Abstract
In 2012, cancer affected 14.1 million people worldwide and was responsible for 8.2 million deaths. The disease predominantly affects aged populations and is one of the leading causes of death in most western countries. In tumors, the aggressive growth of the neoplastic cell population and associated overexpression of pro-angiogenic factors lead to the development of disorganized blood vessel networks that are structurally and functionally different from normal vasculature. A disorganized labyrinth of vessels that are immature, tortuous and hyperpermeable typifies tumor vasculature. Functionally, the ability of the tumor vasculature to deliver nutrients and remove waste products is severely diminished. A critical consequence of the inadequate vascular networks in solid tumors is the development of regions of hypoxia [low oxygen tensions typically defined as oxygen tensions (pO2 values) < 10 mm Hg]. Tumor cells existing in such hypoxic environments have long been known to be resistant to anticancer therapy, display an aggressive phenotype, and promote tumor progression and dissemination. This review discusses the physiological basis of hypoxia, methods of detection, and strategies to overcome the resulting therapy resistance.
The Oxygen Effect
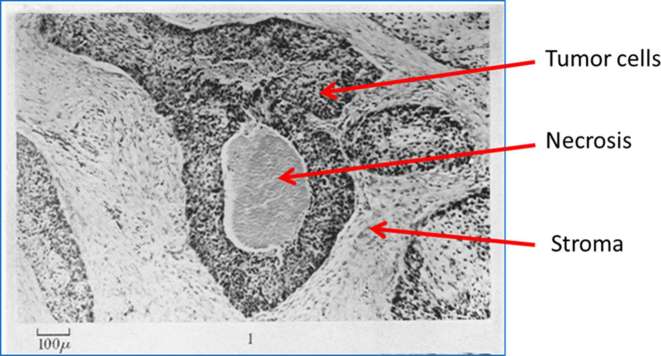
Radiation therapy has been used since the end of the 19th century as a means to control tumor burden, both with curative and palliative intent. Following the discovery in 1896 by German physicist Wilhelm Conrad Roentgen, X-rays were used for diagnosis, and within 3 years radiation was used to treat cancer. To test the status of the radiotherapy machine, radiologists would expose the skin of their arms to the instrument. The telltale burn would indicate the instrument was at the proper setting. Unfortunately, the frequent exposure to radiation resulted in many of these individuals developing leukemia. Interestingly, it was reported by Schwarz that clamping the skin to reduce blood flow to the arm decreased the skin irritation effects of ionizing radiation.1 However, it was not until the 1920 and 30s that the absence of oxygen was found to be the culprit behind the lack of radiation-induced damage.2 In the 1950s, Hal Gray’s research team explored the potential impact of oxygen in radiotherapy in vitro and in vivo.3–5 The landmark publication of Thomlinson and Gray, which evaluated histological structures of human lung cancers (Figure 1), concluded that, “there must exist a falling gradient in oxygen tension between the periphery and the centre of each tumour cord”.6 These findings implied that tumor cells near the limit of oxygen diffusion would survive at lower than normal oxygen tensions, rendering them resistant to radiation therapy.
Figure 1.
Fixed section of a human squamous cell carcinoma of the bronchus stained by hematoxylin and eosin. Modified with permission from Thomlinson and Gray.6
Initially, evidence of the impact of oxygen on radiation sensitivity was discovered in 1956, utilizing experiments that exposed Escherichia coli to radiation while in hypoxic conditions.7 The same radiation-enhancing effect of oxygen was soon observed in mammalian cells, with the use of the clonogenic cell survival assay developed by Puck and Marcus.8 In the 1970s, Adams demonstrated that oxygen had to be present during or within submilliseconds after radiation damage in order to observe the “oxygen effect”.9 Radiation causes DNA damage in cells, most importantly double strand breaks, by both direct and indirect effects.10 In the presence of oxygen, free radical attack enhances radiation-induced DNA damage such that tumor cells lacking oxygen were found to be 2.5–3 times more resistant to radiation than their normoxic counterparts.10 This phenomenon occurred primarily for oxygen tensions < 20 mm Hg, with little additional radiation sensitizing effect at values > 20 mm Hg.11 The “oxygen effect” was not confined to tumor cells in tissue culture. Pre-clinical studies in mice12 demonstrated that even tumors as small as 0.6 mm3 may contain areas of hypoxia that are resistant to radiation treatment, and that such resistance could be mitigated by oxygen breathing prior to tumor irradiation.13 Indeed, it is now well-established that hypoxia is a common feature of most, if not all, experimental solid tumors, and that there exists a direct correlation between tumor cell survival and the level of hypoxia within a tumor.11, 14
Support for the hypothesis that oxygen-deficient cells in patient tumors could affect the ability to control malignant disease at the local treatment site comes from three types of radiotherapy investigations; (i) studies of physiological variables associated with hypoxia, (ii) the direct assessments of oxygenation in situ, and (iii) outcomes of hypoxia targeted therapeutic interventions.
Physiological Variables Associated with Hypoxia
Tumor oxygenation status was indirectly linked to radiosensitivity, when anemia was identified as a negative prognostic factor in cervical cancer patients receiving radiotherapy in the 1960s.15 Subsequently, hemoglobin levels were negatively associated with outcomes of patients receiving radiotherapy in a number of cancer types, including uterine cervix,16 head and neck,17 bronchus,18 bladder,19 and prostate.20 These observations coupled with the finding that anemic patients treated with radiotherapy responded favorably after receiving blood transfusions21 strongly supported the belief that oxygenation status influenced the tumor response to radiation treatment.
Measuring Hypoxia in Tumors
Early measurements of tumor oxygenation began in the late 1960s, using glass electrodes. Studies from this time demonstrated a relationship between tumor oxygenation and response to radiation therapy in both cervical and head and neck cancer.22, 23 The Eppendorf pO2 histograph, also referred to as the polarographic electrode, was introduced in the early 1990s and revolutionized in vivo oxygenation measurements.24 It quickly became the “gold-standard” in oxygen tension measurements because of its ability to directly measure tissue oxygen tensions as low as 1–2 mm Hg and, importantly, because hypoxic measurements made with the electrodes in tumor-bearing mice correlated with the tumor response to radiation.11 The Eppendorf electrode became widely used to assess oxygen tensions in head and neck,25 breast,24 cervical26 and prostate cancer.27 Importantly, several studies confirmed that patients whose tumors were characterized as hypoxic by electrode measurement made prior to radiotherapy, had worse outcomes (local tumor control and/or survival).28, 29
One shortcoming of the polarographic electrode method of determining oxygen tensions was that it was limited to accessible tumors. Even more concerning was the invasive nature of the procedure, which required insertion of the probe along multiple tracks in order to collect sufficient readings. Consequently, the procedure became unpopular with both patients and physicians. Electrode measurements of oxygen tensions in tumors fell out of favor and were largely discontinued in the early 2000s.
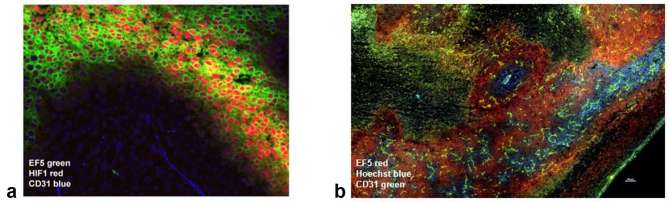
The most widely applied approach for hypoxic tumor cell detection has been the development of nitroimidazole hypoxic markers. Nitroheterocyclic agents, originally developed as radiation sensitizers, contain an RNO2 side chain that becomes rapidly reduced in the absence of oxygen30, 31 and binds to cellular adducts. Detection of these adducts permits quantification of hypoxia by histochemical analysis or flow cytometry.30 Nitroimidazole-based hypoxic markers, such as EF532 and pimonidazole hydrochloride,33 are capable of identifying regions of hypoxia in tumors (Figure 2) in preclinical30, 33,34 and clinical34, 35 evaluations.
Figure 2.
(a) Distribution of hypoxia, identified by the bioreductive nitroimidazole agent EF5 (green) and the endogenous hypoxia marker HIF-1 (red), in human cervical carcinoma (SiHa) xenograft with respect to blood vessel localization (CD31, blue). Image courtesy of D. Hedley. (b) Murine mammary carcinoma (4T1) after intravenous injection with Hoechst-33342 (40 mg kg–1, blue) as a surrogate marker for perfusion, and stained with EF5 (red) and CD31 (green). Please see the online version of this publication for colour images.
Metabolic markers of tumor hypoxia also have been assessed. Because expression of hypoxia inducible factors 1 and 2 (HIF-1 and HIF-2), Carbonic anhydrase IX (CA-IX), and glucose transporter-1 (GLUT-1) can correlate with areas of pimonidazole staining,36 these markers have been used to quantify low oxygen tensions.37 However, the expression of metabolic markers also can be triggered by factors other than hypoxia, including changes in pH, decreased levels of iron and other metabolites, or activation by endogenous ligands. Hence, the use of metabolic markers to identify tumor hypoxia has considerable limitations and consequently, they are more commonly used in pre-clinical assessments of hypoxia than in patient tumor evaluations.
A more recent development in the attempt to establish tumor oxygenation profiles is the determination of genomic signatures; a topic discussed in detail by West et al in this current issue of B r J R adiol. However, there are concerns. As of 2015, there were 32 different gene signatures in use, with no generally accepted “gold-standard” hypoxia signature.38 A further complicating issue is that the majority of genomic information was obtained from late-stage cancer patients. This raises the question of whether such information translates to early-stage patients treated with a curative intent. Furthermore, it is conceivable that the genome changes in time, as the tumor progresses and/or responds during the course of the treatment.
A significant impediment to the clinical application of many of the hypoxia detecting approaches has been the invasive nature of probes or the need to utilize biopsy samples to determine the extent of hypoxic marker expression. To address this issue, various nitroimidazole hypoxia markers have been developed for non-invasive detection methods that can determine spatial and temporal changes in oxygenation, such as PET (Figure 3), MRI, or CT imaging.39, 40 Recent results with non-invasive imaging are highly encouraging, particularly because they allow longitudinal evaluations of tumors throughout the course of therapy. However, these techniques are less precise than invasive techniques such as the oxygen probe,41 as PET, MRI and CT do not allow detailed image resolution at distances relevant to the oxygen diffusion distance (100 µm).
Figure 3.
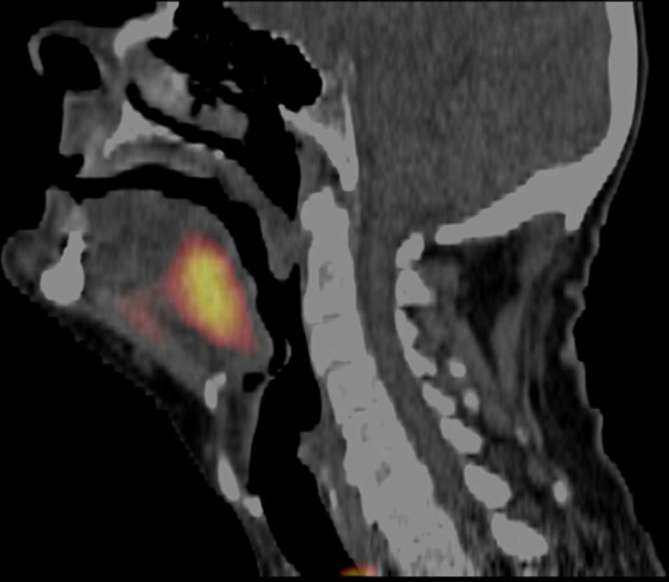
Patient with a FAZA PET positive oropharyngeal cancer. Reprinted with permission from Mortensen et al.39 FAZA is a PET tracer developed as a hypoxia identification method for preclinical and clinical application.39 FAZA, 18F-Fluoroazomycin arabinoside.
Heterogeneity is a major issue regardless of hypoxia measurement technique. Gene expression signatures are based on biopsies and therefore, are representative of a very small sampling of the tumor. Eppendorf electrode measurements do sample multiple locations to account for tumor heterogeneity; however, they also measure only a small part of the tumor unless multiple needle tracks are employed. Imaging techniques, while having the benefit of being non-invasive, have difficulty distinguishing between necrosis and hypoxia and are unable to measure fluctuating (acute) hypoxia. Furthermore, imaging resolution is too low to detect the small regions of hypoxia that frequently occur at the microenvironmental level.
While there has been a great deal of interest in methodologies designed to identify patients whose tumors may contain hypoxic cells, many of these methods show conflicting and inconsistent results when simultaneously assessed. For example, there are reports that demonstrate no correlation between oxygen electrode measurements and CAIX, GLUT-1 measurements, or pimonidazole staining.42, 43 Inconsistencies have also been observed between PET and oxygen electrode measurements41,42,44–46 and direct comparison between hypoxic signatures and other means of determining tumor oxygenation are lacking. Thus, many unsolved questions remain regarding when and how to measure tumor hypoxia.
Hypoxia and Patient Response to Radiotherapy
Despite the difficulties associated with determining the oxygenation status of tumors, it is clear from studies employing a range of methodologies that hypoxia exists in human tumors and that it can influence radiation response.47 Results have shown hypoxia to have a significant influence on both local tumor control and overall survival in a variety of tumor types.39,48–50 Interestingly, in cervical cancer patients who were treated with surgery or radiotherapy,28 all patients who had tumor pO2 values < 10 mm Hg (even those treated only with surgery) had a significantly lower overall and disease-free survival than patients whose tumors were better oxygenated.26 These findings not only corroborated in vitro data on the effect of hypoxia on radiation sensitivity, but also suggested a role for hypoxia in tumor aggressiveness. This belief was subsequently confirmed in prostate cancer studies in which hypoxia correlated with local recurrence and early biochemical relapse, supporting a role for hypoxia in radioresistance, increased aggressiveness, and enhanced metastatic potential.29, 51,52 Indeed, hypoxia now is considered an independent predictor of disease progression, treatment failures, and greater metastatic potential in sarcoma, cervix, breast, and prostate cancer.28,29,52–55
Chronic and Acute Hypoxia
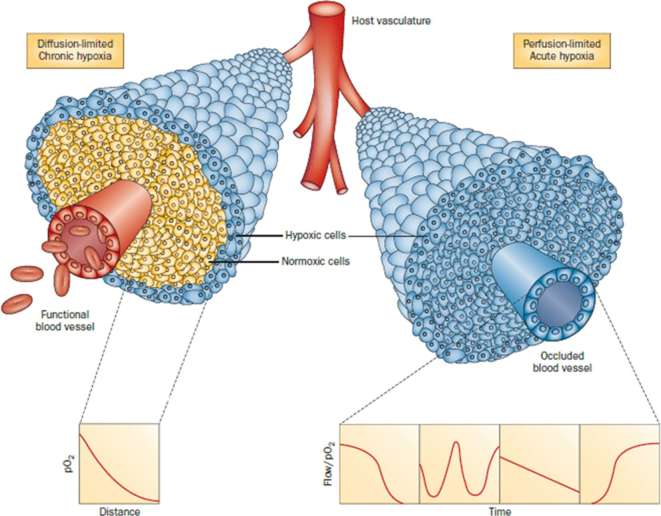
Hypoxia can be broadly classified as chronic or acute (Figure 4).56 Chronic hypoxia is a frequent characteristic of cancer cells residing at the limit of oxygen diffusion.6 This diffusion-limited hypoxia occurs as tumor cells proximal to the blood vessel metabolize available oxygen, leaving the distal cells in a state of oxygen deprivation.6, 47,57 Acute hypoxia58 is characterized as short-term oxygen deprivation due to transient fluctuations in blood flow that may occur when vessels are temporarily partially or totally occluded. Typically, tumor cells may experience acute hypoxia as relatively short hypoxic-oxic cycles lasting for several minutes to a few hours.47, 57 However, designating tumor hypoxia simply as acute or chronic is likely a significant oversimplification. Hypoxia in tumors is dynamic, varying temporally and in severity, and influenced by a variety of factors including blood flow variation, cellular oxygen consumption rates, and differences in inherent tumor cell sensitivities to oxygen deprivation.
Figure 4.
Illustration of tumor cells growing as a cord around blood vessels from which they obtain oxygen and nutrients. The left side illustrates oxygen diffusion and utilization from the vessel resulting in the development of chronically hypoxic cells at the outer edge of the cord. The right side shows perfusion through the vessel that has been transiently compromised and results in the development of acute hypoxia; examples of the types of flow/oxygen changes reported during a 60-min period are illustrated in the four panels below. Reprinted with permission from Horsman et al56.
Hypoxia influences many aspects of cancer biology through the activation of transcriptional factors, primarily members of the HIF family.59 In the presence of oxygen, HIF is quickly degraded.60 However, in low oxygen tensions, HIF stabilizes and translocates to the nucleus, where it upregulates the expression of a multitude of genes involved in the survival and escape from the hypoxic microenvironment. The phenotypic effects of HIF activation are broad, including epithelial-to-mesenchymal transition, resistance to therapy, enhanced invasive capacities, initiation of angiogenesis, potentiation of metastasis, and modulation of stemness.61, 62 As stemness is involved in cancer therapeutic failure and metastatic disease and the latter is the primary cause of cancer patient deaths,63 the consequences of tumor hypoxia on cancer therapy outcomes are significant.
Hypoxia also contributes directly to replication stress and genomic instability (for review see Bristow and Hill,64 Riffle and Hedge65). Under conditions of diminished oxygenation, DNA repair pathways including homologous recombination, non-homologous end joining, and mismatch repair are downregulated, leading to increased genetic instability.64, 66,67 Additionally, hypoxia contributes to microsatellite and chromosomal instability.68 In terms of radiosensitivity, chronically hypoxic tumor cells generally are more radiosensitive than acutely hypoxic cells;13, 69 a finding typically ascribed to changes in energy metabolism and suppression of DNA repair capacity under prolonged hypoxia.66, 70 Beyond the molecular, the cellular and functional consequences of tumor hypoxia also are strongly dependent on the degree and duration of hypoxia. HIF-1α regulates a large number of genes associated with tumor invasion and progression induced by short-term hypoxia, while HIF-2α expression during prolonged low oxygen tensions drive and maintain stemness.47, 71,72 Since both acute and chronic hypoxia can exist in solid tumors, the presence of one and/or the other and the relative extent to which each occurs, can significantly affect the functional consequences of oxygen deprivation. This is readily apparent when assessing their relative impact on a cancer cell's metastatic behavior. While some studies appear to favor chronic hypoxia as the key factor,73 others suggest that transient hypoxia mediates the dissemination of tumor cells.74, 75
Impact of Hypoxia on Immune Cells
Normally, the immune system is effective at recognizing and destroying abnormal cells within the body. However, tumors have the ability to circumvent destruction by the immune system by suppressing or evading immune cells. The field of cancer immunology has rapidly expanded over the last few years, leading to some very promising discoveries and therapeutics.
In order to maintain homeostasis and prevent an inappropriate response, immune checkpoint proteins regulate the function of immune cells. One method of immune escape utilized by the tumor cells involves suppressing immune cells by interaction with checkpoint proteins. Checkpoint inhibitors, such as CTLA4 or PD-1/PD-L1 inhibitors, have resulted in remarkable improvement in outcomes of patients with certain types of cancer, such as melanoma and lung cancer.76 Furthermore, combining radiation therapy with immunotherapy may offer a novel treatment strategy in the care of cancer patients.77 However, recent findings indicate that hypoxic regions in tumors may foster an immunosuppressive environment. PD-L1 is upregulated in response to hypoxia, and is a direct target of HIF-1.78 Hypoxic zones in tumors are found to be infiltrated with regulatory T-cells,79 myeloid-derived suppressor cells,78 and macrophages,80 cells which promote immune suppression and angiogenesis. HIF-1 is reported to be involved in macrophage-mediated inhibition of T cells. Taken together, current data support the notion that hypoxic microenvironments in tumors may negatively affect this emerging anticancer therapy.
Approaches to Modulate Tumor Oxygenation
The consequences of both chronic and acute hypoxia lead to therapeutic barriers that create a significant hindrance to the efficacy of radiotherapy as well as other conventional anticancer therapies, particularly those requiring blood-borne delivery.81 Thus, approaches to overcome hypoxia in tumors could increase sensitivity to anticancer therapies and improve overall survival. Initial studies focused on strategies that sought to directly improve oxygenation through methods including modulating blood flow, administering blood transfusions, or high oxygen content breathing. Others have taken advantage of the properties of hypoxic cells to target them with hypoxic cell sensitizers or bioreductive agents. Since high linear energy transfer (LET) radiations have low oxygen enhancement ratio (OER) values, the utilization of such particles in radiotherapy would reduce the relevance of tumor hypoxia. Alternatively, as hypoxic regions in tumors develop because of aberrant vascular networks, vascular targeting approaches, such as antiangiogenic (AAs) or vascular disrupting agents (VDAs), seek to improve tumor oxygenation through blood vessel normalization (AAs) or hypoxic cell elimination via induction of tumor cell death (VDAs). Furthermore, evidence exists that modifiable lifestyle behaviors may influence tumor hypoxia.
Radiation Dosing Schedule
In conventional fractionated radiotherapy, small radiation doses are given on a daily basis for periods of weeks or months. Such a treatment schedule may elicit the process of tumor reoxygenation,82 by which tumor cells that were resistant to radiation due to being hypoxic at the time of irradiation with a given dose become oxygenated and hence, sensitive to radiation when the next dose is given. Consequently, reoxygenation could reduce the impact of hypoxia on conventional fractionated radiotherapy outcomes. However, the extent to which tumors reoxygenate and the time course of reoxygenation is highly variable among tumors and thus likely to be insufficient to overcome the detriment of hypoxia. Recently, stereotactic body radiation therapy (SBRT), which employs high doses of radiation given in a single or a few treatment sessions, has become increasingly popular. The impact of SBRT on the relevance of tumor hypoxia as a determinant of treatment outcome remains somewhat controversial but pre-clinical studies and limited clinical findings suggest that tumor hypoxia is likely to be more important for SBRT than for conventional fractionation.83, 84 Furthermore, our aforementioned lack of knowledge of the relative contributions of acutely and chronically hypoxic cell fractions in tumors, coupled with the modulation of genomic instability and DNA repair capacities under varying hypoxic conditions, makes predicting the impact of reoxygenation exceedingly difficult.
Overcoming Anemia
Although anemic patients who received blood transfusions prior to radiotherapy had improved treatment outcomes, issues arose in treating cancer patients with this approach because of the HIV epidemic in the 1980s, when many individuals were unwittingly infected with the virus through blood transfusions.85 Additional complications of blood transfusions include immunomodulation86 and a low supply of donated blood. Erythropoietin therapy was developed as an alternative method to stimulate hemoglobin levels in anemic patients. However, in cancer patients, several clinical trials indicated that erythropoietin therapy not only did not improve response to radiation treatment, it was tentatively associated with a decrease in survival.87, 88
Blood Flow Modulators
Modulation of tumor blood flow to increase tissue oxygenation offers another means of reducing tumor hypoxia. Noradrenaline was shown to significantly improved blood flow and drug delivery in a rat model of liver cancer.89 Benzyl nicotinate improved the radiation response of a preclinical murine tumor model, likely due to an increase in tumor pO2.90 A number of other agents evaluated preclinically, including pentoxifylline and nicotinamide, also improved tumor blood flow and response to radiotherapy.91–93 However, clinical trials of nicotinamide have encountered considerable toxicities, with some patients experiencing nausea and vomiting that was unresponsive to antiemetics.91
High oxygen content breathing
Hyperbaric chambers
Oxygen delivery to tissues is dependent on several factors, mainly blood flow, oxygen content, and oxygen tension.94 Recognizing the deficiencies of oxygen delivery in solid tumors, the landmark publication of Thomlinson and Gray6 ushered in the era of hyperbaric oxygen use in the treatment of cancer. Hyperbaric chambers were designed to alter both oxygen content and partial pressure by treating patients with 100% oxygen at three atmospheres pressure. Under these conditions, the amount of oxygen dissolved in plasma is significantly increased independently of hemoglobin saturation.95 However, despite initial positive results, hyperbaric chambers were discontinued in favor of alternate methods due to patient discomfort, cases of oxygen poisoning, and fires that occurred inside the chambers.96
Carbogen breathing
100% oxygen breathing has been shown to elicit deleterious and toxic effects in the body, mainly to the central nervous system, lungs, red blood cell morphology, and peripheral vision.97 Furthermore, in response to this level of oxygen, arteries and arterioles actively vasoconstrict. To mitigate the latter, carbogen (95% O2, 5% CO2) was introduced as an alternate breathing gas. Indeed, preclinical investigations demonstrated that carbogen improved tumor oxygenation and response to radiotherapy.98, 99 In the clinic, carbogen largely, and somewhat surprisingly, did not demonstrate improvements in radiotherapy outcomes.100 However, a trial using carbogen breathing in advanced head and neck cancer patients did suggest that carbogen breathing may be an alternative for patients who are unfit to receive concomitant chemotherapy with the radiation treatments.101
Pre-clinical investigations have shown that carbogen effectively reduces chronic hypoxia in tumors, but has little or no impact on acute hypoxia. Nicotinamide reduces acute hypoxia, though the mechanism remains unknown.102 Thus, to overcome both types of hypoxia, nicotinamide and carbogen breathing were evaluated in combination. In pre-clinical tumor models, the combination showed substantial improvements in radiosensitivity by reducing both acute and chronic hypoxia.103, 104 However, it is important to note that these improvements were time and dose-dependent, making the duration of breathing carbogen, the dose of nicotinamide, and the timing of the radiotherapy crucial to the therapy success.98, 105,106 When evaluated in the clinic, combining carbogen and nicotinamide with conventional or accelerated radiotherapy protocols in bladder and laryngeal cancer demonstrated improvements in outcome.107, 108 However, despite showing promising results, the clinical usage of carbogen as a means to improve tumor oxygenation has fallen out of favor.
Hypoxic Cell Sensitizers
Hypoxic cell radiation sensitizers mimic the effects of oxygen to increase radiation-induced DNA damage. Such agents are extremely effective in sensitizing hypoxic cells in vitro and in rodent tumors to radiation.109–111 Several agents reached clinical evaluation. However, many clinical trials encountered dose-limiting toxicities. These precluded administration of the agents at doses sufficient for effective radiosensitization during courses of multidose fractionated radiotherapy.109, 110 Indeed, rodent studies mimicking the clinical treatment regimens showed no or very little improvement in tumor response.110 To date, the most significant effects of combining hypoxic cell sensitizers with radiotherapy are those reported by Overgaard et al which combined nimorazole and radiotherapy to treat head and neck cancer patients, who experienced improvements in disease-free survival with no major side effects.18 Consequently, the addition of nimorazole to radiotherapy has now become standard of care for head and neck cancer patients in Denmark.
Bioreductive Therapies
In contrast to hypoxic cell sensitizers, bioreductive agents selectively kill hypoxic cells. These agents become cytotoxic by redox processes under hypoxic conditions.112 A number of different classes of agents including quinones, nitroimidazoles, and benzotriazines demonstrate hypoxic selectivity.113–115 One of the most effective, tirapazamine, causes DNA single- and double-strand breaks under low oxygen conditions, and significantly enhances the efficacy of radiotherapy in in vitro and in preclinical mouse models.116, 117 However, several clinical trials investigations combining tirapazamine with chemotherapy or radiotherapy failed to provide an improvement in survival compared to standard care.118, 119 Still, the area of deploying cytotoxic agents to eliminate treatment resistant hypoxic tumor cells remains an active area of research.120 Given that tumor cells exposed to acute and chronic hypoxic conditions can initiate differences in genomic instability and repair capacities, it would appear imperative that the efficacy of future agents be evaluated under both short term and prolonged oxygen deprivation.
High Linear Energy Transfer
An alternate method to reduce the loss of radiation sensitivity of cells existing in tumors in areas of low oxygen tensions is to use a radiation that is less dependent on oxygen for its efficacy. It is believed that as the LET for radiation increases, the OER decreases.121, 122 Charged particles, such as protons, have a high LET yet provide only a modest improvement in OER.123 Heavier particles, such as carbon, hold promise for further decreasing the OER. While high-LET radiation can reduce the impact of hypoxia, currently used particle radiotherapy machines cannot completely overcome the detrimental impact of hypoxia on tumor response.
Targeting the Tumor Vasculature
Antiangiogenic therapy
In order for a tumor to grow beyond a small size, it must induce its own vascular network.124, 125 However, the neoplastic cell demands are so severe the resultant tumor vasculature is functionally and structurally abnormal.126 Due to constant stimulation with pro-angiogenic growth factors such as VEGF,127 the tumor vasculature never achieves maturation, lacks coverage by pericyte or smooth muscle cells, and is leaky and prone to collapse.128
Several AA therapeutics have been developed as a strategy to target tumor vasculature.129 Targeting the vasculature is appealing, as normal cells will not rapidly mutate and develop resistance, as tumor cells do. By blocking the effects of VEGF or other pro-angiogenic growth factors, the vasculature has an opportunity to mature and obtain pericyte and smooth muscle cell coverage in a process known as normalization. This normalization allows more effective blood delivery, reducing tumor hypoxia.130 As a result, the addition of AA therapy to radiation can improve tumor response.131 However, such normalization of the tumor vasculature after AA therapy is not a universal occurrence being dependent on the agent, schedule, phase of growth and tumor type.47
Vascular disrupting agents
VDAs do not target hypoxic cells, per se, rather, this class of drug disrupts established tumor vasculature and elicits a tumor cell death cascade due to prolonged ischemia. VDAs induce vascular shutdown lasting hours to days leading to extensive necrosis in the core and hypoxic regions of a variety of tumor types,132, 133 and consequently, leave behind viable well-oxygenated tumor cells that are sensitive to radiation and other conventional anticancer therapies.47, 132,133
While both AA and VDA treatments can affect tumor oxygenation and therapy response, the combination of the two classes may be required to realize the full antitumor potential of vascular targeting therapy. Preclinical and initial clinical data support the therapeutic potential of such a combination.133
Modifiable Lifestyle Behaviors
Smoking and obesity
Modifiable lifestyle behaviors are also known impact tumor hypoxia. Patients who smoke cigarettes often have lower oxygen concentrations in blood as well as tumors, due to the reduction in arterial oxygen saturation. Pre-clinical investigations showed that carboxyhemoglobin levels such as those observed in smokers could significantly impair the efficacy of radiation treatment.134 Non-small cell lung cancer patients, who smoke during radiotherapy have worse outcomes than their non-smoking counterparts.135 Adipose tissue secretes a variety of cytokines, including many implicated in cancer progression, including TNF-alpha and IL-6.136 Simple changes made to modifiable behaviors including obesity and smoking have the potential to modulate tumor oxygenation and tumor response to radiation therapy.
Exercise
Systemic interventions, such as aerobic exercise, may be a possible therapeutic adjuvant to conventional anticancer therapies by improving treatment efficacy, decreasing therapeutic side effects and improving overall quality of life. The idea that exercise may prevent cancer development was first postulated early in the 20th century,137 and several elegant reviews have focused on physical activity and cancer risk,138 tumor progression139 and cardiovascular disease in cancer patients.140 Preclinical data are beginning to accumulate on the effects of prolonged exercise training on the tumor microenvironment.139 Results indicate that during exercise, the increased perfusion pressure to the tumor (and the inability of tumor blood vessels to constrict) increases tumor blood flow and reduces tumor hypoxia.141 Given its potential to modulate tumor physiology, the use of physical exercise in conjunction with conventional anticancer therapies is under active investigation and a recent report indicates that exercise could improve cyclophosphamide efficacy.142 In addition to the hypothesis that prolonged exercise may normalize tumor vasculature and its favorable immunological effects,139, 141,142 exercise training as an adjuvant to conventional anticancer therapy incurs little risk of negative side effects.
Modulation of Tumor Hypoxia Summary
Pre-clinical studies have clearly established a variety of interventional approaches that can be utilized to overcome tumor hypoxia and reduce its detrimental impact on conventional as well as emerging anticancer therapies. However, the translation of such approaches to the clinical management of cancer has been difficult. Enthusiasm for developing radiosensitizers and bioreductive agents has declined after multiple trials failed to demonstrate a positive impact. However, said trials did not include assessments of tumor hypoxia in the patients prior to enrolment. As with any targeted therapeutic, presence of the target is essential for therapeutic efficacy. Evaluating an agent in a setting where some of the patients lack the target will result in a high likelihood of failure to achieve statistically significant results. Moreover, as these interventions come with risks, it is imperative to deliver them only to patients who could benefit.
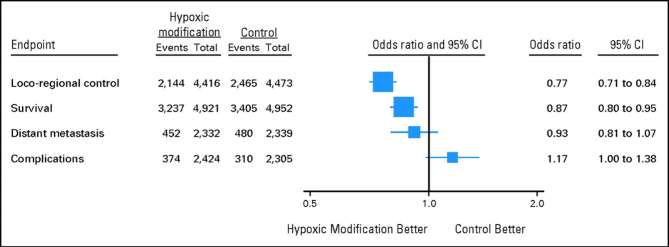
Despite limitations and difficulties, the comprehensive meta-analysis review by Overgaard143 of more than 80 trials and 10,000 patients showed that hypoxia modification in solid tumors undergoing radiotherapy leads to improved outcomes (Figure 5). Clearly, these findings underscore the need for continued efforts to identify accurately those patients whose tumors may contain treatment refractory hypoxic cells. Most notably, it is essential to develop techniques that can quantify and monitor both acute and chronic hypoxia in solid tumors in patients. Until such an ideal can be achieved, non-invasive imaging that allows longitudinal evaluations of tumors throughout the course of therapy may prove most advantageous despite limitations.
Figure 5.
Effect of hypoxic modification in cancer patients treated with primary radiotherapy. Data are from 86 randomized trials including 10,108 patients with either bladder, cervical, head and neck, CNS, lung or other types of cancer such as esophagus and pancreas. Reprinted with permission from Overgaard143.
Molecular, cellular, and functional behavior of cancer cells as well as their ability to cope with oxygen deprivation and therapeutic insults, differ greatly depending upon the extent and duration of the hypoxia exposure. It makes sense that therapeutic interventions and molecular targeting strategies aimed at overcoming detrimental effects of hypoxia or seeking to exploit hypoxia-associated cancer cell vulnerabilities are assessed under conditions of both acute and prolonged oxygen deprivation. Once obtained, such information will establish targeted molecules that allow the development of novel therapeutic intervention strategies to provide more effective treatment options for cancer patients.
Conclusions
Extensive data derived from preclinical investigations now exist to clearly demonstrate that hypoxic microenvironments in solid tumors significantly impair tumor response to anticancer therapies, promote tumor aggression and progression, and facilitate the spread of cancer cells. Much interest in this topic originated from the scientific investigations of the “oxygen effect” by Sir Oliver Scott and colleagues at the Gray Laboratories in the 1950s that led to the translation from the laboratory to the pioneering application of hyperbaric oxygen in combination with radiation therapy. Furthermore, his research set the stage for numerous subsequent laboratory and clinical investigations seeking to understand and overcome the detrimental role of hypoxia in the treatment of cancer.
Contributor Information
Veronica S Hughes, Email: nikkisaf@ufl.edu.
Jennifer M Wiggins, Email: jmwiggins@ufl.edu.
Dietmar W Siemann, Email: siemadw@ufl.edu.
REFERENCES
- 1. Schwarz G. Ueber Desensibilisierung gegen röntgen- und radiumstrahlen. Munchener Medizinische Wochenschrift 1909; 24: 1–2. [Google Scholar]
- 2. Mottram JC. A factor of importance in the radio sensitivity of tumours. Br J Radiol 1936; 9: 606–14. doi: 10.1259/0007-1285-9-105-606 [DOI] [Google Scholar]
- 3. Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26: 638–48. doi: 10.1259/0007-1285-26-312-638 [DOI] [PubMed] [Google Scholar]
- 4. Scott OC. Some aspects of the effect of ionizing radiation on tumors in experimental animals. Adv Biol Med Phys 1958; 6: 121–73. [DOI] [PubMed] [Google Scholar]
- 5. Scott OC. A model system for examining the radiosensitivity of metabolising layers of cells. Br J Cancer 1957; 11: 130–6. doi: 10.1038/bjc.1957.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955; 9: 539–49. doi: 10.1038/bjc.1955.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ALPER T, HOWARD-FLANDERS P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature 1956; 178: 978–9. doi: 10.1038/178978a0 [DOI] [PubMed] [Google Scholar]
- 8. Puck TT, MARCUS PI. Action of X-rays on mammalian cells. J Exp Med 1956; 103: 653–66. doi: 10.1084/jem.103.5.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michael BD, Adams GE, Hewitt HB, Jones WB, Watts ME. A posteffect of oxygen in irradiated bacteria: a submillisecond fast mixing study. Radiat Res 1973; 54: 239–51. doi: 10.2307/3573702 [DOI] [PubMed] [Google Scholar]
- 10. Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Philadelphia, PA, USA: The British Institute of Radiology.; 2006. 546. [Google Scholar]
- 11. Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med 2009; 9: 442–58. doi: 10.2174/156652409788167087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suit H, Maeda M. Oxygen effect factor and tumor volume in the C3H mouse mammary carcinoma. A preliminary report. American Journal of Roentgenology 1966; 96: 177–82. doi: 10.2214/ajr.96.1.177 [DOI] [PubMed] [Google Scholar]
- 13. Shrieve DC, Harris JW. The in vitro sensitivity of chronically hypoxic EMT6/SF cells to X-radiation and hypoxic cell radiosensitizers. Int J Radiat Biol Relat Stud Phys Chem Med 1985; 48: 127–38. doi: 10.1080/09553008514551131 [DOI] [PubMed] [Google Scholar]
- 14. Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and predicting radiation response. Semin Radiat Oncol 2015; 25: 260–72. doi: 10.1016/j.semradonc.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 15. Evans JC, BERGSJO P. The influence of anemia on the results of radiotherapy in carcinoma of the cervix. Radiology 1965; 84: 709–17. doi: 10.1148/84.4.709 [DOI] [PubMed] [Google Scholar]
- 16. Takeshi K, Katsuyuki K, Yoshiaki T, Teppei S, Tadayoshi M, Akira M, et al. . Definitive radiotherapy combined with high-dose-rate brachytherapy for Stage III carcinoma of the uterine cervix: retrospective analysis of prognostic factors concerning patient characteristics and treatment parameters. Int J Radiat Oncol Biol Phys 1998; 41: 319–27. doi: 10.1016/S0360-3016(98)00053-4 [DOI] [PubMed] [Google Scholar]
- 17. Stadler P, Becker A, Feldmann HJ, Hänsgen G, Dunst J, Würschmidt F, et al. . Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 44: 749–54. doi: 10.1016/S0360-3016(99)00115-7 [DOI] [PubMed] [Google Scholar]
- 18. Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. . A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish head and neck cancer study (DAHANCA) protocol 5-85. Radiother Oncol 1998; 46: 135–46. doi: 10.1016/S0167-8140(97)00220-X [DOI] [PubMed] [Google Scholar]
- 19. Greven KM, Solin LJ, Hanks GE. Prognostic factors in patients with bladder carcinoma treated with definitive irradiation. Cancer 1990; 65: 908–12. doi: [DOI] [PubMed] [Google Scholar]
- 20. Dunphy EP, Petersen IA, Cox RS, Bagshaw MA. The influence of initial hemoglobin and blood pressure levels on results of radiation therapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys 1989; 16: 1173–8. doi: 10.1016/0360-3016(89)90277-0 [DOI] [PubMed] [Google Scholar]
- 21. Girinski T, Pejovic-Lenfant MH, Bourhis J, Campana F, Cosset JM, Petit C, et al. . Prognostic value of hemoglobin concentrations and blood transfusions in advanced carcinoma of the cervix treated by radiation therapy: results of a retrospective study of 386 patients. Int J Radiat Oncol Biol Phys 1989; 16: 37–42. doi: 10.1016/0360-3016(89)90007-2 [DOI] [PubMed] [Google Scholar]
- 22. Kolstad P. Intercapillary distance, oxygen tension and local recurrence in cervix cancer. Scand J Clin Lab Invest Suppl 1968; 106: 145–57. [PubMed] [Google Scholar]
- 23. Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH, et al. . Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 1988; 14: 831–8. doi: 10.1016/0360-3016(88)90002-8 [DOI] [PubMed] [Google Scholar]
- 24. Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 1991; 51: 3316–22. [PubMed] [Google Scholar]
- 25. Gabalski EC, Adam M, Pinto H, Brown JM, Bloch DA, Terris DJ. Pretreatment and midtreatment measurement of oxygen tension levels in head and neck cancers. Laryngoscope 1998; 108: 1856–60. doi: 10.1097/00005537-199812000-00017 [DOI] [PubMed] [Google Scholar]
- 26. Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–15. [PubMed] [Google Scholar]
- 27. Parker C, Milosevic M, Toi A, Sweet J, Panzarella T, Bristow R, et al. . Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. International journal of radiation oncology, biology. Physics 2004; 58: 750–7. [DOI] [PubMed] [Google Scholar]
- 28. Höckel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, et al. . Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 1993; 26: 45–50. doi: 10.1016/0167-8140(93)90025-4 [DOI] [PubMed] [Google Scholar]
- 29. Milosevic M, Warde P, Ménard C, Chung P, Toi A, Ishkanian A, et al. . Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res 2012; 18: 2108–14. doi: 10.1158/1078-0432.CCR-11-2711 [DOI] [PubMed] [Google Scholar]
- 30. Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)a cet amide]: analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer 1995; 72: 869–74. doi: 10.1038/bjc.1995.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raleigh JA, Franko AJ, Koch CJ, Born JL. Binding of misonidazole to hypoxic cells in monolayer and spheroid culture: evidence that a side-chain label is bound as efficiently as a ring label. Br J Cancer 1985; 51: 229–35. doi: 10.1038/bjc.1985.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lord EM, Harwell L, Koch CJ. Detection of hypoxic cells by monoclonal antibody recognizing 2-nitroimidazole adducts. Cancer Res 1993; 53: 5721–6. [PubMed] [Google Scholar]
- 33. Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res 1999; 151: 580–9. doi: 10.2307/3580034 [DOI] [PubMed] [Google Scholar]
- 34. Evans SM, Hahn SM, Magarelli DP, Koch CJ. Hypoxic heterogeneity in human tumors: EF5 binding, vasculature, necrosis, and proliferation. Am J Clin Oncol 2001; 24: 467–72. [DOI] [PubMed] [Google Scholar]
- 35. Koch CJ, Hahn SM, Rockwell K, Covey JM, McKenna WG, Evans SM. Pharmacokinetics of EF5 [2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide] in human patients: implications for hypoxia measurements in vivo by 2-nitroimidazoles. Cancer Chemother Pharmacol 2001; 48: 177–87. [DOI] [PubMed] [Google Scholar]
- 36. Gillies RM, Robinson SP, McPhail LD, Carter ND, Murray JF. Immunohistochemical assessment of intrinsic and extrinsic markers of hypoxia in reproductive tissue: differential expression of HIF1α and HIF2α in rat oviduct and endometrium. J Mol Histol 2011; 42: 341–54. doi: 10.1007/s10735-011-9338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim B, Cho H, Chung J-Y, Conway C, Ylaya K, Kim J-H, et al. . Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. J Transl Med 2013; 11: 185. doi: 10.1186/1479-5876-11-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris BH, Barberis A, West CM, Buffa FM. Gene expression signatures as biomarkers of tumour hypoxia. Clin Oncol 2015; 27: 547–60. doi: 10.1016/j.clon.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 39. Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. . FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol 2012; 105: 14–20. doi: 10.1016/j.radonc.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 40. Rasey JS, Grunbaum Z, Magee S, Nelson NJ, Olive PL, Durand RE, et al. . Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat Res 1987; 111: 292–304. doi: 10.2307/3576986 [DOI] [PubMed] [Google Scholar]
- 41. Mortensen LS, Buus S, Nordsmark M, Bentzen L, Munk OL, Keiding S, et al. . Identifying hypoxia in human tumors: a correlation study between 18F-FMISO PET and the eppendorf oxygen-sensitive electrode. Acta Oncol 2010; 49: 934–40. doi: 10.3109/0284186X.2010.516274 [DOI] [PubMed] [Google Scholar]
- 42. Bentzen L, Keiding S, Nordsmark M, Falborg L, Hansen SB, Keller J, et al. . Tumour oxygenation assessed by 18F-fluoromisonidazole PET and polarographic needle electrodes in human soft tissue tumours. Radiotherapy and Oncology 2003; 67: 339–44. doi: 10.1016/S0167-8140(03)00081-1 [DOI] [PubMed] [Google Scholar]
- 43. Jankovic B, Aquino-Parsons C, Raleigh JA, Stanbridge EJ, Durand RE, Banath JP, et al. . Comparison between pimonidazole binding, oxygen electrode measurements, and expression of endogenous hypoxia markers in cancer of the uterine cervix. Cytometry B Clin Cytom 2006; 70B: 45–55. doi: 10.1002/cyto.b.20086 [DOI] [PubMed] [Google Scholar]
- 44. Gagel B, Piroth M, Pinkawa M, Reinartz P, Zimny M, Kaiser HJ, et al. . pO polarography, contrast enhanced color duplex sonography (CDS), [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron emission tomography: validated methods for the evaluation of therapy-relevant tumor oxygenation or only bricks in the puzzle of tumor hypoxia? BMC Cancer 2007; 7: 113. doi: 10.1186/1471-2407-7-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gagel B, Reinartz P, Dimartino E, Zimny M, Pinkawa M, Maneschi P, et al. . pO2 polarography versus positron emission tomography ([18F] fluoromisonidazole, [18F]-2-fluoro-2’-deoxyglucose). An appraisal of radiotherapeutically relevant hypoxia. Strahlenther Onkol 2004; 180: 616–22. doi: 10.1007/s00066-004-1229-y [DOI] [PubMed] [Google Scholar]
- 46. Zimny M, Gagel B, DiMartino E, Hamacher K, Coenen HH, Westhofen M, et al. . FDG—a marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur J Nucl Med Mol Imaging 2006; 33: 1426–31. doi: 10.1007/s00259-006-0175-6 [DOI] [PubMed] [Google Scholar]
- 47. Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther 2015; 153: 107–24. doi: 10.1016/j.pharmthera.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, et al. . Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 2002; 8: 2595–604. [PubMed] [Google Scholar]
- 49. Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle PO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys 2012; 82: e433–e439. doi: 10.1016/j.ijrobp.2011.05.037 [DOI] [PubMed] [Google Scholar]
- 50. Mayr NA, Wang JZ, Zhang D, Grecula JC, Lo SS, Jaroura D, et al. . Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. Int J Radiat Oncol Biol Phys 2010; 77: 502–8. doi: 10.1016/j.ijrobp.2009.04.084 [DOI] [PubMed] [Google Scholar]
- 51. Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Greenberg RE, Stobbe C, et al. . Hypoxic prostate/muscle PO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology 2002; 60: 634–9. doi: 10.1016/S0090-4295(02)01858-7 [DOI] [PubMed] [Google Scholar]
- 52. Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick ACP. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int 2010; 105: 8–13. doi: 10.1111/j.1464-410X.2009.08921.x [DOI] [PubMed] [Google Scholar]
- 53. Chaudary N, Hill RP. Hypoxia and metastasis in breast cancer. Breast Dis 2006; 26: 55–64. doi: 10.3233/BD-2007-26105 [DOI] [PubMed] [Google Scholar]
- 54. Pitson G, Fyles A, Milosevic M, Wylie J, Pintilie M, Hill R. Tumor size and oxygenation are independent predictors of nodal diseases in patients with cervix cancer. Int J Radiat Oncol Biol Phys 2001; 51: 699–703. doi: 10.1016/S0360-3016(01)01662-5 [DOI] [PubMed] [Google Scholar]
- 55. Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996; 41: 31–9. doi: 10.1016/S0167-8140(96)91811-3 [DOI] [PubMed] [Google Scholar]
- 56. Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012; 9: 674–87. doi: 10.1038/nrclinonc.2012.171 [DOI] [PubMed] [Google Scholar]
- 57. Bayer C, Shi K, Astner ST, Maftei CA, Vaupel P. Acute versus chronic hypoxia: why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys 2011; 80: 965–8. doi: 10.1016/j.ijrobp.2011.02.049 [DOI] [PubMed] [Google Scholar]
- 58. Chaplin DJ, Olive PL, Durand RE. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res 1987; 47: 597–601. [PubMed] [Google Scholar]
- 59. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32. doi: 10.1038/nrc1187 [DOI] [PubMed] [Google Scholar]
- 60. Kaelin WG. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 2008; 8: 865–73. doi: 10.1038/nrc2502 [DOI] [PubMed] [Google Scholar]
- 61. Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer 2010; 102: 789–95. doi: 10.1038/sj.bjc.6605551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev 2004; 23: 293–310. doi: 10.1023/B:CANC.0000031768.89246.d7 [DOI] [PubMed] [Google Scholar]
- 63. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 64. Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. doi: 10.1038/nrc2344 [DOI] [PubMed] [Google Scholar]
- 65. Riffle S, Hegde RS. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J Exp Clin Cancer Res 2017; 36: 102. doi: 10.1186/s13046-017-0570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan N, Koritzinsky M, Zhao H, Bindra R, Glazer PM, Powell S, et al. . Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res 2008; 68: 605–14. doi: 10.1158/0008-5472.CAN-07-5472 [DOI] [PubMed] [Google Scholar]
- 67. Powell SN, Kachnic LA. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene 2003; 22: 5784–91. doi: 10.1038/sj.onc.1206678 [DOI] [PubMed] [Google Scholar]
- 68. Luoto KR, Kumareswaran R, Bristow RG. Tumor hypoxia as a driving force in genetic instability. Genome Integr 2013; 4: 5. doi: 10.1186/2041-9414-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zölzer F, Streffer C. Increased radiosensitivity with chronic hypoxia in four human tumor cell lines. Int J Radiat Oncol Biol Phys 2002; 54: 910–20. doi: 10.1016/S0360-3016(02)02963-2 [DOI] [PubMed] [Google Scholar]
- 70. Ling CC, Robinson E, Shrieve DC. Repair of radiation induced damage-dependence on oxygen and energy status. Int J Radiat Oncol Biol Phys 1988; 15: 1179–86. doi: 10.1016/0360-3016(88)90201-5 [DOI] [PubMed] [Google Scholar]
- 71. Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. . HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 2006; 20: 557–70. doi: 10.1101/gad.1399906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bae KM, Dai Y, Vieweg J, Siemann DW. Hypoxia regulates SOX2 expression to promote prostate cancer cell invasion and sphere formation. Am J Cancer Res 2016; 6: 1078–88. [PMC free article] [PubMed] [Google Scholar]
- 73. Alqawi O, Wang HP, Espiritu M, Singh G. Chronic hypoxia promotes an aggressive phenotype in rat prostate cancer cells. Free Radic Res 2007; 41: 788–97. doi: 10.1080/10715760701361531 [DOI] [PubMed] [Google Scholar]
- 74. Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res 2004; 64: 2054–61. doi: 10.1158/0008-5472.CAN-03-3196 [DOI] [PubMed] [Google Scholar]
- 75. Dai Y, Bae K, Siemann DW. Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int J Radiat Oncol Biol Phys 2011; 81: 521–8. doi: 10.1016/j.ijrobp.2011.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33: 1974–82. doi: 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Loi M, Desideri I, Greto D, Mangoni M, Sottili M, Meattini I, et al. . Radiotherapy in the age of cancer immunology: current concepts and future developments. Crit Rev Oncol Hematol 2017; 112: 1–10. doi: 10.1016/j.critrevonc.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 78. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. . PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211: 781–90. doi: 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, et al. . Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A 2012; 109: E2784–E2793. doi: 10.1073/pnas.1202366109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan L-J, et al. . Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 2010; 120: 2699–714. doi: 10.1172/JCI39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006; 6: 583–92. doi: 10.1038/nrc1893 [DOI] [PubMed] [Google Scholar]
- 82. Kallman RF. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology 1972; 105: 135–42. doi: 10.1148/105.1.135 [DOI] [PubMed] [Google Scholar]
- 83. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014; 88: 254–62. doi: 10.1016/j.ijrobp.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hong B-J, Kim J, Jeong H, Bok S, Kim Y-E, Ahn G-O. Tumor hypoxia and reoxygenation: the yin and yang for radiotherapy. Radiat Oncol J 2016; 34: 239–49. doi: 10.3857/roj.2016.02012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Donegan E, Stuart M, Niland JC, Sacks HS, Azen SP, Dietrich SL, et al. . Infection with human immunodeficiency virus type 1 (HIV-1) among recipients of antibody-positive blood donations. Ann Intern Med 1990; 113: 733–9. doi: 10.7326/0003-4819-113-10-733 [DOI] [PubMed] [Google Scholar]
- 86. Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol 1994; 1: 457–61. [PubMed] [Google Scholar]
- 87. Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, et al. . Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 2007; 25: 1027–32. doi: 10.1200/JCO.2006.07.1514 [DOI] [PubMed] [Google Scholar]
- 88. Shenouda G, Zhang Q, Ang KK, Machtay M, Parliament MB, Hershock D, et al. . Long-term results of radiation therapy oncology group 9903: a randomized phase 3 trial to assess the effect of erythropoietin on local-regional control in anemic patients treated with radiation therapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2015; 91: 907–15. doi: 10.1016/j.ijrobp.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shankar A, Loizidou M, Burnstock G, Taylor I. Noradrenaline improves the tumour to normal blood flow ratio and drug delivery in a model of liver metastases. Br J Surg 1999; 86: 453–7. doi: 10.1046/j.1365-2168.1999.01045.x [DOI] [PubMed] [Google Scholar]
- 90. Hou H, Abramovic Z, Lariviere JP, Sentjurc M, Swartz H, Khan N. Effect of a topical vasodilator on tumor hypoxia and tumor oxygen guided radiotherapy using EPR oximetry. Radiat Res 2010; 173: 651–8. doi: 10.1667/RR1947.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kaanders JHAM, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol 2002; 3: 728–37. doi: 10.1016/S1470-2045(02)00929-4 [DOI] [PubMed] [Google Scholar]
- 92. Collingridge DR, Rockwell S. Pentoxifylline improves the oxygenation and radiation response of BA1112 rat rhabdomyosarcomas and EMT6 mouse mammary carcinomas. Int J Cancer 2000; 90: 256–64. doi: [DOI] [PubMed] [Google Scholar]
- 93. Horsman MR, Chaplin DJ, Brown JM. Tumor radiosensitization by nicotinamide: a result of improved perfusion and oxygenation. Radiat Res 1989; 118: 139–50. doi: 10.2307/3577429 [DOI] [PubMed] [Google Scholar]
- 94. Collins J-A, Rudenski A, Gibson J, Howard L, O’Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin–oxygen dissociation curve. Breathe 2015; 11: 194–201. doi: 10.1183/20734735.001415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moen I, Stuhr LEB. Hyperbaric oxygen therapy and cancer—a review. Target Oncol 2012; 7: 233–42. doi: 10.1007/s11523-012-0233-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tewarson A. Nonmetallic material flammability in oxygen enriched atmospheres. J Fire Sci 2000; 18: 183–214. doi: 10.1177/073490410001800303 [DOI] [Google Scholar]
- 97. Chawla A, Lavania AK. Oxygen toxicity. Med J Armed Forces India 2001; 57: 131–3. doi: 10.1016/S0377-1237(01)80133-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Siemann DW, Hill RP, Bush RS. The importance of the pre-irradiation breathing times of oxygen and carbogen (5% CO2: 95% O2) on the in vivo radiation response of a murine sarcoma. Int J Radiat Oncol Biol Phys 1977; 2: 903–11. doi: 10.1016/0360-3016(77)90188-2 [DOI] [PubMed] [Google Scholar]
- 99. Siemann DW, Johansen IM, Horsman MR. Radiobiological hypoxia in the KHT sarcoma: predictions using the Eppendorf histograph. Int J Radiat Oncol Biol Phys 1998; 40: 1171–6. doi: 10.1016/S0360-3016(98)00004-2 [DOI] [PubMed] [Google Scholar]
- 100. Rubin P, Hanley J, Keys HM, Marcial V, Brady L. Carbogen breathing during radiation therapy-the radiation therapy oncology group study. Int J Radiat Oncol Biol Phys 1979; 5: 1963–70. doi: 10.1016/0360-3016(79)90946-5 [DOI] [PubMed] [Google Scholar]
- 101. Vees H, Allal AS. Carbogen breathing combined with radical radiotherapy in advanced head and neck cancer patients with severe co-morbidities. Clin Oncol 2006; 18: 493–6. doi: 10.1016/j.clon.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 102. Horsman MR. Nicotinamide and other benzamide analogs as agents for overcoming hypoxic cell radiation resistance in tumours. A review. Acta Oncol 1995; 34: 571–87. doi: 10.3109/02841869509094031 [DOI] [PubMed] [Google Scholar]
- 103. Horsman MR, Nordsmark M, Khalil AA, Hill SA, Chaplin DJ, Siemann DW, et al. . Reducing acute and chronic hypoxia in tumours by combining nicotinamide with carbogen breathing. Acta Oncol 1994; 33: 371–6. doi: 10.3109/02841869409098431 [DOI] [PubMed] [Google Scholar]
- 104. Siemann DW, Horsman MR, Chaplin DJ. The radiation response of KHT sarcomas following nicotinamide treatment and carbogen breathing. Radiother Oncol 1994; 31: 117–22. doi: 10.1016/0167-8140(94)90391-3 [DOI] [PubMed] [Google Scholar]
- 105. Horsman MR, Siemann DW, Chaplin DJ, Overgaard J. Nicotinamide as a radiosensitizer in tumours and normal tissues: the importance of drug dose and timing. Radiother Oncol 1997; 45: 167–74. doi: 10.1016/S0167-8140(97)00127-8 [DOI] [PubMed] [Google Scholar]
- 106. Bussink J, Kaanders JH, Strik AM, van der Kogel AJ. Effects of nicotinamide and carbogen on oxygenation in human tumor xenografts measured with luminescense based fiber-optic probes. Radiother Oncol 2000; 57: 21–30. doi: 10.1016/S0167-8140(00)00275-9 [DOI] [PubMed] [Google Scholar]
- 107. Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol 2010; 28: 4912–8. doi: 10.1200/JCO.2010.28.4950 [DOI] [PubMed] [Google Scholar]
- 108. Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. . Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 2012; 30: 1777–83. doi: 10.1200/JCO.2011.35.9315 [DOI] [PubMed] [Google Scholar]
- 109. Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol 1996; 6: 10–21. doi: 10.1016/S1053-4296(96)80032-4 [DOI] [PubMed] [Google Scholar]
- 110. Moulder JE, Dutreix J, Rockwell S, Siemann DW. Applicability of animal tumor data to cancer therapy in humans. Int J Radiat Oncol Biol Phys 1988; 14: 913–27. doi: 10.1016/0360-3016(88)90014-4 [DOI] [PubMed] [Google Scholar]
- 111. Adams GE. Hypoxic cell sensitizers for radiotherapy. Int J Radiat Oncol Biol Phys 1978; 4: 135–41. doi: 10.1016/0360-3016(78)90129-3 [DOI] [PubMed] [Google Scholar]
- 112. Kennedy KA, Teicher BA, Rockwell S, Sartorelli AC. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol 1980; 29: 1–8. doi: 10.1016/0006-2952(80)90235-X [DOI] [PubMed] [Google Scholar]
- 113. Whitmore GF, Varghese AJ. The biological properties of reduced nitroheterocyclics and possible underlying biochemical mechanisms. Biochem Pharmacol 1986; 35: 97–103. doi: 10.1016/0006-2952(86)90565-4 [DOI] [PubMed] [Google Scholar]
- 114. Keyes SR, Heimbrook DC, Fracasso PM, Rockwell S, Sligar SG, Sartorelli AC. Chemotherapeutic attack of hypoxic tumor cells by the bioreductive alkylating agent mitomycin C. Adv Enzyme Regul 1985; 23: 291–307. doi: 10.1016/0065-2571(85)90053-6 [DOI] [PubMed] [Google Scholar]
- 115. Laderoute K, Wardman P, Rauth AM. Molecular mechanisms for the hypoxia-dependent activation of 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233). Biochem Pharmacol 1988; 37: 1487–95. doi: 10.1016/0006-2952(88)90010-X [DOI] [PubMed] [Google Scholar]
- 116. Brown JM. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer 1993; 67: 1163–70. doi: 10.1038/bjc.1993.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Brown JM. The hypoxic cell: a target for selective cancer therapy--eighteenth Bruce F. Cain memorial award lecture. Cancer Res 1999; 59: 5863–70. [PubMed] [Google Scholar]
- 118. Reddy SB, Williamson SK. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin Investig Drugs 2009; 18: 77–87. doi: 10.1517/13543780802567250 [DOI] [PubMed] [Google Scholar]
- 119. Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. . Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010; 28: 2989–95. doi: 10.1200/JCO.2009.27.4449 [DOI] [PubMed] [Google Scholar]
- 120. Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer 2011; 11: 504. doi: 10.1186/1471-2407-11-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wenzl T, Wilkens JJ. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys Med Biol 2011; 56: 3251–68. doi: 10.1088/0031-9155/56/11/006 [DOI] [PubMed] [Google Scholar]
- 122. Barendsen GW. Responses of cultured cells, tumours, and normal tissues to radiations of different linear energy transfer : Current topics in radiation research quarterly. Rijswijk, Netherlands: The British Institute of Radiology.; 1968. 293–356. [Google Scholar]
- 123. Kanemoto A, Hirayama R, Moritake T, Furusawa Y, Sun L, Sakae T, et al. . RBE and OER within the spread-out Bragg peak for proton beam therapy: in vitro study at the proton medical research center at the University of Tsukuba. J Radiat Res 2014; 55: 1028–32. doi: 10.1093/jrr/rru043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial award lecture. Cancer Res 1986; 46: 467–73. [PubMed] [Google Scholar]
- 125. Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 1977; 14: 53–65. doi: 10.1016/0026-2862(77)90141-8 [DOI] [PubMed] [Google Scholar]
- 126. Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer Res 1991; 51: 265–73. [PubMed] [Google Scholar]
- 127. Siemeister G, Martiny-Baron G, Marmé D. The pivotal role of VEGF in tumor angiogenesis: molecular facts and therapeutic opportunities. Cancer Metastasis Rev 1998; 17: 241–8. doi: 10.1023/A:1006027124696 [DOI] [PubMed] [Google Scholar]
- 128. Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005; 7: 452–64. doi: 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016; 388: 518–29. doi: 10.1016/S0140-6736(15)01088-0 [DOI] [PubMed] [Google Scholar]
- 130. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 2017; 20: 409–26. doi: 10.1007/s10456-017-9562-9 [DOI] [PubMed] [Google Scholar]
- 131. Brazelle WD, Shi W, Siemann DW. VEGF-associated tyrosine kinase inhibition increases the tumor response to single and fractionated dose radiotherapy. Int J Radiat Oncol Biol Phys 2006; 65: 836–41. doi: 10.1016/j.ijrobp.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 132. Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev 2011; 37: 63–74. doi: 10.1016/j.ctrv.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Siemann DW, Chaplin DJ, Horsman MR. Realizing the potential of vascular targeted therapy: the rationale for combining vascular disrupting agents and anti-angiogenic agents to treat cancer. Cancer Invest 2017; 35: 519–34. doi: 10.1080/07357907.2017.1364745 [DOI] [PubMed] [Google Scholar]
- 134. Siemann DW, Hill RP, Bush RS. Smoking: the influence of carboxyhemoglobin (HbCO) on tumor oxygenation and response to radiation. Int J Radiat Oncol Biol Phys 1978; 4: 657–62. doi: 10.1016/0360-3016(78)90189-X [DOI] [PubMed] [Google Scholar]
- 135. Fox JL, Rosenzweig KE, Ostroff JS. The effect of smoking status on survival following radiation therapy for non-small cell lung cancer. Lung Cancer 2004; 44: 287–93. doi: 10.1016/j.lungcan.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 136. Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 2001; 60: 349–56. doi: 10.1079/PNS2001110 [DOI] [PubMed] [Google Scholar]
- 137. Cherry TA. A theory of cancer. Medical Journal of Australia 1922; 1: 425–38. [Google Scholar]
- 138. Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. . Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016; 176: 816. doi: 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res 2016; 76: 4032–50. doi: 10.1158/0008-5472.CAN-16-0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Scott JM, Adams SC, Koelwyn GJ, Jones LW. Cardiovascular late effects and exercise treatment in breast cancer: current evidence and future directions. Can J Cardiol 2016; 32: 881–90. doi: 10.1016/j.cjca.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wiggins JM, Opoku-Acheampong AB, Baumfalk DR, Siemann DW, Behnke BJ. Exercise and the tumor microenvironment: potential therapeutic implications. Exerc Sport Sci Rev 2018; 46: 56–64. doi: 10.1249/JES.0000000000000137 [DOI] [PubMed] [Google Scholar]
- 142. Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. . Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst 2015; 107. doi: 10.1093/jnci/djv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol 2007; 25: 4066–74. doi: 10.1200/JCO.2007.12.7878 [DOI] [PubMed] [Google Scholar]