Abstract
OBJECTIVES:
Children with multiple complex chronic conditions (MCCs) represent a small fraction of our communities but a disproportionate amount of health care cost and mortality. Because the temporal trends of children with MCCs within a geographically well-defined US pediatric population has not been previously assessed, health care planning and policy for this vulnerable population is limited.
METHODS:
In this population-based, repeated cross-sectional study, we identified and enrolled all eligible children residing in Olmsted County, Minnesota, through the Rochester Epidemiology Project, a medical record linkage system of Olmsted County residents. The pediatric complex chronic conditions classification system version 2 was used to identify children with MCCs. Five-year period prevalence and incidence rates were calculated during the study period (1999–2014) and characterized by age, sex, ethnicity, and socioeconomic status (SES) by using the housing-based index of socioeconomic status, a validated individual housing-based SES index. Age-, sex-, and ethnicity-adjusted prevalence and incidence rates were calculated, adjusting to the 2010 US total pediatric population.
RESULTS:
Five-year prevalence and incidence rates of children with MCCs in Olmsted County increased from 1200 to 1938 per 100 000 persons and from 256 to 335 per 100 000 person-years, respectively, during the study period. MCCs tend to be slightly more prevalent among children with a lower SES and with a racial minority background.
CONCLUSIONS:
Both 5-year prevalence and incidence rates of children with MCCs have significantly increased over time, and health disparities are present among these children. The clinical and financial outcomes of children with MCCs need to be assessed for formulating suitable health care planning given limited resources.
The National Academy of Medicine reported that 1% and 5% of the US population spent 23% and 50%, respectively, of the total health care expenditure in 2014, highlighting that a small proportion consumes a large proportion of health care resources.1 Children with multiple complex chronic conditions (MCCs) are recognized as a focus population for improvement in health care systems because they constitute a small fraction of our communities yet represent a disproportionate amount of health care costs and mortality.2–21 Children with MCCs represented more than one-quarter of children in the top 5% of Medicaid spending in 2010.20 In addition, children who are hospitalized with MCCs have a threefold longer hospital length of stay, 11-fold greater charges, and 15-fold higher inpatient mortality compared with children who are hospitalized without MCCs.3 Therefore, addressing the needs of children with MCCs and improving their outcomes while containing costs are national and local research priorities.22,23
Despite the burden of MCCs on children, families, communities, and health care systems, the epidemiological assessment of children with MCCs within a geographically defined pediatric US population has not been previously performed. Although valuable, the extant literature is limited to cohorts derived from databases originating from health care use,* payer source,4,7–10,14,20,30–33 death certificates,34–37 and national surveys.38,39 Furthermore, the reported prevalence for chronic disease in children is widely variable (0.2%–44%) because of considerable heterogeneity in study definitions, designs, populations, and conditions.40 In studies in which the lower range of prevalence estimates of children with chronic disease are reported, the authors use definitions that do not include all chronic diseases (complex chronic disease, etc). Previous population-based studies include work by Ralston et al,7 in which the authors assessed hospital variation in health care use by children with medical complexity in northern New England, and work by Cohen et al,29,41 in which the authors described both patterns and costs of health care use of children with medical complexity and residential movement patterns of families of young children with chronic conditions in Ontario, Canada. At present, the epidemiological profile for children with MCCs over time in a well-defined pediatric population by using comprehensive electronic medical records (EMRs) and individual-level socioeconomic status (SES) background has not been assessed. Because epidemiological profiles are often used to determine population needs, this knowledge provides an important basis for understanding the nature of children with MCCs to mitigate the burden and improve outcomes.
To address this knowledge gap, we sought to determine the prevalence and incidence of MCCs in children living in a mixed urban-rural community and examine temporal trends over the past 15 years. To address the limitations of previous studies, children in our study population were characterized by age, sex, ethnicity, and SES by using a unified records-linkage system,42,43 an accepted and multidimensional measure of pediatric chronic disease (pediatric complex chronic conditions [CCCs] classification system version 2),34,44 and the housing-based index of socioeconomic status (HOUSES), a validated, individualized SES measure.45
Methods
Population and Setting
Olmsted County, Minnesota is a mixed urban-rural county located 90 miles southeast of Minneapolis, Minnesota, and is an ideal setting to conduct population-based epidemiological surveillance because 98% of medical care received by county residents is delivered through 2 hospitals and affiliated health care facilities. The Rochester Epidemiology Project (REP) has been used to electronically index all patient care episodes since its inception in 1966 through continuous funding by the National Institutes of Health.42 Population counts obtained by the REP are similar to those obtained by the US Census Bureau.43,46 Patients are categorized as Olmsted County residents on the basis of address information at the time of each health care visit. Although residents of Olmsted County have historically represented the US white population, more recent 2010 census data reveal increasing diversification, and 24% of the Olmsted County youth population (0–17 years of age) were classified as people of color.47
Design
The study was designed as a population-based repeated cross-sectional study used to assess the temporal trends of 5-year prevalence and incidence rates of MCCs between 1999 and 2014 among all eligible children residing in Olmsted County, Minnesota. The eligible study subjects were identified through the National Institutes of Health–funded REP records-linkage system. The REP includes all patient encounters (ie, inpatient and outpatient). We calculated age-, sex-, and ethnicity-adjusted incidence rates and prevalence using the pediatric population of the 2010 US Census for standardization. We characterized incidence and prevalence by age, sex, ethnicity, and SES as measured by HOUSES (described below). Institutional review boards (IRBs) approved this study (Mayo Clinic IRB [16-00128] and Olmsted Medical Center IRB [052-OMC-16]).
Study Subjects
Four historical cohorts were defined from all eligible children 0 to 17 years of age who were residents of Olmsted County on April 1 of 4 study years (1999, 2004, 2009, and 2014) to calculate 5-year period prevalence and incidence rates on the basis of the 5-year capture frame. Prevalence rates were calculated by using the 2004, 2009, and 2014 cohorts, whereas the incidence rates were calculated by using the 1999, 2004, and 2009 cohorts. The 1999 cohort was not used for 5-year prevalence rate calculation because the cohort did not have sufficient medical records for the 5-year capture frame through the REP. Similarly, the 2014 cohort was not used for 5-year incidence calculation because the cohort did not have 5-year follow-up information yet. The exclusion criteria for each of the 4 historical cohorts were the following: (1) non–Olmsted County residents on April 1 of each study year time point (1999, 2004, 2009, and 2014; REP has a function of checking the residency data for a specific person at any given date from 1966 to the present date) and (2) children without research authorization.
MCCs
We adopted the definition of MCCs as any child with ≥2 CCC categories previously defined by Feudtner et al.34,44 This definition has been widely used in the pediatric literature, uses International Classification of Diseases, Ninth Revision (ICD-9) diagnostic and procedure codes, and is accepted as a measure of chronic disease in children.† The index date of MCCs was defined as the date the first ICD-9 code was used to identify a child with a second CCC category. CCCs were assessed by using ICD-9 codes from all settings.
Sociodemographic Variables
Children with MCCs were characterized by age, sex, ethnicity, and SES as measured by HOUSES. HOUSES was developed and validated in Olmsted County, Minnesota; Jackson County, Missouri; and Sioux Falls, South Dakota; to address the absence of SES variables in commonly used data sources for clinical research (eg, medical record).45 Study participants’ addresses were geocoded to geographic reference (latitude and longitude) and real property data available from the local government assessor’s office on April 1 of each study year described above. A standardized HOUSES score (z score) was formulated from 4 real property variables (housing value, square footage, number of bedrooms, and number of bathrooms). A high HOUSES score correlates with high SES. Previous research has been used to document the successful application of HOUSES as an effective SES measure in clinical research,55–62 and HOUSES was recently found to be a useful surrogate for SES to assess risk stratification and hospitalization in adults with multiple chronic conditions.63 Children with missing address information were excluded from the HOUSES analyses. For ethnicity classification, self-reported ethnicity was categorized into 2 groups: non-Hispanic white and other (African American, Asian American, Hispanic, and other and/or unknown).
Statistical Analyses
Descriptive statistics were used to summarize demographic characteristics of each cohort (1999, 2004, 2009, and 2014). This is a population-based cross-sectional study based on all eligible children in our community (Olmsted County, MN) for a given time point, not based on a sampling frame; thus, results were reported on the basis of only descriptive statistics without a P value.
Crude Prevalence Rates of MCCs
Five-year prevalence was estimated for the 2004, 2009, and 2014 cohorts, separately. For example, to determine the 5-year prevalence64 of MCCs for the 2004 cohort, the 2004 REP cohort was used, which included all children 0 to 17 years of age living in Olmsted County, Minnesota, on April 1, 2004, with Minnesota research authorization. Children in the 2004 cohort with ≥2 CCCs indicated within the previous 5 years (April 1, 1999–March 31, 2004) were considered to have prevalent MCCs on April 1, 2004. These analyses were repeated on the 2009 and 2014 cohorts to get the prevalence rates, respectively.
Crude Incidence Rates of MCCs
Five-year–period incidence rates were determined for the 1999, 2004, and 2009 cohorts. After excluding cases of prevalent MCCs that occurred as of the index date, incidence cases of MCCs were determined by having index MCCs occurring in the subsequent 5-year period. The index date of MCCs for those who had at least 2 CCC categories was defined as the date the first ICD-9 code was used to identify a second CCC category. For example, to determine the 5-year incidence rate of MCCs for the 1999 cohort, the 1999 REP cohort was used, which included all eligible children 0 to 17 years of age living in Olmsted County, Minnesota, on April 1, 1999, with Minnesota research authorization. Prevalent MCCs cases, based on 2 CCC categories in the previous 5 years (April 1, 1994–March 31, 1999), were excluded from this analysis. All children who developed de novo MCCs from April 1, 1999, to March 31, 2004, were considered to have incident cases of MCCs. The denominator was person-years of follow-up for children 0 to 17 years of age at risk for index MCCs in the 1999 cohort. Follow-up ended at the index date of MCCs, 18th birthday, death, or on March 31, 2004, whichever came first. These analyses were repeated on the 2004 and 2009 cohorts.
Adjusted Prevalence and Incidence Rates
For each cohort, age-, sex-, and ethnicity-specific prevalence and incidence rates were first estimated by using the number of patients with MCCs in each age group (<1, 1–4, 5–9, and 10–17 years), sex group, and ethnicity group (non-Hispanic white and other), with corresponding age-, sex-, and ethnicity-specific person-years at risk as denominators. All rates were standardized to the 2010 US Census pediatric total population.64 Overall 5-year prevalence and incidence rates were calculated by adjusting age, sex, and ethnicity distribution to the 2010 US Census pediatric total population.64,65 For the adjusted rates, 95% confidence intervals (CIs) were constructed by using the assumption that the number of cases follow a Poisson distribution.66
Association of HOUSES With Prevalence and Incidence of Pediatric MCCs
HOUSES z scores were determined for each cohort in which address information was known and analyzed in quartiles. Poisson regression was applied to assess the linear trend of HOUSES quartiles and prevalence and incidence rates of pediatric MCCs.65 Statistical significance was tested at a 2-sided α error of .05. All statistical analyses were computed by using SAS statistical software (version 9.4; SAS Institute, Inc, Cary, NC).
Results
Demographic Characteristics
Eligible study participants in each cohort were as follows: 31 390 subjects in 1999, 32 490 subjects in 2004, 32 942 subjects in 2009, and 33 097 subjects in 2014; characteristics of study subjects are summarized in Table 1. The proportion of children in each age group across the 4 cohorts was similar. Olmsted County, Minnesota, increasingly became more diverse. In 1999, 24.7% of the pediatric population identified as a non-Hispanic white, whereas this grew to 33.1% by 2014.
TABLE 1.
Demographics of Children Living in Olmsted County, Minnesota
| 1999 (n = 31 390), n (%) | 2004 (n = 32 490), n (%) | 2009 (n = 32 942), n (%) | 2014 (n = 33 097), n (%) | |
|---|---|---|---|---|
| Age, y | ||||
| <1 | 1768 (5.6) | 2016 (6.2) | 2087 (6.3) | 1985 (6.0) |
| 1–4 | 6755 (21.5) | 7337 (22.6) | 8396 (25.5) | 7825 (23.6) |
| 5–9 | 8689 (27.7) | 8518 (26.2) | 8945 (27.2) | 9789 (29.6) |
| 10–17 | 14 178 (45.2) | 14 619 (45.0) | 13 514 (41.0) | 13 498 (40.8) |
| Sex | ||||
| Girls | 15 200 (48.4) | 15 814 (48.7) | 16 058 (48.7) | 16 105 (48.7) |
| Boys | 16 190 (51.6) | 16 676 (51.3) | 16 884 (51.3) | 16 992 (51.3) |
| Ethnicity | ||||
| Non-Hispanic white | 23 645 (75.3) | 24 131 (74.3) | 23 393 (71.0) | 22 137 (66.9) |
| Othera | 7745 (24.7) | 8359 (25.7) | 9549 (29.0) | 10 960 (33.1) |
| African American | 1373 (4.4) | 2392 (7.4) | 2876 (8.7) | 3380 (10.2) |
| Asian American | 1410 (4.5) | 1714 (5.3) | 2036 (6.2) | 2229 (6.7) |
| Hispanic | 1119 (3.6) | 1825 (5.6) | 2392 (7.3) | 2743 (8.3) |
| Other and/or unknown | 3843 (12.2) | 2428 (7.5) | 2245 (6.8) | 2608 (7.9) |
| SES (HOUSES)b,c,d | ||||
| First quartile | 5990 (24.7) | 6862 (24.7) | 6661 (24.4) | 6248 (24.5) |
| Second quartile | 6023 (24.8) | 6899 (24.9) | 6868 (25.2) | 6360 (24.9) |
| Third quartile | 6091 (25.1) | 6967 (25.1) | 6846 (25.1) | 6412 (25.1) |
| Fourth quartile | 6163 (25.4) | 7029 (25.3) | 6909 (25.3) | 6496 (25.5) |
| Unknown | 7123 | 4733 | 5658 | 7581 |
| No. CCCse | ||||
| 0 | 29 367 (93.6) | 29 905 (92.0) | 30 246 (91.8) | 29 896 (90.3) |
| 1 | 1761 (5.6) | 2183 (6.7) | 2219 (6.7) | 2552 (7.7) |
| 2 | 194 (0.6) | 293 (0.9) | 332 (1.0) | 449 (1.4) |
| 3 | 44 (0.1) | 72 (0.2) | 82 (0.2) | 113 (0.3) |
| 4 | 16 (0.1) | 24 (0.1) | 35 (0.1) | 52 (0.2) |
| 5 | 5 (0.0) | 8 (0.0) | 19 (0.1) | 17 (0.1) |
| 6 | 2 (0.0) | 3 (0.0) | 6 (0.0) | 9 (0.0) |
| 7 | 1 (0.0) | 2 (0.0) | 2 (0.0) | 6 (0.0) |
| 8 | 0 (0.0) | 0 (0.0) | 1 (0.0) | 2 (0.0) |
| 9 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) |
| CCCse | 273 (0.9) | 354 (1.1) | 423 (1.3) | 574 (1.7) |
| Neurologic and/or neuromuscular | ||||
| Cardiovascular | 362 (1.2) | 446 (1.4) | 673 (2.0) | 862 (2.6) |
| Respiratory | 51 (0.2) | 135 (0.4) | 181 (0.5) | 227 (0.7) |
| Renal and/or urologic | 159 (0.5) | 184 (0.6) | 244 (0.7) | 261 (0.8) |
| Gastrointestinal | 88 (0.3) | 126 (0.4) | 142 (0.4) | 185 (0.6) |
| Hematologic and/or immunologic | 89 (0.3) | 147 (0.5) | 165 (0.5) | 258 (0.8) |
| Metabolic | 370 (1.2) | 369 (1.1) | 336 (1.0) | 494 (1.5) |
| Other congenital or genetic | 633 (2.0) | 918 (2.8) | 817 (2.5) | 888 (2.7) |
| Malignancy | 318 (1.0) | 397 (1.2) | 328 (1.0) | 350 (1.1) |
| Premature and/or neonatal | 46 (0.1) | 77 (0.2) | 113 (0.3) | 104 (0.3) |
| Technology dependence | 130 (0.4) | 175 (0.5) | 200 (0.6) | 219 (0.7) |
| Transplant | 8 (0.0) | 18 (0.1) | 20 (0.1) | 26 (0.1) |
Population living in Olmsted County, Minnesota on April 1, 1999, 2004, 2009, and 2014.
Self-reported ethnicity was categorized into 2 groups: non-Hispanic white and other (African American, Asian American, Hispanic, and other and/or unknown).
The first quartile is the lowest quartile, and the fourth quartile is the highest quartile.
Children with missing HOUSES data were excluded (7123 in 1999, 4733 in 2004, 5658 in 2009, and 7581 in 2014).
HOUSES data for the 2014 cohort were from 2012.
Pediatric CCCs classification system version 2.
The 5-Year Prevalence of MCCs
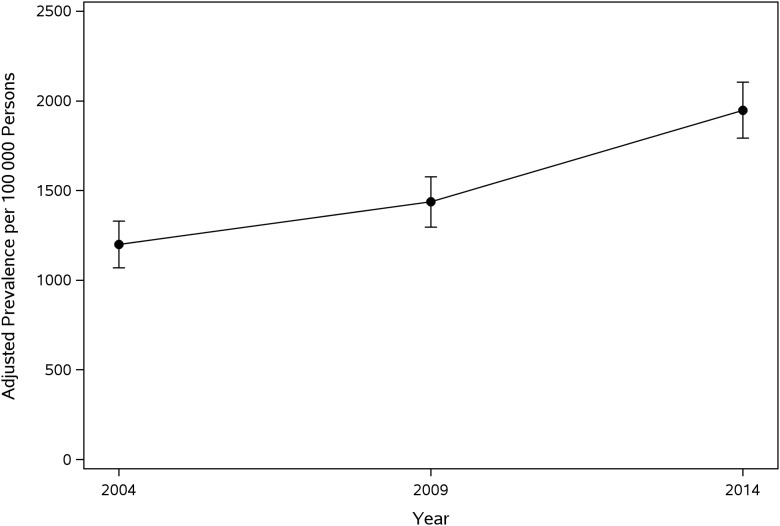
Prevalence rates of MCCs among children living in Olmsted County, Minnesota, are summarized in Table 2. Overall, crude prevalence of pediatric MCCs significantly increased from 1237 (95% CI: 1119–1364) per 100 000 persons in the 2004 cohort to 1961 (95% CI: 1813–2118) in the 2014 cohort. The age-, sex-, and ethnicity-adjusted prevalence rates of MCCs in children living in Olmsted County, Minnesota (standardized to the 2010 US Census pediatric population), significantly increased during the study period from 1200 to 1948 per 100 000 persons from 2000–2004 to 2010–2014 (Fig 1). In addition, all age-, sex-, and ethnicity-adjusted prevalence rates also increased in each period (Table 2).
TABLE 2.
The 5-Year Prevalence and Incidence Rates of Children With MCCs Living in Olmsted County, Minnesota, During the Study Period (1999–2014)
| Prevalence at Each Time Pointa (95% CI) | Incidence at Each Time Pointa (95% CI) | |||||
|---|---|---|---|---|---|---|
| 2004 | 2009 | 2014 | 1999 | 2004 | 2009 | |
| Overall | 1237 (1119–1364) | 1448 (1321–1584) | 1961 (1813–2118) | 250 (223–280) | 273 (245–304) | 333 (310–366) |
| Age, y | ||||||
| <1 | 893 (529–1411) | 1437 (970–2052) | 1259 (815–1859) | 491 (134–1256) | 1306 (675–2282) | 315 (65–920) |
| 1–4 | 1649 (1358–1971) | 1858 (1578–2174) | 2441 (2107–2813) | 231 (166–313) | 150 (101–214) | 303 (235–385) |
| 5–9 | 1057 (850–1299) | 1196 (980–1446) | 1798 (1542–2084) | 115 (83–155) | 144 (108–187) | 213 (172–262) |
| 10–17 | 1183 (1014–1373) | 1361 (1172–1573) | 1904 (1678–2152) | 330 (287–377) | 375 (329–425) | 426 (376–481) |
| Age-adjusted rate | 1235 (1114–1356) | 1429 (1300–1558) | 1958 (1807–2110) | 258 (221–295) | 312 (264–359) | 335 (299–371) |
| Sex | ||||||
| Boys | 1355 (1184–1544) | 1534 (1353–1733) | 2119 (1905–2349) | 240 (203–282) | 270 (231–314) | 333 (289–380) |
| Girls | 1113 (955–1290) | 1358 (1183–1550) | 1794 (1594–2014) | 260 (221–305) | 277 (236–322) | 333 (289–381) |
| Sex-adjusted rate | 1237 (1116–1358) | 1448 (1318–1578) | 1960 (1809–2111) | 250 (222–278) | 273 (244–303) | 333 (301–364) |
| Ethnicity | ||||||
| Non-Hispanic white | 1264 (1126–1414) | 1415 (1267–1576) | 1933 (1755–2126) | 253 (222–287) | 287 (254–323) | 334 (297–374) |
| Other | 1160 (941–1416) | 1529 (1291–1798) | 2016 (1759–2300) | 238 (180–308) | 229 (177–291) | 329 (272–395) |
| Ethnicity-adjusted rate | 1216 (1085–1347) | 1468 (1327–1609) | 1972 (1814–2129) | 246 (213–279) | 260 (229–292) | 332 (297–366) |
| Age-, sex-, and race-adjusted rate | 1200 (1070–1329) | 1436 (1297–1576) | 1948 (1792–2104) | 256 (213–298) | 300 (250–350) | 335 (296–373) |
MCCs were defined as ≥2 CCCs by using the pediatric CCCs classification system version 2.
Prevalence and incidence rates per 100 000 person-y (based on a 5-y capture frame) were calculated by using the population-based cohort at each time period (2004, 2009, and 2014 for prevalence; 1999, 2004, and 2009 for incidence).
FIGURE 1.
Age-, sex-, and ethnicity-adjusted prevalence of MCCs among children living in Olmsted County, Minnesota.
The 5-Year Incidence of MCCs
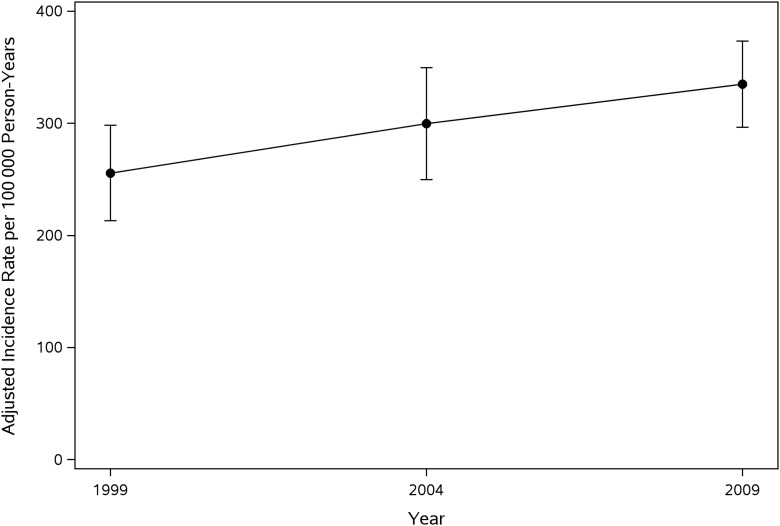
Incidence rates of MCCs among children living in Olmsted County, Minnesota, are listed in Table 2. The age-, sex-, and ethnicity-adjusted incidence rates of MCCs in children living in Olmsted County, Minnesota (standardized to the 2010 US Census pediatric population), increased during the study period from 256 (95% CI: 213–298) in the 1999 cohort to 335 (95% CI: 296–373) per 100 000 person-years in the 2014 cohort, respectively (Table 2, Fig 2). In addition, all age-, sex-, and ethnicity-adjusted incidence rates also increased each period (Table 2).
FIGURE 2.
Age-, sex-, and ethnicity-adjusted incidence rates of MCCs among children living in Olmsted County, Minnesota.
Association of HOUSES and Ethnicity With Prevalence and Incidence Rates of Children With MCCs
Children with a lower SES (measured by HOUSES) tend to have a slightly higher prevalence rate in all 3 cohorts (Table 3). For example, the 5-year prevalence of MCCs for the lowest HOUSES group (the first quartile) in the 2014 cohort was 34% higher compared with that of those in the highest HOUSES group (the fourth quartile). HOUSES was not associated with the prevalence rate in the 2009 cohort (P = .58), although the rate was slightly higher in the lowest HOUSES group. The 5-year incidence rates were not associated with HOUSES in all 3 cohorts (P > 0.7) but was slightly higher among non-Hispanic white participants.
TABLE 3.
Association of HOUSES With Prevalence and Incidence Rates of Children With MCCs
| Prevalence at Each Time Pointa (95% CI) | Incidence at Each Time Pointa (95% CI) | |||||
|---|---|---|---|---|---|---|
| 2004 | 2009 | 2014 | 1999 | 2004 | 2009 | |
| HOUSESb | ||||||
| First quartile | 1443 (1173–1756) | 1576 (1289–1908) | 2225 (1870–2627) | 262 (202–334) | 273 (214–343) | 363 (293–445) |
| Second quartile | 1435 (1166–1747) | 1587 (1303–1914) | 2327 (1967–2734) | 237 (181–305) | 290 (230–362) | 278 (219–349) |
| Third quartile | 1134 (898–1413) | 1563 (1281–1889) | 2339 (1980–2745) | 305 (241–380) | 304 (243–377) | 346 (279–425) |
| Fourth quartile | 1124 (890–1400) | 1462 (1191–1776) | 1663 (1364–2007) | 224 (170–288) | 267 (130–283) | 362 (294–442) |
| Pc | .039 | .583 | .038 | .726 | .982 | .655 |
MCCs were defined as ≥2 CCCs by using the pediatric CCCs classification system version 2.
Prevalence and incidence rates per 100 000 person-y (based on 5-y capture frame) were calculated using the population-based cohort at each time period (2004, 2009, and 2014 for prevalence; 1999, 2004, and 2009 for incidence).
The first quartile is the lowest quartile, and the fourth quartile is the highest quartile.
The P value is used to test the linear trend of HOUSES quartiles with prevalence and incidence rates within each period.
Discussion
Children with MCCs compose a high-risk patient population in which significant improvement is needed in all dimensions of the Institute of Healthcare Improvement Triple Aim.23 To better understand the natural history of MCCs in children, we sought to describe the first temporal trends and epidemiological profile of MCCs in children living in a mixed urban-rural community. We found that both the prevalence and the incidence of MCCs among children living in Olmsted County, Minnesota, have increased during the 15-year study period, and MCCs are more prevalent among children with a low SES and minority background.
The overall age-, sex, and ethnicity-adjusted period prevalence of pediatric MCCs in our study setting increased from 1200 to 1948 per 100 000 persons (62% increase in prevalence). The overall age-, sex-, and ethnicity-adjusted incidence rate for pediatric MCCs in our study setting increased from 256 to 335 per 100 000 person-years (31% increase in incidence). To our knowledge, this is the first population-based study in which temporal trends of both prevalence and incidence rates of pediatric MCCs are reported over a 15-year period in a geographically defined population in a non–inner-city setting by using EMRs, a pediatric chronic disease measure, and an individualized SES index. Therefore, there is no current literature available with which we can directly compare our estimates. The only data available to date are limited by an adult chronic disease measure applied to a pediatric population or pediatric chronic disease measures applied to specific populations not representing the general pediatric population. For example, in our same study setting, researchers determined the prevalence and incidence rates of MCCs (≥2 chronic conditions) in children 0 to 19 years of age, using 20 common adult chronic conditions recommended by the US Department of Health and Human Services, to be 132864 and 750 per 100 000 person-years,67 respectively. These estimates appear different from our study because of the chronic disease measure. In a different geographic region, among children 0 to 20 years of age who died between 2007 and 2008 and were enrolled in California Medicaid, the prevalence rate of MCCs was 55 940 per 100 000 persons8; however, this study was limited to children who were insured and children who were deceased. At the national level, among only US children 0 to 18 years of age who were hospitalized and participated in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database, the prevalence rate of MCCs was 1507 per 100 000 persons.3 Therefore, estimates of prevalence and incidence significantly vary among previous studies depending on classification of MCCs and study population.
Many speculations may explain the increasing prevalence and incidence of MCCs in Olmsted County, Minnesota. First, advances in medical technology, health care delivery, and public health interventions may have decreased mortality in children with CCCs, leading to improved longevity and opportunity to develop MCCs. In a secondary data analysis of the National Health Interview Survey, rising prevalence rates of childhood disability were partly explained as being due to neurodevelopmental problems.39 Advances in surgical techniques and mechanical cardiopulmonary support have led to improved outcomes in children with congenital heart disease.68 Second, we found that children with prevalent MCCs were significantly more likely to have a minority background; thus, a growing minority population in Olmsted County may contribute to the rising trends of pediatric MCCs. Third, we used ICD-9 billing codes, of which provider awareness and use may have increased over time. Nonetheless, the precise reasons for the rising trends of prevalence and incidence of pediatric MCCs are unknown.
The rising prevalence and incidence rates of pediatric MCCs in Olmsted County have a few implications on both national and local levels. First, our population-based estimates of pediatric MCCs should be used to inform the health care policy at national and local levels because these children have high health care expenditures‡ and payer source is more likely to be public.69 Second, given our study finding that children of low SES were more likely to have prevalent MCCs, prevalence rates of pediatric MCCs may be higher in other parts of the United States. At present, the impact of pediatric MCCs on financial outcomes of families and the total national health care costs are not fully recognized but should be assessed. Third, we found that children of low SES and a minority background had more prevalent MCCs, a finding consistent with the extant literature. Authors of a recent national survey study found that the percentage of children with parent-reported disability rose 16% between 2001 and 2011, and children who were economically disadvantaged had the highest disability rates.39 Among US children who were hospitalized, rural children had higher rates of medical complexity and often resided in low-income and medically underserved areas.15 Finally, the authors of a survey of >40 000 children with special health needs found that 57% of the families reported financial problems, and 54% reported unemployment due to a child’s health.70 This finding requires further investigation because achieving health equity is an overreaching goal of Healthy People 2020.71
The main strengths of this study are its population-based study design and epidemiological advantages of our study setting, including a self-contained health care environment enabling use of an EMR linkage system. In addition, we applied objective, validated, and individualized measures for both pediatric chronic disease and SES.
Potential limitations include our classification of MCCs and a study setting limited to a mixed urban-rural community. Although our study setting is becoming more diverse, it may not be generalizable to an inner-city setting with significant racial diversity. The pediatric CCCs classification system does not include non-CCCs, including asthma, obesity, and attention-deficit/hyperactivity disorder and is only 1 of many tools developed to identify children with medical complexity.72 Other tools include the 3M Health Information Systems Clinical Risk Groups, the Agency for Healthcare Research and Quality Chronic Condition Indicator, and the Seattle Children’s Hospital Center of Excellence on Quality of Care Measures for Children with Complex Needs Pediatric Medical Complexity Algorithm. Nonetheless, CCCs have been extensively used in pediatric research and are strongly associated with mortality, functional limitations, and health care use.72 Because CCCs do not include all chronic conditions, this study’s reported period prevalence and incidence rates likely represent the lower range of MCCs within this population. Furthermore, we acknowledge the inherent limitations in combining all minority groups for statistical analyses because all minority groups are uniquely heterogeneous. However, we chose to combine all minorities into 1 group because our end points are rare. Furthermore, because our reporting of incidence trends per 5-year period included a fixed population at risk for each period, perturbation in birth rates may affect study results; however, we standardized our results to the US Census population for effective comparison. Finally, there are many unobserved factors that influence where children with MCCs live that are unaccounted for in this study and limit the generalizability of the data.
The REP has been used to assess and report generalizability of study findings from the Olmsted County, Minnesota, population.46 Briefly, age, sex, and ethnic characteristics of Olmsted County were similar to those of the state of Minnesota and the Upper Midwest from 1970 to 2000. However, Olmsted County was less ethnically diverse than the entire US population (90.3% vs 75.1% white), more highly educated (91.1% vs 80.4% high school graduates), and wealthier ($51 316 vs $41 994 median household income; 2000 US Census data). Age- and sex-specific mortality rates were similar for Olmsted County, the state of Minnesota, and the entire United States. In the report, it was suggested that generalizations from studies in any single selected population must be judged on a study-by-study basis. Our study covers the entire local pediatric population, and all eligible children with all race and/or ethnicity and socioeconomic strata were included. Our study findings, based on a geographically well-defined local pediatric population and on ethnic and socioeconomic factors, provide an important insight into the epidemiology of CCCs at a population level. In addition, although our study setting lacks racial and ethnic diversity, our local population represents a rural population that is 1 of special populations designated by the National Center for Advancing Translational Sciences and is, otherwise, overlooked (and the needs of this population is likely to be overlooked). We believe that the study results in the local context, especially representing a rural population, will complement and enrich the national perspective in the epidemiology of CCCs despite the lack of racial and ethnic diversity.
Conclusions
Both the prevalence and the incidence of MCCs among children living in this mixed urban-rural US community are increasing. Health disparities are present among children with MCCs and require further assessment to guide health care planning on both national and local levels.
Acknowledgments
We acknowledge and thank Dr Chris Feudtner for creating and sharing the pediatric CCCs classification system version 2, which was used to identify children in this study with MCCs.
Footnotes
Dr Bjur conceptualized and designed the study, interpreted the data, and drafted the initial manuscript; Drs Wi, Crow, and Juhn conceptualized and designed the study, interpreted the data, and reviewed and revised the manuscript; Dr Ryu conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection, and reviewed and revised the manuscript; Ms King conducted the initial analyses and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the Mayo Clinic Children’s Research Center, Department of Pediatric and Adolescent Medicine, and housing-based index of socioeconomic status research program. Also, this study was made possible by using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under award R01AG034676. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Juhn is the principal investigator of the 2015 Innovative Methods for Asthma Award, which is supported by Genentech and a steering committee member for the Real World Evidence Project for Asthma (supported by Genentech); the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Dzau VJ, McClellan MB, McGinnis JM, et al. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA. 2017;317(14):1461–1470 [DOI] [PubMed] [Google Scholar]
- 2.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay*. Crit Care Med. 2012;40(7):2196–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezaee ME, Pollock M. Multiple chronic conditions among outpatient pediatric patients, southeastern Michigan, 2008-2013. Prev Chronic Dis. 2015;12:E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646 [DOI] [PubMed] [Google Scholar]
- 6.Ananth P, Melvin P, Feudtner C, Wolfe J, Berry JG. Hospital use in the last year of life for children with life-threatening complex chronic conditions. Pediatrics. 2015;136(5):938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralston SL, Harrison W, Wasserman J, Goodman DC. Hospital variation in health care utilization by children with medical complexity. Pediatrics. 2015;136(5):860–867 [DOI] [PubMed] [Google Scholar]
- 8.Lindley LC, Lyon ME. A profile of children with complex chronic conditions at end of life among Medicaid beneficiaries: implications for health care reform. J Palliat Med. 2013;16(11):1388–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong W, Finnie DM, Shah ND, et al. Effect of multiple chronic diseases on health care expenditures in childhood. J Prim Care Community Health. 2015;6(1):2–9 [DOI] [PubMed] [Google Scholar]
- 10.Gray SH, Trudell EK, Emans SJ, Woods ER, Berry JG, Vernacchio L. Total direct medical expenses and characteristics of privately insured adolescents who incur high costs. JAMA Pediatr. 2015;169(10):e152682. [DOI] [PubMed] [Google Scholar]
- 11.Murtagh Kurowski E, Byczkowski T, Grupp-Phelan JM. Comparison of emergency care delivered to children and young adults with complex chronic conditions between pediatric and general emergency departments. Acad Emerg Med. 2014;21(7):778–784 [DOI] [PubMed] [Google Scholar]
- 12.Berry JG, Hall M, Dumas H, et al. Pediatric hospital discharges to home health and postacute facility care: a national study. JAMA Pediatr. 2016;170(4):326–333 [DOI] [PubMed] [Google Scholar]
- 13.Chan T, Di Gennaro J, Wechsler SB, Bratton SL. Complex chronic conditions among children undergoing cardiac surgery. Pediatr Cardiol. 2016;37(6):1046–1056 [DOI] [PubMed] [Google Scholar]
- 14.Stephens JR, Steiner MJ, DeJong N, et al. Healthcare utilization and spending for constipation in children with versus without complex chronic conditions. J Pediatr Gastroenterol Nutr. 2017;64(1):31–36 [DOI] [PubMed] [Google Scholar]
- 15.Peltz A, Wu CL, White ML, et al. Characteristics of rural children admitted to pediatric hospitals. Pediatrics. 2016;137(5):e20153156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold JM, Hall M, Shah SS, et al. Long length of hospital stay in children with medical complexity. J Hosp Med. 2016;11(11):750–756 [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Das P, Roberson DW, et al. Hospitalizations in children with preexisting tracheostomy: a national perspective. Laryngoscope. 2015;125(2):462–468 [DOI] [PubMed] [Google Scholar]
- 18.Stone BL, Boehme S, Mundorff MB, Maloney CG, Srivastava R. Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255 [DOI] [PubMed] [Google Scholar]
- 19.Edwards JD, Houtrow AJ, Lucas AR, et al. Children and young adults who received tracheostomies or were initiated on long-term ventilation in PICUs. Pediatr Crit Care Med. 2016;17(8):e324–e334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal R, Hall M, Cohen E, et al. Trends in health care spending for children in Medicaid with high resource use. Pediatrics. 2016;138(4):e20160682. [DOI] [PubMed] [Google Scholar]
- 21.Kuo DZ, Houtrow AJ; Council on Children With Disabilities. Recognition and management of medical complexity. Pediatrics. 2016;138(6):e20163021. [DOI] [PubMed] [Google Scholar]
- 22.Parekh AK, Goodman RA, Gordon C, Koh HK; HHS Interagency Workgroup on Multiple Chronic Conditions. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126(4):460–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mery G, Majumder S, Brown A, Dobrow MJ. What do we mean when we talk about the Triple Aim? A systematic review of evolving definitions and adaptations of the framework at the health system level. Health Policy. 2017;121(6):629–636 [DOI] [PubMed] [Google Scholar]
- 24.Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics. 2015;135(1). Available at: www.pediatrics.org/cgi/content/full/135/1/e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Doren BA, Roy D, Noone JM, Blanchette CM, Arthur ST. Cachexia & debility diagnoses in hospitalized children and adolescents with complex chronic conditions: evidence from the Kids’ Inpatient Database. Drugs Context. 2015;4:212277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez MA, Cruz AT, Kowalkowski MA, Raphael JL. Trends in hospitalizations and resource utilization for pediatric pertussis. Hosp Pediatr. 2014;4(5):269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. 2014;33(9):907–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry JG, Hall M, Hall DE, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatr. 2013;167(2):170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6). Available at: www.pediatrics.org/cgi/content/full/130/6/e1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindley LC, Mack JW, Bruce DJ. Clusters of multiple complex chronic conditions: a latent class analysis of children at end of life. J Pain Symptom Manage. 2016;51(5):868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindley LC, Mixer SJ, Mack JW. Home care for children with multiple complex chronic conditions at the end of life: the choice of hospice versus home health. Home Health Care Serv Q. 2016;35(3–4):101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brittan MS, Sills MR, Fox D, et al. Outpatient follow-up visits and readmission in medically complex children enrolled in Medicaid. J Pediatr. 2015;166(4):998–1005.e1 [DOI] [PubMed] [Google Scholar]
- 33.Agrawal R, Smith T, Li Y, Cartland J. Rate of spending on chronic conditions among Medicaid and CHIP recipients. Pediatrics. 2014;134(1). Available at: www.pediatrics.org/cgi/content/full/134/1/e80 [DOI] [PubMed] [Google Scholar]
- 34.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1, pt 2):205–209 [PubMed] [Google Scholar]
- 35.Feudtner C, Silveira MJ, Christakis DA. Where do children with complex chronic conditions die? Patterns in Washington State, 1980-1998. Pediatrics. 2002;109(4):656–660 [DOI] [PubMed] [Google Scholar]
- 36.Jamorabo DS, Belani CP, Martin EW. Complex chronic conditions in Rhode Island’s pediatric populace: implications for palliative and hospice services, 2000-2012. J Palliat Med. 2015;18(4):350–357 [DOI] [PubMed] [Google Scholar]
- 37.Feudtner C, DiGiuseppe DL, Neff JM. Hospital care for children and young adults in the last year of life: a population-based study. BMC Med. 2003;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feinstein JA, Feudtner C, Kempe A. Adverse drug event-related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133(6). Available at: www.pediatrics.org/cgi/content/full/133/6/e1575 [DOI] [PubMed] [Google Scholar]
- 39.Houtrow AJ, Larson K, Olson LM, Newacheck PW, Halfon N. Changing trends of childhood disability, 2001-2011. Pediatrics. 2014;134(3):530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Lee JH, Mokkink LB, Grootenhuis MA, Heymans HS, Offringa M. Definitions and measurement of chronic health conditions in childhood: a systematic review. JAMA. 2007;297(24):2741–2751 [DOI] [PubMed] [Google Scholar]
- 41.Cohen E, Yantzi N, Guan J, Lam K, Guttmann A. Residential movement patterns of families of young children with chronic conditions in Ontario, Canada: a population-based cohort study. Int J Equity Health. 2013;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., III History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88(5):933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.United States Census Bureau. Olmsted County, Minnesota community facts. 2010. Available at: https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml. Accessed January 29, 2019
- 48.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e99 [DOI] [PubMed] [Google Scholar]
- 49.Feudtner C, Feinstein JA, Satchell M, Zhao H, Kang TI. Shifting place of death among children with complex chronic conditions in the United States, 1989-2003. JAMA. 2007;297(24):2725–2732 [DOI] [PubMed] [Google Scholar]
- 50.Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen E, Patel H. Responding to the rising number of children living with complex chronic conditions. CMAJ. 2014;186(16):1199–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindley LC, Newnam KM. Hospice use for infants with life-threatening health conditions, 2007 to 2010. J Pediatr Health Care. 2017;31(1):96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith AG, Andrews S, Bratton SL, et al. Pediatric palliative care and inpatient hospital costs: a longitudinal cohort study. Pediatrics. 2015;135(4):694–700 [DOI] [PubMed] [Google Scholar]
- 54.Kim B, Kim SZ, Lee J, et al. Clinical profiles of adverse drug reactions spontaneously reported at a single Korean hospital dedicated to children with complex chronic conditions. PLoS One. 2017;12(2):e0172425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. 2013;141(4):880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wi CI, St Sauver JL, Jacobson DJ, et al. Ethnicity, socioeconomic status, and health disparities in a mixed rural-urban US community-Olmsted County, Minnesota. Mayo Clin Proc. 2016;91(5):612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med. 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghawi H, Crowson CS, Rand-Weaver J, Krusemark E, Gabriel SE, Juhn YJ. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case-control study. BMJ Open. 2015;5(4):e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bang DW, Manemann SM, Gerber Y, et al. A novel socioeconomic measure using individual housing data in cardiovascular outcome research. Int J Environ Res Public Health. 2014;11(11):11597–11615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammer R, Capili C, Wi CI, Ryu E, Rand-Weaver J, Juhn YJ. A new socioeconomic status measure for vaccine research in children using individual housing data: a population-based case-control study. BMC Public Health. 2016;16(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wi CI, Gauger J, Bachman M, et al. Role of individual-housing-based socioeconomic status measure in relation to smoking status among late adolescents with asthma. Ann Epidemiol. 2016;26(7):455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryu E, Wi CI, Crow SS, et al. Assessing health disparities in children using a modified housing-related socioeconomic status measure: a cross-sectional study. BMJ Open. 2016;6(7):e011564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc. 2014;89(10):1336–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62(6):1576–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergstralh EJ, Offord KP, Chu CP, Beard CM, O’Fallon WM, Melton LJ., III Calculating Incidence, Prevalence and Mortality Rates for Olmsted County, Minnesota: An Update. Technical Report No 49. Rochester, MN: Mayo Clinic; 1992 [Google Scholar]
- 67.St Sauver JL, Boyd CM, Grossardt BR, et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open. 2015;5(2):e006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holst KA, Said SM, Nelson TJ, Cannon BC, Dearani JA. Current interventional and surgical management of congenital heart disease: specific focus on valvular disease and cardiac arrhythmias. Circ Res. 2017;120(6):1027–1044 [DOI] [PubMed] [Google Scholar]
- 69.Kuo DZ, Melguizo-Castro M, Goudie A, Nick TG, Robbins JM, Casey PH. Variation in child health care utilization by medical complexity. Matern Child Health J. 2015;19(1):40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuo DZ, Cohen E, Agrawal R, Berry JG, Casey PH. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165(11):1020–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.US Department of Health and Human Services. Secretary’s Advisory Committee on national health promotion and disease prevention objectives for 2020. Available at: www.healthypeople.gov/2010/hp2020/advisory/societaldeterminantshealth.htm. Accessed January 29, 2019 [DOI] [PubMed]
- 72.Berry JG, Hall M, Cohen E, O’Neill M, Feudtner C. Ways to identify children with medical complexity and the importance of why. J Pediatr. 2015;167(2):229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]