Abstract
Background:
Inequality in health outcomes in relation to Americans’ socioeconomic position is rising.
Objectives:
First, to evaluate the spatial relationship between neighborhood disadvantage and major atherosclerotic cardiovascular disease (ASCVD)-related events; and second, to evaluate the relative extent to which neighborhood disadvantage and physiological risk account for neighborhood-level variation in ASCVD event rates.
Design:
Observational cohort analysis of geocoded longitudinal electronic health records.
Setting:
A single academic health center and surrounding neighborhoods in Northeast Ohio.
Patients:
109,793 Cleveland Clinic Health System (CCHS) patients who had had an outpatient lipid panel drawn between 2007 and 2010. The date of the first qualifying lipid panel served as study baseline.
Measurements:
Time from baseline to the first occurrence of a major ASCVD event (myocardial infarction, stroke, or cardiovascular death) within 5 years, modeled as a function of 1) a locally-derived neighborhood disadvantage index (NDI) and 2) the predicted 5-year ASCVD event rate from the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Model (PCERM). Outcome data were censored if there were no CCHS encounters for two consecutive years or when state death data were no longer available (i.e., 2015 onward).
Results:
The PCERM systematically under-predicted ASCVD event risk among patients from disadvantaged communities. Model discrimination was poorer among these patients (concordance index [95% confidence interval]: 0.70 [0.67 – 0.74]) than among patients from the most affluent communities (0.80 [0.78 – 0.81]). The NDI alone accounted for 32.0% of census-tract-level variation in ASCVD event rates, compared to 10.0% accounted for by the PCERM.
Limitation:
Patients from affluent communities were over-represented. Outcomes of patients treated for cardiovascular disease diagnoses at Cleveland Clinic were assumed to be independent of whether patients came from a disadvantaged or affluent neighborhood.
Conclusions:
Neighborhood disadvantage may be a powerful regulator of ASCVD event risk. In addition to supplemental risk models and clinical screening criteria, population-based solutions to ameliorating the deleterious effects of neighborhood disadvantage on health outcomes are needed.
Americans who reach age 50 and who are in the lowest decile of career earnings now live over a decade less than their counterparts in the highest decile.[1] Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of death for most Americans. Even modest reductions in cardiovascular health disparities have the potential to substantially improve the health and well-being of socioeconomically challenged populations.
Accurate risk assessment of atherosclerotic cardiovascular disease (ASCVD)-related events such as myocardial infarction and stroke is important in identifying high risk patients and appropriately applying interventions.
Such risk varies by race and socioeconomic position (SEP) but, with the exception of the UK population, SEP is not generally considered in cardiovascular risk assessment.[2] In 2014, the American College of Cardiology/American Heart Association Task Force on Practice Guidelines released the Pooled Cohort Equations Risk Model (PCERM) for 10-year atherosclerotic cardiovascular disease (ASCVD) risk.[3] The PCERM was based on data from several large, racially and geographically diverse cohort studies, and modeled risk of major ASCVD events (defined as nonfatal myocardial infarction, death due to coronary heart disease, or stroke). While the goal of the PCERM was to establish more demographically representative models for ASCVD events, it did not incorporate variation in risk directly related to SEP.
We sought to evaluate relationships between neighborhood socioeconomic conditions, clinical assessments of atherosclerotic risk and major ASCVD events in a large observational cohort derived from routinely-collected electronic health data. Specifically, we sought to: quantify predictive accuracy of the PCERM with respect to neighborhood SEP; and characterize the extent to which the PCERM and neighborhood SEP account for local variation in ASCVD event rates.
METHODS
Data Sources and Study Inclusion Criteria
With approval from the Cleveland Clinic Institutional Review Board, we analyzed data pertaining to all Cleveland Clinic Health System (CCHS) patients who stated they were of Caucasian or African American race, who had at least one outpatient lipid panel performed between January 1, 2007 and December 31, 2010; and who, on the date of the first such blood draw (which we classified as the study baseline time point, T0), were over 35 years old and resided in one of 21 Northeast Ohio counties. The restriction on race was necessary due to the fact that the PCERM is applicable only to Caucasians and African Americans. Patients with Post Office boxes or patients who otherwise had missing or inaccurate information on place of residence (including individuals who were documented as being homeless) were excluded from the analysis*.
Patients with a history of myocardial infarction (International Classification of Diseases and Injuries, 9th Revision, Clinical Modification, ICD-9-CM: 410.xx); stroke (ICD-9-CM: 434.9x); heart valve disorder (U.S. Agency for Healthcare Research and Quality’s Clinical Classifications Software†; single-level diagnosis category #96‡); or pericarditis, endocarditis, myocarditis, or cardiomyopathy (U.S. Agency for Healthcare Research and Quality’s Clinical Classifications Software single-level diagnosis category #97) prior to T0 were excluded. Variables used in generating the estimate of 5-year risk of major ASCVD events from the PCERM were sex, age, race, diabetes mellitus, smoking, total cholesterol, high-density lipoprotein cholesterol, and systolic blood pressure, which we defined at baseline as the median of all measurements taken within 3 months of T0. Patients with missing baseline data necessary for PCERM calculation were excluded.
All clinical data were extracted from CCHS electronic medical records via Structured Query Language programs. Patients’ locations of residence were geocoded and matched to environmental characteristics tied to the census tract in which they lived at T0. Census tract-level variables were extracted from the U.S. Census Bureau’s website§.
Study Variables
Our primary time-to-event outcome, incident major ASCVD event, was defined as the first occurrence of myocardial infarction, stroke, or cardiovascular death subsequent to T0. Myocardial infarction and stroke were defined using ICD-9-CM diagnosis codes (details above) from all CCHS encounters during the follow-up period, and cardiovascular death was defined based on International Classification of Diseases and Injuries, 10th Revision (ICD-10) cause of death codes I00-I79, which were obtained from the Ohio Department of Health**. Outcome data were considered to be censored at the earlier of i) the start date of any contiguous two-year time period with no CCHS encounters, ii) the date of non-cardiac death or death due to an unspecified cause, and iii) January 1, 2014 for any patient with T0 after January 1, 2010. (The third censoring condition was due to the fact that Ohio cause of death data were available only through 2013 at the time of our analysis.) The follow-up period for our study was 5 years.
The PCERM was originally published with respect to 10-year mortality risk. To obtain 5-year estimates from the PCERM, information on the baseline hazard function from the underlying Cox regression models is required. This baseline hazard function was not published; however, the authors of the PCERM did provide 5-year cumulative baseline hazard function estimates to Muntner et al. (2014)[4] for their validation study. In particular, the required formulas for computing 5-year PCERM risk estimates are published in an online supplement to the Muntner et al. article. We used those formulas instead of the original 10-year risk equations.
To analyze aspects of neighborhood SEP associated with patients’ location of residence at T0, we created a neighborhood disadvantage index (NDI). This index served as a single-factor representation of multiple variables that reflect neighborhood SEP, which we used to distribute and analyze risk associated with SEP within our particular sample. We derived the NDI as a specific measure of neighborhood disadvantage across Northeast Ohio.
The NDI was defined at the census tract level based on the following U.S. Census 2010 variables: percent white, non-Hispanic; percent with high school degree; percent with Medicaid, age 18–64; percent uninsured, age 18–64; median income; percent of households below federal poverty level; percent of children living in households receiving supplemental security income, cash public assistance income, or food stamps/SNAP benefits; and percent of households that are headed by an unmarried mother. We transformed these variables into their principal components, and used the first principal component as the NDI (i.e., a single-factor latent variable model). With the exception of race, all of the above characteristics are reflected in the Area Deprivation Index, a national index of neighborhood-level disadvantage based on 2000 U.S. Census data.
Statistical Analysis
We assessed prognostic accuracy of the PCERM-estimated 5-year ASCVD event rates within subgroups of patients defined according to selected quantiles of the NDI. Discrimination was assessed using the concordance index for censored outcomes.[5] Calibration was assessed visually, by comparing observed 5-year ASCVD event rates against those predicted by the PCERM within progressive risk strata i.e., patients with predicted risk <2.5%, patients with predicted risk between 2.5% and 5.0%, patients with predicted risk between 5.0% and 7.5%, etc. (We selected these risk thresholds so that they corresponded with the American College of Cardiology/American Heart Association guidelines for treatment of blood cholesterol.[6]) Observed event rates with respect to this calibration analysis were obtained from Kaplan-Meier curves.
Census tract-level ASCVD event rates were estimated under the Bayesian framework using the integrated nested Laplace approximation procedure by Rue et al.[7] as implemented in the R package INLA.[8] This allowed for implementation of a conditional autoregressive Weibull time-to-event model which incorporated the Besag-York-Mollié covariance structure.[9, 10] This model can be thought of as a spatial analogue of the more common autoregressive time series model, because it incorporates correlation among estimates (in our case, hazard ratio estimates for ASCVD) for neighboring geographical areas (census tracts) the same way the time series model allows for correlation among neighboring time points.
We began with a null model (Model 1) consisting of only random effects for each census tract (characterized using the the Besag-York-Mollié structure). Fixed effects were added to this model – namely, PCERM-estimated risk and/or NDI. We estimated a model which added the PCERM-estimated 5-year probability of major ASCVD events to the null model (Model 2), a model which added a fixed effect for the NDI to the null model (Model 3), and a model which included fixed effects for both PCERM risk and NDI (Model 4). In comparing two models (e.g., Model 3 vs. Model 1), the degree by which the random effect estimates from the model (i.e., census tract-level log-hazard ratio estimates after adjustment for any fixed effects) are reduced by adding the covariate is the amount of spatial variation accounted for by that covariate. Details of these calculations are given in Appendix 1.
RESULTS
Of 125,449 unique patients living in Northeast Ohio who had a qualifying outpatient lipid panel between 2007 and 2010 and who were aged 35+ on the date of that lipid panel, 15,153 were excluded due to medical history (n=3,473 with history of myocardial infarction, n=1,852 with history of stroke, n=9,178 with history of heart valve disorders, and n=2,761 with history of pericarditis, endocarditis, myocarditis, or cardiomyopathy). After excluding an additional 503 with missing baseline data on required elements of the PCERM, our final sample consisted of 109,793 unique patients.
The NDI accounted for 65.2% of the variability in the eight census-tract-level indicators. The formula for the NDI is given in Appendix 2. Figure 1 displays the spatial distribution of the NDI across Northeast Ohio. Relative to persons in low-NDI neighborhoods, individuals living in greater-NDI neighborhoods at baseline were more likely to be female; were more likely to be black; had slightly higher average blood pressure; were more likely to have diabetes; were more likely to have been prescribed antihypertensive medication or statins; were more likely to have coronary artery disease and peripheral vascular disease; and had higher 5-year predicted ASCVD event risk as defined by the PCERM (Table 1).
Figure 1:
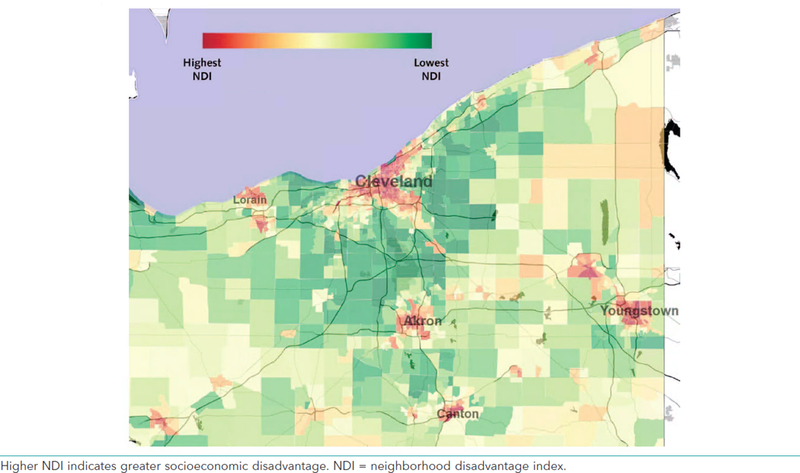
Distribution of neighborhood disadvantage index (NDI, defined at the census tract-level) across Northeast Ohio. Higher NDI indicates greater socioeconomic disadvantage.
Table 1:
Baseline characteristics of 109,793 patients included in the analysis, stratified by neighborhood disadvantage index (NDI) levels. Summary statistics are presented as prevalence (as a percentage of the patients in a given NDI group), mean ± standard deviation, or median [first quartile, third quartile].
| Neighborhood Disadvantage Associated with Patients’ Location of Residence at Baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| Highest 5% (Least Affluent) |
95–90% | 90–75% | 75–25% | 25–10% | 10–5% | Lowest 5% (Most Affluent) |
||
| N = 1,961 | N = 2,019 | N = 7,474 | N = 41,199 | N = 23,631 | N = 14,072 | N = 19,437 | ||
| Race/Sex | Black Female | 54.3 | 49.4 | 38.6 | 7.5 | 2.3 | 1.8 | 1.3 |
| Black Male | 31.4 | 29.4 | 23.3 | 4.8 | 1.8 | 1.3 | 1.1 | |
| White Female | 8.2 | 11.6 | 21.3 | 48.1 | 51.6 | 50.1 | 50.7 | |
| White Male | 6.2 | 9.6 | 16.8 | 39.5 | 44.3 | 46.7 | 46.9 | |
| Age (years) | 57 ± 13 | 56 ± 13 | 56 ± 13 | 56 ± 13 | 56 ± 13 | 56 ± 12 | 56 ± 12 | |
| Systolic BP (mmHg) | 132 ± 16 | 131 ± 17 | 130 ± 16 | 127 ± 15 | 125 ± 15 | 124 ± 14 | 123 ± 14 | |
| Diastolic BP (mmHg) | 79 ± 10 | 79 ± 11 | 79 ± 10 | 77 ± 9 | 77 ± 9 | 77 ± 9 | 76 ± 9 | |
| Total Cholesterol (mg/dL) | 194 [166, 224] | 195 [166, 224] | 194 [168, 224] | 196 [170, 224] | 197 [171, 224] | 197 [172, 224] | 198 [172, 223] | |
| HDL Cholesterol (mg/dL) | 54 [44, 66] | 53 [43, 65] | 53 [43, 64] | 51 [42, 62] | 53 [43, 65] | 54 [44, 66] | 55 [45, 67] | |
| LDL Cholesterol (mg/dL) | 113 [90, 142] | 114 [91, 138] | 113 [91, 140] | 115 [93, 140] | 115 [93, 139] | 115 [93, 139] | 115 [93, 138] | |
| Triglycerides (mg/dL) | 99 [72, 143] | 103 [73, 148] | 106 [75, 154] | 115 [80, 169] | 111 [77, 161] | 108 [76, 158] | 105 [74, 153] | |
| VLDL Cholesterol (mg/dL) | 20 [14, 28] | 20 [14, 29] | 21 [15, 30] | 23 [16, 33] | 22 [15, 32] | 21 [15, 32] | 21 [15, 30] | |
| Antihypertensive Prescribed | 67.2 | 65.0 | 62.2 | 50.6 | 46.3 | 41.9 | 39.1 | |
| Statin Prescribed | 37.9 | 37.9 | 36.8 | 34.6 | 34.6 | 32.3 | 32.0 | |
| Type II DM | No | 70.4 | 70.0 | 74.4 | 83.1 | 87.1 | 89.2 | 91.1 |
| Uncomplicated | 0.8 | 0.6 | 0.7 | 0.9 | 0.7 | 0.8 | 0.5 | |
| With Complications | 28.8 | 29.4 | 24.9 | 16.1 | 12.2 | 10.0 | 8.4 | |
| Smoking | 28.4 | 26.9 | 23.3 | 16.6 | 12.5 | 11.3 | 9.0 | |
| Coronary Artery Disease | 8.5 | 7.0 | 7.8 | 7.1 | 6.5 | 5.6 | 5.9 | |
| Peripheral Vascular Disease | 5.8 | 4.3 | 4.1 | 2.8 | 2.2 | 2.0 | 2.0 | |
| Family History of CVD | 8.5 | 10.2 | 9.2 | 9.6 | 11.1 | 11.9 | 11.7 | |
| Family History of DM | 8.0 | 8.5 | 7.6 | 4.9 | 4.8 | 4.4 | 4.3 | |
|
Primary Source of Payment |
Private/Managed Care | 39.2 | 41.9 | 44.7 | 53.6 | 57.3 | 60.3 | 61.8 |
| Medicare | 44.4 | 43.8 | 43.7 | 41.2 | 40 | 37.1 | 36.3 | |
| Medicaid | 11.1 | 8.9 | 7.7 | 2.8 | 1.3 | 0.9 | 0.6 | |
| Military | 0.6 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.4 | |
| Self-pay/Other | 4.8 | 5.2 | 3.5 | 1.9 | 1.0 | 1.1 | 0.8 | |
|
ACC/AHA PCERM 5-year Probability of Major ASCVD Events† (%) |
||||||||
| Overall | 4.1 [1.5, 9.0] | 3.8 [1.3, 8.7] | 3.4 [1.2, 7.8] | 2.2 [0.7, 6.1] | 1.9 [0.6, 5.5] | 1.6 [0.5, 4.7] | 1.5 [0.5, 4.6] | |
| Among Black Females | 3.3 [1.1, 7.8] | 3.1 [0.9, 7.9] | 2.9 [0.8, 6.9] | 1.7 [0.4, 5.0] | 1.3 [0.3, 4.0] | 1.5 [0.5, 4.2] | 1.5 [0.3, 3.7] | |
| Among Black Males | 6.3 [3.1, 11.1] | 6.4 [3.2, 11.0] | 5.8 [2.9, 10.2] | 4.7 [2.2, 8.7] | 4.4 [2.2, 8.5] | 3.6 [1.8, 6.8] | 4.9 [2.2, 8.5] | |
| Among White Females | 1.5 [0.5, 4.6] | 1.3 [0.5, 3.4] | 1.5 [0.5, 4.5] | 1.1 [0.4, 3.6] | 0.9 [0.3, 3.0] | 0.8 [0.3, 2.5] | 0.7 [0.2, 2.3] | |
| Among White Males | 4.5 [1.9, 9.8] | 4.5 [1.7, 8.9] | 4.0 [1.7, 8.7] | 3.7 [1.5, 8.4] | 3.3 [1.4, 7.8] | 3.0 [1.2, 6.6] | 2.8 [1.2, 6.5] | |
BP = blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; VLDL = very low-density lipoprotein; DM = diabetes mellitus;CVD = cardiovascular disease; ACC/AHA = American College of Cardiology/American Heart Association; PCERM = pooled cohort equations risk model; ASCVD = atherosclerotic cardiovascular disease; ACC/AHA = American College of Cardiology/American Heart Association
Major ASCVD events are defined as any of the following: myocardial infarction, stroke and death due to cardiovascular disease.
Across the six strata defined according to the NDI, stroke was the most commonly observed event, followed by acute myocardial infarction and finally cardiovascular death (see Table 2). The most common cause of censoring was unavailability of death data from the State of Ohio beyond January 1, 2014.
Table 2:
Summary of observed outcomes and censoring types, stratified by neighborhood disadvantage index (NDI) levels.
| Neighborhood Disadvantage Associated with Patients’ Location of Residence at Baseline | |||||||
|---|---|---|---|---|---|---|---|
| Highest 5% (Least Affluent) |
5–10% | 10–25% | 25–75% | 75–90% | 90–95% | Lowest 5% (Most Affluent) |
|
| Patient Outcome | N = 1,961 | N = 2,019 | N = 7,474 | N = 41,199 | N = 23,631 | N = 14,072 | N = 19,437 |
| 5-year follow-up without ASCVD event | 721 (36.8%) | 731 (36.2%) | 2,903 (38.8%) | 19,257 (46.7%) | 11,751 (49.7%) | 6,772 (48.1%) | 9,909 (51.0%) |
| AMI | 41 (2.1%) | 53 (2.6%) | 184 (2.5%) | 673 (1.6%) | 327 (1.4%) | 163 (1.2%) | 235 (1.2%) |
| Stroke | 94 (4.8%) | 96 (4.8%) | 319 (4.3%) | 941 (2.3%) | 514 (2.2%) | 277 (2.0%) | 364 (1.9%) |
| Cardiovascular Death | 19 (1.0%) | 27 (1.3%) | 60 (0.8%) | 265 (0.6%) | 138 (0.6%) | 63 (0.4%) | 80 (0.4%) |
| Censored at death date due to non-cardiac death | 49 (2.5%) | 39 (1.9%) | 145 (1.9%) | 730 (1.8%) | 360 (1.5%) | 171 (1.2%) | 215 (1.1%) |
| Censored at death date due to unknown cause of death | 1 (0.1%) | 5 (0.2%) | 21 (0.3%) | 70 (0.1%) | 28 (0.1%) | 26 (0.2%) | 20 (0.1%) |
| Censored at January 1, 2014 due to unavailability of Ohio death data | 596 (30.4%) | 612 (30.3%) | 2,394 (32.0%) | 12,134 (29.5%) | 7,162 (30.3%) | 4,556 (32.4%) | 6,058 (31.2%) |
| Censored at the beginning of a 2-year contiguous interval without health system encounters | 440 (22.4%) | 456 (22.6%) | 1,448 (19.4%) | 7,129 (17.3%) | 3,351 (14.2%) | 2,044 (14.5%) | 2,556 (13.2%) |
Performance of the PCERM with respect to discrimination and calibration is given in Figure 2. The PCERM discriminated events from non-events reasonably well among patients from affluent communities – with an estimated concordance index (C) and 95% confidence interval of 0.80 [0.78, 0.81] for the lowest (most affluent) 10% of communities with respect to the NDI – while discrimination was poorer among socioeconomicaly challenged neighborhoods (C = 0.70 [0.67, 0.74] for the highest 10% of communities). Calibration performance was good among patients from affluent communities, while the PCERM systematically under-estimated risk among patients from socioeconomicaly challenged communities.
Figure 2:
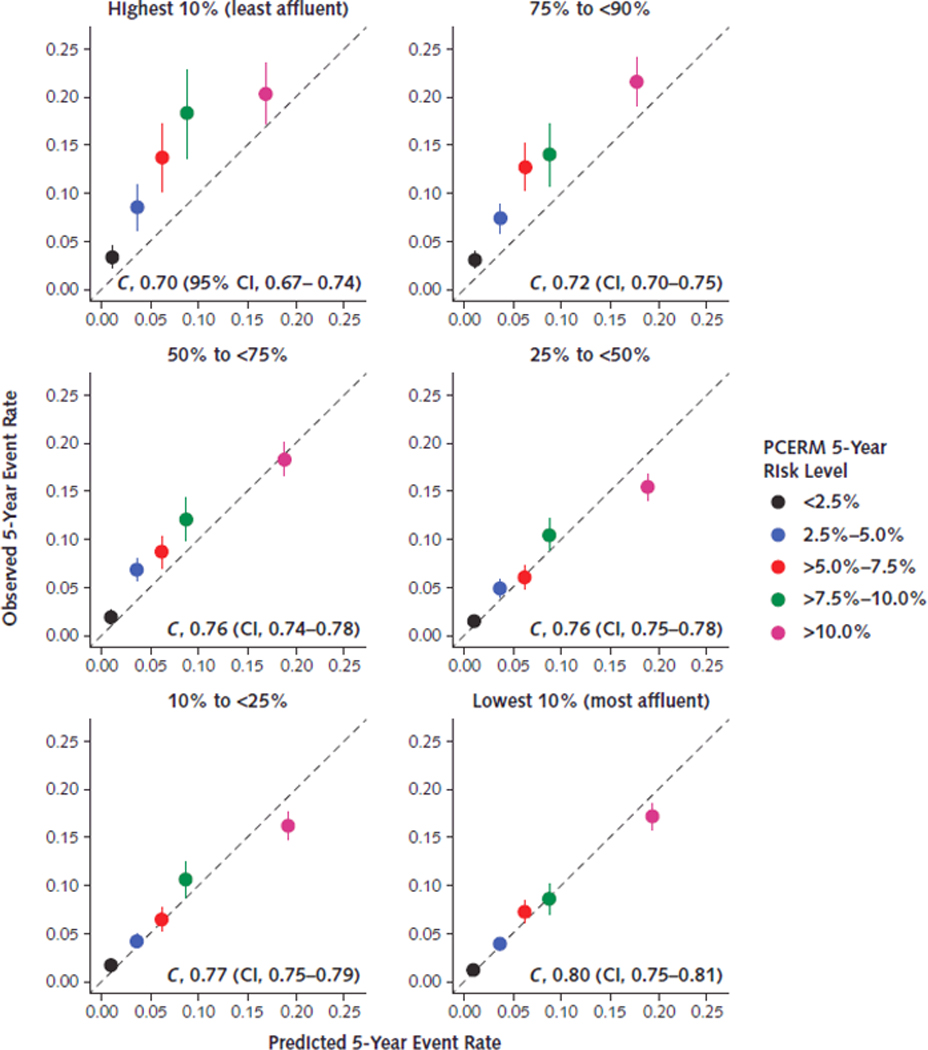
Prognostic accuracy of the PCERM across strata defined according to percentile groups of the neighborhood disadvantage index (highest percentiles correspond to the least affluent communities). Perfect calibration of the PCERM is represented along the line y = x; points above this line indicate under-estimation of risk by the PCERM in relation to observed event rates, and points below this line indicate over-estimation of risk. Concordance indices (C) and corresponding 95% confidence intervals are displayed within each panel. The concordance index ranges from 0.5 to 1.0, where a value of 0.5 represents no discrimination of events from non-events and a value of 1.0 represents complete separation of outcomes. NDI = neighborhood disadvantage index; PCERM = Pooled Cohort Equations Risk Model.
Based on our null model without covariates (Model 1), we found substantial geographic variation in major ASCVD event rates (Figure 3) that largely corresponded with the distribution of the NDI. ASCVD hazard rates in inner-city Cleveland were over three times that observed among the most affluent suburbs. The PCERM (Model 2) accounted for 10.0% of census tract-level variability in ASCVD event rates, while the NDI (Model 3) accounted for 32.0%. Incrementally, the PCERM accounted for 6.9% of census tract-level variation beyond that of the NDI, for a total of 38.9% of variation accounted for by the two measures (Model 4). Census-tract-level hazard ratio estimates from Model 4 – the final model, which adjusted for both the PCERM and the NDI – are displayed in Figure 4.
Figure 3:
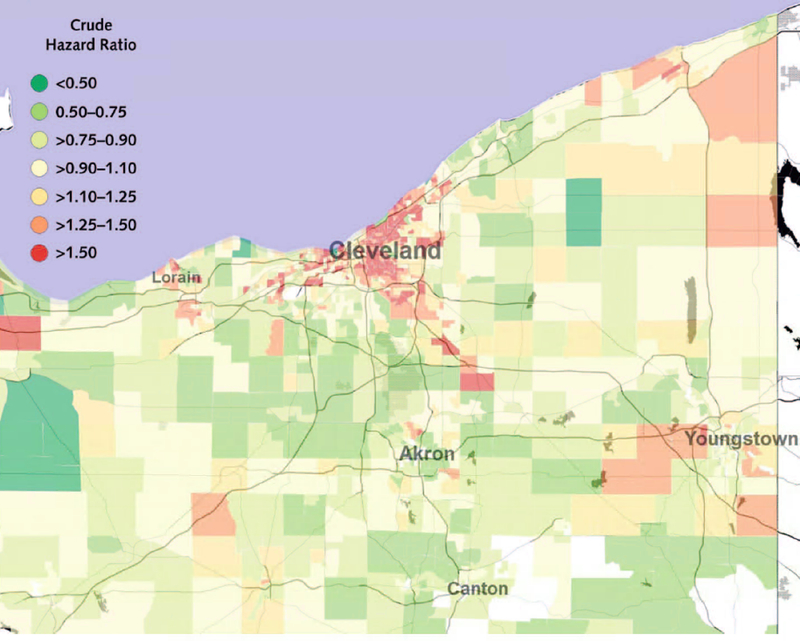
Hazard ratios for major atherosclerotic cardiovascular disease events (myocardial infarction, stroke, or cardiovascular death) across Northeast Ohio, from the null model without covariates (Model 1).
Figure 4:
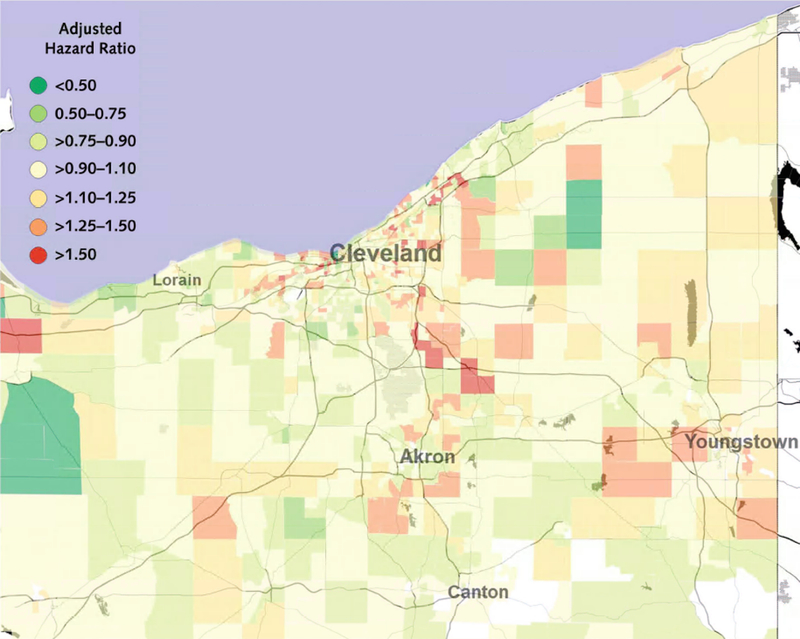
Hazard ratios for major atherosclerotic cardiovascular disease events (myocardial infarction, stroke, or cardiovascular death) across Northeast Ohio, from the model that adjusted for estimated 5-year risk from the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Model and our neighborhood socioeconomic status index (Model 4).
CONCLUSIONS
In this large, retrospective cohort study, we found that performance of the PCERM worsens among patients living in resource-challenged neighborhoods and that neighborhood disadvantage accounts for over three times the amount of geographic variability in major ASCVD event rates than one widely-accepted risk assessment tool for atherosclerotic disease (PCERM). Our study is not the first to evaluate performance of the PCERM per se, but it is the first to evaluate performance within a large, heterogeneous cohort of patients that is representative of routine care practices and the first to evaluate performance across the socioeconomic spectrum.
Current understanding of the determinants of ASCVD outcomes, for the most part, assumes that clinical indicators directly predict individual risk and can be used to inform clinical decisions. While it may be the case that the relationships between traditional risk factors and outcomes are different among individuals from socioeconomically challenged neighborhoods, an alternative or perhaps additional explanation is that this “clinical/physiological” model for understanding disease progression, risk and outcomes is incomplete. The PCERM is stratified by sex and race, but does not include a direct measure of SEP. Sex, race/ethnicity and SEP are intersecting, in that individuals can experience disadvantage in ways that are specific to distinct combinations of these three characteristics. [11]
The finding is compelling, especially when taken in the context of the immense challenges facing individuals living in disadvantaged neighborhoods. In addition to the personal challenges associated with impoverishment, these individuals face a variety of neighborhood-level stressors. Comparatively speaking, disadvantaged neighborhoods lack options for exercising (including limited access to parks, trails and sport and fitness facilities)[12] and use of available exercise facilities in these communities is negatively affected by lack of pedestrian access, litter, vandalism, homelessness and perceptions of lack of safety associated with high rates of violent crime.[13, 14] Moreover, healthy food is less available††and more costly[15] within low-SEP communities. Finally, individuals from disadvantaged communities may either not have access to or not seek quality preventative cardiovascular care.
All of these relationships, and perhaps others, may explain why the PCERM performed more poorly among patients from disadvantaged neighborhoods. In particular, the PCERM systematically under-estimated ASCVD event risk across the entire risk spectrum for patients from high-NDI neighborhoods. On the other end of the socioeconomic spectrum, calibration was much better, although we did observe slight over-estimates of risk among high-risk patients from affluent communities. Others have found that the PCERM may over- or underestimate risk depending on the subpopulation being evaluated [4, 16–18]; the present data suggests that this relationship varies according to neighborhood SEP. In particular, we observed slight over-estimates among patients from low-NDI neighborhoods and substantial under-estimates among patients from high-NDI neighborhoods.
There are at least three possibilities in terms of explaining why prediction performance of the PCERM might be poorer among patients from disadvantaged communities. First, there might be variation in the nature of the relationships between clinical risk factors and ASCVD outcomes across the socioeconomic spectrum. Second, there might be environmental and other neighborhood-level exposures (e.g., resource-poor schools, sources of chronic stress, noise, air pollution, heavy metals, etc.) that are external to the model and that differentially impact individuals from disadvantaged communities. Third, certain individual exposures (epigenetic changes, untoward prenatal exposures and serious mental illness) might be more prevalent among people from disadvantaged communities than among people from more affluent communities.
Our results indicating PCERM miscalibration by neighborhood SEP have implications for performance assessment: to the extent that an institutions’ (or physicians’) case mixes are differentially oriented toward either end of the NDI spectrum, expected event rates are biased. Efforts to incentivize health systems to improve population health – such as the U.S. Center for Medicare & Medicaid Innovation’s Million Hearts® Cardiovascular Disease Risk Reduction Model[19, 20], which assigns reimbursement rates for preventative cardiovascular services based on the PCERM – may inappropriately lead to penalizing certain providers and hospitals that manage the health of socioeconomically challenged populations.
We created a new index of neighborhood disadvantage, based upon available data from the US Census that are relevant to the challenges faced across the spectrum of socioeconomic status in Northeast Ohio. Similar in nature to other indices such as the Area Deprivation Index[21] or the CDC’s Social Vulnerability Index[22], our measure includes disadvantages due to poverty, family structure, health insurance coverage and segregation. Although those indices are more commonly used in spatial analyses of neighborhood disadvantage, we felt that a locally-derived index would allow for relationships among neighborhood indicators that might be unique to Northeast Ohio.
A limitation of our analysis is that, given our use of an index as opposed to specific measures of each of these social/environmental determinants, we are unable to identify and articulate possible simple causative pathways and the contributions of specific factors in the current analysis. To begin to understand the relative contribution of race/ethnicity and neighborhood disadvantage, however, we conducted a post-hoc analysis of the disparity in event rates (i.e., hazard ratio) between African Americans and whites, adjusting for PCERM-estimated risk and/or the NDI (Appendix 3). We found that the NDI played a larger role in accounting for the racial disparity in event rates than the PCERM, and that African Americans had increased event rates even after adjusting for these factors. Further work is necessary to evaluate how race, ethnicity and neighborhood factors combine to produce health disadvantage.
Since our study involved routinely-collected electronic health data, its results may be vulnerable to certain forms of sampling bias. The analyzed study cohort contained ten times as many patients from the top 5% of census tracts (with respect to the NDI) than it did from the bottom 5%. Also, it contained many more patients from the Cleveland metropolitan area than it did from outlying communities. More comprehensive data will be needed to identify whether finding increased ASCVD event rates in more distant low-SEP communities (such as those in Akron, Youngstown, Warren, and Canton) was due to sampling bias as opposed to actual differences in risk patterns. Patients from low-SEP neighborhoods were more likely to be censored due to lack of CCHS encounters (e.g., leaving the health system or not seeking medical care) for at least 2 years. Given that censored individuals were similarly healthy to individuals who were not censored (see Appendices 4 and 5), we believe that any net effect of differential censoring on our findings would likely be directional in nature: complete ascertainment of outcomes would have only caused the observed miscalibration of the PCERM among patients from disadvantaged communities to be amplified. Finally, we did not analyze the effect of residential mobility or other temporal phenomena.[11] Finally, our study assumes that outcomes of patients treated for cardiovascular disease diagnoses at Cleveland Clinic were independent from the patients’ respective NDI values.
In summary, neighborhood socioeconomic position appears to be an important determinant[23] of PCERM accuracy. Efforts to enhance risk prediction by incorporating aspects of neighborhood socioeconomic position and by discerning its system effects on individuals are needed. Such efforts are particularly important in the context of health disparities in ASCVD, whereby the mechanisms involved in progression of ASCVD may qualitatively differ among subpopulations defined according to social strata. In addition to supplemental risk models and clinical screening criteria, it is necessary to collectively work toward grass-root and policy-oriented approaches that ameliorate the deleterious effects of neighborhood conditions on health outcomes.
Supplementary Material
Acknowledgments
Role of the Funding Source
This work was supported by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440, from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
FUNDING SOURCE
This work was supported by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Reproducible Research Statement:
Study protocol and statistical code: Available from Dr. Dalton. Data set: Limited data may be available under strict conditions; send inquires to Dr. Dalton.
In general, approximately 0.3% of the electronic health records within the Cleveland Clinic Health System have address information that cannot be geocoded.
HCUP CCS. Healthcare Cost and Utilization Project (HCUP). March 2016. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 2016–03-30.
Two diagnoses were excluded from the U.S. Agency for Healthcare Research and Quality’s Clinical Classifications Software diagnosis code list for heart valve disorder: 785.2 (undiagnosed cardiac murmurs) and 785.3 (other abnormal heart sounds).
United States Census Bureau. American FactFinder. URL: http://factfinder.census.gov. Accessed 2016–01-13.
The Ohio Department of Health receives certificates for all deaths occurring in the state and also for deaths to Ohio residents which occurred outside the state. Details are given on the Ohio Department of Health’s website: http://www.odh.ohio.gov/en/healthstats/vitalstats/deathstat.
Economic Research Service (ERS), U.S. Department of Agriculture (USDA). Food Environment Atlas. URL: http://www.ers.usda.gov/data-products/food-environment-atlas.aspx. Accessed 2016–04-29
All Authors: No conflicts of interest
REFERENCES
- 1.Bosworth B, Burtless G, and Zhang K, Later Retirement, Inequality in Old Age, and the Growing Gap in Longevity Between Rich and Poor. 2016, Washington, DC: Brookings Institution. [Google Scholar]
- 2.Stringhini S, et al. , Socioeconomic status and the 25× 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1· 7 million men and women. The Lancet, 2017. 389(10075): p. 1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff JDC, et al. , 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 2014. 63(25b): p. 2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P, et al. , Validation of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations. Journal of the American Medical Association, 2014. 311(14): p. 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrell F, Regression Modeling Strategies: with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2015: Springer. [Google Scholar]
- 6.Stone NJ, et al. , 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation, 2014. 129(25 suppl 2): p. S1–S45. [DOI] [PubMed] [Google Scholar]
- 7.Rue H, Martino S, and Chopin N, Approximate Bayesian inference for Latent Gaussian Models by using Integrated Nested Laplace Approximations. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 2009. 71(2): p. 319–392. [Google Scholar]
- 8.Lindgren F and Rue H, Bayesian Spatial Modelling with R-INLA. Journal of Statistical Software, 2015. 63(19). [Google Scholar]
- 9.Besag J, York J, and Mollié A, Bayesian Image Restoration, with Two Applications in Spatial Statistics. Annals of the Institute of Statistical Mathematics, 1991. 43(1): p. 1–20. [Google Scholar]
- 10.Blangiardo M, et al. , Spatial and spatio-temporal models with R-INLA. Spatial and spatio-temporal epidemiology, 2013. 7: p. 39–55. [DOI] [PubMed] [Google Scholar]
- 11.Schulz AJ and Mullings L, Gender, race, class, and health: Intersectional approaches. 2006, San Francisco, CA: Jossey-Bass. [Google Scholar]
- 12.Estabrooks PA, Lee RE, and Gyurcsik NC, Resources for Physical Activity Participation: Does Availability and Accessibility Differ by Neighborhood Socioeconomic Status? Annals of Behavioral Medicine, 2003. 25(2): p. 100–104. [DOI] [PubMed] [Google Scholar]
- 13.McCormack GR, et al. , Characteristics of Urban Parks Associated with Park Use and Physical Activity: a Review of Qualitative Research. Health Place, 2010. 16(4): p. 712–26. [DOI] [PubMed] [Google Scholar]
- 14.Griffin SF, et al. , Physical Activity Influences in a Disadvantaged African American Community and the Communities’ Proposed Solutions. Health Promotion Practice, 2008. 9(2): p. 180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball K, Timperio A, and Crawford D, Neighbourhood Socioeconomic Inequalities in Food Access and Affordability. Health & Place, 2009. 15(2): p. 578–585. [DOI] [PubMed] [Google Scholar]
- 16.Cook NR and Ridker PM, Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA internal medicine, 2014. 174(12): p. 1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFilippis AP, et al. , An Analysis of Calibration and Discrimination among Multiple Cardiovascular Risk Scores in a Modern Multiethnic Cohort. Annals of Internal Medicine, 2015. 162(4): p. 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFilippis AP, et al. , Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. European Heart Journal, 2017. 38(8): p. 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frieden TR and Berwick DM, The “Million Hearts” initiative--preventing heart attacks and strokes. N Engl J Med, 2011. 365(13): p. e27. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, et al. , Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool. Journal of the American College of Cardiology, 2017. 135(13): p. e793–e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh GK, Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health, 2003. 93(7): p. 1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flanagan BE, et al. , A social vulnerability index for disaster management. Journal of Homeland Security and Emergency, 2011. 8(1): p. Article 3. [Google Scholar]
- 23.Glass TA and McAtee MJ, Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med, 2006. 62(7): p. 1650–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


