Abstract
Oxidative stress (OS) is a common characteristic of several neurodegenerative disorders, including Parkinson disease (PD). PD is more prevalent in men than in women, indicating the possible involvement of androgens. Androgens can have either neuroprotective or neurodamaging effects, depending on the presence of OS. Specifically, in an OS environment, androgens via a membrane-associated androgen receptor (mAR) exacerbate OS-induced damage. To investigate the role of androgens on OS signaling and neurodegeneration, the effects of testosterone and androgen receptor activation on the major OS signaling cascades, the reduced form of NAD phosphate (NADPH) oxidase (NOX)1 and NOX2 and the Gαq/inositol trisphosphate receptor (InsP3R), were examined. To create an OS environment, an immortalized neuronal cell line was exposed to H2O2 prior to cell-permeable/cell-impermeable androgens. Different inhibitors were used to examine the role of G proteins, mAR, InsP3R, and NOX1/2 on OS generation and cell viability. Both testosterone and DHT/3-O-carboxymethyloxime (DHT)–BSA increased H2O2-induced OS and cell death, indicating the involvement of an mAR. Furthermore, classical AR antagonists did not block testosterone’s negative effects in an OS environment. Because there are no known antagonists specific for mARs, an AR protein degrader, ASC-J9, was used to block mAR action. ASC-J9 blocked testosterone’s negative effects. To determine OS-related signaling mediated by mAR, this study examined NOX1, NOX2, Gαq. NOX1, NOX2, and the Gαq complex with mAR. Only NOX inhibition blocked testosterone-induced cell loss and OS. No effects of blocking either Gαq or G protein activation were observed on testosterone’s negative effects. These results indicate that androgen-induced OS is via the mAR–NOX complex and not the mAR–Gαq complex.
Parkinson disease (PD) is the second most common neurodegenerative disease (1), and the prevalence of this disorder is expected to increase to ∼2 million by the year 2030 (2). The two major hallmark neuropathological characteristics of PD are the presence of Lewy bodies and the loss of dopaminergic neurons in the substantia nigra pars compacta of the brain. The established motor symptoms (resting tremor, rigidity, bradykinesia) manifest when ∼80% of the dopaminergic neurons within the substantia nigra are lost (3, 4). Because no laboratory biomarker or imaging exists for PD diagnosis, diagnosis of PD is a clinical diagnosis that requires the presence of at least two established motor symptoms.
Although the etiology of PD remains elusive (5), oxidative stress (OS) has been linked to PD development and progression (6). OS is an early event in PD pathogenesis and can precede dopaminergic neurodegeneration in the substantia nigra (7–9). The substantia nigra pars compacta is rich in dopaminergic neurons (∼25,000 cells) (10–12), and this composition may underlie its vulnerability to OS insults. Dopamine and its metabolites are highly reactive owing to the auto-oxidation of dopamine that can generate free radicals, leading to cellular vulnerability of the substantia nigra (13, 14). In addition to dopamine auto-oxidation, major cellular OS signaling cascades can contribute to dopaminergic neuronal vulnerability, such as the reduced form of NAD phosphate (NADPH) oxidase (NOX) and calcium (Ca2+) neurotoxicity (15, 16). NOX1 and NOX2 isoforms can increase OS generation and play a role in neurodegeneration (17–21), including dopaminergic neuronal loss in PD (22, 23). Indeed, NOX can affect Ca2+ release and vice versa (24–33). For example, OS can modulate ryanodine receptors and inositol trisphosphate receptors (InsP3Rs) that are involved in intracellular Ca2+ release from the endoplasmic reticulum via the canonical G protein–coupled receptor (GPCR) Gαq signaling pathway (30–33). Furthermore, NOX inhibition can block Ca2+ signaling (28). Because these signaling pathways can induce a feed-forward loop, dysregulation could lead to substantia nigra vulnerability and, ultimately, PD. Therefore, determining mechanisms that can affect this feed-forward loop is paramount.
Sex differences in PD incidence and prevalence have also been recognized (34–38). Men are 1.5- to 2-fold more likely to develop PD than are women (36, 39, 40). Furthermore, sex differences have been observed in OS signaling. Overall, men have a higher level of circulating OS and higher levels of NOX1 and NOX2 than do women (41–45). In fact, sex hormones (estrogens, androgens) can influence OS generation. It is well established that estrogens are protective against OS insults (46, 47). However, the role of androgens, such as testosterone and DHT, in neurodegeneration is not extensively studied.
Current findings on the effects of androgens on cells are equivocal, wherein androgens were found to be protective or damaging to cells (48–52). Low testosterone is a risk factor for neurodegenerative disorders in men (53–55). However, other studies have found that testosterone can induce neuronal loss (56–60). Our laboratory observed that testosterone can have either neuroprotective or neurodamaging effects, depending on the presence of OS in the cellular environment (58, 60). Specifically, we found that testosterone is an oxidative stressor in both in vivo and in vitro studies (58–64). Depending on the level of OS in the cell, testosterone-induced OS could be neuroprotective via a preconditioning mechanism (58, 65, 66) or damaging by exacerbating existing OS damage (58–60, 64, 67–69). Using cell-impermeable androgens (e.g., testosterone bound to BSA), we found that testosterone can increase cellular OS via a membrane-associated androgen receptor (mAR), specifically an androgen receptor (AR) variant, AR45, that is missing its regulatory N-terminal domain (58, 60, 70). However, the specific OS signaling pathways initiated by mAR are not fully understood. To address this gap in knowledge, this study investigates the mechanisms underlying mAR-induced neurodegeneration in the N27 dopaminergic cell line. Specifically, the role of NOX1/NOX2 and Gαq/InsP3R in mediating androgen-induced neurodegeneration in an OS environment was investigated.
Materials and Methods
Reagents
The AR antagonist bicalutamide (no. B9061) was obtained from ApexBio Tech. AR degrader (ASC J9, HY-15194) and NOX2 inhibitor (no. GSK2795039) were purchased from MedChem Express. Apocynin (no. 4663) and diphenyleneiodonium chloride (DPI; no. 0504) were obtained from Tocris Bioscience. NOX1 inhibitor (no. ML171) was purchased from EMD Millipore. InsP3R inhibitor (2-APB; no. 100065) and Gαq G protein inhibitor (BIM-46187; no. 533299) were purchased from Calbiochem. GDPβS trilithium salt (no. G7637) was obtained from Sigma-Aldrich/Millipore. Antibodies, including MOX1 (sc-25545 for NOX1) (71), NOX2 (sc-130543) (72), goat anti-rabbit (sc-2004) (73), goat anti-mouse (sc-2005) (74), AR C19 (sc-815) (75), and Gαq (sc-365906) (76), were from Santa Cruz Biotechnology. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (GTX627408) (77) was purchased from GeneTex. Dimethyl sulfoxide (DMSO) was obtained from VWR International, and PBS was from Quality Biological. A Pierce™ bicinchoninic acid protein assay kit was purchased from Thermo Scientific. Protein gels (no. 456-1093) and Mini-PROTEAN TGX (no. 456-9036) were acquired from Bio-Rad Laboratories. Protein A–Sepharose (CL-4B) was obtained from GE Healthcare. RPMI 1640 was purchased from HyClone. Penicillin/streptomycin and TrypLE select (10×) were acquired from Gibco. Fetal bovine serum (FBS) and l-glutamine were obtained from Corning, and charcoal-stripped FBS was from Atlanta Biologicals. Tert-butyl H2O2 (A13926) and 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT; no. L11939) were purchased from Alfa Aesar. A fluorescent thiol detection kit (no. FLTHIO100) was obtained from Cell Technology. Super Signal West Femto chemiluminescent substrate was purchased from Thermo Scientific. Testosterone (no. A6950-000) and DHT/3-O-carboxymethyloxime/BSA (DHT-BSA, no. A2574-050) were obtained from Steraloids. Testosterone was made from a stock solution in 100% ethanol, whereas DHT-BSA was prepared in media. All inhibitors, except Gαq protein, were made from a stock solution in DMSO. Final concentrations of DMSO and ethanol were <0.001% in all vehicle controls and treatment groups.
Cell culture
The immortalized 1RB3AN27 (N27) dopaminergic neuronal cell line was harvested from fetal female rat mesencephalic tissue (78). This cell line expresses tyrosine hydroxylase (TH), a marker for dopaminergic neurons, and a membrane-associated splice variant of the AR, AR45, that is missing the regulatory N terminus domain (58, 69, 70, 79–81). N27 cells were cultured as previously published (60). Cells were grown in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37°C in 5% CO2. Cells were seeded at a density of 6 × 104 per well in 96-well cell culture plates (for cell viability assay), 6 × 104 per 100-mm dish (for OS assays), and 1 × 106 per 100-mm dish (for coimmunoprecipitation and Western blot studies). To ensure the quality and integrity of the N27 cell line, we only used passages between 16 and 19 for all experiments. We also characterized these cells based on morphology, doubling time, and a well-characterized response to tert-butyl H2O2 and testosterone (58, 60).
Experimental design
Cells were plated into 96-well cell culture plates at a density of 6.0 × 104 cells per well in RPMI 1640 media supplemented with 10% FBS and 1% penicillin/streptomycin and incubated for 24 hours at 37°C, 5% CO2. At ∼80% confluency, media were changed to RPMI 1640 medium with charcoal-stripped FBS to remove confounding variables (e.g., hormones) from the serum. Maintaining cells in charcoal-stripped FBS does not negatively impact cell viability, as the media still contain salt, glucose, amino acids, and other nutrients (58, 60, 82). Inhibitors (and associated vehicle controls) used in this study were applied 2 hours prior to H2O2. Cells were exposed to 10 μM H2O2 for 2 hours. This concentration of H2O2 is sufficient to achieve 20% cell loss, which is necessary to achieve the OS threshold for androgens to negatively impact cells. After H2O2 exposure, androgens were applied to cells for 4 hours. Based on our previous studies (58, 60), androgen concentrations used in this study were 100 nM testosterone and 500 nM DHT-BSA. A fivefold increase in the DHT-BSA concentration (i.e., 500 nM) was used to account for decreased DHT receptor binding due to the structure of DHT-BSA (11 DHT molecules bound to 1 BSA molecule). Furthermore, these androgen concentrations are appropriate to examine the AR splice variant, AR45, which exhibits a similar affinity as the full-length AR to androgens (83).
Cell viability
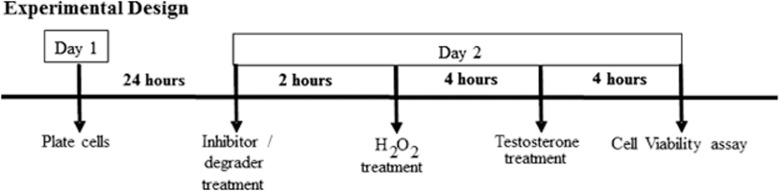
Cell viability was determined by an MTT assay as previously described (60). Briefly, N27 cells were plated into 96-well cell culture plates at a density of 6.0 × 104 cells/mL in each well. Treatments were carried out as shown in Fig. 1. Cell viability was examined by aspirating the media and incubating the cells in each well with 100 μL of phenol red–free RPMI 1640 medium with charcoal-stripped FBS, followed by 20 μL of 5 mg/mL MTT at 37°C and 5% CO2 for a 3-hour period. Absorbance was read at 595 nm. Experiments were replicated at least three times on three separate plates; each n is a mean of eight wells per treatment group on one plate. The colorimetric intensity is directly proportional to the number of live cells.
Figure 1.
Experimental design. N27 cells were plated in 96-well cell culture plates and incubated at 37°C in 5% CO2 for 24 h. Two-hour pretreatment was conducted for all inhibitors and AR degrader. At 80% confluency, cells were exposed to the OS or, H2O2, for 4 h followed by testosterone treatment.
Oxidative stress
A fluorescent thiol detection kit was used to measure the levels of reduced thiols, as previously published (60, 69). Cells were plated in 100-mm × 20-mm cell culture dishes at a density of 6.0 × 104 cells per plate. Similar to our cell viability experiments, at 80% confluency, cells were treated with the inhibitor or vehicle for 2 hours prior to H2O2 exposure, but they were only exposed to testosterone for 2 hours instead of 4 hours. This method allows OS to be measured prior to cell loss. Afterward, cells were lysed with a mild lysis buffer (10× TrypLE select) on ice, collected into tubes, centrifuged for 1 minute at high speed, and then trypsin was removed. Fluorescent thiol lysis buffer (200 µL) was added to each tube, homogenized, and centrifuged (10,000 rpm) for 5 minutes. Supernatants were collected. Reduced thiols, an inverse measure of OS, were quantified in the cell lysates (1.0 × 105 cells/mL). At least three independent experiments were performed, and fluorescence was measured at 488 nm excitation and 525 nm emission wavelengths.
Cell lysates and homogenization
Following our experimental design (Fig. 1), cells were placed on ice, washed with PBS, and lysed using Nonidet P-40 lysis buffer with a cocktail of dithiothreitol (1 µM), EDTA (1 mM), and phosphatase and protease inhibitors (1:100). The lysates were homogenized and centrifuged at 12,753 rpm at 4°C for 20 minutes. The supernatant was then removed and assayed for protein concentration. Protein concentrations were measured by using the Pierce bicinchoninic acid protein assay kit, according to the manufacturer’s instructions.
Coimmunoprecipitation
For coimmunoprecipitation studies to determine protein–protein interactions of proteins with molecular masses ∼40 to 50 kDa, the protocol was slightly modified to move the IgG bands from 45 kDa to 100 kDa. Cell lysate in Nonidet P-40 lysis buffer was incubated with various primary antibodies overnight at 4°C with agitation. The next day, the cell lysate plus primary antibody mixture was placed on ice, and a bead slurry (protein A–Sepharose beads with PBS) was added. The mixture was incubated at 4°C for 4 hours, centrifuged at maximum speed for 2 minutes, and the supernatant was aspirated. To separate the proteins attached to the primary antibody from the beads, 2× Laemmli sample loading buffer, containing β-mercaptoethanol, was added to the mixture and allowed to incubate at 37°C for 30 minutes in a water bath. After centrifugation at high speed for 2 minutes, protein–protein interactions were determined by Western blotting using respective antibodies specific to the proteins of interest.
Western blot
For all experiments 20 µg of protein was loaded on either Bio-Rad AnykD or 4% to 20% precast gels, except for coimmunoprecipitation that used 37 5µg of protein. Experiments were performed according to our previously published protocols (70). Proteins were separated by SDS-PAGE at room temperature at 25 mA. Next, proteins were transferred overnight at 50 V onto a polyvinylidene difluoride membrane at 4°C. Membranes were quickly washed under agitation with Tris-buffered saline with Tween 20 (TBST) and incubated in 5% nonfat milk in TBST at room temperature to block nonspecific binding. Afterward, the membranes were incubated with specific primary antibodies (1:500 dilution for MOX1, NOX2, and ARC19, and 1:1000 dilution for Gαq) (71, 72, 75, 76) in TBST with 1% nonfat milk overnight at 4°C. The membranes were washed twice with TBST for 10 minutes on a shaker and incubated for 30 minutes in the corresponding secondary antibody (1:1000 dilution for goat anti-rabbit and goat anti-mouse) (73, 74) in TBST with 1% nonfat milk at room temperature. After washing membranes twice for 10 minutes, the protein bands were detected using a Super Signal West Femto chemiluminescence assay. Protein band intensities were imaged using the Syngene G:BOX system together with FluorChem HD2 AIC software. Protein band densities were measured through densitometry with National Institutes of Health ImageJ densitometer software and normalized to GAPDH (1:10,000 dilution).
Statistical analysis
All analyses were performed using IBM SPSS Statistics version 21 software. Results were expressed as mean ± SEM, and a P value ≤0.05 indicates statistically significant differences. Comparisons were made by two- or three-way ANOVA using inhibitors, oxidative stressor, and hormone as independent factors. Fisher least significant difference post hoc analysis was used to assess differences between the various groups. Each experiment was replicated at least three times with different cell cultures.
Results
mAR interacts with NOX1, NOX2, and Gαq
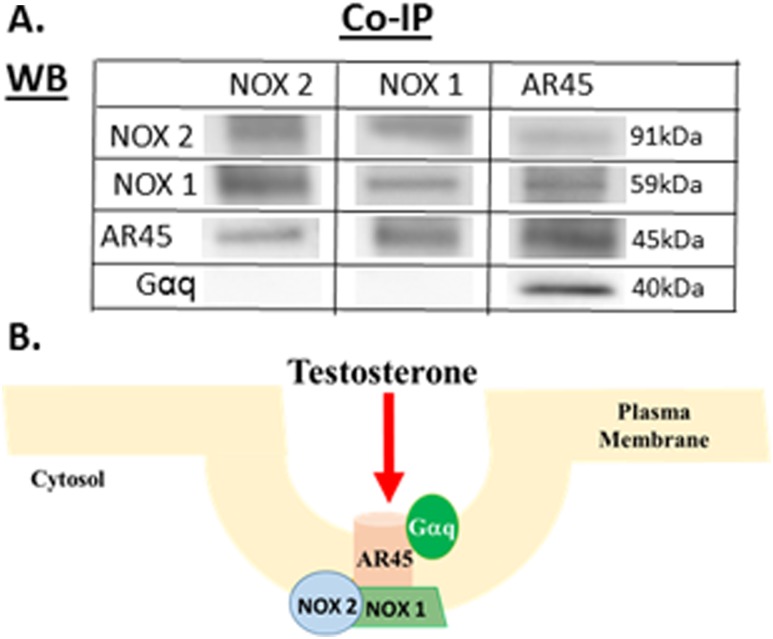
Previously, we discovered that the mAR, AR45 splice variant, was localized to lipid rafts within the plasma membrane (70), indicating that the mAR is a component of a communication hub that could facilitate its interaction with membrane-associated proteins to initiate cellular signaling (e.g., OS signaling). To determine whether the mAR (i.e., AR45) complexes with membrane-associated NOX1/NOX2 and Gαq/InsP3R OS signaling cascades, we performed coimmunoprecipitation on N27 cell lysate. To immunoprecipate specific proteins of interest, primary antibodies for NOX1 (i.e., MOX1), NOX2, and AR45 (i.e., AR-C19) (71, 72, 75) were used. Next, we probed for protein–protein interactions by using primary antibodies for Gαq, NOX1, NOX2, and AR45 (71, 72, 75, 76), which showed AR45 complexed with NOX1 and NOX2. Consistent with our prior publication, AR45 complexed with Gαq (70). Although AR45 coupled with Gαq, no protein interactions between NOX1 or NOX2 with Gαq were observed (Fig. 2). Only the 45-kDa AR45 protein was observed, indicating that the coimmunoprecipitation studies were determining protein interactions with mAR.
Figure 2.
Coimmunoprecipitation of AR45, NOX1, and NOX2 to determine the protein complex. NOX1, NOX2, and AR45 proteins were precipitated using specific antibodies and then probed with respective antibodies for NOX1, NOX2, and AR45 to determine protein–protein interactions. NOX1, NOX2, and AR45 interact to form a protein complex. (A) Gαq couples with AR45 but does not interact with either NOX1 or NOX2. (B) Testosterone can bind to the mAR, AR45, and activate multiple signaling pathways via its protein interactions with NOX1, NOX2, and Gαq in a lipid raft. Caveolin (purple), flotillin (blue), and phospholipids (orange) are shown. Co-IP, coimmunoprecipitation; WB, Western blot.
Testosterone’s detrimental effects are not mediated through the classical genomic pathway
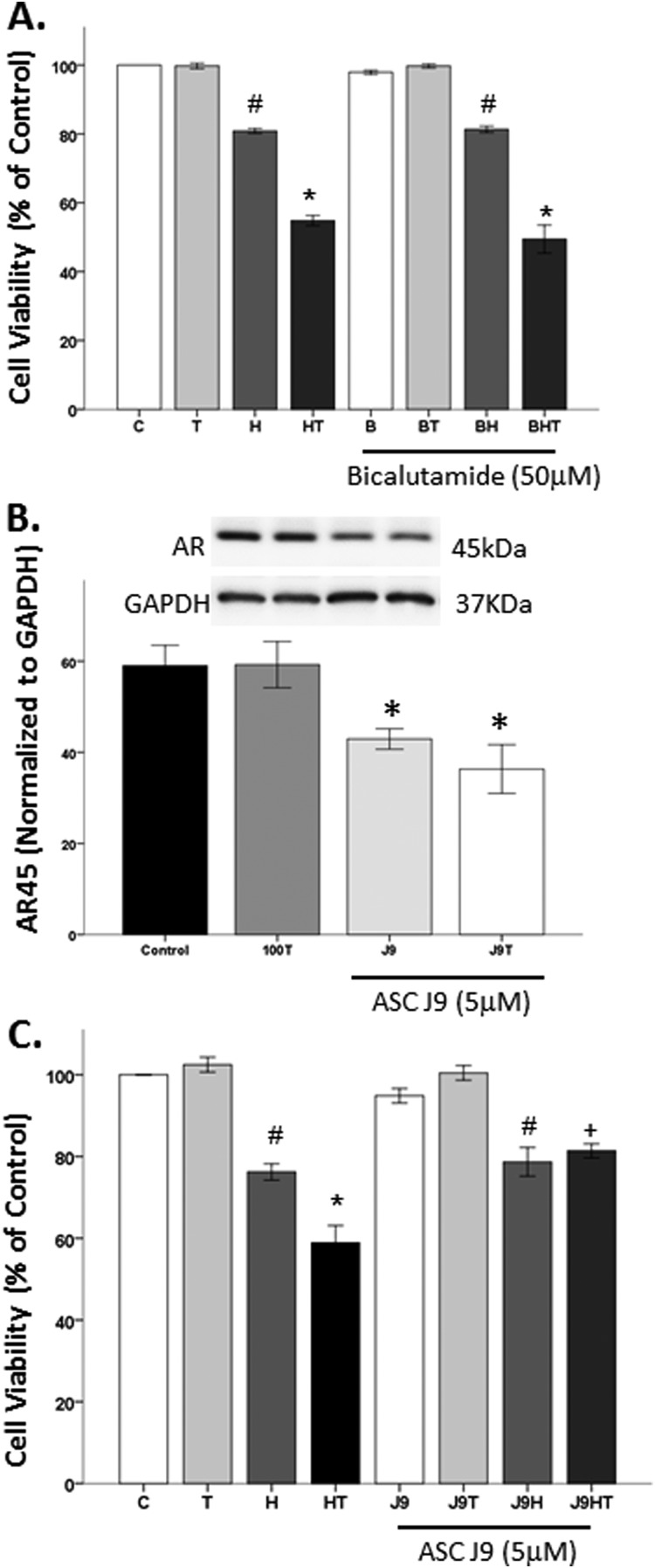
We previously published that flutamide, a classical AR antagonist, did not block testosterone’s negative effects (58). In this study, we strengthened our findings using a classical AR antagonist, bicalutamide. Bicalutamide binds to the ligand-binding pocket of AR and inhibits AR by failing to induce the correct conformational change (84, 85). Therefore, to confirm that testosterone’s detrimental effects are not mediated through the classical cytosolic AR, bicalutamide AR antagonist was used. As expected, testosterone did not affect cell viability (Fig. 3A), and H2O2 significantly decreased cell viability (F1,16 = 799.2, P < 0.05). In the presence of OS, testosterone further decreased cell viability, as indicated by a significant interaction between OS and hormone (F1,16 = 165, P < 0.05). The AR antagonist bicalutamide had no effect on cell viability, regardless of the OS environment. Furthermore, bicalutamide did not block testosterone’s negative actions on cell viability in an OS environment.
Figure 3.
Testosterone’s detrimental effects are not mediated through the classical genomic pathway. Testosterone alone does not affect cell viability. H2O2 induced ∼20% cell loss, which was exacerbated by testosterone. (A) The AR antagonist bicalutamide did not block testosterone’s negative effects in an OS environment. (B) Testosterone did not alter AR45 expression. The AR degrader, ASC J9, significantly decreased the expression of AR45, irrespective of the presence of testosterone. (C) ASC J9 blocked testosterone-induced cell loss in an OS environment, but it did not influence H2O2-induced cell loss. Results are reported as mean ± SEM. Results were determined by ANOVA followed by a Fisher least significant difference post hoc test. *P ≤ 0.05 vs all groups; #P ≤ 0.05 vs control; +P ≤ 0.05 vs HT groups. B, bicalutamide; C, vehicle control; H, H2O2; HT, posttreatment T; J9, ASC J9; T, 100 nM testosterone.
Because classical AR antagonists did not influence testosterone’s effects, an AR degrader, ASC J9, was used to degrade both cytosolic and mARs. ASC J9 selectively promotes AR degradation by disrupting the interaction between AR and AR coregulators, without impacting AR mRNA expression (86). To ensure that ASC J9 degraded mAR protein (45 kDa) expression, ASC J9 was used with and without testosterone, as previous reports stated that testosterone can stabilize AR (70, 87, 88). Consistent with our prior findings (70), testosterone did not alter mAR protein expression (Fig. 3B). In contrast, ASC J9 significantly reduced mAR expression (F1,8 = 10.6, P < 0.05), regardless of testosterone exposure (Fig. 3B). To determine whether degrading the mAR impacts testosterone’s negative effects in an OS environment, N27 cells were pretreated with ASC J9 for 2 hours prior to exposure to H2O2 and testosterone (Fig. 3C). As expected, significant negative effects from OS exposure on cell viability were observed (F1,28 = 167.1, P < 0.05). Additionally, significant interactions between oxidative stressor and testosterone treatment (F1,28 = 8.2, P < 0.05) and between oxidative stressor, hormone, and degrader (F1,26 = 4.5, P < 0.05) were observed. Specifically, H2O2 induced ∼20% cell loss, and testosterone exacerbated H2O2-induced cell loss. The AR degrader blocked testosterone’s negative effects on cell viability in an OS environment. However, the AR degrader did not impact H2O2-induced cell loss.
The role of G protein and InsP3R in testosterone-induced neurodegeneration
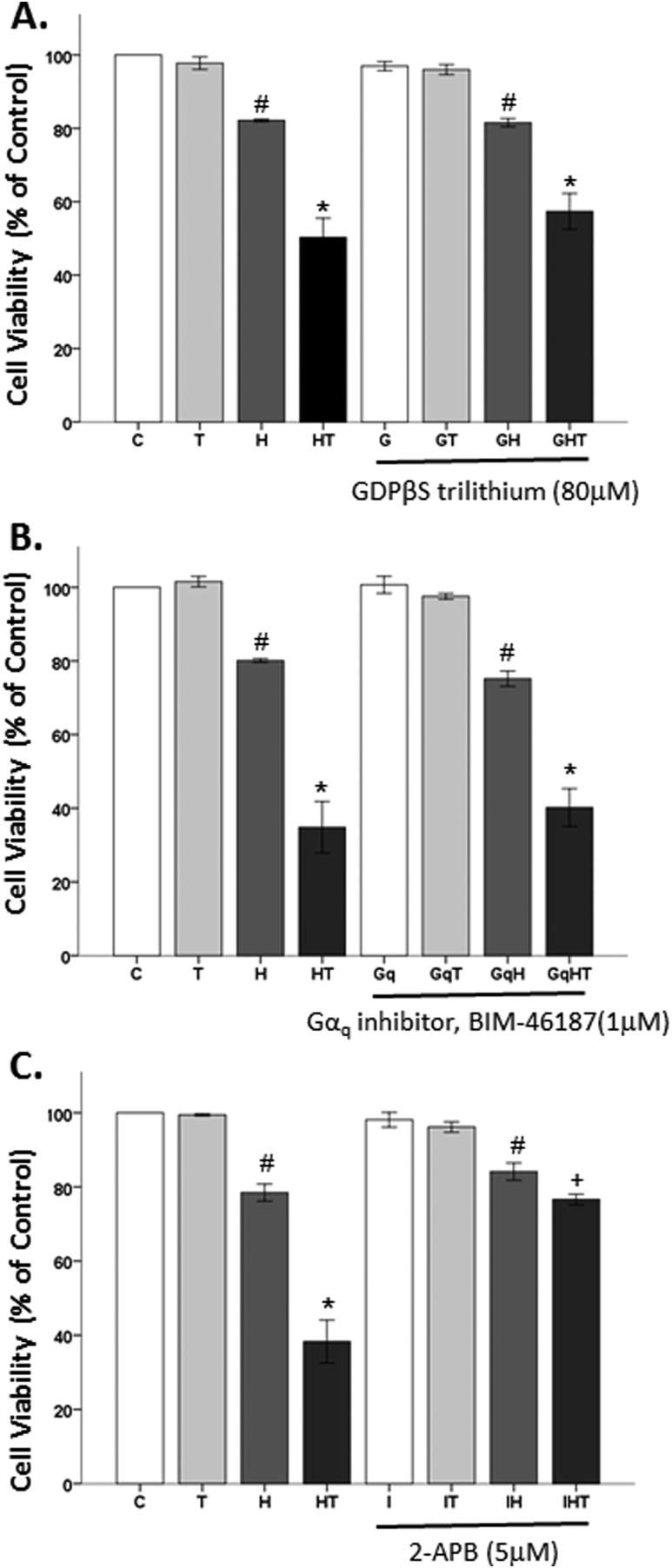
Because AR45 complexes with Gαq (Fig. 2A) (70), it is of interest to determine whether Gαq plays a role in testosterone’s damaging effects in an OS environment. The canonical GPCR Gαq signaling pathway, which is involved in intracellular Ca2+ release from the endoplasmic reticulum via ryanodine receptors and InsP3Rs, can increase OS (89). To examine this pathway, G protein activity was blocked with GDPβS trilithium, Gαq with BIM-46187, and InsP3R was blocked with 2-APB. In Fig. 4A using GDPβS trilithium, a GDP analog, to block G protein activity, significant effects of oxidative stressor (F1,29 = 206.8, P < 0.05), hormone (F1,29 = 51, P < 0.05), and an interaction between oxidative stressor and hormone (F1,29 = 40.6, P < 0.05) were observed. As expected, we observed no effects of testosterone alone and significant effects of H2O2 on the cell viability ∼20% cell loss. Testosterone, in the presence of an oxidative stressor, caused a further decrease in cell viability. However, GDPβS trilithium neither blocked H2O2’s effects nor the negative effects of testosterone in an OS environment.
Figure 4.
The role of G protein and InsP3R receptor in testosterone-induced neurodegeneration. (A and B) GDPβS trilithium, a GDP analog, and BIM-46187, a Gαq inhibitor, did not protect the cells from testosterone’s detrimental effects in an OS environment. (C) The InsP3R inhibitor 2-APB was able to block testosterone’s damaging effects in an OS environment. Results are reported as mean ± SEM. Results were determined by ANOVA followed by a Fisher least significant difference post hoc test. *P ≤ 0.05 vs all groups; #P ≤ 0.05 vs control; +P ≤ 0.05 vs HT groups. C, vehicle control; G, GDPβS trilithium; Gq, BIM-46187; H, H2O2; HT, posttreatment T; I, 2-APB; T, 100 nM testosterone.
Next, Gαq was blocked with BIM-46187. We found significant effects of oxidative stressor (F1,23 = 303.1, P < 0.05), hormone (F1,23 = 70.8, P < 0.05), and an interaction between oxidative stressor and hormone (F1,23 = 65.4, P < 0.05) (Fig. 4B). Again, no effects of testosterone alone were observed. H2O2 significantly induced ∼20% cell loss and testosterone worsened H2O2’s damaging effects. Similar to the GDP analog, the Gαq inhibitor did not block testosterone’s detrimental effects in an OS environment. These results indicate that testosterone’s damaging effects via the mAR are not through the Gαq protein pathway, although AR45 complexes with Gαq.
Lastly, we looked at the effects of InsP3R inhibition on testosterone’s negative effects on cell viability. We found significant effects of oxidative stressor (F1,48 = 314.10, P < 0.05), hormone (F1,48 = 58.8, P < 0.05), inhibitor (F1,48 = 35.1, P < 0.05), an interaction between oxidative stressor and hormone (F1,23 = 65.4, P < 0.05), and an interaction between oxidative stressor, hormone, and InsP3R inhibitor (F1,48 = 27, P < 0.05) (Fig. 4C). Consistent with our prior studies, testosterone alone did not influence cell viability, whereas H2O2 did. Testosterone exacerbated H2O2’s detrimental effects on the cells. InsP3R inhibition with 2-APB, alone, had no effect on cells, regardless of OS environment. In contrast to earlier observations with GDPβS trilithium and BIM-46187, 2-APB was able to protect the cells from testosterone’s negative effects in an OS environment. This finding of InsP3R signaling, which mediates intracellular Ca2+ release, extends our previous work showing that mAR’s negative effects on OS and cell viability in N27 cells was influenced by Ca2+ influx into the mitochondria (58).
The effects of NOX inhibitor on OS generation and cell loss
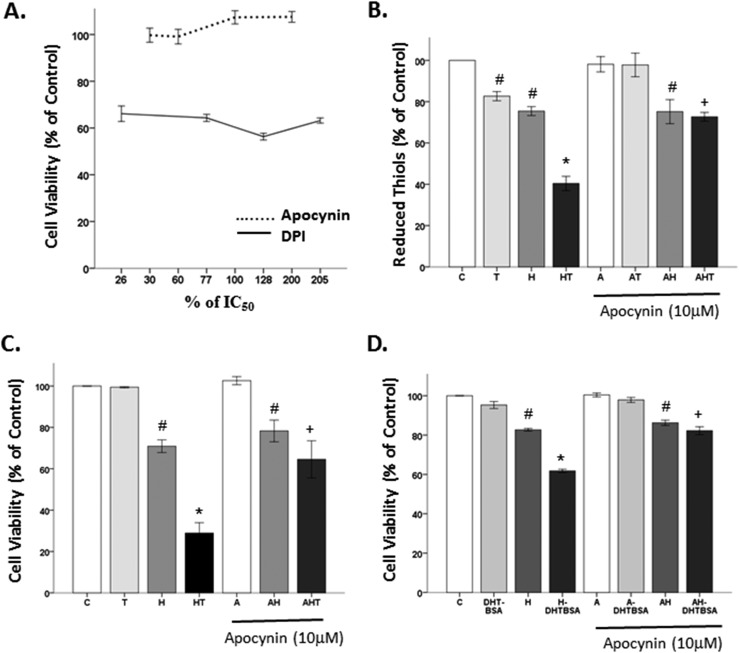
To determine whether NOX is involved in mAR-induced OS, we used the frequently used NOX inhibitors, apocynin and DPI, at various concentrations based on their IC50 (10 μM and 39 nM, respectively) (90, 91). DPI was toxic to the cells, regardless of the concentration (10 to 80 nM) used. However, apocynin did not impact cell viability in any of the doses (3 to 20 μM) investigated (Fig. 5A). Therefore, apocynin was used as a NOX inhibitor in the subsequent experiments.
Figure 5.
The effects of nonspecific NOX inhibitor on OS generation and cell loss. (A) DPI was toxic to N27 cells, regardless of the concentration used. Apocynin did not show toxicity. (B) Testosterone alone increased OS generation, as evidenced by decreased reduced thiols. Apocynin blocked testosterone’s effects on OS generation. (C) Testosterone alone had no effect on cell viability. Apocynin did not protect cells from H2O2’s effects. Apocynin blocked testosterone-induced cell loss in an OS environment. (D) Similarly, apocynin blocked DHT-BSA exacerbation of H2O2-induced cell loss. Results are reported as mean ± SEM. Results were determined by ANOVA followed by a Fisher least significant difference post hoc test. *P ≤ 0.05 vs all groups; #P ≤ 0.05 vs control; +P ≤ 0.05 vs HT groups. A, apocynin; C, vehicle control; H, H2O2; HT, posttreatment testosterone; T, 100 nM testosterone, 500 nm DHT-BSA.
OS was measured by quantifying reduced thiols (an inverse measure of OS). Significant effects of oxidative stressor (F1,16 = 124.2, P < 0.05), hormone (F1,16 = 28.7, P < 0.05), inhibitor (F1,16 = 19.3, P < 0.05), an interaction between oxidative stressor and hormone (F1,16 = 3.7, P < 0.05), and an interaction between oxidative stressor, hormone, and inhibitor (F1,16 = 2.3, P < 0.05) were observed (Fig. 5B). Consistent with our prior publications (58–60, 63), testosterone is an oxidative stressor. Both testosterone and H2O2 significantly decreased the level of reduced thiols, indicating an increase in OS. Furthermore, testosterone exacerbated H2O2-induced OS by further decreasing reduced thiols. The NOX inhibitor apocynin did not alter H2O2-induced cell loss, indicating that H2O2 increases OS via a non-NOX mechanism. However, apocynin blocked the testosterone-induced OS, indicating that NOX mediates testosterone-induced OS generation.
A similar paradigm was observed for the cell viability assay, except that testosterone alone had no effect on cell viability. Significant effects of oxidative stressor (F1,23 = 13.4, P < 0.05), an interaction between oxidative stressor and hormone (F1,23 = 21.4, P < 0.05), and an interaction between oxidative stressor, hormone, and inhibitor (F1,23 = 10.3, P < 0.05) were observed (Fig. 5C). Specifically, H2O2 decreased cell viability, inducing ∼20% cell loss. Testosterone exacerbated H2O2-induced cell death, which was blocked by apocynin. Apocynin had no effect on cell viability, regardless of OS exposure. A similar pattern was observed using DHT-BSA. In Fig. 5D, we show significant effects of oxidative stressor (F1,21 = 513.9, P < 0.05), hormone (F1,21 = 81.8, P < 0.05), inhibitor (F1,21 = 57.8, P < 0.05), and an interaction between oxidative stressor and hormone (F1,21 = 17, P < 0.05). H2O2 induced ∼20% cell loss, and DHT-BSA further increased H2O2-induced cell loss. Apocynin did not alter H2O2’s effects on cell viability. However, apocynin blocked DHT-BSA’s detrimental effects in the presence of OS, implying the involvement of mAR and NOX on androgen’s negative effects in an OS environment.
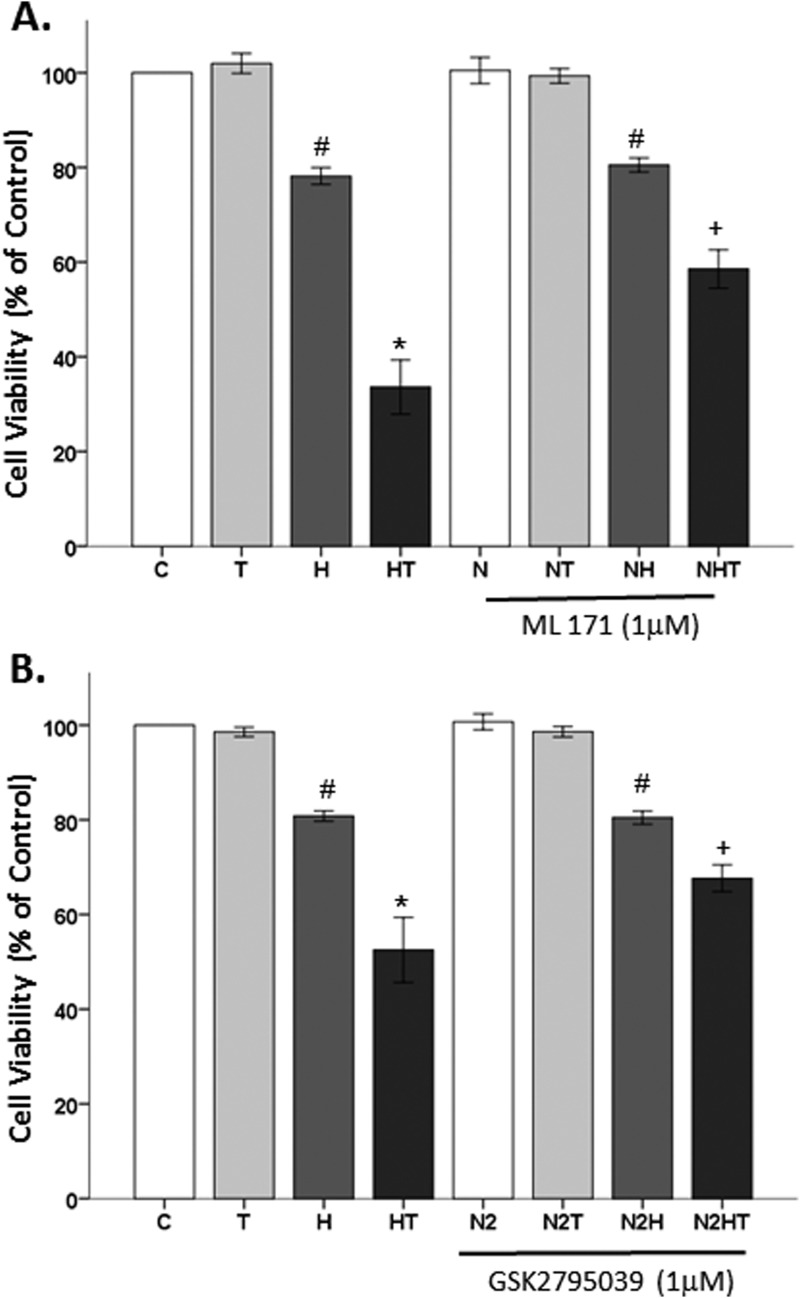
Because apocynin is a nonspecific NOX inhibitor (92, 93), the role of NOX1 and NOX2 in testosterone-mediated cell loss was examined. Cells were pretreated with a selective NOX1 inhibitor, ML171 (94, 95), followed by H2O2 and testosterone. Significant effects of oxidative stressor (F1,47 = 321.5, P < 0.05), hormone (F1,47 = 60.9, P < 0.05), inhibitor (F1,47 = 8.9, P < 0.05), an interaction between oxidative stressor and hormone (F1,47 = 64.1, P < 0.05), and an interaction between oxidative stressor, hormone, and inhibitor (F1,47 = 9.3, P < 0.05) were observed. Similar to previous observations, H2O2 decreased cell viability, and testosterone further exacerbated H2O2-induced cell loss. ML171, however, did not alter cell viability by itself or in the presence of testosterone. Furthermore, ML171 did not protect the cells from H2O2. Contrary to observations with apocynin, ML171 partially protected against testosterone-induced cell loss in an OS environment (Fig. 6A).
Figure 6.
The effects of selective NOX inhibitors on testosterone-induced cell loss. (A) The selective NOX1 inhibitor, ML171, had no effect on cell viability. H2O2-induced cell loss was not blocked by ML171. ML171 partially blocked testosterone-induced cell loss in the presence of OS. (B) The selective NOX2 inhibitor, GSK2795039, had no effect on cell viability. H2O2-induced cell loss was not blocked by GSK2795039. GSK2795039 partially blocked testosterone-induced cell loss in the presence of OS. Results are reported as mean ± SEM. Results were determined by ANOVA followed by a Fisher least significant difference post hoc test. *P ≤ 0.05 vs all groups; #P ≤ 0.05 vs control; +P ≤ 0.05 vs HT groups. C, vehicle control; H, H2O2; HT, posttreatment T; N, ML 171; N2, GSK2795039; T, 100 nM testosterone, 500 nm DHT-BSA.
The role of NOX2 in testosterone-induced neurodegeneration was further examined using a selective NOX2 inhibitor, GSK2795039. Again, H2O2 decreased cell viability, and testosterone further exacerbated H2O2-induced cell loss. However, GSK2795039, alone or in the presence of testosterone, did not alter cell viability. GSK2795039 did not protect the cells from H2O2, indicating that GSK2795039 at this concentration does not exhibit general antioxidant properties. Similar to observations with ML171, GSK2795039 partially blocked testosterone-induced cell loss in the presence of H2O2. Significant effects of oxidative stressor (F1,28 = 233.5, P < 0.05), hormone (F1,28 = 34.6, P < 0.05), inhibitor (F1,28 = 4.3, P < 0.05), an interaction between oxidative stressor and hormone (F1,28 = 24.5, P < 0.05), and an interaction between oxidative stressor, hormone, and inhibitor (F1,28 = 4.6, P < 0.05) were observed (Fig. 6B). Based on these findings using ML171 and GSK2795039, cells were exposed to both NOX1 and NOX2 inhibitors with the aim of achieving a full protection from testosterone’s neurotoxic effects in an OS environment. Interestingly, antioxidant effects in the H2O2 group were observed, indicating nonspecific antioxidant properties. This general antioxidant effect may be due to the phenolic structure of these inhibitors, which can have antioxidant effects at higher levels (96–98). Hence, we were unable to examine the combined effects of these inhibitors on testosterone-induced cell loss in an OS environment.
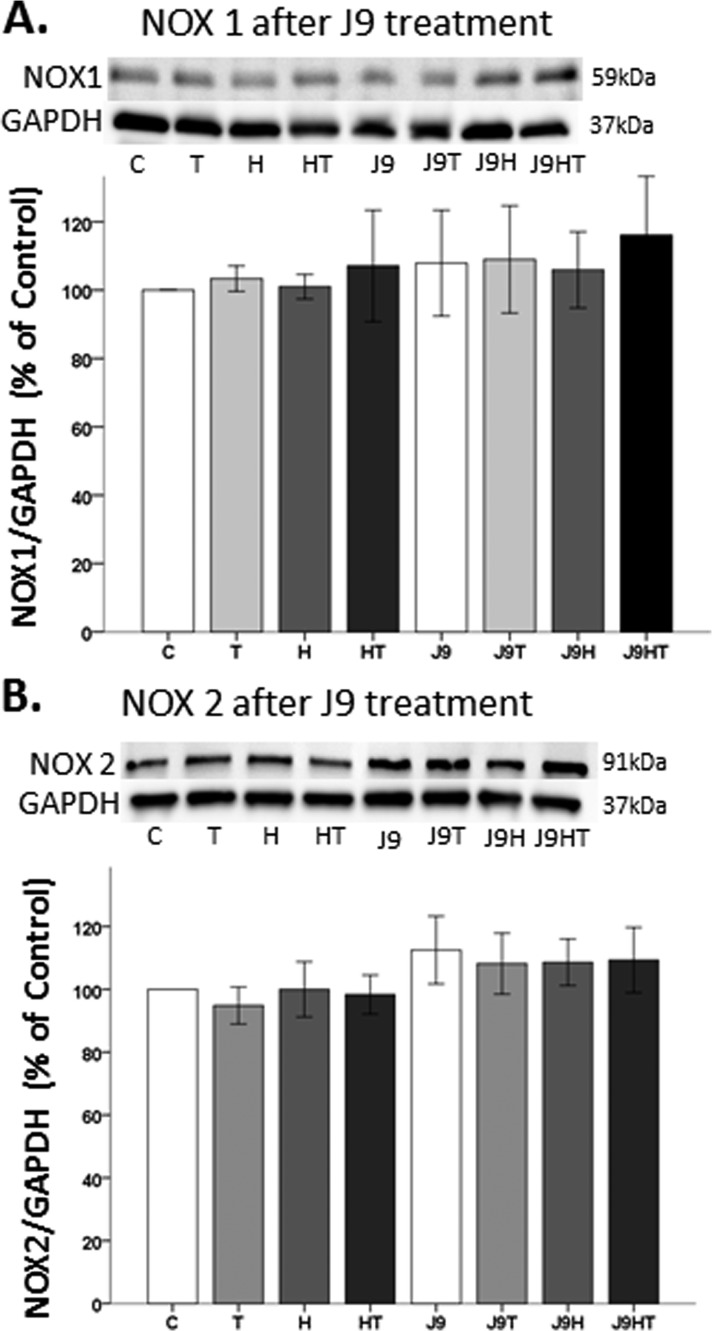
The effects of AR degradation on NOX1 and NOX2 protein expression
Because the mAR (i.e., AR45) complexed with NOX1 and NOX2, we wanted to ensure that mAR protein degradation did not alter NOX1 and NOX2 protein expression. Two-hour pretreatment of N27 cells with the AR degrader, ASC J9, did not alter NOX1 or NOX2 expression, irrespective of OS or testosterone exposure (Fig. 7A). Although it appears mAR degradation with ASC J9 affects NOX2 expression, there was no statistical significance across all treatment groups (F1,24 = 3.95, P = 0.059) (Fig. 7B).
Figure 7.
The effects of AR degradation on NOX1 and NOX2 protein expression. (A and B) Degrading the AR45, using ASC J9, did not affect NOX1 or NOX2 expression. Data are expressed as a normalized ratio of protein band density of (A) NOX1 and (B) NOX2 against GAPDH and presented as mean ± SD. Results were determined by ANOVA followed by a Fisher least significant difference post hoc test. C, vehicle control; H, H2O2; HT, posttreatment T; J9, ASC J9; T, 100 nM testosterone.
Discussion
More men than women are affected by PD, regardless of age (35, 40, 69, 99). Sex hormones have been proposed to be involved in this disparity. Interestingly, the AR in a ligand-dependent manner can induce transcriptional activity of the TH gene (100) and increase TH activity (101). Thus, androgens can modulate dopaminergic neurons and dopamine synthesis, and ultimately OS generation via auto-oxidation of dopamine (13, 14). This pathway may underlie androgen neuroprotection via OS preconditioning (58) and the increased incidence in PD for men. Notably, AR is expressed in the substantia nigra pars compacta, the region affected by PD, and not in the substantia nigra pars reticulata (102). ARs are known to mediate important physiological and biological actions, including mood and libido (103–105), sexual reproductive functions (106), and learning and memory (107, 108). Indeed, in men with PD, decreased testosterone has been associated with poor cognitive abilities (109), reduced sexual libido, and erectile dysfunction (110–112). Testosterone replacement therapy in men with PD significantly improved motor and nonmotor symptoms (113, 114). However, in light of reports that estrogen via the estrogen receptor α can also upregulate TH activity (115, 116), androgen-induced transcriptional regulation of TH does not fully explain the increased PD symptoms after disease onset observed in men compared with women (117–119). Therefore, in this study, other OS signaling cascades that can be modulated by androgens, such as NOX1, NOX2, and Gαq/InsP3R were examined.
Low levels of androgens (1 nmol/L) via a nongenomic mechanism can exacerbate OS–induced damage in dopaminergic neurons (58, 60), supporting the role of a mAR in testosterone’s negative effects in an OS environment. The function of mAR is poorly understood, but it is proposed to mediate fast, nongenomic actions of androgens (120–122) and is unaffected by classical AR antagonists (58). Furthermore, it is unclear whether the mAR is a full-length AR, truncated AR, or an unknown AR localized to the plasma membrane. Recently, our laboratory discovered the presence of an AR splice variant, AR45, localized to lipid rafts within the substantia nigra pars compacta and the dopaminergic N27 cells (70). AR45 lacks the N-terminal regulatory domain, resulting in a molecular mass of 45 kDa, unlike full-length AR with a molecular mass of 110 kDa (84, 106). Neither age nor testosterone affected AR45 expression (70). In this study, degradation of AR45 using ASC J9, unlike classical AR antagonists, did block mAR exacerbation of OS–induced damage, supporting the role of AR45 as an mAR. This is of interest, as currently there are no known mAR antagonists. To the best of our knowledge, this is the first work to show inhibition of an mAR in the central nervous system.
Based on previous publications showing that AR45 interacts with Gαq protein within the lipid rafts (70) and that testosterone increases intracellular Ca2+ release in N27 cells (58), the role of the Gαq/InsP3R/Ca2+ pathway on mAR AR45-induced neurodegeneration was determined. Although AR45 complexes with Gαq protein, Gαq does not influence mAR-induced neurodegeneration, as evidenced by no effects from either Gαq protein inhibitor or GDP analog. Interestingly, inhibition of InsP3R, which mediates intracellular Ca2+ release, did block mAR-induced neurodegeneration. Clinical studies found therapeutic benefits of blocking Ca2+. Dihydropyridine Ca2+ channel blockers have been shown to decrease PD risk, progression, and even mortality (123–128). Furthermore, there is an ongoing exploratory phase 3 clinical trial of isradipine (dihydropyridine Ca2+ channel blocker), which may provide better insights into the association between the Ca2+ pathway and PD and its therapeutic potential (129).
In addition to the canonical GPCR Gαq pathway modulation of InsP3R for intracellular Ca2+ release from the endoplasmic reticulum (30–33), NOX can affect InsP3R-mediated Ca2+ release (24–33, 130). There are seven members of the NOX family: NOX1, NOX2, NOX3, NOX4, NOX5, Duox 1, and Duox 2. It is well known that the NOX enzyme is associated with dopaminergic neurons (131, 132) and plays an important role in normal cell physiology, including differentiation, proliferation, and immune response (133–135). Indeed, several studies have reported that NOX is the predominant source of reactive oxygen species in brain cells (16, 22), aside from the mitochondria. Dysregulation of NOX can induce neuronal dysfunction. NOX1, NOX2, and NOX4 are expressed in neurons (136–139). In fact, NOX1 and NOX2 have been shown to be expressed in dopaminergic neurons from PD patients (20, 140). Postmortem substantia nigra tissue from PD patients showed higher NOX2 expression than did individuals without PD (131). In several PD experimental models [e.g., 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, rotenone, and 6-hydroxydopamine (AR) toxins], including the N27 dopaminergic cell line, NOX1 and NOX2 can mediate dopaminergic neuronal death (20, 22, 141–147).
The N27 cell line expresses NOX1, NOX2, NOX4, Duox 1, and Duox 2 homologs of NOX (20), and this study confirms the presence of NOX1 and NOX2 in N27 cells. This study extends these findings by showing that NOX1 and NOX2 complex with AR45. To determine the role of NOX on mAR neurodegeneration, two widely used nonspecific small molecule NOX inhibitors, apocynin and DPI, were used (91, 142, 148, 149), as both have shown neuroprotective benefits in neurodegenerative diseases (150–155). Although prior studies using DPI observed protection from 6-OHDA–induced OS in N27 cells (20), it was not appropriate for our studies, as DPI was toxic to N27 cells. DPI toxicity has been observed in other cells such as N11 glial cells and cultured cells isolated from rat heart, whereby DPI blocked a major antioxidant pathway, resulting in increased OS and apoptosis (156, 157). In contrast, apocynin proved to be nontoxic in this cell line, making it an ideal NOX inhibitor. This is consistent with publications reporting the effectiveness, yet low toxicity, of apocynin in both in vitro and in vivo models (91, 158). Prior studies have shown that apocynin decreased NOX activation, inflammation, and apoptosis in experimental models of PD (132, 159). Indeed, in this study apocynin blocked testosterone’s neurodamaging effects in an OS environment, suggesting that both NOX1 and NOX2 are involved. Apocynin had no influence on H2O2-induced cell loss. This effect was not unexpected, as tert-butyl H2O2 exerts its effects on OS via two pathways: cytochrome P450 for peroxyl and alkoxyl radical production, and glutathione peroxidase for lipid peroxidation (160). Importantly, note that sublethal concentrations of tert-butyl H2O2 that do not affect NOX protein expression were used in this study.
Apocynin’s mechanism of action is controversial. It can act on NOX1 and NOX2 (21), as well as other flavoprotein enzymes (92, 161, 162). Because increased expression of NOX1, and not NOX2, has been shown in response to 6-OHDA and rotenone PD toxins in N27 cells (20, 147), this study examined both NOX1 and NOX2. NOX1 was examined by using a NOX1-specific inhibitor, ML171, which does not affect NOX2 activity (94, 163). ML171, an unsubstituted phenothiazine has shown very high potency in blocking NOX1-dependent reactive oxygen species generation (94). Contrary to observations with apocynin, this selective NOX1 inhibitor only partially inhibited testosterone’s negative actions in an OS environment in N27 cells. These results indicate that another NOX subunit is involved, such as NOX2, that also complexes with AR45. Indeed, selective NOX2 inhibition by GSK2795039 partially blocked testosterone-induced cell loss in an OS environment. Although NOX2 is generally associated with astrocytes and microglia (16, 131, 164, 165), NOX2 is expressed in dopaminergic neurons (20, 140) and can influence OS (144). Unlike apocynin that is orally bioavailable (166–168), in vivo use of NOX2 inhibitors is limited due to the poor oral bioavailability and the need for intravenous administration (163).
Because there has been a lack of success of mitigating mitochondrial-associated OS in clinical trials (169), NOX inhibitors may be potential therapeutics for PD. The NOX1, NOX4, and NOX5 inhibitor, GKT137831, is orally bioavailable and well tolerated in humans. Furthermore, this is the only NOX inhibitor that has progressed into clinical trials with >170 patients (170, 171). Apocynin has also been proposed as a possible therapeutic. Using the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine PD model, apocynin was shown to protect dopaminergic neurons and improve motor function in nonhuman primates (172). Although apocynin has not been tested in patients with neurodegeneration, it decreased OS production in asthmatic patients (173). These preclinical and clinical studies appear promising. Because NOX1 and NOX2 complex with AR45, NOX inhibition may be a potential therapeutic to slow PD progression in men or in postmenopausal women who exhibit an androgenic hormone profile compared with premenopausal women (174, 175).
In conclusion, this study provides evidence that (i) AR45 located in lipid rafts complexes with NOX1, NOX2, and Gαq; (ii) Gαq does not complex with NOX1 and NOX2; (iii) androgen’s negative effects are mediated via AR45 and NOX; (iv) Gαq does not mediate testosterone’s negative effects; and (v) InsP3R is involved in testosterone-induced neurodegeneration (Fig. 8). The findings of this study help identify key players (e.g., mAR, NOX, and InsP3R-mediated Ca2+) in testosterone-induced neurodegeneration that could serve as potential therapeutic targets for PD.
Figure 8.
Working model. Membrane AR (i.e., AR45) resides in a lipid raft within the plasma membrane and complexes with NOX1, NOX2, and Gαq. Testosterone can bind and activate AR45, which in turn stimulates NOX1 and NOX2, resulting in OS generation. Alternatively, the AR45–NOX complex can upregulate InsP3R activity to increase intracellular calcium release, resulting in increased OS. Dysregulation of this pathway can lead to neurotoxicity, such as during PD. The AR45–Gαq pathway is not involved in testosterone-induced cell loss in OS environments. PLC, Phospholipase C.
Acknowledgments
We thank Dr. Brina Snyder, Dr. George Farmer, Dr. J. Thomas Cunningham, and Nataliya Rybalchenko for technical assistance.
Financial Support: This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant R01 NS0091359 (to R.L.C).
Author Contributions: M.A.A.T. has full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 6-OHDA
6-hydroxydopamine
- AR
androgen receptor
- DHT-BSA
DHT/3-O-carboxymethyloxime/BSA
- DMSO
dimethyl sulfoxide
- DPI
diphenyleneiodonium chloride
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPCR
G protein–coupled receptor
- InsP3R
inositol trisphosphate receptor
- mAR
membrane-associated androgen receptor
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide
- NADPH
reduced form of NAD phosphate
- NOX
reduced form of NAD phosphate oxidase
- OS
oxidative stress
- PD
Parkinson disease
- TBST
Tris-buffered saline with Tween 20
- TH
tyrosine hydroxylase
References
- 1. Sherer TB, Chowdhury S, Peabody K, Brooks DW. Overcoming obstacles in Parkinson’s disease. Mov Disord. 2012;27(13):1606–1611. [DOI] [PubMed] [Google Scholar]
- 2. Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. [DOI] [PubMed] [Google Scholar]
- 3. Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56(4):532–539. [DOI] [PubMed] [Google Scholar]
- 4. Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet. 2014;384(9942):532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlossmacher MG, Tomlinson JJ, Santos G, Shutinoski B, Brown EG, Manuel D, Mestre T. Modelling idiopathic Parkinson disease as a complex illness can inform incidence rate in healthy adults: the PREDIGT score. Eur J Neurosci. 2017;45(1):175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad Sci USA. 1998;95(13):7659–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S, Souza JM, Jackson-Lewis V, Lee VM, Ischiropoulos H. Oxidative post-translational modifications of α-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Neurochem. 2001;76(2):637–640. [DOI] [PubMed] [Google Scholar]
- 9. Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. [DOI] [PubMed] [Google Scholar]
- 10. Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. J Comp Neurol. 1996;366(4):580–599. [DOI] [PubMed] [Google Scholar]
- 11. German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331(3):297–309. [DOI] [PubMed] [Google Scholar]
- 12. Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152(4):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res. 2003;5(3):165–176. [DOI] [PubMed] [Google Scholar]
- 14. Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med Okayama. 2008;62(3):141–150. [DOI] [PubMed] [Google Scholar]
- 15. Isaacs KR, Wolpoe ME, Jacobowitz DM. Vulnerability to calcium-induced neurotoxicity in cultured neurons expressing calretinin. Exp Neurol. 2000;163(2):311–323. [DOI] [PubMed] [Google Scholar]
- 16. Hernandes MS, Café-Mendes CC, Britto LR. NADPH oxidase and the degeneration of dopaminergic neurons in parkinsonian mice. Oxid Med Cell Longev. 2013;2013:157857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10(6):453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104(2):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aguado A, Fischer T, Rodríguez C, Manea A, Martínez-González J, Touyz RM, Hernanz R, Alonso MJ, Dixon DA, Briones AM, Salaices M. Hu antigen R is required for NOX-1 but not NOX-4 regulation by inflammatory stimuli in vascular smooth muscle cells. J Hypertens. 2016;34(2):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi DH, Cristóvão AC, Guhathakurta S, Lee J, Joh TH, Beal MF, Kim YS. NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson’s disease. Antioxid Redox Signal. 2012;16(10):1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rastogi R, Geng X, Li F, Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci. 2017;10:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cristóvão AC, Choi DH, Baltazar G, Beal MF, Kim YS. The role of NADPH oxidase 1–derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal. 2009;11(9):2105–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pal R, Bajaj L, Sharma J, Palmieri M, Di Ronza A, Lotfi P, Chaudhury A, Neilson J, Sardiello M, Rodney GG. NADPH oxidase promotes Parkinsonian phenotypes by impairing autophagic flux in an mTORC1-independent fashion in a cellular model of Parkinson’s disease. Sci Rep. 2016;6(1):22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Putney JW., Jr Excitement about calcium signaling in inexcitable cells. Science. 1993;262(5134):676–678. [DOI] [PubMed] [Google Scholar]
- 25. Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268(5208):239–247. [DOI] [PubMed] [Google Scholar]
- 26. Sée V, Loeffler JP. Oxidative stress induces neuronal death by recruiting a protease and phosphatase-gated mechanism. J Biol Chem. 2001;276(37):35049–35059. [DOI] [PubMed] [Google Scholar]
- 27. Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27(5):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid β peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742(1–3):81–87. [DOI] [PubMed] [Google Scholar]
- 29. Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787(11):1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hidalgo C. Cross talk between Ca2+ and redox signalling cascades in muscle and neurons through the combined activation of ryanodine receptors/Ca2+ release channels. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aarts MM, Tymianski M. TRPM7 and ischemic CNS injury. Neuroscientist. 2005;11(2):116–123. [DOI] [PubMed] [Google Scholar]
- 32. Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7(9-10):1140–1149. [DOI] [PubMed] [Google Scholar]
- 33. Addabbo F, Montagnani M, Goligorsky MS. Mitochondria and reactive oxygen species. Hypertension. 2009;53(6):885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lubomski M, Louise Rushworth R, Lee W, Bertram KL, Williams DR. Sex differences in Parkinson’s disease. J Clin Neurosci. 2014;21(9):1503–1506. [DOI] [PubMed] [Google Scholar]
- 35. Gillies G, Pienaar I, Vohra S, Qamhawi Z.. Sex differences in Parkinson’s disease. Front Neuroendocrinol. 2014;35(3):370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. Neurology. 2000;55(9):1358–1363. [DOI] [PubMed] [Google Scholar]
- 37. Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. [DOI] [PubMed] [Google Scholar]
- 38. Mayeux R, Marder K, Cote LJ, Denaro J, Hemenegildo N, Mejia H, Tang MX, Lantigua R, Wilder D, Gurland B, Hauser A. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142(8):820–827. [DOI] [PubMed] [Google Scholar]
- 39. Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75(4):637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55(1):25–31. [DOI] [PubMed] [Google Scholar]
- 41. Wong PS, Randall MD, Roberts RE. Sex differences in the role of NADPH oxidases in endothelium-dependent vasorelaxation in porcine isolated coronary arteries. Vascul Pharmacol. 2015;72:83–92. [DOI] [PubMed] [Google Scholar]
- 42. Barp J, Araujo AS, Fernandes TR, Rigatto KV, Llesuy S, Bello-Klein A, Singal P. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz J Med Biol Res. 2002;35(9):1075–1081. [DOI] [PubMed] [Google Scholar]
- 43. Xie W, Parker JL, Heaps CL. Effect of exercise training on nitric oxide and superoxide/H2O2 signaling pathways in collateral-dependent porcine coronary arterioles. J Appl Physiol (1985). 2012;112(9):1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38(7):2142–2149. [DOI] [PubMed] [Google Scholar]
- 45. Tenkorang MA, Snyder B, Cunningham RL. Sex-related differences in oxidative stress and neurodegeneration. Steroids. 2018;133:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Regan JC, Partridge L. Gender and longevity: why do men die earlier than women? Comparative and experimental evidence. Best Pract Res Clin Endocrinol Metab. 2013;27(4):467–479. [DOI] [PubMed] [Google Scholar]
- 47. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23(6):1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate β-amyloid levels in male rat brain. J Neurochem. 2003;87(4):1052–1055. [DOI] [PubMed] [Google Scholar]
- 49. Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122(3):573–578. [DOI] [PubMed] [Google Scholar]
- 50. Yang SH, Liu R, Wen Y, Perez E, Cutright J, Brun-Zinkernagel A-M, Singh M, Day AL, Simpkins JW. Neuroendocrine mechanism for tolerance to cerebral ischemia-reperfusion injury in male rats. J Neurobiol. 2005;62(3):341–351. [DOI] [PubMed] [Google Scholar]
- 51. Mitchell E, Thomas D, Burnet R. Testosterone improves motor function in Parkinson’s disease. J Clin Neurosci. 2006;13(1):133–136. [DOI] [PubMed] [Google Scholar]
- 52. Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30(2):239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflammation. 2014;11(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paoletti AM, Congia S, Lello S, Tedde D, Orrù M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62(2):301–303. [DOI] [PubMed] [Google Scholar]
- 55. Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62(2):188–193. [DOI] [PubMed] [Google Scholar]
- 56. Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281(35):25492–25501. [DOI] [PubMed] [Google Scholar]
- 57. Lopes RA, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, Chignalia AZ, Valim YM, Silveira LR, Curti C, Tostes RC. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol. 2014;306(11):H1485–H1494. [DOI] [PubMed] [Google Scholar]
- 58. Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154(11):4281–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Snyder B, Duong P, Trieu J, Cunningham RL. Androgens modulate chronic intermittent hypoxia effects on brain and behavior. Horm Behav. 2018;106:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Holmes S, Singh M, Su C, Cunningham RL. Effects of oxidative stress and testosterone on pro-inflammatory signaling in a female rat dopaminergic neuronal cell line. Endocrinology. 2016;157(7):2824–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Snyder B, Duong P, Tenkorang M, Wilson EN, Cunningham RL. Rat strain and housing conditions alter oxidative stress and hormone responses to chronic intermittent hypoxia. Front Physiol. 2018;9:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5(9):e13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilson EN, Anderson M, Snyder B, Duong P, Trieu J, Schreihofer DA, Cunningham RL. Chronic intermittent hypoxia induces hormonal and male sexual behavioral changes: hypoxia as an advancer of aging. Physiol Behav. 2018;189:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cunningham RL, Macheda T, Watts LT, Poteet E, Singh M, Roberts JL, Giuffrida A. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Horm Behav. 2011;60(5):617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11(4):1285–1291. [DOI] [PubMed] [Google Scholar]
- 66. Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–262. [DOI] [PubMed] [Google Scholar]
- 67. Son SW, Lee JS, Kim HG, Kim DW, Ahn YC, Son CG. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J Neurochem. 2016;136(1):106–117. [DOI] [PubMed] [Google Scholar]
- 68. Zhang L, Wu S, Ruan Y, Hong L, Xing X, Lai W. Testosterone suppresses oxidative stress via androgen receptor-independent pathway in murine cardiomyocytes. Mol Med Rep. 2011;4(6):1183–1188. [DOI] [PubMed] [Google Scholar]
- 69. Cunningham RL, Giuffrida A, Roberts JL. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cδ. Endocrinology. 2009;150(12):5539–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Garza-Contreras J, Duong P, Snyder BD, Schreihofer DA, Cunningham RL. Presence of androgen receptor variant in neuronal lipid rafts. eNeuro. 2017;4(4):ENEURO.0109-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.RRID:AB_2282705, https://scicrunch.org/resolver/AB_2282705.
- 72.RRID:AB_2261483, https://scicrunch.org/resolver/AB_2261483.
- 73.RRID:AB_631746, https://scicrunch.org/resolver/AB_631746.
- 74.RRID:AB_631736, https://scicrunch.org/resolver/AB_631736.
- 75.RRID:AB_630864, https://scicrunch.org/resolver/AB_630864.
- 76.RRID:AB_10842057, https://scicrunch.org/resolver/AB_10842057.
- 77.RRID:AB_11174761, https://scicrunch.org/resolver/AB_11174761.
- 78.RRID:CVCL_D584, https://scicrunch.org/resolver/CVCL_D584.
- 79. Anantharam V, Lehrmann E, Kanthasamy A, Yang Y, Banerjee P, Becker KG, Freed WJ, Kanthasamy AG. Microarray analysis of oxidative stress regulated genes in mesencephalic dopaminergic neuronal cells: relevance to oxidative damage in Parkinson’s disease. Neurochem Int. 2007;50(6):834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Carvour M, Song C, Kaul S, Anantharam V, Kanthasamy A, Kanthasamy A. Chronic low-dose oxidative stress induces caspase-3-dependent PKCδ proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann N Y Acad Sci. 2008;1139(1):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clarkson ED, Edwards-Prasad J, Freed CR, Prasad KN. Immortalized dopamine neurons: a model to study neurotoxicity and neuroprotection. Proc Soc Exp Biol Med. 1999;222(2):157–163. [DOI] [PubMed] [Google Scholar]
- 82. Biswas R, Vonderhaar BK. Role of serum in the prolactin responsiveness of MCF-7 human breast cancer cells in long-term tissue culture. Cancer Res. 1987;47(13):3509–3514. [PubMed] [Google Scholar]
- 83. Ahrens-Fath I, Politz O, Geserick C, Haendler B. Androgen receptor function is modulated by the tissue-specific AR45 variant. FEBS J. 2005;272(1):74–84. [DOI] [PubMed] [Google Scholar]
- 84. Rathkopf D, Scher HI. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer J. 2013;19(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, Pang ST, Li L, Arai Y, Kung HJ, Yeh S, Chang C. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM. Specificity of ligand-dependent androgen receptor stabilization: receptor domain interactions influence ligand dissociation and receptor stability. Mol Endocrinol. 1995;9(2):208–218. [DOI] [PubMed] [Google Scholar]
- 88. Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA. Dihydrotestosterone: biochemistry, physiology, and clinical implications of elevated blood levels. Endocr Rev. 2017;38(3):220–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu J, Holstein JD, Upadhyay G, Lin DT, Conway S, Muller E, Lechleiter JD. Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. J Neurosci. 2007;27(24):6510–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gatto GJ Jr, Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem. 2013;28(1):95–104. [DOI] [PubMed] [Google Scholar]
- 91. Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab. 2008;9(8):686–696. [DOI] [PubMed] [Google Scholar]
- 93. Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol. 2011;164(3):866–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol. 2010;5(10):981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015;23(5):406–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14(4):840–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Myhrstad MCW, Carlsen H, Nordström O, Blomhoff R, Moskaug JØ. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32(5):386–393. [DOI] [PubMed] [Google Scholar]
- 98. Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, de Lourdes Bastos M. Antioxidant activity of Hypericum androsaemum infusion: scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull. 2002;25(10):1320–1323. [DOI] [PubMed] [Google Scholar]
- 99. Schrag A, Ben-Shlomo Y, Quinn NP. Cross sectional prevalence survey of idiopathic Parkinson’s disease and Parkinsonism in London. BMJ. 2000;321(7252):21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jeong H, Kim MS, Kwon J, Kim KS, Seol W. Regulation of the transcriptional activity of the tyrosine hydroxylase gene by androgen receptor. Neurosci Lett. 2006;396(1):57–61. [DOI] [PubMed] [Google Scholar]
- 101. Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89(3):939–954. [DOI] [PubMed] [Google Scholar]
- 102. Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379(2):247–260. [DOI] [PubMed] [Google Scholar]
- 103. Elaut E, Buysse A, De Sutter P, De Cuypere G, Gerris J, Deschepper E, T’Sjoen G. Relation of androgen receptor sensitivity and mood to sexual desire in hormonal contraception users. Contraception. 2012;85(5):470–479. [DOI] [PubMed] [Google Scholar]
- 104. Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105(1):53–68. [DOI] [PubMed] [Google Scholar]
- 105. Donovan KA, Walker LM, Wassersug RJ, Thompson LMA, Robinson JW. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer. 2015;121(24):4286–4299. [DOI] [PubMed] [Google Scholar]
- 106. Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 107. Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18(1):27–36. [DOI] [PubMed] [Google Scholar]
- 108. Edinger KL, Frye CA. Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-β in the hippocampus. Neurobiol Learn Mem. 2007;87(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl. 2003;24(4):568–576. [DOI] [PubMed] [Google Scholar]
- 110. Bronner G, Vodušek DB. Management of sexual dysfunction in Parkinson’s disease. Ther Adv Neurol Disorder. 2011;4(6):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hand A, Gray WK, Chandler BJ, Walker RW. Sexual and relationship dysfunction in people with Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(3):172–176. [DOI] [PubMed] [Google Scholar]
- 112. Okun MS, Fernandez HH, Rodriguez RL, Romrell J, Suelter M, Munson S, Louis ED, Mulligan T, Foster PS, Shenal BV, Armaghani SJ, Jacobson C, Wu S, Crucian G. Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol. 2006;63(5):729–735. [DOI] [PubMed] [Google Scholar]
- 113. Mitchell E, Thomas D, Burnet R. Testosterone improves motor function in Parkinson’s disease. J Clin Neurosci. 2006;13(1):133–136. [DOI] [PubMed] [Google Scholar]
- 114. Okun MS, Walter BL, McDonald WM, Tenover JL, Green J, Juncos JL, DeLong MR. Beneficial effects of testosterone replacement for the nonmotor symptoms of Parkinson disease. Arch Neurol. 2002;59(11):1750–1753. [DOI] [PubMed] [Google Scholar]
- 115. Maharjan S, Serova L, Sabban EL. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors α and β and interactions with cyclic AMP. J Neurochem. 2005;93(6):1502–1514. [DOI] [PubMed] [Google Scholar]
- 116. Maharjan S, Serova LI, Sabban EL. Membrane-initiated estradiol signaling increases tyrosine hydroxylase promoter activity with ERα in PC12 cells. J Neurochem. 2010;112(1):42–55. [DOI] [PubMed] [Google Scholar]
- 117. Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson’s disease symptom profile. Acta Neurol Scand. 2000;102(1):37–43. [DOI] [PubMed] [Google Scholar]
- 118. Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. An examination of male-female differences in progression and mortality of Parkinson’s disease. Neurology. 1990;40(5):763–766. [DOI] [PubMed] [Google Scholar]
- 120. Sato SM, Johansen JA, Jordan CL, Wood RI. Membrane androgen receptors may mediate androgen reinforcement. Psychoneuroendocrinology. 2010;35(7):1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29(2):169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lucas-Herald AK, Alves-Lopes R, Montezano AC, Ahmed SF, Touyz RM. Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications. Clin Sci (London). 2017;131(13):1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Marras C, Gruneir A, Rochon P, Wang X, Anderson G, Brotchie J, Bell CM, Fox S, Austin PC. Dihydropyridine calcium channel blockers and the progression of parkinsonism. Ann Neurol. 2012;71(3):362–369. [DOI] [PubMed] [Google Scholar]
- 124. Pasternak B, Svanström H, Nielsen NM, Fugger L, Melbye M, Hviid A. Use of calcium channel blockers and Parkinson’s disease. Am J Epidemiol. 2012;175(7):627–635. [DOI] [PubMed] [Google Scholar]
- 125. Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann Neurol. 2010;67(5):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gudala K, Kanukula R, Bansal D. Reduced risk of Parkinson’s disease in users of calcium channel blockers: a meta-analysis. Int J Chronic Dis. 2015;2015:697404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lee YC, Lin CH, Wu RM, Lin JW, Chang CH, Lai MS. Antihypertensive agents and risk of Parkinson’s disease: a nationwide cohort study. PLoS One. 2014;9(6):e98961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Becker C, Jick SS, Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurology. 2008;70(16 Pt 2):1438–1444. [DOI] [PubMed] [Google Scholar]
- 129. Biglan KM, Oakes D, Lang AE, Hauser RA, Hodgeman K, Greco B, Lowell J, Rockhill R, Shoulson I, Venuto C, Young D, Simuni T; Parkinson Study Group STEADY‐PD III Investigators. A novel design of a phase III trial of isradipine in early Parkinson disease (STEADY-PD III). Ann Clin Transl Neurol. 2017;4(6):360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191(6):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100(10):6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rodriguez-Pallares J, Parga JA, Muñoz A, Rey P, Guerra MJ, Labandeira-Garcia JL. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem. 2007;103(1):145–156. [DOI] [PubMed] [Google Scholar]
- 133. Skonieczna M, Hejmo T, Poterala-Hejmo A, Cieslar-Pobuda A, Buldak RJ. NADPH oxidases: insights into selected functions and mechanisms of action in cancer and stem cells. Oxid Med Cell Longev. 2017;2017:9420539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang YJ, Wei XY, Jing XQ, Chang YL, Hu CH, Wang X, Chen KM. The fundamental role of NOX family proteins in plant immunity and their regulation. Int J Mol Sci. 2016;17(6):E805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47(9):1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ibi M, Katsuyama M, Fan C, Iwata K, Nishinaka T, Yokoyama T, Yabe-Nishimura C. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic Biol Med. 2006;40(10):1785–1795. [DOI] [PubMed] [Google Scholar]
- 137. Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20(1):RC53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kim MJ, Shin KS, Chung YB, Jung KW, Cha CI, Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040(1–2):178–186. [DOI] [PubMed] [Google Scholar]
- 139. Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132(2):233–238. [DOI] [PubMed] [Google Scholar]
- 140. Zawada WM, Mrak RE, Biedermann J, Palmer QD, Gentleman SM, Aboud O, Griffin WST. Loss of angiotensin II receptor expression in dopamine neurons in Parkinson’s disease correlates with pathological progression and is accompanied by increases in Nox4- and 8-OH guanosine-related nucleic acid oxidation and caspase-3 activation. Acta Neuropathol Commun. 2015;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Choi DH, Lee KH, Kim JH, Seo JH, Kim HY, Shin CY, Han JS, Han SH, Kim YS, Lee J. NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal. 2014;21(4):533–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sharma H, Hirko AC, King MA, Liu B. Role of NADPH oxidase in cooperative reactive oxygen species generation in dopaminergic neurons induced by combined treatment with dieldrin and lindane. Toxicol Lett. 2018;299:47–55. [DOI] [PubMed] [Google Scholar]
- 143. Zawada WM, Banninger GP, Thornton J, Marriott B, Cantu D, Rachubinski AL, Das M, Griffin WS, Jones SM. Generation of reactive oxygen species in 1-methyl-4-phenylpyridinium (MPP+) treated dopaminergic neurons occurs as an NADPH oxidase-dependent two-wave cascade. J Neuroinflammation. 2011;8(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33(5):627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cristóvão AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi DH, Kim Y-S. NADPH oxidase 1 mediates α-synucleinopathy in Parkinson’s disease. J Neurosci. 2012;32(42):14465–14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Exp Neurol. 2014;258:112–120. [DOI] [PubMed] [Google Scholar]
- 147. Zhang FL, He Y, Zheng Y, Zhang WJ, Wang Q, Jia YJ, Song HL, An HT, Zhang HB, Qian YJ, Tong YL, Dong L, Wang XM. Therapeutic effects of fucoidan in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease: role of NADPH oxidase-1. CNS Neurosci Ther. 2014;20(12):1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sovari AA, Morita N, Karagueuzian HS. Apocynin: a potent NADPH oxidase inhibitor for the management of atrial fibrillation. Redox Rep. 2008;13(6):242–245. [DOI] [PubMed] [Google Scholar]
- 149. Massart C, Giusti N, Beauwens R, Dumont JE, Miot F, Sande JV. Diphenyleneiodonium, an inhibitor of NOXes and DUOXes, is also an iodide-specific transporter. FEBS Open Bio. 2013;4(1):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang Q, Chu CH, Oyarzabal E, Jiang L, Chen SH, Wilson B, Qian L, Hong JS. Subpicomolar diphenyleneiodonium inhibits microglial NADPH oxidase with high specificity and shows great potential as a therapeutic agent for neurodegenerative diseases. Glia. 2014;62(12):2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Qian L, Gao X, Pei Z, Wu X, Block M, Wilson B, Hong JS, Flood PM. NADPH oxidase inhibitor DPI is neuroprotective at femtomolar concentrations through inhibition of microglia over-activation. Parkinsonism Relat Disord. 2007;13(Suppl 3):S316–S320. [DOI] [PubMed] [Google Scholar]
- 152. Langley M, Ghosh A, Charli A, Sarkar S, Ay M, Luo J, Zielonka J, Brenza T, Bennett B, Jin H, Ghaisas S, Schlichtmann B, Kim D, Anantharam V, Kanthasamy A, Narasimhan B, Kalyanaraman B, Kanthasamy AG. Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in MitoPark transgenic mice. Antioxid Redox Signal. 2017;27(14):1048–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Simonyi A, Serfozo P, Lehmidi TM, Cui J, Gu Z, Lubahn DB, Sun AY, Sun GY. The neuroprotective effects of apocynin. Front Biosci (Elite Ed). 2012;4(6):2183–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414(3):228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.’t Hart BA, Copray S, Philippens I. Apocynin, a low molecular oral treatment for neurodegenerative disease. BioMed Res Int. 2014;2014:298020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Riganti C, Gazzano E, Polimeni M, Costamagna C, Bosia A, Ghigo D. Diphenyleneiodonium inhibits the cell redox metabolism and induces oxidative stress. J Biol Chem. 2004;279(46):47726–47731. [DOI] [PubMed] [Google Scholar]
- 157. Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. DPI induces mitochondrial superoxide-mediated apoptosis. Free Radic Biol Med. 2003;34(4):465–477. [DOI] [PubMed] [Google Scholar]
- 158. Yu J, Weïwer M, Linhardt RJ, Dordick JS. The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr Vasc Pharmacol. 2008;6(3):204–217. [DOI] [PubMed] [Google Scholar]
- 159. Sharma N, Kapoor M, Nehru B. Apocyanin, NADPH oxidase inhibitor prevents lipopolysaccharide induced α-synuclein aggregation and ameliorates motor function deficits in rats: possible role of biochemical and inflammatory alterations. Behav Brain Res. 2016;296:177–190. [DOI] [PubMed] [Google Scholar]
- 160. Davies MJ. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem J. 1989;257(2):603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Maraldi T. Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev. 2013;2013:271602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chéret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a Nox1-dependent NADPH oxidase. J Neurosci. 2008;28(46):12039–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, Ramachandran S, Bridges A, Chaudry L, Pettman G, Allan C, Duncan S, Lee KC, Lim J, Ma MT, Ong AB, Ye NY, Nasir S, Mulyanidewi S, Aw CC, Oon PP, Liao S, Li D, Johns DG, Miller ND, Davies CH, Browne ER, Matsuoka Y, Chen DW, Jaquet V, Rutter AR. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid Redox Signal. 2015;23(5):358–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25(40):9176–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zhang W, Gao JH, Yan ZF, Huang XY, Guo P, Sun L, Liu Z, Hu Y, Zuo LJ, Yu SY, Cao CJ, Wang XM, Hong JS. Minimally toxic dose of lipopolysaccharide and α-synuclein oligomer elicit synergistic dopaminergic neurodegeneration: role and mechanism of microglial NOX2 activation. Mol Neurobiol. 2018;55(1):619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lull ME, Levesque S, Surace MJ, Block ML. Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)SL mice. PLoS One. 2011;6(5):e20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, van den Berg WB, van Beuningen HM, Smit HF. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur J Pharmacol. 2006;531(1–3):264–269. [DOI] [PubMed] [Google Scholar]
- 168. ’t Hart BA, Simons JM, Knaan-Shanzer S, Bakker NP, Labadie RP. Antiarthritic activity of the newly developed neutrophil oxidative burst antagonist apocynin. Free Radic Biol Med. 1990;9(2):127–131. [DOI] [PubMed] [Google Scholar]
- 169. Schapira AH. Targeting mitochondria for neuroprotection in Parkinson’s disease. Antioxid Redox Signal. 2012;16(9):965–973. [DOI] [PubMed] [Google Scholar]
- 170. Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56(6):2316–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Teixeira G, Szyndralewiez C, Molango S, Carnesecchi S, Heitz F, Wiesel P, Wood JM. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br J Pharmacol. 2017;174(12):1647–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 2000;273(1):5–9. [DOI] [PubMed] [Google Scholar]
- 173. Peters EA, Hiltermann JT, Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med. 2001;31(11):1442–1447. [DOI] [PubMed] [Google Scholar]
- 174. Santoro N, Randolph JF Jr. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am. 2011;38(3):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ala-Fossi SL, Mäenpää J, Aine R, Punnonen R. Ovarian testosterone secretion during perimenopause. Maturitas. 1998;29(3):239–245. [DOI] [PubMed] [Google Scholar]