Abstract
Whether hormone replacement therapy has beneficial metabolic effects in postmenopausal women remains controversial because of between-study differences in menopausal duration, estrogen formulations, and diet. Additionally, animal studies have not reflected the typical human obesogenic, Western-style diet (WSD). In this study, we determined the effects of immediate 17β-estradiol (ImE) or delayed 17β-estradiol treatment on weight and metabolism parameters in old ovo-hysterectomized rhesus macaques consuming a WSD over a 30-month period. The placebo and ImE groups exhibited progressive gains in weight and fat mass, which ImE initially attenuated but did not prevent. Progression of insulin resistance (IR) was lessened by ImE compared with placebo under both fasting and IV glucose–stimulated conditions, plateauing in all groups between 24 and 30 months. Consequently, relative euglycemia was maintained through lower stimulated insulin levels with ImE than with placebo. Bone mineral density decreased in the placebo group but was maintained in the ImE group, whereas bone mineral content was unaffected by placebo and increased with ImE. Daily activity was reduced while macaques consumed a WSD and was not affected by ImE. Over time, total cholesterol, triglyceride, very-low-density cholesterol, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, and IL-8 levels increased or trended upward in all animals, with only the change in HDL-C affected by ImE. Delayed estrogen treatment (months 24 to 30) had no significant impact on body composition or glucometabolic parameters. In summary, detrimental WSD-induced changes in body composition and metabolism were only temporarily ameliorated by ImE, with the important exception of glucose homeostasis, which benefited from E replacement even as body composition worsened.
More than 25 million women enter menopause each year, with the number of postmenopausal women worldwide expected to reach 1.2 billion by 2030 (1). In addition to precipitating disruptive hot flashes in many women, the postmenopausal hormonal decline of estrogen (E) and progesterone (P) (2–4) levels coincides with a rapid reduction in bone density (5), disruption in physiological processes such as sleep-wake cycles, and accelerated onset of chronic diseases of aging (6, 7). For example, the incidences of hypertension (8), multiple sclerosis (9), nonalcoholic fatty liver disease (10), coronary artery calcification (11), and fatal and nonfatal stroke (12) have been shown to increase after menopause, and menopause is also associated with alterations in cardiovascular function and pericardial fat (12, 13), pulmonary function (14), circadian activity rhythms and hormone secretion (15), body composition including increased central (visceral) adiposity in some women (16), and IR (17, 18).
However, the conflicting results (19, 20) or findings of limited benefit of E and P hormone replacement therapy (HRT) on health outcomes in postmenopausal women that have been reported in several prospective, randomized clinical trials suggest that the deterioration of these systems may be the direct consequence of aging per se rather than deficiencies in these hormones. Explanations for these conflicts have focused on methodological inconsistencies between studies. For example, some studies recruited women who were well past menopause onset, when age-related influences have already had major impacts on the natural history of several chronic diseases (such as atherosclerosis). Indeed, recent reanalyses of the Women’s Health Initiative demonstrated that participants treated with HRT nearer to their menopause onset benefited in terms of significant reductions in cardiovascular disease events (21) and progression to type 2 diabetes (22, 23). Other potential explanations for discrepant results have focused on the differing HRT regimens used (including the source of E, route of administration, and timing in relation to menopause onset), many of which typically included oral E, a formulation that may have diminished the likelihood for detecting a cardiovascular benefit through increased thrombotic risk (24).
Consumption of a typical obesogenic American or Western-style diet (WSD) is a major contributor to the increasing rates of obesity and diabetes in the United States and worldwide (25, 26). In fact, interactions between adiposity and HRT have been reported for overall mortality and for the incidence of asthma and endometrial and breast cancer (27–30). Studies in rodents have shown benefits of estrogen replacement therapy (ERT), but rodent models often use low-fat chow diets or extremely high-fat diets whose usual fat content [60% to 70% of total calories as fat (31)] far exceeds that of the WSD, which has a total-calorie fat content closer to 35% (32). Thus, rodent data on the efficacy of ERT may have limited translatability to present-day humans. In contrast, nonhuman primates exposed to an experimental WSD that is similar in fat, protein, and carbohydrate content to WSD display variable weight gain, increased IR, and eventual expression of type 2 diabetes that is very similar to that of human populations (33, 34). Thus, the nonhuman primate is an ideal preclinical model in which to evaluate the effects of ERT under controlled environmental conditions, combining experimental rigor with human-relevant physiology.
For these studies, we used a WSD-fed rhesus macaque model. All enrolled females were premenopausal but at an age considered to be near the perimenopausal transition in humans (35). They underwent ovo-hysterectomy (OvH) to standardize the timing between menopause onset and initiation of HRT, which included random assignment to one of three treatment arms to study the effects of immediate and delayed ERT on long-term metabolic and body composition outcomes.
Materials and Methods
The study protocol was approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and conducted in accordance with the 2011 Eighth Edition of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The Indian-origin rhesus macaques (Macaca mulatta) used in this study were older (>17 years at the start) experimentally-naive retired breeders. Animals were provided with unlimited access to WSD pellets (5L0P; TAD; Laboratory Diet, Inc., St. Louis, MO), containing 36% of calories from fat (equivalent to 15% fat/g), 44% from carbohydrates (18.5% as simple sugars and 18% from protein), plus fresh fruit and vegetables. In comparison, a typical regular monkey chow formulation (i.e., Laboratory Diet 5052) contains 13% fat, 69% complex carbohydrates (6% as simple sugars), and 27% protein. All animals had unlimited access to drinking water via lixit spouts. Animals were socially housed in large indoor pens with feeders, foraging mats, toys, shelves, and a tunnel for separation of individuals as needed for administering medicines, obtaining fasting glucose and insulin measurements, administering ketamine before transport, or obtaining blood samples. At study initiation, three or four animals were housed in each pen. Additional enrichment included watching video programs and interactions with the ONPRC Behavioral Science Unit staff and animal care technicians.
Diet, OvH, and timing of E treatment
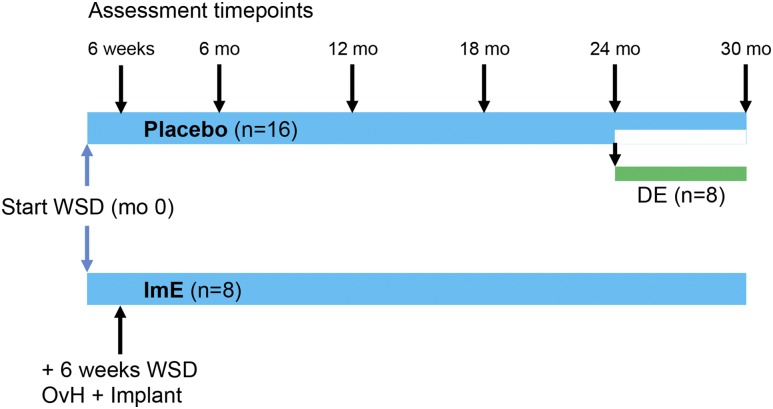
The experimental design is illustrated in Fig. 1. After 6 weeks on the WSD, 24 animals underwent OvH via transabdominal surgery. Because some animals were to receive E for 30 months (2.5 years), a full hysterectomy was performed to reduce the likelihood of endometrial hyperplasia and cancer. After surgical recovery, all animals continued on the WSD for the duration of the study and were assigned to receive either placebo (n = 16) or E immediately after hysterectomy (ImE; n = 8) for 24 months. After 24 months, eight animals in the placebo group were randomly reassigned to receive delayed E (DE) for another 6 months, whereas the remaining animals in the placebo group, and in the ImE group, continued on their previous drug assignment.
Figure 1.
Protocol design. Twenty-four aged ovarian-intact female macaques were initially randomly assigned to a treatment (n = 16 placebo or n = 8 ImE) in staggered cohorts. Each cohort contained a mixture of placebo- and ImE-treated animals and underwent study protocols at baseline (month 0). During subsequent study follow-up visits, the staggered order of study was maintained. After the baseline visit but before OvH, all animals consumed a WSD for 6 weeks. At the end of this initial feeding period, study protocols were repeated and the animals then underwent OvH and immediate placement of implants containing either placebo or E. Study protocols were repeated at 6-month intervals thereafter. At 24 months, half of the placebo group were randomly reassigned to either continue with their placebo assignment or receive E-filled Silastic implants for an additional 6 months. Final protocols were performed after 30 months.
Crystalline E (1,3,5,10-estratrien-3,17-β-diol; Steraloids, Wilton, NH) was administered via Silastic capsules (Dow Corning, Midland, MI) implanted subcutaneously in the periscapular region. Serum E was measured every 2 months starting shortly after hysterectomy, and dosing was adjusted to achieve target levels of 70 to 100 pg/mL. If serum E concentrations were >120 pg/mL in an individual measurement at any time during the protocol, the capsule was replaced with a smaller capsule, which was used for that animal subsequently. If serum E levels declined below 50 pg/mL, implants were replaced. Immediately after implantation, E levels surge before gradually declining and stabilizing, so monitoring every 2 months enabled maintenance of the desired level. Placebo treatment consisted of implantation of an empty Silastic capsule.
At baseline and 6-month intervals, all animals underwent measurement of body weight, an intravenous glucose tolerance test (IVGTT) for calculation of glucometabolic parameters, a dual X-ray absorptiometry (DEXA) scan for assessment of body and bone composition, measurement of serum leptin from morning and evening samples, fasting plasma lipid levels, and activity monitoring for 2 weeks. Cytokine levels were determined during the first 10 months and at the end of the study.
Glucose metabolism
IVGTTs were performed under sedation with Telazol (5 mg/kg IM) to assess insulin secretion, glucose disposal, and insulin sensitivity after an overnight fast (36, 37). Animals received dextrose (600 mg/kg) administered intravenously over 1 minute. Venous blood samples were obtained at baseline and at 1, 3, 5, 10, 20, 40, and 60 minutes after injection for measurement of blood glucose (Freestyle; Abbott Laboratories, Chicago, IL) and insulin levels (Roche Diagnostics Cobas e411 analyzer). Basal serum insulin and glucose concentrations on the day of IVGTT were used to calculate the homeostatic model assessment of insulin resistance (HOMA-IR) values with the equation described in Sarafidis et al. (38). Time concentration curves from baseline to 60 minutes were plotted to generate glucose or insulin area under the curve (AUC) concentrations (39). Glucose disappearance rate (Kg) and insulin sensitivity index (ISI) were derived from timed glucose and insulin values from the IVGTT as previously described (40, 41).
Body composition, truncal fat, and bone parameters
Body composition for the determination of total, lean, and fat mass (including truncal fat), bone density, and bone mineral content (BMC) was assessed by DEXA imaging (QDR Series X-Ray Bone Densitometer, Horizon A; Hologic, Marlborough, MA) under Telazol (5 mg/kg IM) sedation immediately before the IVGTT.
Activity determinations
Each animal was fitted with an Actiwatch activity monitor (Philips Respironics, Bend, OR) worn inside a protective case that was attached to a lightweight, loose-fitting aluminum collar (Primate Products, Inc., Immokalee, FL) (42). At the start of the study, 24-hour activity recordings were made for 14 consecutive days, and a characteristic baseline pattern was established for each animal, and activity was further monitored in the same fashion every 6 months. Actograms were subsequently analyzed in Actiware-Sleep (version 3.4) software (Cambridge Neurotechnology Ltd., Cambridge, UK). The mean total daily activity (defined as the average 24-hour activity) was calculated for each animal, as was the mean daytime activity (defined as activity during the period between 0700 and 1900 hours) and mean nighttime activity (activity between 1900 and 0700 hours). Focal observations on locomotor activity have been described previously (43).
Assays
The ONPRC Endocrine Technologies Core performed estradiol assays with a Roche Diagnostics Cobas e411 analyzer. The sensitivity limit of the assay was 5 pg/mL, and intra-assay and interassay coefficients of variation (CVs) were <10%. Plasma leptin concentrations were determined by RIA (Linco Research, Inc., St. Charles, MO) with a detection limit of 0.25 ng/mL. Intra-assay CVs were 5.6% at 3.5 ng/mL and 6.0% at 21.1 ng/mL. The corresponding interassay CVs were 7.9% and 7.0%, respectively. Cytokines [IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, interferon (IFN)-γ, and TNF-α] were also assayed by the ONPRC Endocrine Technologies Core with a custom 9-plex nonhuman primate Luminex panel (Thermo Fisher, Waltham, MA). Briefly, 25 µL of each serum sample was diluted in assay diluent and incubated overnight with antibody-coated, fluorescent capture microspheres specific for each analyte, followed by detection antibodies and streptavidin-phycoerythrin. Washed microspheres with bound analytes were resuspended in reading buffer and analyzed on a Milliplex LX-200 Analyzer (EMD Millipore, Billerica, MA) bead sorter with XPonent Software version 3.1 (Luminex, Austin, TX). Data were calculated in Milliplex Analyst software version 5.1 (EMD Millipore). A rhesus macaque serum pool was run in quadruplicate as a quality control. Intra-assay CVs were as follows: IL-1β, 1.0%; IL-6, 26.4%; IL-8, 6.9%; IL-12, 13.9%; and IFN-γ, 13.5%. Intra-assay CVs for IL-2, IL-4, IL-10, and TNF-α were not calculated because they were undetectable in the quality control. Because all samples were analyzed in a single assay, no interassay variation was calculated. Lipid levels were analyzed by the Oregon Health & Science University Knight Cardiovascular Institute Lipoprotein Analytical Core as described previously, using an ultracentrifugation method to isolate and quantitate very-low-density lipoprotein cholesterol, with calculated low-density lipoprotein and quantitated HDL-C (44). Serum total and high-molecular-weight (HMW) adiponectin levels were measured by ELISA (Alpco, Salem, NH) in the ONPRC Endocrine Technologies Core according to the manufacturer’s instructions. The assay range for both assays was 0.075 to 4.8 ng/mL. Intra-assay variation, determined by analysis of an in-house macaque serum pool was 2.0% for total adiponectin and 4.3% for HMW adiponectin. Because all samples were analyzed in a single assay, no interassay variation for these samples was determined. The interassay variations for total and HMW adiponectin are 3.7% and 4.4%, respectively.
Statistical analysis
For the longitudinal study protocol with treatment group assignment to two arms (placebo and ImE) for 2 years, two-way ANOVA was used with post hoc pairwise comparisons from months 0 to 24. The two-way ANOVA tested the difference across time, the difference between treatment groups, and the interaction between time and treatment. At 24 months, half of the placebo group received E-filled Silastic capsules, becoming the DE group. Therefore, at month 30, three treatment groups were analyzed with one-way ANOVA. If a Bartlett test revealed a significant difference between the within-group variances, then Kruskal-Wallis nonparametric ANOVA was performed. Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA) was used to perform all analyses. Statistical difference was accepted with P < 0.05. Prism 5.0 produces exact P values >0.0001, but any lesser P value is expressed as P < 0.0001. Because of age-related attrition, the group sizes at the end of the study were 6, 7, and 7, respectively, for the placebo, ImE, and DE groups. However, not all animals are reflected in all outcomes at each time point. Deviation from animal n for any outcome indicates that data are missing at a time point for a clinical reason, because of a technical problem with an assay, or because a blood sample was lost, an animal died, or data from a particular animal met outlier qualification. An outlier value was defined as falling two standard deviations outside the group mean. Two-way ANOVA was used on all outcomes (except adiponectin) instead of repeated-measures analysis for the 0- to 24-month data because of occasional missing data points. Adiponectin was also examined with a two-way ANOVA mixed-effects analysis in which missing data points were allowed.
Results
Validation of treatment
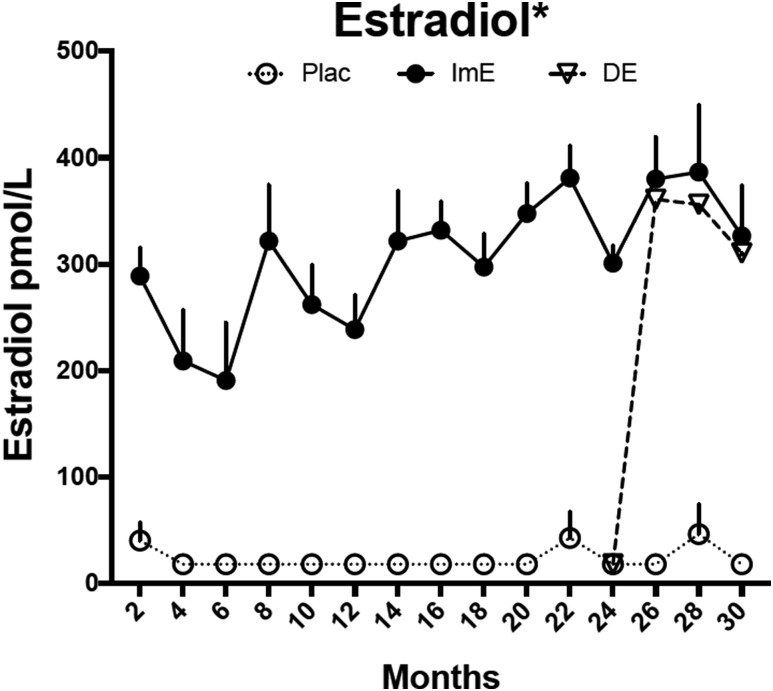
Over the entire 30-month study period, the average bimonthly serum E concentration in the ImE group was significantly higher than among placebo controls and comparable to a concentration during the early follicular phase (22.7 ± 4.4 pmol/L vs 1.73 ± 0.19 pmol/L, respectively, P < 0.0001) (Fig. 2). From months 24 to 30, serum E concentrations in the DE group rose to 25.4 ± 1.19 pmol/L (not different from ImE and significantly greater than placebo controls, P < 0.0001) (Fig. 2).
Figure 2.
Serum E concentrations. Serum E was higher in the ImE group than the placebo group throughout the study. DE treatment caused an immediate increase in serum E concentrations, which were significantly higher than in the placebo-treated group but not different from the ImE group. Data are mean ± SEM. *Groups are different by two-way ANOVA. Except 4- and 6-month points, ImE and DE (during E treatment) were significantly different from the placebo group at all other points by Sidak post hoc pairwise comparison. Plac, placebo.
Effect of E on body weight and composition
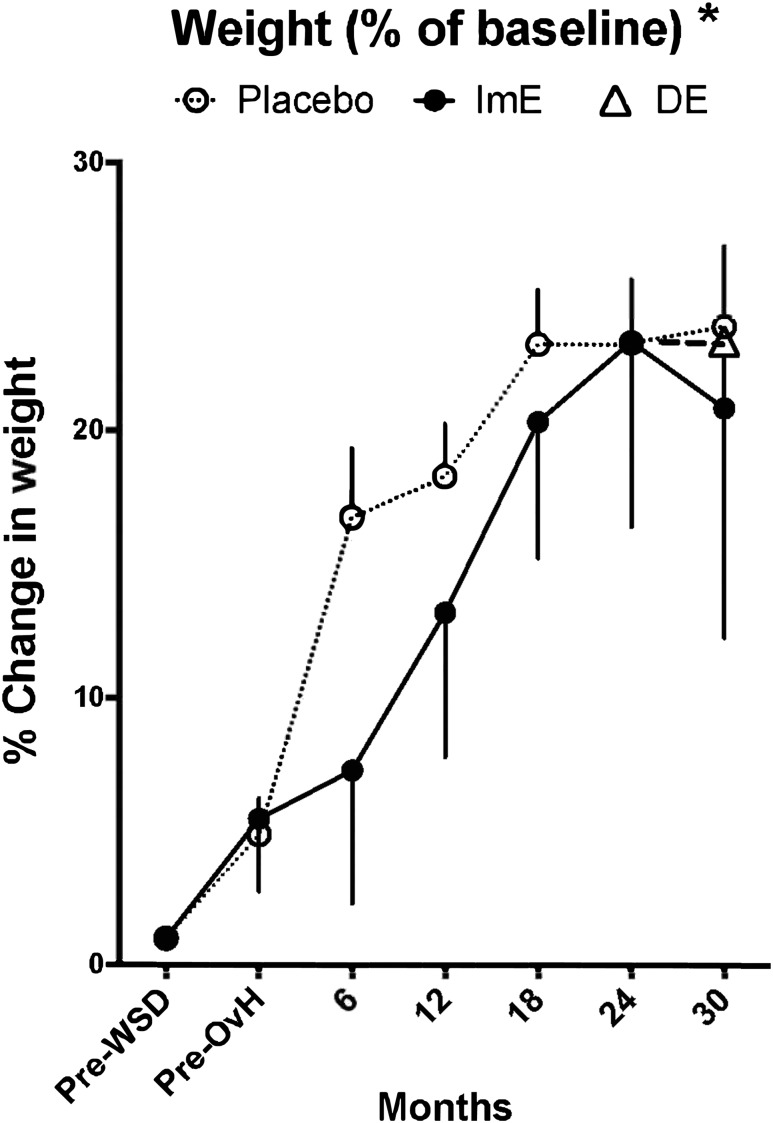
Body weight (in kilograms) is shown in Table 1 and is illustrated in Fig. 3 as percentage change from basal pre-WSD weight. Comparison of ImE treatment to placebo with two-way ANOVA showed significant effects of treatment (P = 0.032) and time (P < 0.005) on body weight, without an interaction between these two variables out to 24 months (Table 1). There was also a significant increase across time in weight expressed as percentage of basal in both groups (P < 0.001; Fig. 3). However, the difference between the treatment groups was eliminated after normalization by baseline weight. Nonetheless, the placebo group exhibited a sharp increase in percentage of basal weight between pre-OvH and 6 months compared with the ImE group, as reflected by the difference in the slope of the curves. There was also a difference in percentage of basal weight between placebo and ImE at 6 months with a t test (P = 0.048) but not with the post hoc test (P = 0.058). Thus, the ImE group appeared to have a slower rate of weight gain initially but caught up to the placebo group by study completion.
Table 1.
Longitudinal Body Composition and Serum Leptin Concentrations of Old OvH Female Monkeys on an Obesogenic Diet and Treated With Placebo, ImE, or Placebo for 24 Months Then DE
| Total Weight (kg) ± SEM | Fat Mass (g) ± SEM | Lean Mass (g) ± SEM | Leptin AM (µg/L) ± SEM | Leptin PM (µg/L) ± SEM | Leptin/Fat × 100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE |
| n | 12 | 7–8 | 14 | 7–8 | 14 | 7–-8 | 12 | 6–7 | 12 | 6–7 | 12 | 6–7 |
| Month | ||||||||||||
| 0 | 7.73 ± 0.36 | 7.57 ± 0.58 | 1300 ± 138 | 1276 ± 275 | 5925 ± 168 | 5574 ± 200 | 6.72 ± 0.87 | 9.56 ± 0.65 | 9.52 ± 1.55 | 9.18 ± 1.11 | 0.47 ± 0.05 | 0.47 ± 0.09 |
| 6 | 8.61 ± 0.41 | 7.67 ± 0.59 | 2686 ± 319 | 1840 ± 383 | 5721 ± 187 | 5374 ± 194 | 11.40 ± 0.90 | 8.90 ± 1.84 | 11.25 ± 1.04 | 10.60 ± 2.50 | 0.50 ± 0.08 | 0.47 ± 0.16 |
| 12 | 8.71 ± 0.36 | 8.11 ± 0.66 | 3087 ± 266 | 2776 ± 490 | 5365 ± 165 | 5025 ± 148 | 10.62 ± 1.07 | 8.11 ± 1.27 | 12.95 ± 1.18 | 10.71 ± 0.95 | 0.39 ± 0.04 | 0.48 ± 0.08 |
| 18 | 9.15 ± 0.40 | 8.63 ± 0.69 | 3871 ± 297 | 3707 ± 548 | 5042 ± 148 | 4664 ± 212 | 13.44 ± 1.37 | 11.29 ± 1.25 | 12.96 ± 1.06 | 10.94 ± 0.88 | 0.32 ± 0.03 | 0.21 ± 0.05 |
| 24 | 9.02 ± 0.30 | 8.79 ± 0.70 | 3846 ± 290 | 3621 ± 466 | 4863 ± 125 | 4706 ± 187 | 13.55 ± 1.41 | 11.08 ± 1.38 | 14.73 ± 1.25 | 11.64 ± 1.01 | 0.36 ± 0.03 | 0.36 ± 0.03 |
| 2-way ANOVA | ||||||||||||
| Interaction | P = 0.96 | P = 0.77 | P = 0.97 | P = 0.29 | P = 0.86 | P = 0.71 | ||||||
| Time | P = 0.005 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P = 0.025 | ||||||
| Treatment | P = 0.032 | P = 0.15 | P = 0.008 | P = 0.027 | P = 0.0518 | P = 0.74 | ||||||
| Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 6 | 6 | 7 | 6 | 7 | 7 | 6 | 7 | 7 | 5 | 6 | 6 | 5 | 6 | 6 | 5 | 6 | 6 |
| Month | ||||||||||||||||||
| 30 | 10.05 ± 0.27 | 8.34 ± 0.50 | 8.61 ± 0.79 | 4218 ± 450 | 3159 ± 326 | 3888 ± 534 | 5257 ± 195 | 4921 ± 277 | 4735 ± 199 | 12.89 ± 0.93 | 9.07 ± 0.94 | 12.28 ± 0.67 | 15.76 ± 1.55 | 9.95 ± 1.35 | 12.93 ± 0.54 | 0.38 ± 0.02 | 0.42 ± 0.03 | 0.39 ± 0.08 |
| One-way ANOVA | P = 0.134 | P = 0.2592 | P = 0.31 | P = 0.014 | P = 0.015 | P = 0.49 | ||||||||||||
Placebo + DE represents the combined groups through 24 months, while both were implanted with empty Silastic capsules.
Figure 3.
Weight changes. Body weight (as a percentage of baseline) increased over time in all groups and reached a similar value at 30 months. However, ImE delayed the rate of increase until 12 to 18 months, after which time both groups exhibited similar weights. Administration of DE had no effect on body weight. Data are average fold changes of individual results normalized by starting value ± SEM. *Groups are different by two-way ANOVA. At the 6-month time point, the placebo group is different from the ImE group by Sidak post hoc pairwise comparison.
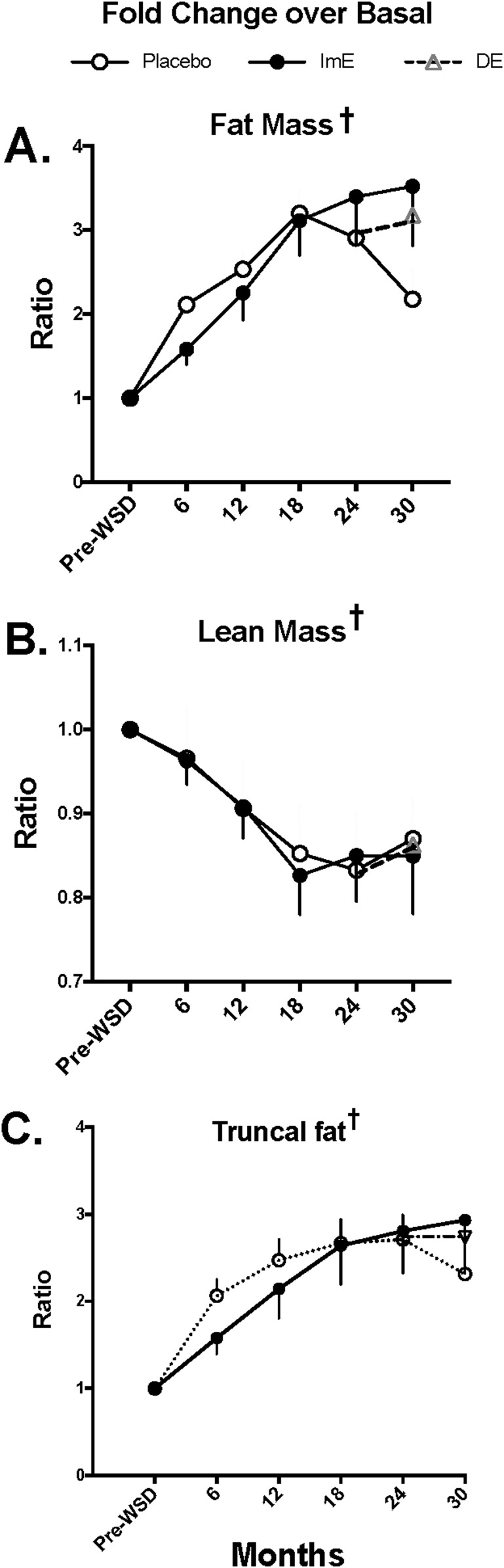
Body composition outcomes are shown in Table 1, and the fold change from basal for each outcome is illustrated in Fig. 4. DEXA scanning revealed that the increase in weight that occurred in both groups was caused primarily by increased fat mass across time, both in terms of kilograms and fold change over basal (two-way ANOVA P < 0.0001; Table 1 and Fig. 4). Likewise, there was a significant longitudinal increase in truncal fat fold change over basal in both groups (two-way ANOVA P < 0.0001; Fig. 4). However, there was no difference in fat mass, fat mass fold change from basal, or truncal fat fold change from basal due to treatment (Table 1 and Fig. 4). ImE treatment slightly delayed the WSD-induced increase in truncal fat fold change during the initial 18 months of the study, but both groups stabilized at a similar and higher fold change in truncal fat after 18 months. Conversely, total lean mass amounts declined across time, which differed by treatment group (two-way ANOVA P < 0.0001 and P = 0.008, respectively; Table 1). Fold change in lean mass over basal also declined across time (P < 0.0001), but there was no difference between treatments after adjustments for baseline differences (Fig. 4). DE did not significantly affect body weight or body composition; measures of weight and body composition were similar in all groups by study end (Table 1 and Figs. 3 and 4).
Figure 4.
Changes in body composition as measured by DEXA scan. (A) Total fat mass increased with time up to 18 months then stabilized for the remainder of the study. (B) Lean mass decreased with time up to 18 months, then stabilized for the remainder of the study. (C) Truncal fat increased with time up to 18 months, then stabilized for the remainder of the study. There were no statistical differences between the treatment groups in any of the measures at any of the study time points. Data are average fold change of individual results normalized by starting value ± SEM. †There was a significant difference across time with two-way ANOVA.
Effect of E on glucose metabolism
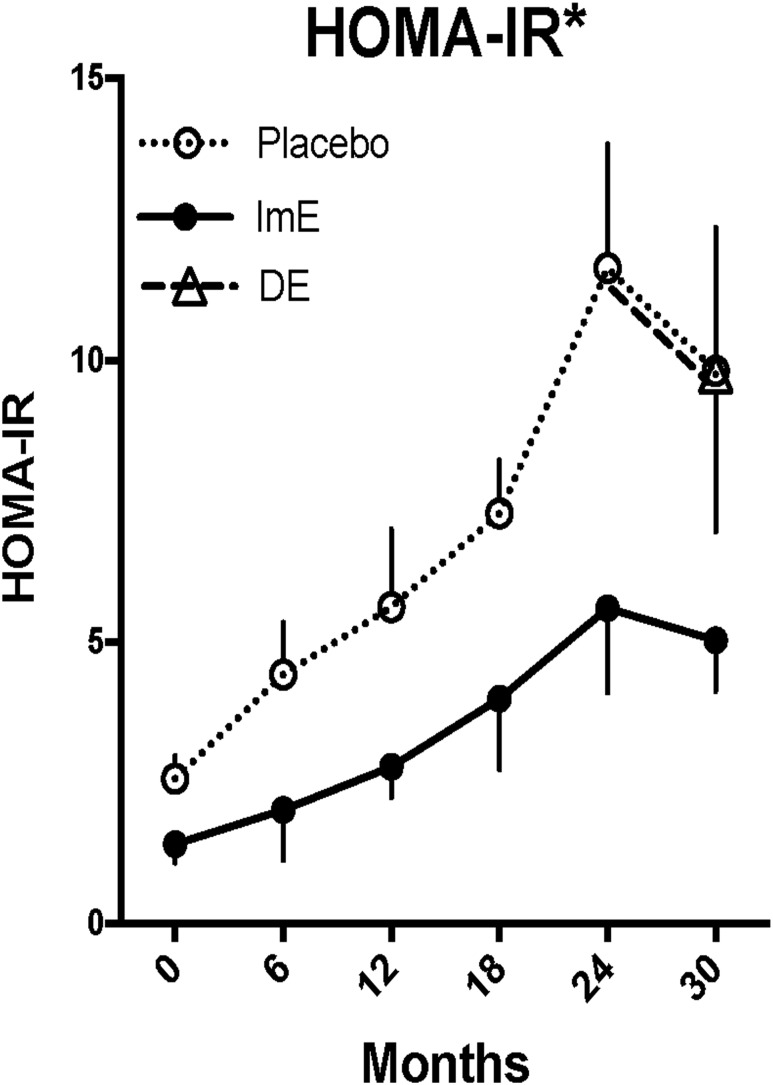
The effects of WSD with and without E replacement on glucose homeostasis are shown in Table 2, Figs. 5 and 6, and an online repository (45). As shown in Table 2, fasting glucose and insulin levels increased over time in both placebo and ImE groups through 24 months, although the increase in fasting insulin was attenuated in the ImE group compared with placebo. There were no further increases or differences in these parameters between placebo, ImE, and DE at 30 months. IR calculated as HOMA-IR increased significantly in both the placebo and ImE groups over 24 months (Fig. 5). ImE markedly attenuated this increase relative to placebo (two-way ANOVA, P < 0.0001 for time and P < 0.0001 for treatment), such that by 24 months HOMA-IR was 50% lower in the ImE group than the placebo group. Addition of E to the DE group from 24 to 30 months had no effect on HOMA-IR.
Table 2.
Fasting Glucose and Insulin Levels and Kg and Sensitivity Parameters Derived From IVGTT Data in Placebo, ImE, and DE Groups
| Fasting Glucose ± (mg/dL) SEM | Fasting Insulin (µIU/mL) ± SEM | Kg Slope of 10’–40’ (%/min) | ISI (hr * mmol − 1) | ISI % Change Baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE |
| n | 15 | 7 | 15 | 7–8 | 13–15 | 7–8 | 14–15 | 7 | 13–114 | 6–8 |
| Month | ||||||||||
| 0 | 54.93 ± 1.81 | 55.71 ± 3.20 | 17.81 ± 2.563 | 13.97 ± 4.85 | 4.56 ± 0.33 | 4.75 ± 0.36 | 0.387 ± 0.03 | 0.618 ± 0.09 | 0 | 0 |
| 6 | 61.50 ± 2.62 | 55.75 ± 2.70 | 28.46 ± 4.97 | 20.85 ± 7.81 | 3.29 ± 0.35 | 4.14 ± 0.39 | 0.376 ± 0.04 | 0.550 ± 0.10 | −0.602 ± 12.7 | −10.73 ± 8.4 |
| 57 | ||||||||||
| 12 | 57.73 ± 2.64 | 57.75 ± 2.74 | 35.88 ± 7.07 | 41.88 ± 22.92 | 3.11 ± 0.22 | 3.92 ± 0.22 | 0.300 ± 0.04 | 0.494 ± 0.12 | −26.18 ± 13.2 | −23.34 ± 14.3 |
| 18 | 59.15 ± 2.46 | 59.38 ± 3.64 | 50.23 ± 6.57 | 54.53 ± 29.16 | 3.10 ± 0.22 | 3.59 ± 0.38 | 0.174 ± 0.2 | 0.330 ± 0.08 | −53.66 ± 4.5 | −44.57 ± 10.3 |
| 24 | 73.62 ± 6.58 | 67.88 ± 5.69 | 66.39 ± 11.19 | 46.73 ± 15.27 | 2.78 ± 0.29 | 3.75 ± 0.39 | 0.199 ± 0.3 | 0.357 ± 0.07 | −44.76 ± 7.6 | −41.84 ± 10.1 |
| 2-way ANOVA | ||||||||||
| Interaction | P = 0.83 | P = 0.82 | P = 0.77 | P = 0.96 | P = 0.93 | |||||
| Time | P = 0.0015 | P = 0.0035 | P = 0.002 | P < 0.0001 | P < 0.0001 | |||||
| Treatment | P = 0.39 | P = 0.56 | P = 0.0004 | P < 0.0001 | P = 0.88 | |||||
| Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 6 | 7 | 7 | 6 | 7 | 6 | 6 | 7 | 7 | 6 | 7 | 7 | 6 | 6 | 7 |
| Month | |||||||||||||||
| 30 | 69.33 ± 3.81 | 67.71 ± 2.67 | 71.86 ± 5.31 | 56.28 ± 12 | 57.95 ± 17.4 | 30.19 ± 4.75 | 2.36 ± 0.55 | 2.89 ± 0.50 | 3.29 ± 0.44 | 0.197 ± 0.38 | 0.239 ± 0.05 | 0.30 ± 0.05 | −42.9 ± 10.4 | −51.19 ± 6.05 | −50.58 ± 7.34 |
| One-way ANOVA | P = 0.76 | P = 0.11 | P = 0.33 (K-W) | P = 0.27 (K-W) | P = 0.74 | ||||||||||
Placebo + DE indicates data from the combined placebo and DE groups that had empty Silastic implants for 24 months, before the DE group was placed on E treatment for the last 6 months of the study.
Abbreviation: K-W, Kruskal-Wallace nonparametric ANOVA.
Figure 5.
Changes in HOMA-IR. HOMA-IR increased with time in all animals, but the rate of increase was slowed by ImE treatment. DE reduced HOMA-IR toward that of the ImE-treated group, although the decline at 30 months in both the ImE and DE groups could be caused by loss of animals with higher values, which is suggested by the large variance from 12 to 24 months in the ImE group. Data are mean ± SEM. *Groups are different by two-way ANOVA. At the 24-month time point, the placebo group is different from the ImE group by Sidak post hoc pairwise comparison.
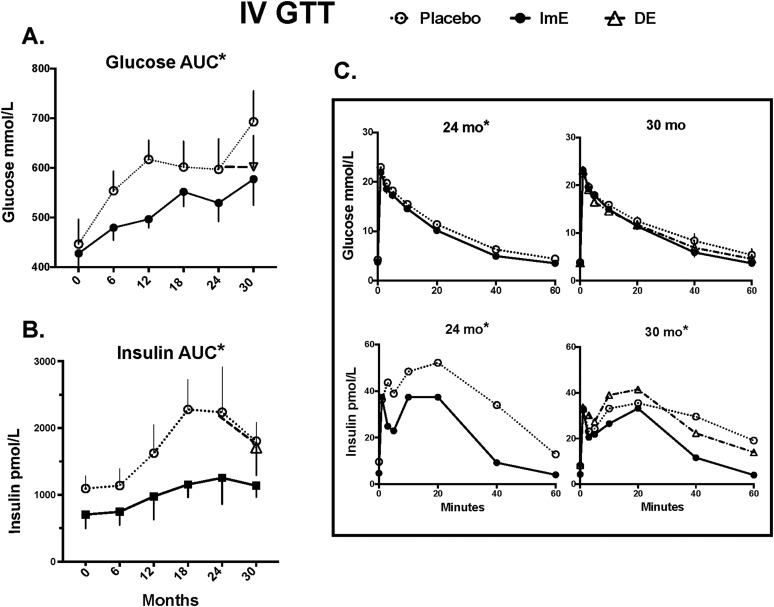
Figure 6.
Glucose and insulin AUCs during IVGTT from baseline through 30 months and glucose and insulin excursions during IVGTT at 24 and 30 months. (A) Glucose: AUC glucose values are significantly lower in the ImE-treated group than the placebo-treated group at all time points after treatment initiation. There was a significant increase in glucose AUC with time in both ImE- and placebo-treated groups. Between 24 and 30 months, DE appeared to reduce AUC glucose to the level observed in the ImE group, but there was no significant difference between the three groups due to variance and number of animals remaining. (B) Insulin: AUC insulin values were significantly lower in the ImE-treated group than the placebo group at all time points after treatment initiation. There were also significant increases in AUC insulin with time. DE had no effect on insulin AUC. (C) Glucose clearance curves (upper graphs) are nearly identical between 24 and 30 months in all three groups, but first- and second-phase insulin levels (lower graphs) decrease in the placebo group at 30 months compared with 24 months, show a slightly improved (increased) curve in the DE group, and remain unchanged in the ImE group. Data are mean ± SEM. *Groups are different by two-way ANOVA.
Paralleling these HOMA-IR findings, the IVGTT-derived parameters of Kg and the insulin sensitivity index (ISI) also declined over the first 24 months in both groups, and there were significant differences between treatment groups (Table 2). That is, ImE significantly ameliorated the decline in both Kg and ISI during this time. However, by 30 months, the groups were not statistically different. It should be noted that the decrease in n or power when the placebo group was split probably contributed to the lack of statistical difference between the groups, with the placebo and DE groups remaining somewhat lower than the ImE group.
Glucose and insulin AUCs determined from the IVGTTs are shown in Fig. 6. In the placebo group, glucose AUC increased from 0 to 12 months before plateauing from 12 to 24 months (Fig. 6A). At the same time, insulin AUCs increased in the placebo group before plateauing after 18 months (Fig. 6B), reflecting a continuously worsening glucose intolerance during the first 2 years in this group. The ImE group also exhibited a progressive impairment in glucose tolerance, but the increases in glucose and insulin AUCs occurred at a slower rate and plateaued at lower levels by 18 months (Fig. 6A and 6B) compared with the placebo group (two-way ANOVA P < 0.0001 for time and P < 0.0001 for treatment). By 30 months, differences in the glucose and insulin AUCs between placebo and ImE remained (Bonferroni P < 0.03), and although the DE group glucose AUC was not statistically different from that of the other groups, it more closely approximated that of the ImE vs the placebo group.
Although insulin AUCs in the ImE group were initially lower and increased little between 6 and 24 months (two-way ANOVA P < 0.0001 for time and P = 0.012 for treatment), by 30 months there was no statistical difference between the groups. However, the pattern of insulin secretion differed as shown in the individual excursions (Fig. 6C). At 24 months, glucose excursions during an IVGTT were similar in the placebo and ImE groups, whereas insulin secretion in the placebo group was significantly higher than that in the ImE group. At 30 months, the placebo group exhibited a delayed first phase and elongated second phase, whereas the DE group showed a somewhat normal pattern, albeit with higher insulin levels than the ImE group.
As shown in more detail in the online repository (45), glucose excursions in the placebo and ImE groups were similar over the first 24 months. Although insulin secretion was initially similar in the placebo and ImE groups, first-phase insulin secretion became progressively delayed and second-phase insulin secretion was extended in duration and amount secreted by 24 months in the placebo group. These detrimental effects on insulin secretion were largely partially prevented by ImE treatment.
Effect of E on adipokine levels
The diurnal pattern of leptin secretion was maintained in all groups consuming a WSD, with evening leptin concentrations generally running higher than morning levels (45). Average morning and evening serum leptin levels increased in both treatment groups and were significantly higher in the placebo than the ImE group in the morning. Treatment differences in evening leptin levels exhibited a trend (two-way ANOVA P < 0.0001 for time and morning P = 0.027, evening P = 0.0518 for treatment) in parallel with increases in weight and fat mass over the study duration (Table 1). At 30 months, the average serum leptin level was significantly lower in the DE group than the placebo group (Bonferroni post hoc test, P < 0.01), and serum leptin correlated with fold change in trunk fat throughout the 30 months in the placebo and ImE groups (placebo r2 = 0.93, P = 0.002; ImE r2 = 0.79, P = 0.0001). After adjustments in leptin levels for the concurrent changes in fat mass (leptin/fat mass), there were decreases over time but no differences between treatment groups (Table 1).
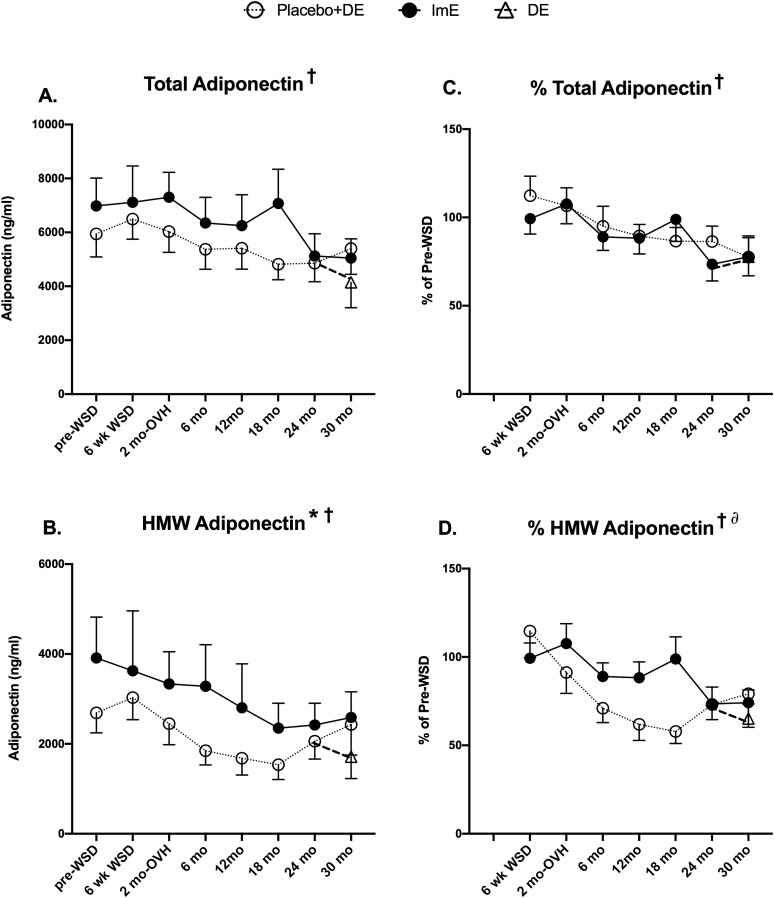
Serum adiponectin concentrations declined from 0 to 24 months in both groups (Fig. 7A and 7B). Total adiponectin concentrations decreased with time in study (mixed-effects ANOVA, P = 0.0003), but there was no difference between treatment groups after adjustment for baseline differences (Fig. 7C). HMW adiponectin concentrations decreased with time (mixed-effects ANOVA, P = 0.0005), and levels in the ImE group remained significantly higher than in the placebo group even after adjustment for baseline differences (two-way ANOVA, P = 0.008) (Fig. 7D). DE had no significant effect on serum adiponectin levels.
Figure 7.
Serum concentrations of total and HMW adiponectin. (A, B) Serum concentrations of total and HMW adiponectin. (C, D) Percentage change in total and HMW adiponectin from pre-WSD. All graphs show a significant decline in adiponectin across time. (B) Serum HMW adiponectin also showed a difference between treatment groups, but (D) this difference was no longer significant after correction of baseline differences. Data are mean ± SEM. *Significant difference between treatment groups with mixed-effects ANOVA. †Significant difference across time with mixed-effects ANOVA. ∂Significant time × treatment interaction.
Effect of E on bone parameters
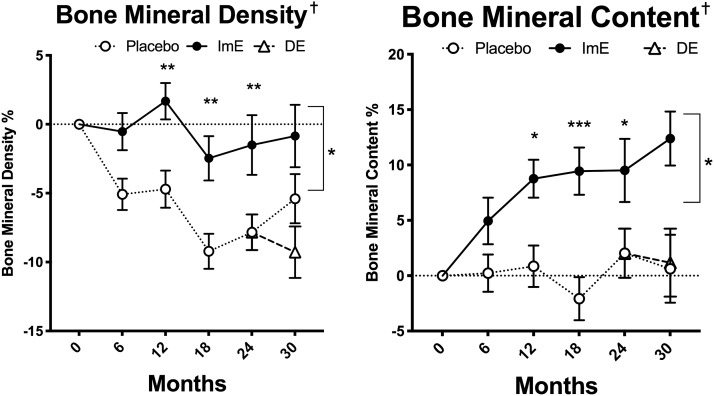
Bone mineral density (BMD) and BMC determined by DEXA scans, because these parameters are known to be sensitive to E status (Fig. 8). BMD expressed as percentage of basal value was stable across time in the ImE-treated group but significantly declined in the placebo group (two-way ANOVA time effect P < 0.0001, treatment effect P < 0.007, interaction P < 0.001). There were significant post hoc differences between ImE- and placebo-treated groups at 12, 18, 24, and 30 months (P < 0.05). BMC remained very low across time in the placebo group, but it significantly increased with ImE treatment (time P < 0.0001, treatment P < 0.018, interaction P < 0.0002). There were also significant post hoc differences between placebo and ImE groups at 12, 18, 24, and 30 months (P < 0.05). DE treatment did not affect BMD or BMC.
Figure 8.
BMD and BMC from DEXA scans. There was a significant difference between the treatment groups, with ImE maintaining BMD and increasing BMC across time. There was no effect of DE. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, difference by Sidak post hoc pairwise comparison; †BMC and BMD groups are different by two-way ANOVA.
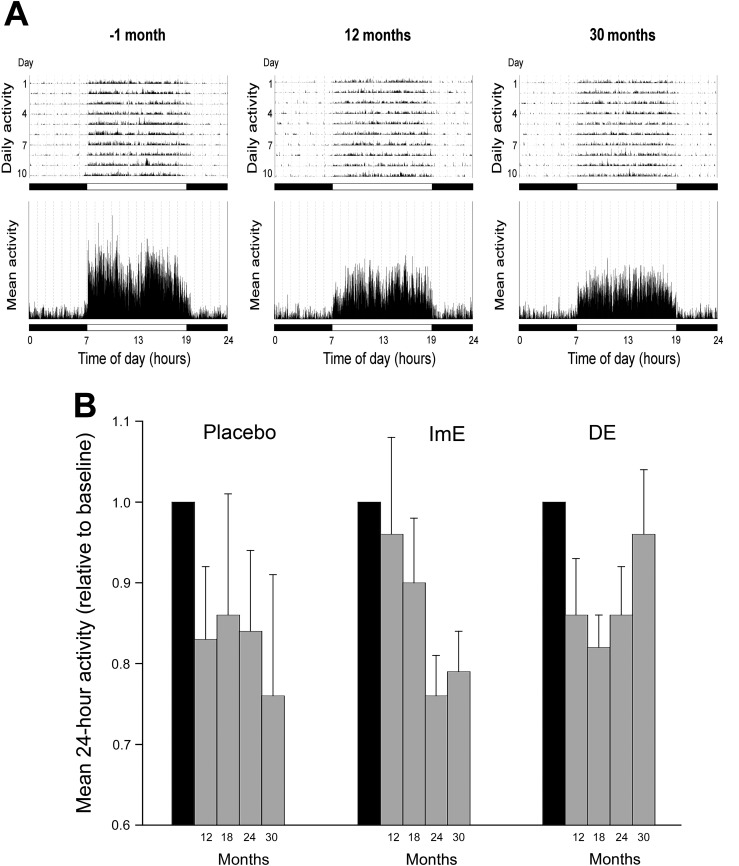
Effect of E on activity levels
A typical animal was more active during the day than at night at both the start and the 24-month time point of the protocol (Fig. 9A). To perform matching comparisons across time, only animals that were alive at 24 months were included in the two-way analysis. From baseline through 24 months, daily activity trended downward in all groups (Fig. 9B). Because of large individual variation in activity, treatment with ImE had no independent effect on these trends. However, some qualitative observations can be made. ImE replacement appeared to delay the magnitude of daily activity reductions until roughly month 24, after which they overlapped with placebo. In addition, an interesting trend emerged at month 30, in which DE reversed the downward daily activity trend (Fig. 9B), which approached baseline levels.
Figure 9.
Activity levels. (A) Representative actograms from a female rhesus macaque obtained over ~10 days, at −1 month and subsequently after 12 and 30 months of exposure to a WSD. In the upper panels, the height of the vertical lines indicates the intensity of physical activity at a particular time of day; the mean 24-hour activity profiles across the 10 consecutive days are depicted in the lower panels. The horizontal black and white bars correspond to the times of night and day, respectively. (B) The average 24-hour daily activity of each treatment group at various time points after exposure to a WSD. Each bar represents the mean activity level (±SEM) normalized against the mean baseline activity level (shown as a gray bar) but includes only animals that survived until 30 months. Although two-way repeated-measures ANOVA showed no significant difference between the treatment groups, there appeared to be a temporary maintenance of elevated activity after estradiol supplementation, seen at months 12 and 30 in the ImE and DE groups, respectively.
Effects of E on lipid and cytokine levels
Significant increases in total cholesterol, HDL-C, and non-HDL-C occurred in both groups from months 0 to 24, with a similar trend in triglyceride levels, but only HDL-C showed a significant difference between placebo and ImE groups (Table 3). However, by 30 months this difference was lost, and ImE had no further impact on any lipid levels.
Table 3.
Longitudinal Lipid Analysis of Old OvH Female Monkeys on a Obesogenic Diet and Treated With Placebo, or ImE, or Estradiol 2 Years After OvH (DE)
| Total Cholesterol (mmol/L) ± SEM | Triglycerides (mmol/L) ± SEM | Very Low–Density Lipoprotein Cholesterol (mmol/L) ± SEM | Low-Density Lipoprotein Cheolesterol (mmol/L) ± SEM | HDL-C (mmol/L) ± SEM | Non-HDL-C (mmol/L) ± SEM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE | Placebo + DE | ImE |
| n | 12 | 7 | 12 | 7 | 12 | 7 | 12 | 7 | 12 | 7 | 12 | 7 |
| Month | ||||||||||||
| 0 | 3.69 ± 0.26 | 4.11 ± 0.39 | 0.48 ± 0.98 | 0.58 ± 0.07 | 0.24 ± 0.04 | 0.29 ± 0.04 | 2.26 ± 0.21 | 2.54 ± 0.29 | 1.18 ± 0.08 | 1.43 ± 0.31 | 2.48 ± 0.22 | 2.74 ± 0.27 |
| 6 | 6.35 ± 0.44 | 6.34 ± 0.58 | 0.76 ± 0.08 | 0.51 ± 0.06 | 0.38 ± 0.04 | 0.26 ± 0.03 | 3.05 ± 0.23 | 3.45 ± 0.32 | 2.95 ± 0.23 | 2.69 ± 0.27 | 3.97 ± 0.24 | 3.61 ± 0.33 |
| 12 | 6.03 ± 0.56 | 5.46 ± 0.52 | 0.69 ± 0.11 | 0.64 ± 0.15 | 0.35 ± 0.05 | 0.33 ± 0.07 | 2.93 ± 0.42 | 2.84 ± 0.29 | 2.78 ± 0.18 | 2.42 ± 0.30 | 3.24 ± 0.43 | 2.92 ± 0.26 |
| 18 | 6.28 ± 0.57 | 5.58 ± 0.27 | 0.85 ± 0.10 | 0.95 ± 0.31 | 0.43 ± 0.05 | 0.48 ± 0.15 | 3.01 ± 0.38 | 3.07 ± 0.33 | 2.87 ± 0.24 | 2.06 ± 0.29 | 3.41 ± 0.37 | 3.38 ± 0.32 |
| 24 | 6.33 ± 0.51 | 5.27 ± 0.38 | 0.77 ± 0.12 | 0.92 ± 0.19 | 0.39 ± 0.06 | 0.46 ± 0.14 | 3.34 ± 0.32 | 2.76 ± 0.27 | 2.63 ± 0.21 | 2.15 ± 0.30 | 3.69 ± 0.34 | 3.46 ± 0.36 |
| % Δ | 177 ± 15% | 130 ± 11%* | 195 ± 36% | 182 ± 60% | 196 ± 36% | 182 ± 60% | 155 ± 16% | 111 ± 6%† | 237 ± 18% | 176 ± 32% | 156 ± 15% | 131 ± 16% |
| 2-way ANOVA | ||||||||||||
| Interaction | P = 0.522 | P = 0.614 | P = 0.608 | P = 0.647 | P = 0.296 | P = 0.891 | ||||||
| Time | P = 0.0001 | P = 0.058 | P = 0.059 | P = 0.142 | P < 0.0001 | P = 0.044 | ||||||
| Treatment | P = 0.311 | P = 0.934 | P = 0.918 | P = 0.947 | P = 0.033 | P = 0.932 | ||||||
| Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | Placebo | DE | ImE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 5 | 6 | 7 | 5 | 6 | 7 | 5 | 6 | 7 | 5 | 6 | 7 | 5 | 6 | 7 | 5 | 6 | 7 |
| Month | ||||||||||||||||||
| 30 | 6.10 ± 1.01 | 4.83 ± 0.46 | 4.98 ± 0.26 | 0.82 ± 0.27 | 1.04 ± 0.15 | 0.99 ± 0.19 | 0.42 ± 0.14 | 0.54 ± 0.07 | 0.50 ± 0.10 | 2.99 ± 0.57 | 2.31 ± 0.29 | 2.72 ± 0.58 | 2.72 ± 0.58 | 2.09 ± 0.24 | 1.76 ± 0.27 | 3.37 ± 0.63 | 2.79 ± 0.24 | 3.46 ± 0.42 |
| One-way ANOVA | P = 0.14 (K-W) | P = 0.56 (K-W) | P = 0.71 | P = 0.42 | P = 0.21 | P = 0.42 | ||||||||||||
Placebo + DE indicates data from the combined placebo and DE groups that had empty Silastic implants for 24 months of the study, before the DE group was placed on E treatment for the last 6 months of the study.
Abbreviations: % Δ, Percentage change from baseline (time 0) to month 24; K-W, Kruskal-Wallis nonparametric ANOVA.
Nine proinflammatory cytokines were also examined. The average serum concentrations of the proinflammatory cytokines IL-1β and IL-8 were 0.18 ± 0.07 pg/mL and 23.7 ± 10.6 pg/mL, respectively, early in the protocol (months 0 to 10). By the end of the study (months 28 to 30), only IL-8 levels were different between the groups (ANOVA P = 0.043). Post hoc testing demonstrated that IL-8 levels in the placebo group were significantly higher than at baseline (97.4 ± 32.8, P < 0.05), whereas levels in the ImE (48.5 ± 20.8) and DE (50.3 ± 16.4) groups were not different from baseline. Although mean IL-1β levels showed nearly twofold variation between groups, these were not statistically different because of large variations in values between animals and reduced group numbers by study end: placebo (0.56 ± 0.28 pg/mL), ImE (0.24 ± 0.12 pg/mL), and DE (0.17 ± 0.02 pg/mL). No significant differences were observed between the groups for IL-2, IL-6, or IL-12 levels. Levels of the remaining cytokines on the array (IL-4, IL-10, IFN-γ, and TNF-α) were undetectable.
Discussion
Controversy still surrounds the health benefits of postmenopausal hormone replacement. To address potential limitations of previous studies, we used a nonhuman primate model of older macaques consuming a WSD (mimicking the human dietary environment) who underwent simultaneous surgical menopause and hysterectomy (to harmonize onset of menopause with timing of hormonal replacement and to avoid risk of endometrial cancer from ERT), and who were provided with either E or placebo via peripheral subcutaneous delivery (to avoid first-pass effects of oral estrogen on liver production of prothrombotic factors). Estradiol treatment began either immediately after surgery or after a 2-year delay to allow quantification of early and later postmenopausal effects.
Key features of this study were the longitudinal study design and the social housing structure, providing opportunity for spontaneous physical activity. The longitudinal study design enabled observation of changes over time due to WSD, as well as effects resulting from ERT. The ability to compare outcomes before and after exposure to WSD is a significant improvement over previous clinical human studies and enabled normalization of the data in each individual animal. This finding proved important, because the baseline values often differed between animals and the raw data could be misleading. Social housing also has significant advantages over previous studies. For example, a study of rhesus macaques demonstrated important differences in weight and metabolic parameters leading to type 2 diabetes depending on diet, housing, age, and sex (46). Notably, single-cage housing was strongly linked to development of diabetes, and the authors attributed this development to constrained activity compared with monkeys in group housing or in free-living circumstances. Also, in cynomolgus macaques, single-cage housing has been associated with increased stress and decreased exercise that, in turn, contributes to obesity, metabolic abnormalities, and other stereotypic and self-injurious behaviors (47, 48). Animals in the current study were housed in social groups, thus providing space to move freely about and reducing the stress of single housing. Interestingly, the WSD still led to detrimental changes in metabolic parameters.
Macaques exhibit similar population heterogeneity with regard to weight gain susceptibility as human populations when exposed to a WSD (39, 49, 50). However, all of the animals on WSD in this study gained weight, even those treated with ImE. Aged animals may be more susceptible to weight gain than younger adults in which heterogeneity has been reported. The placebo group on the WSD experienced rapid weight gain, an increase in body fat, and an increase in truncal fat mass over 2 years, whereas ImE replacement attenuated rapid changes in these parameters for the first 6 months and only delayed subsequent changes in these parameters. The data indicate that the ImE group caught up or added weight at a higher rate after 12 months. The mechanism by which this occurred is unknown and warrants further investigation. Likewise, increases in body fat fold change and in truncal fat fold change were delayed with ImE, and DE had no effect on these parameters. Loss of lean mass during the study occurred in both groups, but the placebo group lost more lean mass than the ImE group. It is notable that the decrease in lean mass has not been previously observed in adult male macaques maintained on WSD (51). Therefore, loss in lean mass may be primarily an aging phenomenon in females. The decrease in activity experienced by all groups with WSD could also have affected lean mass, although the tendency for ImE-treated animals to move more overall did not affect lean mass changes. Finally, bone was significantly benefited by E treatment, indicating that WSD did not generally inactivate E effects in this organ and paralleling benefits found in human studies.
Estrogen replacement demonstrated effects on metabolism that would be predicted from human studies, where benefits on diabetes risk reduction have been consistently demonstrated (52). As expected, exposure to a WSD adversely affected body weight and insulin sensitivity in both the placebo and ImE groups. Although ImE did not ultimately attenuate WSD-induced weight gain, it did delay the progression to maximum weight. However, ImE improved HOMA-IR, glucose tolerance, glucose disappearance rates, and insulin sensitivity as assessed by IVGTT. Both the placebo and ImE groups exhibited stable and similar glucose responses to a glucose challenge over the study period, with differing insulin profiles consistent with beneficial ImE effects on insulin sensitivity. It was not until the final time point that the placebo group displayed hyperglycemia compared with the ImE group. In sum, our data suggest that WSD induced IR increases over time in the presence or absence of E but that the degree of hyperinsulinemia necessary to maintain a given level of glucose disposal is decreased by ImE treatment. Thus, the stable glucose response over time in the ImE group was accomplished at lower insulin secretion rates, consistent with an insulin-sensitizing action with ImE treatment. This conclusion is supported by a recent study demonstrating in an ovariectomy mouse model that subcutaneous E implants improved insulin sensitivity and reduced hepatic gluconeogenesis through a Foxo1-dependent mechanism (53).
In the DE group, glucose metabolism came to more closely resemble that of the ImE group. In addition, DE replacement showed potential weight-independent benefits, mostly in the form of stabilization of a downward trend in glucose disposal and improvement in glucose tolerance as evidenced by a stable glucose AUC despite lower insulin secretion during the IVGTT. In contrast, the placebo group experienced an increase in glucose AUC over the same time period. Comparing the IVGTT AUC data with Kg and ISI suggests improved glucose tolerance (lower glucose excursions per insulin excursion after glucose infusion) in parallel with a marked increase in Kg and reversal of declining ISI with DE compared with placebo. Interestingly, ImE limited the increase in HDL-C that is typically associated with WSD consumption. Our results may not be generalizable to other primates. For example, rhesus macaques differ from cynomolgus macaques in cholesterol metabolism (54) and are uniquely resistant to atherosclerosis unless they consume a diet of 40% peanut oil (55).
The effects of ImE on WSD-induced weight gain were leptin independent, because serum leptin levels (adjusted for fat mass) and circadian leptin secretion were not altered by ImE or DE treatment. Although ImE reduced morning and evening leptin levels relative to placebo, these differences also disappeared after adjustment for fat mass. Similarly, effects of E replacement therapy on markers of inflammation were minimal (the stimulatory effect of WSD on IL-8 was partially ameliorated by ImE and DE) or absent (the remainder of measured cytokines). Serum adiponectin concentrations also declined with time in the study. After adjustment for different baseline values between the groups, there was little effect of ImE on total adiponectin. However, there was a significant time × treatment interaction in HMW adiponectin. Statistically, this interaction reflects a divergence between the groups in normalized HMW adiponectin at months 6, 12, and 18, which was lost at later time points. Overall, it indicates that HMW adiponectin decreased more over time in the placebo group than in the ImE group, but the effect of ImE was lost by 24 months. Given HMW adiponectin’s purported role in governing insulin sensitivity, higher levels of this adipokine may play a mechanistic role in estrogen’s beneficial effects on glucose metabolism.
We observed trends in reduced daily activity due to diet and age that were partially attenuated with ImE. The marked increase in daily activity with DE, although not reaching the level of significance, is in contrast to the slow decline in both parameters with ImE. However, whether these changes with DE would persist in the longer term is unknown. Complementing these activity findings, we have previously published direct observations of these animals’ behavior by trained staff, reporting that ImE animals spent less sedentary time in close social contact and more time moving about on their own, and they exhibited fewer behaviors indicating anxiety (43). With regard to time spent eating or handling food and water, we found increases over time in both the ImE and placebo groups without a treatment effect (43).
Several factors may have limited the findings of our study. First, each group consisted of a small number of animals at baseline that became smaller over the duration of the study because of age-related mortality. Because DE treatment did not begin until close to study conclusion, these smaller numbers may have had a larger impact on potential findings of significance in this group. Analysis of the effects of DE may also have become more apparent if extended >6 months. Finally, although benefits of ImE on body composition and glucose metabolism were found, even if temporary, it was not possible to determine whether these benefits extended to fewer cases of type 2 diabetes. Nonetheless, by 30 months the insulin excursions in the placebo group demonstrated changes indicative of declining islet cell function, which combined with the increasing AUC glucose curve typically heralds diabetes onset. On an individual basis, in one placebo animal that had a stroke, HbA1c levels increased from 4.8% to 6.8%, and in another placebo animal, HbA1c levels increased from 5.1% to 8.6%.
In conclusion, ImE therapy was able to maintain stable glucose control despite consumption of a WSD and weight gain through insulin-sensitizing effects, as reflected in the reduction in the degree of hyperinsulinemia required to maintain relative euglycemia and other parameters derived from fasting and dynamic glucose testing. Delayed estrogen treatment also increased glucose tolerance in the absence of effects on weight or body composition. Declining activity was mitigated to a small degree in ImE animals, as suggested by activity collars and confirmed by observations in another publication (43). Loss of lean mass was not prevented by ImE, but bone measures remained responsive to E for 30 months with WSD and obesity. However, for several important outcomes, including total and truncal fat mass, lipids, and HMW adiponectin levels, early beneficial effects of ImE were lost by 24 to 30 months, and DE showed no benefit. Our data highlight the importance of lifestyle influences on the impact of medical hormonal therapy on health and indicate that diet and obesity reduce the long-term benefits on metabolism. Limiting the adverse impact of a WSD on the potential benefits of hormone therapy could be the focus of future clinical studies. Nonetheless, our data support recent human studies reporting lower risk of type 2 diabetes progression with E therapy and those that have also suggested that the benefits of E therapy may be directly mediated by increasing insulin action (56).
Acknowledgments
We thank the staff of the ONPRC Division of Comparative Medicine, including the Surgical and Pathology units, for their expertise and helpfulness in all aspects of animal management and care. We thank Kevin Mueller for his careful management of the colony and the veterinary staff of ONPRC for their clinical oversight of the resource animals. We also thank the Behavioral Science Unit for help in maintaining the animals, the Obese NHP Resource for the metabolic characterization of the animals, and the ONPRC Endocrine Technologies Core for hormone assays.
Financial Support: This study was supported by National Institutes of Health Grants R24 OD11895 (to C.L.B.) and P51 OD11092 for operation of the Oregon National Primate Research Center.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- BMC
bone mineral content
- BMD
bone mineral density
- CV
coefficient of variation
- DE
delayed estrogen
- DEXA
dual X-ray absorptiometry
- E
estrogen
- ERT
estrogen replacement therapy
- HDL-C
high-density lipoprotein cholesterol
- HMW
high molecular weight
- HOMA-IR
homeostatic model assessment of insulin resistance
- HRT
hormone replacement therapy
- IFN
interferon
- ImE
estrogen immediately after hysterectomy
- IR
insulin resistance
- ISI
insulin sensitivity index
- IVGTT
intravenous glucose tolerance test
- Kg
glucose disappearance rate
- ONPRC
Oregon National Primate Research Center
- OvH
ovo-hysterectomy
- P
progesterone
- WSD
Western-style diet
References
- 1. Hill K. The demography of menopause. Maturitas. 1996;23(2):113–127. [DOI] [PubMed] [Google Scholar]
- 2. Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57(1):257–275. [DOI] [PubMed] [Google Scholar]
- 3. Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta). Biol Reprod. 2006;75(4):539–546. [DOI] [PubMed] [Google Scholar]
- 4. McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 2008;61(1–2):4–16. [DOI] [PubMed] [Google Scholar]
- 5. Dai J, Yu X, Huang S, Fan L, Zhu G, Sun H, Tang X.. Relationship between sagittal spinal alignment and the incidence of vertebral fracture in menopausal women with osteoporosis: a multicenter longitudinal follow-up study. Eur Spine J. 2015;24(4):737–743. [DOI] [PubMed] [Google Scholar]
- 6. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109(48):19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarti CD, Chiantera A, Graziottin A, Ognisanti F, Sidoli C, Mincigrucci M, Parazzini F; Gruppo di Studio IperAOGOI . Hormone therapy and sleep quality in women around menopause. Menopause. 2005;12(5):545–551. [DOI] [PubMed] [Google Scholar]
- 8. Son MK, Lim NK, Lim JY, Cho J, Chang Y, Ryu S, Cho MC, Park HY. Difference in blood pressure between early and late menopausal transition was significant in healthy Korean women. BMC Womens Health. 2015;15(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christianson MS, Mensah VA, Shen W. Multiple sclerosis at menopause: Potential neuroprotective effects of estrogen. Maturitas. 2015;80(2):133–139. [DOI] [PubMed] [Google Scholar]
- 10. Ryu S, Suh BS, Chang Y, Kwon MJ, Yun KE, Jung HS, Kim CW, Kim BK, Kim YJ, Choi Y, Ahn J, Cho YK, Kim KH, Ahn Y, Park HY, Chung EC, Shin H, Cho J. Menopausal stages and non-alcoholic fatty liver disease in middle-aged women. Eur J Obstet Gynecol Reprod Biol. 2015;190:65–70. [DOI] [PubMed] [Google Scholar]
- 11. Värri M, Tuomainen TP, Honkanen R, Rikkonen T, Niskanen L, Kröger H, Tuppurainen MT. Carotid intima-media thickness and calcification in relation to bone mineral density in postmenopausal women: the OSTPRE-BBA study. Maturitas. 2014;78(4):304–309. [DOI] [PubMed] [Google Scholar]
- 12. Piskorz A, Brzostek T.. Comparison of SCORE-predicted risk of death due to cardiovascular events in women before and after menopause. Prz Menopauzalny = Menopause review. 2015;14(3):168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, Everson-Rose SA, Powell LH, Matthews KA. Cardiovascular fat, menopause, and sex hormones in women: the SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab. 2015;100(9):3304–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeon YK, Shin MJ, Kim WJ, Kim SS, Kim BH, Kim SJ, Kim YK, Shin YB, Kim IJ. The relationship between pulmonary function and bone mineral density in healthy nonsmoking women: the Korean National Health and Nutrition Examination Survey (KNHANES) 2010. Osteoporos Int. 2014;25(5):1571–1576. [DOI] [PubMed] [Google Scholar]
- 15. Chen G, Chen L, Wen J, Yao J, Li L, Lin L, Tang K, Huang H, Liang J, Lin W, Chen H, Li M, Gong X, Peng S, Lu J, Bi Y, Ning G. Associations between sleep duration, daytime nap duration, and osteoporosis vary by sex, menopause, and sleep quality. J Clin Endocrinol Metab. 2014;99(8):2869–2877. [DOI] [PubMed] [Google Scholar]
- 16. Elisha B, Messier V, Karelis A, Coderre L, Bernard S, Prud'homme D, Rabasa-Lhoret R.. The Visceral Adiposity Index: relationship with cardiometabolic risk factors in obese and overweight postmenopausal women—a MONET group study. Appl Physiol Nutr Metab. 2013;38(8):892–899. [DOI] [PubMed] [Google Scholar]
- 17. Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med. 2009;71(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller C, Larkey L, Distefano JK, Boehm-Smith E, Records K, Robillard A, Veres S, Al-Zadjali M, O’Brian AM. Perimenopausal obesity. J Womens Health (Larchmt). 2010;19(5):987–996. [DOI] [PubMed] [Google Scholar]
- 19. Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol. 2010;118(4-5):304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV; Women’s Health Initiative Investigators . Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. [DOI] [PubMed] [Google Scholar]
- 23. Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, Limacher MC, Liu JH, Mason E, Oberman A, O’Sullivan MJ, Phillips LS, Prineas RJ, Tinker L. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia. 2006;49(3):459–468. [DOI] [PubMed] [Google Scholar]
- 24. Mohammed K, Abu Dabrh AM, Benkhadra K, Al Nofal A, Carranza Leon BG, Prokop LJ, Montori VM, Faubion SS, Murad MH. Oral vs transdermal estrogen therapy and vascular events: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4012–4020. [DOI] [PubMed] [Google Scholar]
- 25. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. [DOI] [PubMed] [Google Scholar]
- 26. NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez C, Calle EE, Patel AV, Tatham LM, Jacobs EJ, Thun MJ. Effect of body mass on the association between estrogen replacement therapy and mortality among elderly US women. Am J Epidemiol. 2001;153(2):145–152. [DOI] [PubMed] [Google Scholar]
- 28. Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3119–3130. [DOI] [PubMed] [Google Scholar]
- 29. Gómez Real F, Svanes C, Björnsson EH, Franklin KA, Gislason D, Gislason T, Gulsvik A, Janson C, Jögi R, Kiserud T, Norbäck D, Nyström L, Torén K, Wentzel-Larsen T, Omenaas E. Hormone replacement therapy, body mass index and asthma in perimenopausal women: a cross sectional survey [published correction appears in Thorax. 2006;61(3):274] Thorax. 2006;61(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE. Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2004;13(2):220–224. [DOI] [PubMed] [Google Scholar]
- 31. Small L, Brandon AE, Turner N, Cooney GJ. Modeling insulin resistance in rodents by alterations in diet: what have high-fat and high-calorie diets revealed? Am J Physiol Endocrinol Metab. 2018;314(3):E251–E265. [DOI] [PubMed] [Google Scholar]
- 32. Last AR, Wilson SA. Low-carbohydrate diets. Am Fam Physician. 2006;73(11):1942–1948. [PubMed] [Google Scholar]
- 33. Harwood HJ Jr, Listrani P, Wagner JD. Nonhuman primates and other animal models in diabetes research. J Diabetes Sci Technol. 2012;6(3):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pound LD, Kievit P, Grove KL. The nonhuman primate as a model for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21(2):89–94. [DOI] [PubMed] [Google Scholar]
- 35. Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57(2):335–340. [DOI] [PubMed] [Google Scholar]
- 36. Andersen B, Straarup EM, Heppner KM, Takahashi DL, Raffaele V, Dissen GA, Lewandowski K, Bödvarsdottir TB, Raun K, Grove KL, Kievit P. FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. Int J Obes. 2018;42(6):1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62(2):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarafidis PA, Lasaridis AN, Nilsson PM, Pikilidou MI, Stafilas PC, Kanaki A, Kazakos K, Yovos J, Bakris GL. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley’s indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007;21(9):709–716. [DOI] [PubMed] [Google Scholar]
- 39. Hansen BC, Bodkin NL. Heterogeneity of insulin responses: phases leading to type 2 (non-insulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia. 1986;29(10):713–719. [DOI] [PubMed] [Google Scholar]
- 40. Hansen BC, Bodkin NL. Standardization of IVGTT. Importance of method used to calculate glucose disappearance. Diabetes Care. 1993;16(5):847. [DOI] [PubMed] [Google Scholar]
- 41. Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4(4):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology. 2011;93(4):211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coleman K, Robertson ND, Maier A, Bethea CL. Effects of immediate or delayed estradiol on behavior in old menopausal macaques on obesogenic diet. J Obes. 2018;2018:1810275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ordóñez-Llanos J, Wägner AM, Bonet-Marqués R, Sánchez-Quesada JL, Blanco-Vaca F, González-Sastre F. Which cholesterol are we measuring with the Roche direct, homogeneous LDL-C Plus assay? Clin Chem. 2001;47(1):124–126. [PubMed] [Google Scholar]
- 45. Purnell JQ, Urbanski HF, Kievit P, Charles T, Roberts J, Bethea CL. Data from: Estradiol replacement timing and obesogenic diet effects on body composition and metabolism in post-menopausal macaques. figshare 2019. Deposited 30 January 2019.https://figshare.com/s/f35b9c650c9c96835692. [DOI] [PMC free article] [PubMed]
- 46. Yue F, Zhang G, Quintero JE, Gash DM, Zhang Z. Role of social interaction, exercise, diet, and age on developing and untreated diabetes in cynomolgus monkeys. Exp Gerontol. 2017;96:82–88. [DOI] [PubMed] [Google Scholar]
- 47. Crockett CM, Bowers CL, Shimoji M, Leu M, Bowden DM, Sackett GP. Behavioral responses of longtailed macaques to different cage sizes and common laboratory experiences. J Comp Psychol. 1995;109(4):368–383. [DOI] [PubMed] [Google Scholar]
- 48. Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann N Y Acad Sci. 1999;896(1):145–161. [DOI] [PubMed] [Google Scholar]
- 49. Bauer SA, Arndt TP, Leslie KE, Pearl DL, Turner PV. Obesity in rhesus and cynomolgus macaques: a comparative review of the condition and its implications for research. Comp Med. 2011;61(6):514–526. [PMC free article] [PubMed] [Google Scholar]
- 50. Harris RA, Alcott CE, Sullivan EL, Takahashi D, McCurdy CE, Comstock S, Baquero K, Blundell P, Frias AE, Kahr M, Suter M, Wesolowski S, Friedman JE, Grove KL, Aagaard KM. Genomic variants associated with resistance to high fat diet induced obesity in a primate model. Sci Rep. 2016;6(1):36123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li S, Kievit P, Robertson AK, Kolumam G, Li X, von Wachenfeldt K, Valfridsson C, Bullens S, Messaoudi I, Bader L, Cowan KJ, Kamath A, van Bruggen N, Bunting S, Frendéus B, Grove KL. Targeting oxidized LDL improves insulin sensitivity and immune cell function in obese Rhesus macaques. Mol Metab. 2013;2(3):256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, Han G, Newell-Fugate A, Tian Y, Majeti R, Liu W, Xu Y, Wu C, Allred K, Allred C, Sun Y, Guo S. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes. 2019;68(2):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10(6):1051–1057. [DOI] [PubMed] [Google Scholar]
- 55. Bond MG, Bullock BC, Bellinger DA, Hamm TE. Myocardial infarction in a large colony of nonhuman primates with coronary artery atherosclerosis. Am J Pathol. 1980;101(3):675–692. [PMC free article] [PubMed] [Google Scholar]
- 56. Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res. 2015;2015:916585. [DOI] [PMC free article] [PubMed] [Google Scholar]