing open shoulder surgery. Patients received standard antimicrobial preparation. Three dermal swabs were taken from each patient: swab 1 following skin incision; swab taken 2 minutes to 5 minutes post-application of aqueous chlorhexidine to the dermis; and swab 3 taken 60 minutes post-application.
Results
Mean age was 57.5 years (22 males, 28 females). There were 21 patients (42%) with P. acnes present on any dermal swab. There were significantly more P. acnes positive cultures identified at swab 3 compared to swab 1 (p = 0.043). In nine patients with positive P. acnes at cultures swab 1, eight also isolated P. acnes after at swabs 2 or 3. Males were significantly more likely to have P. acnes on any swab (p < 0.001). Positive P. acnes cultures were significantly more common in patients ≤50 years (p < .001). None of the patients had any clinical signs of infection at a minimum of 1 year following surgery.
Conclusions
Dermal application of aqueous chlorhexidine during open shoulder surgery fails to eradicate or reduce P. acnes on deep cultures.
Keywords: chlorhexidine, dermal; infection, microbiology, Proprionibacterium acnes, shoulder
Introduction
Proprionibacterium acnes is a Gram-positive anaerobic rod recognized as a common pathogen in deep shoulder infection.1–3 Proprionibacterium acnes commonly colonizes the pilosebaceous glands of the dermis and is therefore more common in the trunk, neck and shoulders.4 Although highly sensitive to antimicrobials in the laboratory setting, P. acnes is the subject of extensive research as a result of challenges in its detection and eradication in vivo.5 The rapid production of a biofilm layer in vivo has been postulated as a cause for this.5
Chlorhexidine is a commonly-used antiseptic used for shoulder surgery with proven efficacy at reducing bacterial loads on the epidermis.6 Our previous study showed that skin preparation with alcoholic chlorhexidine reduced positive P. acnes cultures on the epidermis; however, it was ineffective at reducing P. acnes lying within the dermis.7 This is of concern because deep infection with P. acnes is considered to result from deep colonization within the dermal pilosebaceous glands and intra-operative seeding as a result of surgical trauma.1,8 It is not routine operating practice to apply a further antiseptic preparation to the dermis and it therefore remains unknown whether further application to the dermis intra-operatively would reduce P. acnes colonization within the surgical wound.
The aim of the present study was to determine whether application of aqueous chlorhexidine solution to the dermal layer following skin incision decreased P. acnes colonization during open shoulder surgery.
Materials and methods
Patients
We received state ethics committee approval for the present study. The study enrolled 50 consecutive patients who were undergoing elective or acute open shoulder surgery. Patients were given an information sheet and all patients provided their written informed consent. Patients aged<16 years were excluded.
Mean age was 57.5 years (range 17 years to 85 years). There were 22 males and 28 females. Details of the procedures performed are provided in Table 1.
Table 1.
Patient demographic data and results of microbiological swabs.
| Patient | Age | Sex | Operation | Dermal pre-prep | 5 min post-prep | 60 min post-prep |
|---|---|---|---|---|---|---|
| 1 | 78 | F | RTSR | – | – | – |
| 2 | 85 | F | RTSR | – | – | – |
| 3 | 81 | M | RTSR | – | P. acnes | P. acnes |
| 4 | 21 | F | Latarjet | – | P. acnes | P. acnes |
| 5 | 66 | M | RTSR | P. acnes | P. acnes | P. acnes |
| 6 | 71 | F | RTSR | P. acnes | – | P. acnes |
| 7 | 76 | F | RTSR | – | – | – |
| 8 | 64 | F | RTSR | – | – | – |
| 9 | 30 | M | ORIF proximal humerus | – | – | – |
| 10 | 46 | F | RTSR | – | – | – |
| 11 | 79 | F | RTSR | – | – | – |
| 12 | 21 | F | Latarjet | – | – | P. acnes |
| 13 | 37 | M | ACJ reconstruction | P. acnes | P. acnes | P. acnes |
| 14 | 46 | F | Open RCR | – | – | – |
| 15 | 57 | F | Open RCR | – | – | – |
| 16 | 73 | F | RTSR | – | – | – |
| 17 | 69 | F | Open RCR | – | – | – |
| 18 | 67 | M | Hemiarthroplasty | – | – | – |
| 19 | 62 | M | RTSR | – | – | P. acnes |
| 20 | 18 | M | Latarjet | P. acnes | P. acnes | P. acnes |
| 21 | 45 | M | ACJ stabilization | – | P. acnes | – |
| 22 | 79 | F | RTSR | – | – | P. acnes |
| 23 | 71 | F | Open RCR | – | – | – |
| 24 | 24 | M | ACJ stabilization | – | P. acnes | P. acnes |
| 25 | 68 | M | RTSR | – | – | – |
| 26 | 81 | M | RTSR | – | – | P. acnes |
| 27 | 18 | M | ACJ stabilization | P. acnes | P. acnes | P. acnes |
| 28 | 76 | F | RTSR | – | – | – |
| 29 | 63 | F | RTSR | – | – | – |
| 30 | 57 | M | Open RCR | – | – | – |
| 31 | 47 | M | Open RCR | P. acnes | P. acnes | P. acnes |
| 32 | 68 | M | Open RCR | – | – | – |
| 33 | 63 | F | ORIF proximal humerus | – | – | – |
| 34 | 22 | F | Open RCR | – | – | – |
| 35 | 81 | F | RTSR | – | – | – |
| 36 | 59 | F | Open RCR | – | – | – |
| 37 | 31 | M | GT ORIF | P. acnes | P. acnes | P. acnes |
| 38 | 72 | M | RTSR | – | – | P. acnes |
| 39 | 53 | M | RTSR | – | – | – |
| 40 | 70 | F | RTSR | – | – | – |
| 41 | 56 | F | ORIF scapula | – | – | – |
| 42 | 65 | F | RTSR | – | – | – |
| 43 | 34 | M | Latarjet | P. acnes | – | – |
| 44 | 80 | F | RTSR | – | – | – |
| 45 | 66 | F | RTSR | P. acnes | P. acnes | – |
| 46 | 44 | F | Shoulder hemiarthroplasty | – | – | – |
| 47 | 76 | M | RTSR | – | – | P. acnes |
| 48 | 68 | F | RTSR | – | – | – |
| 49 | 73 | M | RTSR | – | – | P. acnes |
| 50 | 17 | M | Bristow | – | – | P. acnes |
ACJ, acromioclavicular joint; F, female; GT, greater tuberosity; M, male; ORIF, open reduction and internal fixation; P. acnes, Proprionibacterium acnes; RCR, rotator cuff repair; RTSR, reverse total shoulder replacement.
Antimicrobiological prophylaxis
In accordance with local antibiotic protocols, all patients received 2 g of intravenous cefazolin 30 minutes before the skin incision. An electrical surgical hair clipper (Medline Industries, King’s Park, NSW, Australia) was used to remove excess hair in the axilla and operative site. The skin was then ‘pre-prepped’ with 200 mL of a solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (Chloraprep; Orion Laboratories Pty Ltd, Balcatta, WA, Australia) and allowed to dry. A further application of 200 mL of Chloraprep was applied immediately prior to draping of the surgical field. Ioban 2 Antimicrobial Incise Drapes were applied to the surgical field prior to incision (Ioban; 3 M, St Paul, MN, USA).
Microbiological sampling and application of dermal antisepsis
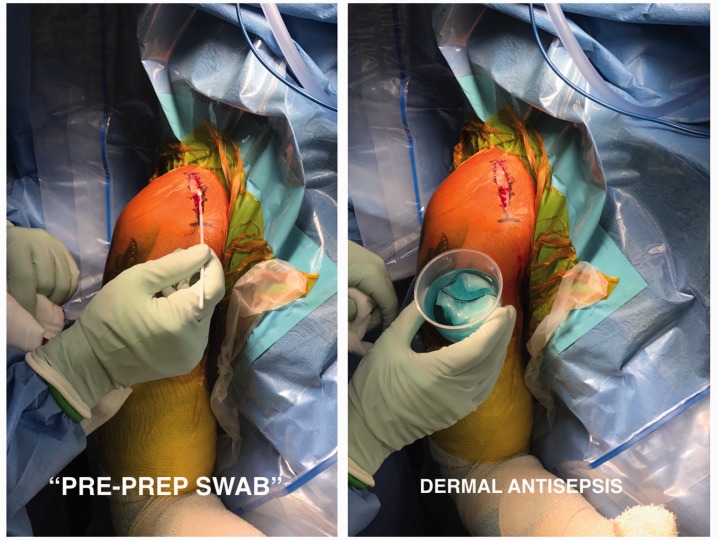
Three swabs were taken for each patient. The first swab termed ‘dermal swab pre-’ was taken from the exposed dermal surface within the wound immediately after the skin had been incised.
As a result of concerns with higher concentrations of chlorhexidine solution on fibroblast cytotoxicity (see Discussion), 0.1% aqueous chlorhexidine (McFarlane Medical Equipment, Surrey Hills, VIC, Australia) was then applied to the dermis using surgical gauze sponge soaked in the solution. Five minutes following this, a second swab termed ‘5 mins post-prep’ was taken from the exposed dermis (Fig. 1).
Figure 1.
Intra-operative sampling technique for ‘pre-prep swab’ prior to application of aqueous chlorhexidine to the exposed dermis.
A third dermal swab termed ’60 mins post-prep’ was taken at 60 minutes following dermal application of aqueous chlorhexidine, or at the time of wound closure, whichever was sooner.
In vitro, chlorhexidine has been shown to eradicate up to 100% of Gram-positive and Gram-negative bacteria within 30 seconds.3 Time points ‘5’ and ‘60’ minutes were chosen because it was hypothesized that, if chlorhexidine was effective at eradicating dermal P. acnes, then there would be more ‘negative swabs’ at these time points. If ineffective, then there would be more ‘positive’ swabs at time ‘60’, as a result of further bacterial seeding intra-operatively.
All samples were sent for microbiological analysis, specifically looking for P. acnes with anaerobic cultures for a minimum of 14 days. Colonies were identified by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI TOF-MS) (Bruker, Bremen, Germany).
Statistical analysis
Categorical variables and baseline demographic data were described using frequencies and percentages. Continuous variables are presented using the means (SD). Nonparametric tests (chi-squared and Fisher’s exact test) were used to compare differences between groups. p ≤ 0.05 was considered statistically significant.
Results
Overall cultures
All 50 patients had three intra-operative swabs taken, yielding 150 total swabs (Table 1). Thirty-eight swabs of these swabs isolated P. acnes (38/150, 25%). Of the 38 positive swabs, nine were ‘pre-prep’ (swab 1), 11 were at ‘5-min post-prep’ (swab 2) and 18 were at ‘60-min post-prep’ (swab 3). There were 21 patients with P. acnes present on any swab (21/50 = 42%). There was a statistically significant difference between the number of positive cultures between swabs 3 and 1 (n = 18 and 9, respectively, p = 0.043). There was a greater number of positive cultures at swab 3 compared to swab 2, although this did not reach statistical significance (n = 18 and 11, respectively, p = 0.123). There was no significant difference between the number of positive cultures at swab 2 and 1 (n = 11 and 9, respectively, p = 0.617).
In nine patients with a positive ‘pre-prep swab’, eight also isolated P. acnes at swabs 2 or 3. Therefore, dermal chlorhexidine eradicated P. acnes in only one out of nine of these patients (11%). From the 41 patients with negative ‘pre-prep swabs’, four isolated P. acnes on swab 2 and 11 isolated P. acnes on swab 3.
Comparison between groups
Table 2 reports the comparison between sub-groups. Positive P. acnes samples were significantly more common in males on any swab and overall (swab 1: seven of 22 versus two of 28, respectively, p = 0.032; swab 2: nine of 22 versus two of 28, respectively, p = 0.006; swab 3: 14 of 22 versus four of 28, respectively, p < 0.001; overall: 30 of 66 versus eight of 84, respectively, p < 0.001). The odds ratio of having a positive P. acnes sample as a male was 7.88 (95% confidence interval = 3.06 to 20.25).
Table 2.
Subgroup analysis of positive Proprionibacterium acnes samples.
| Variable | Number | Positive P. acnes samples, n (%) |
|||
|---|---|---|---|---|---|
| Pre-prep – 1 | 5 min post – 2 | 60 min post – 3 | Overall | ||
| Overall | 50 | 9 (18) | 11 (22) | 18 (36) | 38 (25) |
| Sex | |||||
| Male | 22 | 7 (32) | 9 (41) | 14 (64) | 30 (45) |
| Female | 28 | 2 (7) | 2 (7) | 4 (14) | 8 (10) |
| p value | 0.032 | 0.006 | <0.001 | <0.001 | |
| Age | |||||
| ≤50 years | 17 | 6 (35) | 8 (47) | 9 (53) | 23 (45) |
| >50 years | 33 | 3 (9) | 3 (9) | 9 (27) | 15 (15) |
| p value | 0.047 | 0.004 | 0.073 | <0.001 | |
| Surgical approach | |||||
| Deltopectoral | 40 | 7 (18) | 9 (23) | 15 (38) | 32 (27) |
| Deltoid split | 10 | 2 (20) | 2 (20) | 2 (20) | 6 (20) |
| p value | 0.854 | 0.864 | 0.296 | 0.453 | |
Positive P. acnes samples were significantly more common in patients≤50 years on swabs 2 and 3 and overall (swab 1: six of 17 versus three of 33, respectively, p = 0.047; swab 2: eight of 17 versus three of 33, respectively, p = 0.004; overall: 23 of 51 versus 15 of 99, respectively, p < 0.001). The odds ratio of having a positive P. acnes sample if≤50 years was 1.99 (95% confidence interval = 0.87 to 4.52).
For males ≤ 50 years, 9/10 (90%) had a positive culture. For males> 50 years, seven of 12 (58%) had a positive culture. For females≤ 50 years, two of six (33%) had a positive culture. For females > 50 years, three of 22 (14%) had a positive culture. Males ≤50 years were 57 times more likely than females >50 years to have a positive culture for P. acnes (odds ratio = 57.0, 95% confidence interval = 5.18 to 627.17).
One patient grew Enterococcus faecalis on swab 1. No other organisms were cultured. At a minimum 1 year of follow-up in clinic, no patients in the present study developed clinical signs of a postoperative wound or deep shoulder infection.
Discussion
Despite growing awareness of P. acnes as a pathogen for deep shoulder infection, both the present study and our previous one7 have shown that chlorhexidine application to either the epidermis or directly to the dermis is ineffective at reducing deep rates of P. acnes colonization. We also showed that P. acnes rates were higher at 60 minutes, suggesting that surgical seeding as a cause. Seeding can occur as a result of surgical insult; initially, the scalpel blade and, subsequently, other instruments, including retractors, gloves and the prosthesis itself. Falconer et al.13 recently looked at the source of contamination of P. acnes in the surgical field during shoulder surgery. They found that the commonest site of growth was the subdermal layer followed by the forceps, tip of glove, outside blade and inside blade respectively.13 We routinely change scalpel blade following skin incision. On the basis of the present study, we are now working on the design of a physical barrier that can be placed easily within the wound to prevent seeding. We recommend glove change before handling and implanting the prosthesis. Further work should aim to investigate the application of antibacterial coatings to surgical instruments after or during the sterilization process to improve eradication and minimize seeding to deep tissues.
Chlorhexidine is a broad-spectrum antibiotic, effective against most Gram-positive and Gram-negative bacteria and fungi. It has both bacteriostatic and bactericidal properties. In vitro, it has been shown to eradicate up to 100% of bacteria within 30 seconds.9 Its other advantages are that it exhibits substantivity (prolonged action) up to 48 hours and, unlike povidone-iodine, chlorhexidine is less affected by the presence of body fluids.10,11 Despite its prolonged action, more positive cultures for P. acnes were present an hour after chlorhexidine application. This suggests that P. acnes has innate resistance to chlorhexidine in vivo. This is shown in the literature, where alcoholic chlorhexidine has been shown in some studies to be ineffective as a sole agent in reducing P. acnes.12–14 Despite this and concerns surrounding P. acnes infection, it continues to be used commonly by shoulder surgeons as a sole topical prep. Howlin et al.5 showed success by using absorbable calcium sulphate antibiotic-loaded cement beads in vitro (with tobramycin or vancomycin). In their study, P. acnes colonization was reduced, colony-forming units were eradicated, and biofilm was reduced.5 Other studies have shown that other topical preparations can be used to augment chlorhexidine as an antiseptic. Niazi et al.15 showed that the addition of a protease, (1% trypsin) with chlorhexidine was more effective at reducing biofilm production. We used aqueous chlorhexidine because of concerns about the cytotoxic effect of using an alcoholic antiseptic on the dermis. This may have affected the antimicrobial property of the antisepsis, although aqueous chlorhexidine has proven efficacy in other fields of medicine.16,17
In concordance with other studies, we found a similar proportion of positive swabs and a significant presence of P. acnes in males and patients under 50 years of age.7,13,14 The presence of hair may also be a factor and has been shown to be a positive predictor for the presence of P. acnes.14,18 Hair presence on the shoulder girdle is more likely in males. These factors are likely to be inter-related. The size and frequency of pilosebaceous glands were shown to be higher in males.18 Pilosebaceous gland secretion was shown to decrease with age and this may relate to decrease in circulating androgen levels.19 We take care to shave the surgical site of excess hair prior to application of topical antisepsis. We found that males under 50 years were 57 times more likely to develop positive P. acnes swabs compared to females aged over 50 years. Future strategies of reducing P. acnes infection should in particularly target this high-risk group. Once the optimal P. acnes eradication therapy has been clarified, prophylactic treatment should also be considered prior to surgery.
Identifying P. acnes remains a significant clinical challenge. The bacteria is indolent, slow-growing and there is no agreed ‘gold standard’ as a result of difficulties in identifying the bacteria.20 In the presence of P. acnes infection, symptoms are often vague, although pain and stiffness are considered the most common features.21 We used extended 14-day cultures and MALDI TOF-MS to isolate the organism. Most studies report that at least 1 week of culture is needed, although some report up to 1 month.22,23 Matsen et al.24 recommended a period of 17.4 days to ensure that 95% of positive cultures are identified. Synovial marker methods used in the present study, such as MALDI TOF-MS have also been shown to be effective for the identification of P. acnes, although markers such as alpha-defensin may have higher sensitivity and specificity.8,25 In the presence of infection, erythrocyte sedimentation rate and C-reactive protein have low sensitivity and specificity.26 Recent developments, such as polymerase chain reaction-restriction fragment length polymorphism testing from biopsy specimens, may allow an accurate diagnosis within 24 hours, leading to the early initiation of treatment
The clinical implication of positive P. acnes culture is controversial. In their series, Padegimas et al.27 reported that almost one quarter of patients undergoing revision shoulder arthroplasty had unexpected positive P. acnes cultures. Despite this, most further surgeries occurred in the group with negative cultures and only one patient in the positive culture developed a recurrent infection. In our series, none of our patients developed signs or symptoms of infection postoperatively. Further long-term follow will be needed, particularly in patients with positive cultures, to screen for infection. We know that many of these infections fail to develop clinical features of infection initially but may develop nonspecific signs and symptoms many years following surgery.
The main limitation to the present study is that the relationship of a positive swab to the development of clinical infection is unknown. As a result of challenges in detection of the bacteria in the laboratory, there is also a likelihood of false negative swabs occurring in our cohort. We did not take dermal biopsies because our previous study showed that there was a higher rate of cultures on dermal swabs in comparison to dermal biopsies.12 This may be a result of swab cultures being taken from the length of the wound rather than from one area. We acknowledge that dermal antiseptic prep is an unorthodox method to reduce P. acnes but consider that innovative strategies are needed because skin antiseptics in vivo have so far proven to be ineffective. A further limitation is that the aqueous concentration of chlorhexidine we applied to the dermis was significantly lower than that applied to the alcoholic equivalent on the epidermis. We used the standard aqueous solution provided for use within wounds because there is potential concern on fibroblast cytotoxicity and wound healing in animal studies when using concentrations of chlorhexidine > 0.1%.28 Despite this, most literature shows chlorhexidine is effective as a bacteriostatic agent even at these lower concentrations.29,30 We did not take ‘pre-prep’ swabs from the epidermis because this was performed in our previous study, where we showed that alcoholic chlorhexidine was effective at reducing P. acnes from the epidermis, and not from the dermis.12
Conclusions
Chlorhexidine, whether applied to the epidermis or dermis, is ineffective at reducing P. acnes colonization from deep tissues during open shoulder surgery. Other topical agents or adjuncts should be considered or anti-bacterial barrier methods should be used to prevent seeding of the dermis during surgery. In addition, meticulous surgical technique and change of gloves and instruments may reduce the risk of P. acnes infection.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical review and patient consent
Ethics board approval was obtained for this study (South Australia Clinical Human Research Ethics Committee). Patient consent was obtained during the study.
References
- 1.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Deep infections after rotator cuff repair. J Shoulder Elb Surg 2007; 16: 306–311. [DOI] [PubMed] [Google Scholar]
- 2.Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elb Surg 2013; 22: 620–627. [DOI] [PubMed] [Google Scholar]
- 3.Sperling JW, Kozak TK, Hanssen D, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res 2001; 382: 206–216. [DOI] [PubMed] [Google Scholar]
- 4.Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elb Surg 2009; 18: 897–902. [DOI] [PubMed] [Google Scholar]
- 5.Howlin RP, Winnard C, Angus EM, et al. Prevention of Propionibacterium acnes biofilm formation in prosthetic infections in vitro. J Shoulder Elb Surg December 2017; 26: 553–563. [DOI] [PubMed] [Google Scholar]
- 6.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am 2009; 91: 1949–1953. [DOI] [PubMed] [Google Scholar]
- 7.Phadnis J, Gordon D, Krishnan J, Bain GI. Frequent isolation of Propionibacterium acnes from the shoulder dermis despite skin preparation and prophylactic antibiotics. J Shoulder Elb Surg 2016; 25: 304–310. [DOI] [PubMed] [Google Scholar]
- 8.Barreau M, Pagnier I, La Scola B. Improving the identification of anaerobes in the clinical microbiology laboratory through MALDI-TOF mass spectrometry. Anaerobe 2013; 22: 123–125. [DOI] [PubMed] [Google Scholar]
- 9.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999; 12: 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim K-S, Kam PCA, Lim K.S, Kam PCA. Chlorhexidine-pharmacology and clinical applications. Anaesth Intensive Care 2008; 36: 502–512. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi Z. Chlorhexidine gluconate, its properties and applications in endodontics. Iran Endod J 2008; 2: 113–125. [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang MJ, Jancosko JJ, Mendoza V, Nottage WM. The incidence of Propionibacterium acnes in shoulder arthroscopy. Arthroscopy 2015; 31: 1702–1707. [DOI] [PubMed] [Google Scholar]
- 13.Falconer TM, Baba M, Kruse LM, et al. Contamination of the surgical field with Propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am 2016; 98: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 14.Koh CK, Marsh JP, Drinković D, Walker CG, Poon PC. Propionibacterium acnes in primary shoulder arthroplasty: rates of colonization, patient risk factors, and efficacy of perioperative prophylaxis. J Shoulder Elb Surg 2016; 25: 846–852. [DOI] [PubMed] [Google Scholar]
- 15.Niazi SA, Al-Ali WM, Patel S, Foschi F, Mannocci F. Synergistic effect of 2% chlorhexidine combined with proteolytic enzymes on biofilm disruption and killing. Int Endod J 2015; 48: 1157–1167. [DOI] [PubMed] [Google Scholar]
- 16.Darouiche RO, Wall Jr MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010; 362: 18–26. [DOI] [PubMed] [Google Scholar]
- 17.Merani R, McPherson ZE, Luckie AP, et al. Aqueous chlorhexidine for intravitreal injection antisepsis: a case series and review of the literature. Ophthalmology 2016; 123: 2588–2594. [DOI] [PubMed] [Google Scholar]
- 18.Hudek R, Sommer F, Kerwat M, Abdelkawi AF, Loos F, Gohlke F. Propionibacterium acnes in shoulder surgery: true infection, contamination, or commensal of the deep tissue? J Shoulder Elb Surg 2014; 23: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 19.Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol 2011; 49: 2490–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields MV, Abdullah L, Namdari S. The challenge of Propionibacterium acnes and revision shoulder arthroplasty: a review of current diagnostic options. J Shoulder Elb Surg 2016; 25: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 21.Millett PJ, Yen YM, Price CS, Horan MP, Van Der Meijden OA, Elser F. Propionobacter acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res 2011; 469: 2824–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elb Surg 2010; 19: 303–307. [DOI] [PubMed] [Google Scholar]
- 23.Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Jt Surg Am 2012; 94: 2075–2083. [DOI] [PubMed] [Google Scholar]
- 24.Matsen FA, Butler-Wu S, Carofino BC, Jette JL, Bertelsen A, Bumgarner R. Origin of Propionibacterium in surgical wounds and evidence-based approach for culturing Propionibacterium from surgical sites. J Bone Joint Surg Am 2013; 95: e1811–e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frangiamore SJ, Saleh A, Grosso MJ, et al. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elb Surg 2015; 24: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 26.Piggott DA, Higgins YM, Melia MT, et al. Characteristics and treatment outcomes of Propionibacterium acnes prosthetic shoulder infections in adults. Open Forum Infect Dis 2016; 31: ofv191–ofv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padegimas EM, Lawrence C, Narzikul AC, et al. Future surgery after revision shoulder arthroplasty: the impact of unexpected positive cultures. J Shoulder Elb Surg 2017; 26: 975–981. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez IR, Nusbaum KE, Swaim SF, Hale AS, Henderson RA, McGuire JA. Chlorhexidine diacetate and povidone-iodine cytotoxicity to canine embryonic fibroblasts and Staphylococcus aureus. Vet Surg 1988; 17: 182–185. [DOI] [PubMed] [Google Scholar]
- 29.Karpiński TM, Szkaradkiewicz AK. Chlorhexidine – pharmaco-biological activity and application. Eur Rev Med Pharmacol Sci 2015; 19: 1321–1326. [PubMed] [Google Scholar]
- 30.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J 2001; 34: 424–428. [DOI] [PubMed] [Google Scholar]