Abstract
Cannabinoid/endocannabinoid signaling is primarily mediated by cannabinoid receptor type 1 (CB1; encoded by Cnr1) and/or type 2 (CB2; encoded by Cnr2). Here, we show that Cnr1−/−Cnr2−/− mice are subfertile as a result of compromised implantation. Upon implantation, the epithelium is smooth and adhered to the blastocyst trophectoderm within the implantation chamber (crypt) in wild-type mice, whereas the epithelium in Cnr1−/−Cnr2−/− mice is ruffled, which compromises appropriate blastocyst-uterine interactions. The suboptimal implantation leads to higher incidence of pregnancy failure in Cnr1−/−Cnr2−/− mice. Histological analysis revealed heightened edema around the implantation chamber in these deleted females. With the use of a reporter mouse line, we observed that CB2 is present on endothelial cells of uterine blood vessels, and its absence leads to blood vessel leakage during implantation. These results suggest that appropriately regulated uterine edema is important to optimal implantation.
A generalized uterine edema occurs before blastocyst implantation in rodents, which is considered to facilitate the luminal closure. The luminal closure promotes a close interaction between the trophectoderm and the luminal epithelium (1). Luminal closure occurs throughout the uterus before implantation. Occurrence of the luminal closure in both pregnancy and pseudopregnancy suggests that the presence of blastocysts is not critical. However, the luminal closure does not occur in the absence of progesterone priming of the uterus (2). The vascular permeability is further increased at the site of embryo implantation. Histamine and prostaglandins (PGs) have been considered to be critical for vascular permeability increases. However, normal implantation in mice lacking histidine decarboxylase, a key enzyme to histamine synthesis, or histamine type 2 receptor raises a question regarding histamine’s role in increased vascular permeability in implantation (3, 4). In contrast, PGs are critical to implantation, as mice with deletion of Ptgs2 [encoding cyclooxygenase 2 (COX2)], a rate-limiting enzyme in biosynthesis of PGs, show implantation failure (5); COX2 derived PGI2 partially restores implantation defects in Ptgs2−/− mice (6). Nonetheless, the regulatory mechanism of vascular permeability around the implantation site is largely unknown.
Natural cannabinoids and endocannabinoids signal through two membrane receptors: cannabinoid receptor type 1 (CB1; encoded by Cnr1) and type 2 (CB2; encoded by Cnr2). Cnr1 and Cnr2 share over 40% sequence homology and are coupled to G protein–coupled receptors (GPCRs) of the Gi/o and Gq families. CB1 is expressed in the central nervous system and peripheral tissues, including heart, testis, liver, small intestine, and uterus. In contrast, CB2 (Cnr2) was first thought to be expressed by immune cells (7). Later studies showed that CB2 is also found in microglial and neuron cells in brains of both rodents (8, 9) and humans (10, 11); its function in the brain has been revealed (12–15). However, as a result of low expression of CB2 and lack of research tools, it has been difficult to study the roles of CB2 in peripheral systems (16). To study the expression of CB2 better, two reporter mouse lines were created using green fluorescent protein (GFP) (17, 18).
Previously, we have shown that tightly regulated endocannabinoid signaling is critical to multiple pregnancy events, including peri-implantation embryo development, oviductal transport of embryos, and placentation (19). These studies implicate that dysregulated endocannabinoid signaling makes pregnancy suboptimal. There is also evidence that implantation in the mouse is a target of cannabinoid signaling. For example, levels of an endocannabinoid anandamide are decreased after the uterus enters the receptive phase (20), suggesting that lower endocannabinoid signaling is beneficial to uterine receptivity for implantation. This observation was further reinforced by pharmacological observations that administration of (−)-Δ9-tetrahydrocannabinol, a potent cannabinoid, in wild-type (WT) mice from day 2 of pregnancy causes implantation failure (21). Studies also suggest that elevated cannabinoid signaling is harmful to blastocyst competency to implantation. The expression of CB1 in blastocysts is downregulated with the onset of blastocyst activation before implantation, whereas CB1 levels remain high in dormant blastocysts during delayed implantation (21). In search for the molecular mechanism, we found that MAPK signaling is activated by anandamide at a lower concentration (7 nM), whereas at a higher concentration (28 nM), anandamide inhibits Ca2+ channel activity and blastocyst competency for implantation (22). Taken together, these observations suggest that elevated cannabinoid signaling is detrimental to mouse implantation. However, the physiological role to maintain the activation of endocannabinoid signaling during implantation still is not clear.
In this study, roles of endocannabinoid signaling in implantation are investigated using mouse models with suppression of CB1, CB2, or both. The results show that CB1/CB2 double-mutant mice have a compromised implantation process, resulting in pregnancy loss in midgestational stage.
Materials and Methods
Animals and treatments
Cnr1−/− and Cnr2−/− mice were generated as described (23, 24), and Cnr1−/−Cnr2−/− females were generated by the crossing of Cnr1−/− and Cnr2−/− mice. CB2-enhanced GFP (EGFP) reporter mice were generated by the insertion of an EGFP gene, preceded by an internal ribosomal entry site sequence in the 3′-untranslated region of the mouse Cnr2 gene (18). All genetically modified mice and WT controls were maintained on a C57BL/6 mixed background and were housed in the animal care facility at the Cincinnati Children’s Hospital Medical Center, according to the National Institutes of Health and institutional guidelines for laboratory animals. All protocols of the current study were approved by the Cincinnati Children’s Hospital Research Foundation Institutional Animal Care and Use Committee. All mice were housed in wall-mount, negative-airflow, polycarbonate cages with corn-cob bedding. They were provided ad libitum double-distilled, autoclaved water and a rodent diet (LabDiet 5010).
Female mice were mated with WT fertile males to induce pregnancy (vaginal plug = day 1 of pregnancy). Embryos at the morula or blastocyst stage were recovered by the flushing of uteri on day 4. Implantation sites were visualized by an intravenous injection of 0.1 mL of 1% Chicago blue dye solution in saline, 4 minutes before euthanasia, and the number of implantation sites—demarcated by distinct blue bands—was recorded. Mice were euthanized by cervical dislocation right before tissue collection under deep anesthesia.
In situ hybridization
In situ hybridization was performed as previously described (25). Both radioactive (35S-GTP) and digoxigenin (DIG) labeling methods were used. In brief, frozen sections (12 μm) were mounted onto poly-l-lysine-coated slides and fixed in cold 4% paraformaldehyde in PBS. The sections were prehybridized and hybridized at 45°C for 4 hours in 50% (v/v) formamide hybridization buffer containing 35S-labeled antisense RNA probes (PerkinElmer). RNase A-resistant hybrids were detected by autoradiography. For DIG labeling, sections were hybridized at 65°C overnight in 50% (v/v) formamide hybridization buffer containing DIG-labeled probes (Roche). All sections were poststained with hematoxylin and eosin (H&E).
Immunofluorescence
Antibodies for Epithelial-cadherin (E-cad; Cell Signaling Technology) (26), GFP (Thermo Fisher Scientific) (27), platelet endothelial cell adhesion molecule (PECAM; BD Pharmingen) (28), CD45 (BioLegend) (29), and vascular endothelial-cadherin (VECAD; BD Pharmingen) (30) were used for immunofluorescence staining. Actin staining was performed using rhodamine phalloidin (R415; Invitrogen) (31). All fluorophore-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories. Nuclear staining was performed using Hoechst 33342 (H1399; 2 µg/mL; Molecular Probes). Immunofluorescence was performed on fresh-frozen sections. Sections were fixed in 10% neutral-buffered formalin and incubated with primary antibody at 4°C overnight, followed by incubation in secondary antibody for 1 hour in PBS. Immunofluorescence was visualized under a confocal microscope (Nikon Eclipse TE2000).
Immunohistochemical staining
Immunolocalization was performed in 10% neutral-buffered, formalin-fixed, paraffin-embedded sections using specific antibodies to Ki67 (SP-6; Thermo Fisher Scientific) (32). A Histostain-Plus (DAB) kit (Thermo Fisher Scientific) was used to visualize specific antigens.
RT-PCR
RNAs from WT and Cnr2−/− uterine samples were analyzed, as described previously (33, 34). In brief, total RNA was extracted with Trizol (Invitrogen), according to the manufacturer’s protocol. After DNase treatment (Ambion), 1 µg total RNA was reverse transcribed with Superscript II (Invitrogen). RT-PCR was performed using primers 5′-ACAAGGCTCCACAAGACCCT-3′ (sense) and 5′-CCGTTGGTCACTTCTGTCT-3′ (antisense) for Cnr2.
Statistical analysis
Data were analyzed by the Student t tests or χ2 tests, as indicated in figure legends. Data are shown as means ± SEM.
Results
Deletion of CB2 superimposed on CB1 deletion impairs fertility
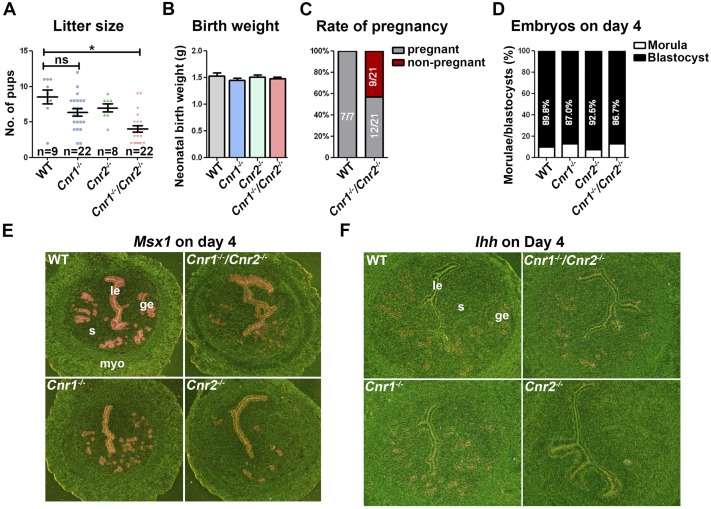
Our previous work showed that Cnr1−/− and Cnr1−/−Cnr2−/− mice retain embryos in the oviducts (35). However, after our laboratory was relocated to Cincinnati Children’s in 2008, the pregnancy phenotypes became subtle in Cnr1−/− pregnant females, perhaps by environmental and dietary changes, as these cues are potential sources of variations (36, 37). Intriguingly, when bred with WT males, Cnr1−/−Cnr2−/− females, but not Cnr1−/− or Cnr2−/− females, still show substantially smaller litter sizes compared with WT (Fig. 1A). Although the neonatal weights are comparable in WT and Cnr1−/−Cnr2−/− females (Fig. 1B), the pregnancy rates are substantially lower in Cnr1−/−Cnr2−/− females (Fig. 1C).
Figure 1.
Deletion of CB2 superimposed on CB1 deletion impairs fertility. (A) The litter size of Cnr1−/−Cnr2−/− females was substantially smaller than WT. Values are means ± SEM; *P < 0.05, Student t tests. (B) Neonatal birth weights in WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females are comparable. (C) The pregnancy rate of Cnr1−/−Cnr2−/− females is lower than that of WT mice. (D) The rates of blastocysts and morulae vs total numbers of embryos are comparable in WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females. (E and F) The expression patterns of uterine receptivity genes critical for implantation show no substantial difference in Cnr1−/−Cnr2−/− females. In situ hybridization of Msx1 and Ihhin uteri on day 4 of pregnancy. ge, glandular epithelium; le, luminal epithelium; myo, myometrium; ns, not significant; s, stroma.
To investigate the causes of this compromised fertility in Cnr1−/−Cnr2−/− females, we examined the number and quality of embryos in uteri on day 4 of pregnancy by mating WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females with WT males. The results show that there are no substantial differences in the rate of blastocysts recovered from uteri of all females examined (Fig. 1D). Given that all embryos are either WT or heterozygous, the results are consistent to our previous observation that embryonic, but not maternal CB1, is important for normal preimplantation embryo development (35). Uterine receptivity was then investigated in these females on day 4 of pregnancy, using two well-established marker genes: Msx1 and Ihh (38, 39). The in situ hybridization results show that Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− uteri have comparable RNA levels of Msx1 and Ihh (Fig. 1E and 1F). These data suggest that Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females have no obvious defects in the acquisition of uterine receptivity to implantation and support of preimplantation embryo development.
Blastocyst implantation in Cnr1−/−Cnr2−/− females is suboptimal
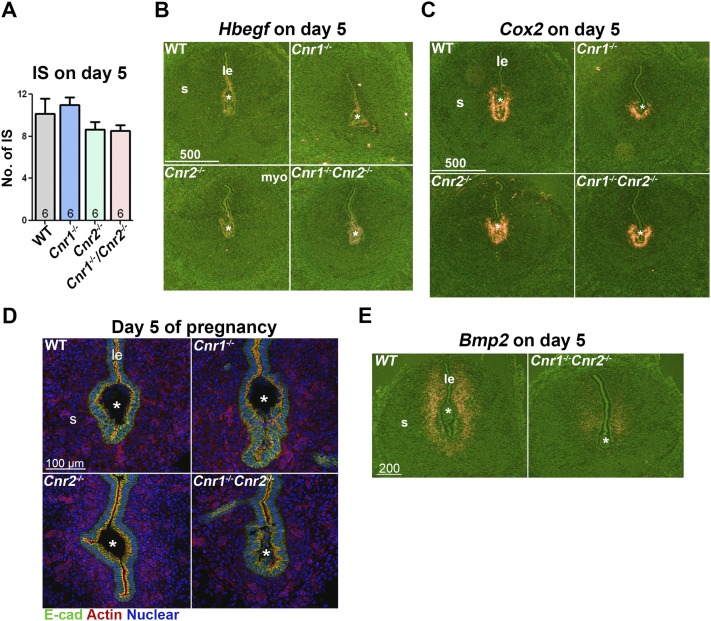
To address the issue of suboptimal implantation, we examined the implantation sites on day 5. Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females show comparable numbers of implantation sites as WT females (Fig. 2A). Blastocysts initiate the attachment in uteri in the midnight of day 4 of pregnancy, and the attachment is associated with the induction of Hbegf expression in the embryo and surrounding luminal epithelia (40); the attachment reaction further induces Cox2 expression in the epithelium lining the implantation chamber and adjacent subepithelial stromal cells (5). We examined the expression of Hbegf and Cox2 in implantation sites on day 5 of pregnancy and found that attachment reaction appears normal in Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− implantation sites, as is evident from comparable expression levels of Hbegf and Cox2 (Fig. 2B and 2C). However, a careful examination of the epithelium lining the implantation chambers (crypts) revealed that crypts appear spear shaped in WT, Cnr1−/−, and Cnr2−/− females, whereas the crypts in Cnr1−/−Cnr2−/− females have a distorted, apical surface of the epithelium, as demarcated by actin staining, suggesting abnormal tension of Cnr1−/−Cnr2−/− epithelia (Fig. 2D). Consequently, the compromised implantation in Cnr1−/−Cnr2−/− females causes weaker decidual responses, as shown by decidualization marker Bmp2 on day 5 of pregnancy (Fig. 2E). All of these data suggest that the implantation process is compromised in Cnr1−/−Cnr2−/− females, which further causes adverse effects on decidualization.
Figure 2.
Cnr1−/−Cnr2−/− females show suboptimal blastocyst implantation. (A) The numbers of implantation sites (IS) in WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females are comparable on day 5 of pregnancy. Values are means ± SEM. (B and C) In situ hybridization of Hbegf and Cox2in uteri on day 5 of pregnancy. The expression patterns of genes critical for implantation show no substantial difference in WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females. (D) Immunofluorescent staining of epithelial cadherin (E-cad) and actin on implantation chambers on day 5 of pregnancy. (E) In situ hybridization of Bmp2in uteri on day 5 of pregnancy. Decidual responses in Cnr1−/−Cnr2−/− females are weaker than those in WT females. *Positions of embryos. IS, implantation site; le, luminal epithelium; s, stroma.
Defective implantation triggers increased incidence of resorptions at midgestation
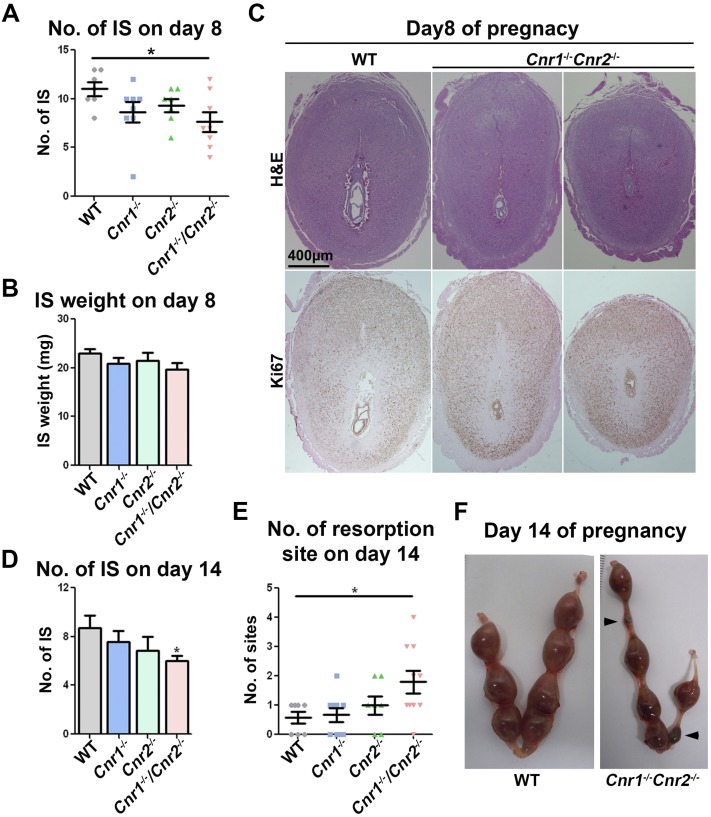
To explore the reason for Cnr1−/−Cnr2−/− females showing smaller litter sizes, we examined the implantation sites on day 8 of pregnancy when decidual responses are maximal and on day 14 of pregnancy when placenta is undergoing maturation. On day 8, the number of implantation sites in Cnr1−/−Cnr2−/− females is significantly lower than those in WT females; the number of implantation sites in Cnr1−/− or Cnr2−/− females shows no substantial differences (Fig. 3A). Interestingly, the average weight of implantation sites in Cnr1−/−Cnr2−/− females appears normal with slight variations (Fig. 3B). However, histological analysis of implantation sites on day 8 shows that embryo development in Cnr1−/−Cnr2−/− females is impeded compared with that in WT mice (Fig. 3C). On day 14, the number of implantation sites is lower in Cnr1−/−Cnr2−/− females with a higher number of resorptions (Fig. 3D–3F). These results suggest that defects in implantation in Cnr1−/−Cnr2−/− females have adverse ripple effects on subsequent embryo development, resulting in a higher rate of resorptions.
Figure 3.
Incidence of resorptions in Cnr1−/−Cnr2−/− females is increased in midgestation. (A and B) Numbers and weights of implantation sites in WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females on day 8 of pregnancy. The number of implantation sites in Cnr1−/−Cnr2−/− females is lower than that of WT females. Values are means ± SEM; *P < 0.05, Student t tests. (C) H&E and Ki67 staining in implantation sites on day 8 of pregnancy. (D and E) Numbers of implantation and resorption sites in WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females on day 14 of pregnancy. Cnr1−/−Cnr2−/− females have fewer implantation sites and higher numbers of resorption sites. Values are means ± SEM; *P < 0.05, Student t tests. (F) Implantation sites in WT and Cnr1−/−Cnr2−/− females on day 14 of pregnancy. Arrowheads point to resorption sites. IS, implantation site.
Abnormal luminal epithelial organization in the implantation chamber accompanies increased edema in double-mutant mice
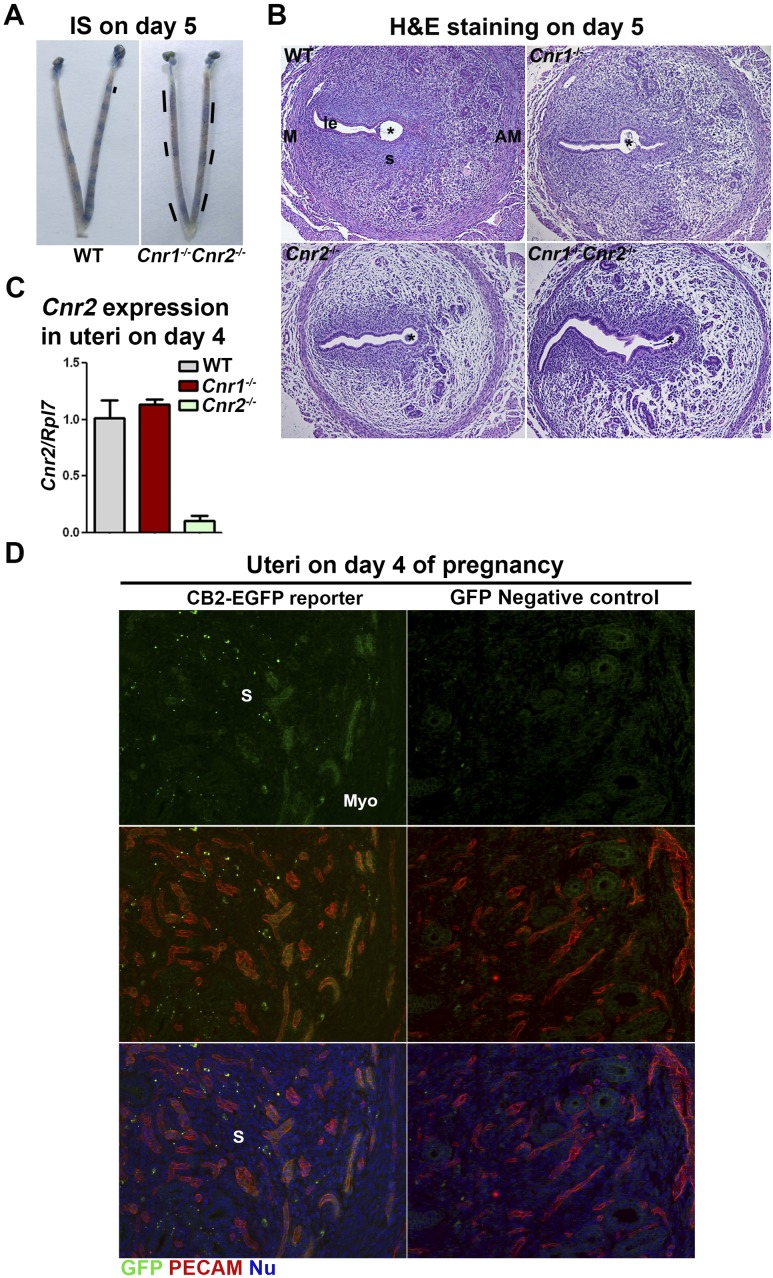
In search for this compromised implantation in Cnr1−/−Cnr2−/− females, we examined the location of implantation sites by giving intravenously a Chicago blue dye solution on day 5 of pregnancy. Distinct blue bands are observed in WT females, but bands with a faint, disseminated blue color are observed in Cnr1−/−Cnr2−/− females (Fig. 4A). This result suggests that the uterine vascular permeability is increased more than normal in Cnr1−/−Cnr2−/− females. H&E staining of implantation sites on day 5 of pregnancy also suggests that edema is increased in Cnr2−/− and Cnr1−/−Cnr2−/− uteri compared with WT uteri (Fig. 4B).
Figure 4.
Cnr1−/−Cnr2−/− females show increased edema in implantation sites on day 5 of pregnancy. (A) Implantation sites in WT and Cnr1−/−Cnr2−/− females on day 5 of pregnancy. Lines indicate the uterine domain with blue color. (B) H&E staining in implantation sites on day 5 of pregnancy. (C) RT-PCR of Cnr2 using RNA collected from uteri on day 4 of pregnancy. Cnr2 is expressed in uterine endothelial cells. Values are means ± SEM. (D) Immunostaining of GFP and PECAM in uteri of CB2-EGFP reporter and control mice on day 4 of pregnancy. GFP-positive signals are observed on PECAM-positive endothelial cells. *Positions of embryos. AM, antimesometrial side; IS, implantation site; M, mesometrial side; Myo, myometrium; Nu, nuclear; S, stroma.
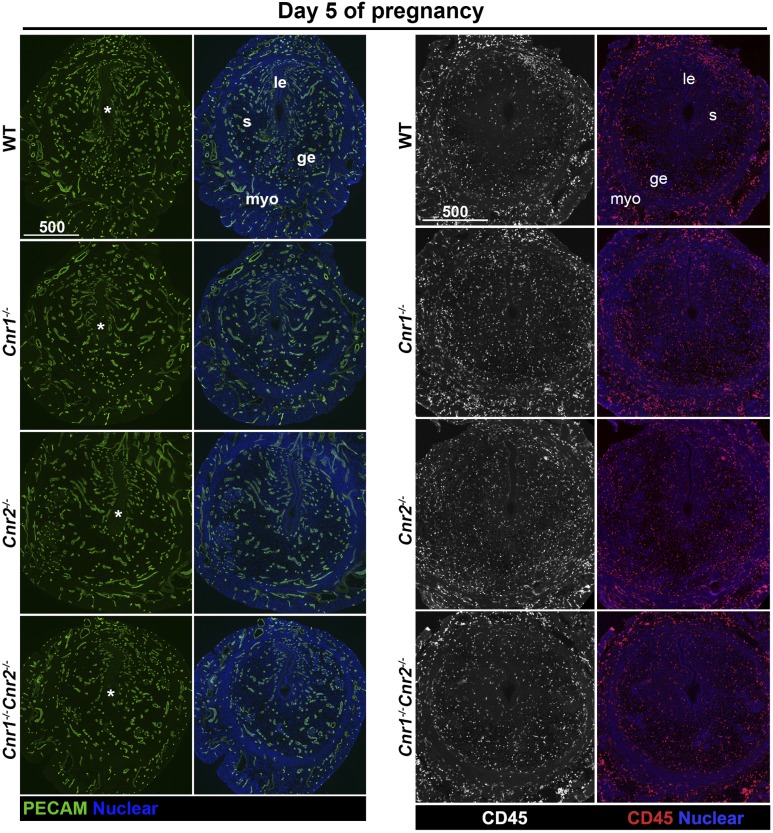
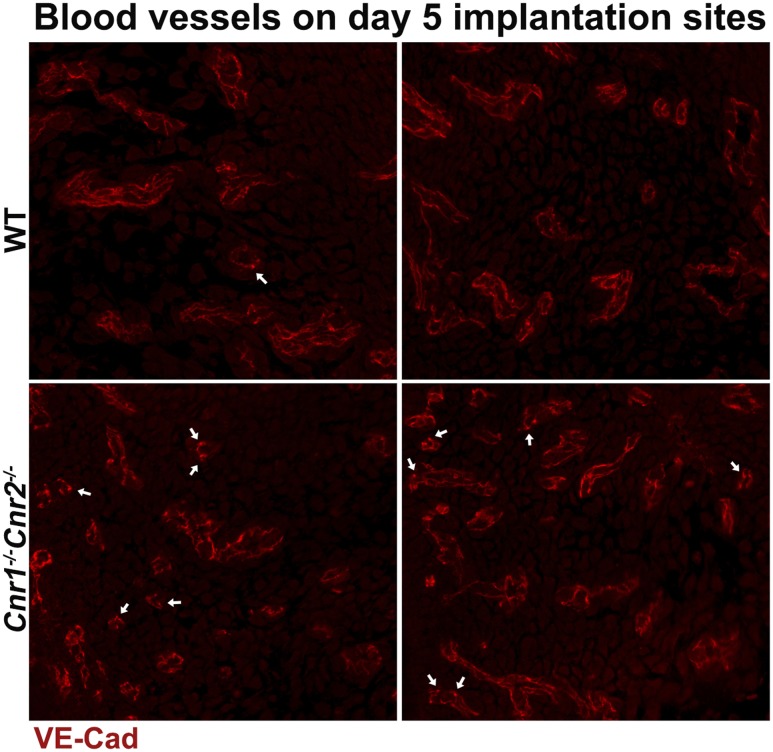
Our previous studies showed that CB1 is expressed in blastocysts, oviducts, and uteri (34, 35, 41). However, the expression of CB2 in uterine cells is debatable, as a result of its low abundance, lack of reliable antibodies, and confounding expression in infiltrating leukocytes in the stroma. We examined mRNA levels of Cnr2 in uterine tissues on day 4 of pregnancy. The results show that Cnr2 is present in WT and Cnr1−/− uteri, and the levels of Cnr2 are significantly lower in Cnr2−/− uteri (Fig. 4C). To locate the specific cell types expressing CB2, we used CB2-EGFP reporter mice (18). Our results show that besides the expression of CB2 in leukocytes, positive signals of CB2-EGFP are also observed in endothelial cells on day 4 of pregnancy (Fig. 4D). Given the presence of CB2 on endothelial cells and leukocytes, we examined whether uteri, deficient in Cnr2, show abnormal distribution of blood vessels or the infiltration of leukocytes by staining endothelial cells by PECAM and hematopoietic cells by CD45. The results show that the pattern and density of blood vessels appear comparable in uteri of WT, Cnr1−/−, Cnr2−/−, and Cnr1−/−Cnr2−/− females on day 5 of pregnancy, and no substantial differences in infiltrated CD45+ cells (Fig. 5). Vascular permeability is maintained by VECAD, which shows punctate staining in activated vessels with higher permeability (42, 43). The immunostaining of VECAD in Cnr1−/−Cnr2−/− vessels shows more punctate staining compared with that in WT females (Fig. 6) (44). These data suggest that the deletion of Cnr2 in leukocytes and endothelial cells causes increased edema during implantation in Cnr1−/−Cnr2−/− females, which is associated with defective implantation.
Figure 5.
Deletion of Cnr1 and Cnr2 does not affect vascular structure and leukocyte infiltration. Blood vessels in implantation sites on day 5 of pregnancy are marked by endothelial marker PECAM. Leukocytes are highlighted by CD45, which is expressed on all leukocytes. *Positions of embryos. ge, glandular epithelium; le, luminal epithelium; myo, myometrium; s, stroma.
Figure 6.
Immunostaining of VECAD (VE-Cad) in implantation sites of WT and Cnr1−/−Cnr2−/− females on day 5 of pregnancy. Arrows point to the loci with concentrated VECAD signals.
Discussion
In this study, we show that embryo implantation in Cnr1−/−Cnr2−/− females is compromised. Despite the occurrence of on-time implantation, the structure of implantation chambers (crypt) appears abnormal, which ultimately leads to resorption of embryos at the midgestational stage. Our recent studies reported that planar cell polarity (PCP) is critical for appropriate formation of the implantation chamber. Implantation chambers are normally “spear shaped” in WT mice (45), and the apical surface of the chamber is smooth. Defects in PCP signaling disrupt this structure and cause pregnancy failure (46). In Cnr1−/−Cnr2−/− females, the implantation chamber projecting toward the antimesometrial pole appears oval shaped, and the apical surface of the crypt (implantation chamber) epithelia is distorted, heralding the signs of compromised pregnancy outcomes. Whether PCP signaling is a cause for this abnormal implantation chamber formation will require further investigation.
CB2 is expressed on hematopoietic cells and endothelial cells (Fig. 4B), and so, the definitive cell type responsible for increased uterine edema in pregnant Cnr1−/−Cnr2−/− females is not clear. However, features of increased endometrial vascular permeability suggest that expression of CB2 in endothelial cells plays a role. CB2 is a GPCR associated with Gi (47). GPCRs in endothelial cells are necessary in maintaining vascular permeability. For example, activation of sphingosine-1 phosphate receptor 1, a Gi-coupled GPCR in endothelial cells, stabilizes adherens junctions and maintains endothelial permeability (48). In addition, receptors for PGs are coupled to a variety of G proteins and are involved in tissue edema (49). Our results indicate that infiltration of leukocytes into the endometrium is not severely affected during implantation in Cnr1−/−Cnr2−/− females (Fig. 5). However, we cannot rule out the possibility that deletion of CB2 in leukocytes plays a role in uterine edema.
The expression of CB2 in endothelial cells has previously been reported (50, 51). The activation of CB2 reduces tissue edema in mice (52). JWH133, a selective CB2 agonist, prevents neurogenic pulmonary edema and downregulates the expression of tight junctional adhesion molecule A (53), and in brain trauma, administration of JWH133 significantly attenuates brain edema (54). In the context of blood vessels, CB2 activation attenuates TNF-α-induced endothelial cell activation, transendothelial migration of monocytes, and augmented monocyte-endothelial adhesion (50). In the current study, we also observed that the deletion of Cnr2 superimposed on Cnr1 deletion causes increased uterine edema, suggesting the role of CB2 in restraining tissue edema.
Endocannabinoid signaling plays regulatory roles in multiple pregnancy events. Preimplantation embryo development is regulated by endocannabinoid signaling. Embryos exposed to high levels of endocannabinoid signaling via CB1 show retarded development (20, 41, 55). The observation that Cnr1−/− but not Cnr1+/− embryos recovered from Cnr1−/− females shows aberrant development compared with WT embryos (35), suggests that appropriate cannabinoid signaling via embryonic CB1 is beneficial to normal embryo development. Cannabinoid signaling is critical to oviductal embryo transport. A reciprocal embryo transfer experiment between Cnr1−/− and WT female mice suggests that the absence of maternal CB1 causes oviductal retention of embryos, irrespective of embryonic genotypes (35). Faah−/− mice with higher oviductal anandamide levels and WT mice exposed to (−)-Δ9-tetrahydrocannabinol or methanandamide (anandamide analog) also show oviductal retention of embryos (55). These observations suggest that a finely regulated endocannabinoid tone mediated by CB is critical to normal embryo development and oviductal transport. The current study suggests suppression of endocannabinoid signaling compromises mouse implantation. Our previous work showed that increased endocannabinoid signaling is also detrimental to implantation (20, 21). Therefore, mouse implantation also requires a finely regulated endocannabinoid tone.
Endocannabinoid signaling is operative during human pregnancy (56, 57). Several studies on endocannabinoid signaling in human reproduction are consistent with studies in mice. For example, our study in Faah−/− females with elevated anandamide levels showing oviductal embryo retention is consistent with a report of ectopic pregnancy in humans with high anandamide levels in the fallopian tube (55, 58). In the similar vein, higher cannabinoid signaling is detrimental to uterine receptivity in mouse (21), which is also relevant to implantation in humans (59, 60). In the present investigation, we show that suppressed endocannabinoid signaling compromises mouse implantation. Whether this finding is important for human implantation will require further investigation. With the consideration of the observations in mice, it is possible that a finely tuned endocannabinoid signaling is also important for human pregnancy success.
Acknowledgments
We thank Cecilia J. Hillard (Medical College of Wisconsin) for sharing with us the CB2-EGFP reporter mouse line.
Financial Support: This work was supported, in part, by the National Institutes of Health Grants DA006668 and HD068524 (to S.K.D.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- COX2
cyclooxygenase 2
- DIG
digoxigenin
- EGFP
enhanced green fluorescent protein
- GFP
green fluorescent protein
- GPCR
G protein–coupled receptor
- H&E
hematoxylin and eosin
- PCP
planar cell polarity
- PECAM
platelet endothelial cell adhesion molecule
- PG
prostaglandin
- VECAD
vascular endothelial-cadherin
- WT
wild-type
References
- 1. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. [DOI] [PubMed] [Google Scholar]
- 2. Huet-Hudson YM, Dey SK. Requirement for progesterone priming and its long-term effects on implantation in the mouse. Proc Soc Exp Biol Med. 1990;193(4):259–263. [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi T, Tonai S, Ishihara Y, Koga R, Okabe S, Watanabe T. Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J Clin Invest. 2000;105(12):1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzás E, Kovács P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502(1-2):53–56. [DOI] [PubMed] [Google Scholar]
- 5. Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 6. Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13(12):1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108(1-2):169–190. [DOI] [PubMed] [Google Scholar]
- 8. Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2(1):69–82. [DOI] [PubMed] [Google Scholar]
- 9. Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. [DOI] [PubMed] [Google Scholar]
- 10. Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23(35):11136–11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Núñez E, Benito C, Pazos MR, Barbachano A, Fajardo O, González S, Tolón RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53(4):208–213. [DOI] [PubMed] [Google Scholar]
- 12. Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. [DOI] [PubMed] [Google Scholar]
- 13. Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13(4):492–497. [DOI] [PubMed] [Google Scholar]
- 14. Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111(46):E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ. Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-Dependent inhibition of dopamine release. Neuron. 2016;91(6):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160(3):467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmöle AC, Lundt R, Gennequin B, Schrage H, Beins E, Krämer A, Zimmer T, Limmer A, Zimmer A, Otte DM. Expression analysis of CB2-GFP BAC transgenic mice [published correction appears in PLoS One. 2015;10(12):e0145472] PLoS One. 2015;10(9):e0138986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. López A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vázquez C, Amores M, Ruiz-Pérez G, García-García E, Beatka M, Tolón RM, Dittel BN, Hillard CJ, Romero J. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation. 2018;15(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chem Neurosci. 2012;3(5):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 1997;94(8):4188–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, Bonner TI, Zimmer A, Dey SK. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276(23):20523–20528. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc Natl Acad Sci USA. 2003;100(25):14914–14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96(10):5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96(24):14136–14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140(11):5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. RRID: AB_2291471, https://scicrunch.org/resolver/AB_2291471.
- 27. RRID: AB_221569, https://scicrunch.org/resolver/AB_221569.
- 28. RRID: AB_394816, https://scicrunch.org/resolver/AB_394816.
- 29. RRID: AB_312966, https://scicrunch.org/resolver/AB_312966.
- 30. RRID: AB_2244723, https://scicrunch.org/resolver/AB_2244723.
- 31. RRID: AB_2572408, https://scicrunch.org/resolver/AB_2572408.
- 32. RRID: AB_149707, https://scicrunch.org/resolver/AB_149707.
- 33. Sun X, Terakawa J, Clevers H, Barker N, Daikoku T, Dey SK. Ovarian LGR5 is critical for successful pregnancy. FASEB J. 2014;28(5):2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci USA. 1995;92(10):4332–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos [published correction appears in Nat Med. 2004;10(12):1397] Nat Med. 2004;10(10):1074–1080. [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102(28):9960–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA. 2006;103(44):16364–16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R, Dey SK. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell. 2011;21(6):1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204–1209. [DOI] [PubMed] [Google Scholar]
- 40. Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA. 2007;104(46):18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci USA. 1995;92(21):9460–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams RH. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 2015;6(1):6429. [DOI] [PubMed] [Google Scholar]
- 43. Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56(9):2380–2387. [DOI] [PubMed] [Google Scholar]
- 44. Li Y, Bian F, Sun X, Dey SK. Data from: Mice missing Cnr1 and Cnr2 show implantation defects. figshare 2019. Accessed 8 February 2019.https://figshare.com/articles/Cnr1-_-Cnr2-_-_uteri_have_higher_vascular_permeability/7695665.
- 45. Yuan J, Deng W, Cha J, Sun X, Borg JP, Dey SK. Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat Commun. 2018;9(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yuan J, Cha J, Deng W, Bartos A, Sun X, Ho HH, Borg JP, Yamaguchi TP, Yang Y, Dey SK. Planar cell polarity signaling in the uterus directs appropriate positioning of the crypt for embryo implantation. Proc Natl Acad Sci USA. 2016;113(50):E8079–E8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Slipetz DM, O’Neill GP, Favreau L, Dufresne C, Gallant M, Gareau Y, Guay D, Labelle M, Metters KM. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol Pharmacol. 1995;48(2):352–361. [PubMed] [Google Scholar]
- 48. Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23(3):600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33(3):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Huffman JW, Csiszar A, Ungvari Z, Mackie K, Chatterjee S, Pacher P. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293(4):H2210–H2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schley M, Ständer S, Kerner J, Vajkoczy P, Schüpfer G, Dusch M, Schmelz M, Konrad C. Predominant CB2 receptor expression in endothelial cells of glioblastoma in humans. Brain Res Bull. 2009;79(5):333–337. [DOI] [PubMed] [Google Scholar]
- 52. Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, Selley DE, Imad Damaj M, Lichtman AH. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60(2-3):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fujii M, Sherchan P, Soejima Y, Doycheva D, Zhao D, Zhang JH. Cannabinoid receptor type 2 agonist attenuates acute neurogenic pulmonary edema by preventing neutrophil migration after subarachnoid hemorrhage in rats. Acta Neurochir Suppl (Wien). 2016;121:135–139. [DOI] [PubMed] [Google Scholar]
- 54. Tao Y, Tang J, Chen Q, Guo J, Li L, Yang L, Feng H, Zhu G, Chen Z. Cannabinoid CB2 receptor stimulation attenuates brain edema and neurological deficits in a germinal matrix hemorrhage rat model. Brain Res. 2015;1602:127–135. [DOI] [PubMed] [Google Scholar]
- 55. Wang H, Xie H, Guo Y, Zhang H, Takahashi T, Kingsley PJ, Marnett LJ, Das SK, Cravatt BF, Dey SK. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006;116(8):2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leconte M, Nicco C, Ngô C, Arkwright S, Chéreau C, Guibourdenche J, Weill B, Chapron C, Dousset B, Batteux F. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am J Pathol. 2010;177(6):2963–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor AH, Abbas MS, Habiba MA, Konje JC. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem Cell Biol. 2010;133(5):557–565. [DOI] [PubMed] [Google Scholar]
- 58. Gebeh AK, Willets JM, Marczylo EL, Taylor AH, Konje JC. Ectopic pregnancy is associated with high anandamide levels and aberrant expression of FAAH and CB1 in fallopian tubes. J Clin Endocrinol Metab. 2012;97(8):2827–2835. [DOI] [PubMed] [Google Scholar]
- 59. Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet. 2000;355(9212):1326–1329. [DOI] [PubMed] [Google Scholar]
- 60. Maccarrone M, Bisogno T, Valensise H, Lazzarin N, Fezza F, Manna C, Di Marzo V, Finazzi-Agrò A. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol Hum Reprod. 2002;8(2):188–195. [DOI] [PubMed] [Google Scholar]