Abstract
Objective:
The purpose of this study was to prospectively evaluate the safety and effectiveness of radiofrequency ablation (RFA) by using a multiple-electrode switching system to treat unresectable medium-large (3.1–6.0 cm) HCC nodules.
Methods:
RFA using a multiple-electrode switching system was performed for HCC nodules with size > 3.0 < 6.0 cm in nonsurgical candidates. Two electrodes were consecutively placed for 3.1–4.0 cm tumours, and three electrodes for 4.1–5.9 cm tumours, with a 2.0–2.5 cm spacing. The power was switched from one electrode to the next automatically when the impedance reached 30 Ω above the baseline level. 25 patients (M/F = 9/16; median age 76 years, range 61-84) with liver cirrhosis (20 HCV-positive) in Child’s Class A (22 cases) and B (3 cases) and 26 HCC nodules (median diameter 4.0 cm; range 3.2–5.5 cm) underwent treatment in 25 sessions from 2013 and 2018. Therapeutic effectiveness was assessed through CT or MRI exam at 30–40 days post-ablation.
Results:
No procedure-related death or major complications occurred. Complete ablation was obtained in all nodules (100%). At a median follow up of 30 months, local tumor progression occurred in five out of 26 nodules (19.2%). Overall survival at 4 years was 49%.
Conclusion:
RFA with a multiple-electrode switching system may be a safe, quick and effective therapeutic option for treatment of 3.1-6.0 cm unresectable HCC tumours.
Advances in knowledge:
RFA with multiple electrodes provides favourable clinical results in patients with medium-large HCC nodules who are not suitable for surgery
Introduction
Image-guided thermal ablation as a minimally invasive technique has been widely used for treatment of primary liver cancer in the past two decades and is now considered among curative treatments for very early hepatocellular carcinoma (HCC) and a valid alternative to surgery in selected HCC patients within the Milan criteria.1,2 Radiofrequency ablation (RFA) still represents the current standard ablative technique and a recent meta-analysis demonstrated that RFA is more cost-effective than resection in the presence of HCC nodules (up to 3) equal to or less than 3 cm.3 Nonetheless, hepatic resection provided better life expectancy and was more cost-effective than RFA for single HCCs of 3–5 cm.3 The latter results are not unexpected since RFA effectiveness dramatically drops when medium–large nodules are considered. In fact, the complete ablation rate decreases to 60% with increasing size (even when multiple insertions are carried out)4 and local tumour progression (LTP) may be as high as 31% if tumour size exceeds 5 cm.5
In spite of improvements in surgical technique and perioperative care allowing treatment even in patients previously considered unsuitable for surgery, e.g. cases allocated in the stage B or C of the Barcelona therapeutic algorithm,6,7 aggressive surgical treatment of medium-large tumours is still hampered by several factors such as advanced age, heavy comorbidities, poor liver function, and portal hypertension. For this vast subgroup of patients, microwave ablation (MW)8,9 and combined therapy (i.e. RFA treatment coupled with either percutaneous ethanol injection (PEI)10 or transcatheter arterial chemoembolization (TACE) have been proposed to overcome these limitations11–14; in particular the combination of TACE and RFA proved to be superior to either RFA or TACE alone in terms complete ablation rate, overall survival, and LTP.11,14 Moreover, combined therapy achieved a 3-year overall survival rate similar to liver resection.12,13
A different approach to treat medium-large nodules entails the positioning of multiple sources of energy (RF or MW) into15–17 or around (“no-touch technique”) the tumours.18,19
Clinical15–19 and experimental20–22 research has demonstrated good results in terms of achieving a wider and rounder area of ablation with a sufficient safety margin, directly resulting in decreased LTP (which is the main parameter to assess ablative technique effectiveness in terms of local tumour control).
The aim of this study was to report on our single-centre experience of using a multiple radiofrequency (RF) electrode switching system to treat cirrhotic patients with unresectable HCC nodules between 3.1 and 6.0 cm over a long-term follow-up period.
methods and Materials
Patient selection
Between September 2013 and February 2018, patients with single or multiple (up to three, the largest up to 5.0 cm) unresectable HCC nodule/s 3.1–6.0 cm were prospectively selected for percutaneous RFA with multiple needles. Institutional review board approval was obtained for this prospective study, and all patients provided written informed consent before interventional procedures.
The patients were considered suitable for percutaneous approach if the following selection criteria were met: (1) lack of portal invasion (including segmental branches); (2) preserved liver function (Child’s A and B); (3) no extrahepatic metastasis; (4) permissive coagulative status (platelet count >50.000 mmc; prothrombin activity >50%); and 5) feasibility and safety of percutaneous approach under ultrasound guidance.
HCC diagnosis was based on either histological findings (2 nodules), or the non-invasive criteria proposed by AASLD and EASL1,2 (24 nodules). Liver tumours were considered unresectable because of: severe cardiopulmonary disease (8 cases); advanced age >75 years (7 cases); recent history or coexistence of another primary tumour (5 cases); severe portal hypertension (2 cases); poor hepatic reserve (1 case); and patient refusal (1 case).
US-guided RFA
All interventional procedures were performed by the same operator (GF), who has more than 20 years of experience in liver ablation.
Monopolar RFA with a multiple-electrode switching system (Covidien, Cool-tip™ RF ablation system E Series) was performed under US guidance using either free-hand technique or needle guiding systems in an operating theatre under conscious sedation. A multichannel RF system (maximum power of 200 W at a frequency of 480 kHz) was used to switch RF energy between the two or three internally cooled 17 g electrodes for a total of 15–20 min in the active tip portion of the electrode. The electrodes were activated alternatively and switched off automatically once the impedance reached 30 Ω above baseline. Two to three return pads were placed on the patients’ thighs to complete the circuit.
The number of electrodes, their spacing, and the exposed tip of the cool-tip needles varied according to lesion size: two needles spaced 1.5–2.0 cm were positioned into tumours up to 4.0 cm, and three needles placed 2.0 cm apart in as close to a triangular configuration as possible in tumours > 4.0 cm. Generally speaking, two needles were inserted as much parallel as possible using the same angle of introduction whereas, in case a third needle was needed for larger lesions, a different plane was chosen. For right-sided lesions we preferred an intercostal approach trying to stick the needles through two adjacent intercostal spaces (Figure 1b).
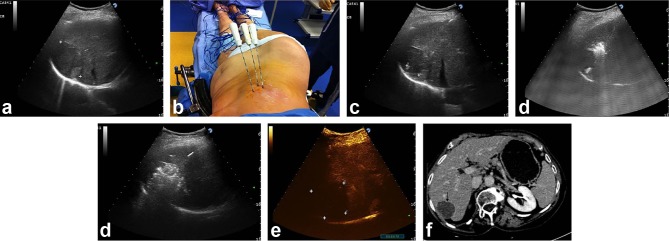
Figure 1.
A 5.5 cm HCC tumour (between markers) is displayed in the seventh hepatic segment at US intercostal scan in a 77-year-old female with HCV-positive hepatic cirrhosis (a). Three RF needles have been sequentially positioned in a triangular configuration through two adjacent intercostal spaces (b). Ultrasound counterpart: two electrodes (4 cm exposed tip) are seen in the same US plane (needle tips identified by the markers) spaced 2 cm apart (c), whereas the third needle is positioned more cranially (white spots due to vaporization gases signal beginning of ablation) (d). The entire tumour (black arrow) is obscured by vaporization clouds at the end of two cycles of ablation: the electrodes had been pulled back 1.5 cm after the first 20 min of energy delivery (e). CEUS performed 5 minutes after completion of ablation shows a wide area of complete ablation (between markers) (f). Follow-up CT scan at 4 years post-RFA with multiple needles: no viable areas are shown in the arterial phase within the index tumour (black arrow), which also appears to have decreased in size (g).
An exposed tip of 4.0 cm was used only if nodules size was >4.5 cm. One cycle of ablation lasted 15–20 min and the number of cycles needed to obtain complete ablation varied according to the nodule size. A second ablation cycle was obtained by pulling back the electrodes so as to cover the entire tumour volume. Whenever possible, the operator tried to obtain an ablative margin of at least 5 mm around the index tumour. Additional insertions of a single needle were considered if immediate post-procedural control of RFA effectiveness by means of contrast enhanced ultrasound (CEUS) demonstrated residual viable tissue. To this end, a second-generation contrast agent (SonoVue, Bracco, Milan, Italy) was injected intravenously as a single bolus of 2.4 ml into an antecubital vein, through an 18–20 gauge cannula, followed by 5 ml of saline. The US equipment machines (MyLab™ Twice, Esaote, Genova, Italy; Resona 7 system, Shenzhen Mindray Bio- Medical Electronic Co, Shenzhen, China) used throughout the study had dedicated software for CEUS-specific imaging.
Track ablation was performed during each needle withdrawal by maintaining the electrode tip at 90°C to prevent post-procedural bleeding and neoplastic seeding.
Contrast-enhanced CT or MR scans were performed to assess treatment success 30–40 days after RFA: complete ablation was considered if no enhanced area/s were demonstrated at the site of the index tumour. Enhanced MR or CT and CEUS were then alternated every 3 months so that patients were screened with each modality every 6 months for the first 2 years. Thereafter CEUS and CT/MR were alternatively performed every 6 months.
Clinical and laboratory evaluation (including alpha-fetoprotein serum levels) paralleled the imaging assessment.
Statistical analysis
Continuous variables were described by their medians and value ranges, and categorical variables were summarized by frequencies.
Time to LTP was calculated as the interval between no evidence of neoplastic disease at CT/MR after RFA, and the reappearance of tumour foci during follow-up.
Overall survival (OS) was calculated as the time from the beginning of treatment until death or last follow-up date.
Survival and local tumour progression in the treatment group was assessed using the log-rank test.
The data were analysed using SPSS (v. 13.0, SPSS Inc., Chicago, IL).
Results
25 patients with 26 HCC nodules were treated with multiple RF needles in a single session. The main characteristics of the patients studied are shown in Table 1.
Table 1.
Main demographic and clinical characteristics of the patients studied
| No. Patients | 25 |
| M/F | 9/16 |
| Median age (range) years | 76 (61–84) |
| Number of Nodules | 26 |
| Median size (range) cm | 4.0 (3.2–5.5) |
| Number of Nodules ≥ 4.0 cm (%) | 18 (69.2) |
| HCV+/HBV+/NAFDL/Crypto | 20/1/2/2 |
| Naive/LTP/NL | 21/3/2 |
| Child’s Class A/B | 22/3 |
| Mean value of serum AFP (SD) ng/ml | 28.9 (56) |
HVC, Hepatitis C Virus; HBV, Hepatitis B Virus; NAFLD, non-alcoholic liver disease; LTP, local tumor progression; NL, new lesion; AFP, alpha-fetoprotein.
Two needles were used for the treatment of 22 nodules (four of those nodules required a second ablation cycle) and three needles were used for the treatment of the other four nodules. In the latter group with HCC tumour size >4.0 cm, two nodules required a second ablation cycle, along with one more insertion of a single electrode (deemed necessary to obtain complete ablation on the basis of immediate post-procedural CEUS results). CT or MR performed after 1 month to verify treatment effectiveness showed absence of viable foci in all 26 nodules.
Median duration of the ablative session was 15 min (range 15–30 min). Figure 1 demonstrates a paradigmatic treatment with three needles for a 5.5 cm HCC nodule.
No peri-operative mortality or major complications occurred. Minor complications requiring only medical therapy were observed in 8 patients (32%): transient paralytic ileus (1), ascites (1), pleural effusion (1), post-ablation syndrome (1), and intense abdominal pain (4). No tumour seeding along the electrode tracks was observed during the available follow-up.
The median follow-up of patients was 30 months (range 6–57 months). Five out of 26 treated nodules demonstrated LTP (19.2%). Regrowth of neoplastic tissue occurred only in nodules with baseline diameter equal to or greater than 4.0 cm (5 out 18 cases). Nothwistanding, maybe as a consequence of the small samples, the difference in LTP between nodules ≥ 4.0 (cm) 27.7% and tumors < 4.0 cm (0%) was not significant (p = 0.16). The cumulative incidence of LTP at 2, 3 and 4 years was 13% (95%CI 6–19) and 29% (95%CI 22–36) and 29% (95%CI 22–36) respectively (Figure 2). Only three relapsing HCCs could be treated by means of RFA (2) and TACE (1), whereas the remaining two had best supportive care owing to concurrent neoplastic intra hepatic spread.
Figure 2.
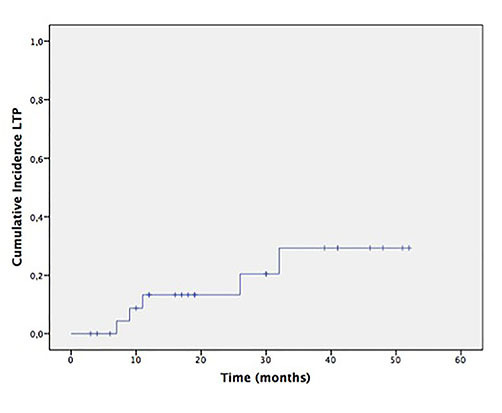
Cumulative local tumour progression probability after RFA. RFA, radio frequency ablation.
None of the patients were lost to follow-up and the estimated 2-, 3-, and 4-year overall survival was 81% (95% CI 77–85), 64% (95% CI 59–69), and 49% (CI 95% 42–56) respectively (Figure 3). Nine patients died due to tumour progression (55.5%, n = 5), liver failure (11.1%; n = 1), or extra hepatic causes (33.4%; n = 3).
Figure 3.
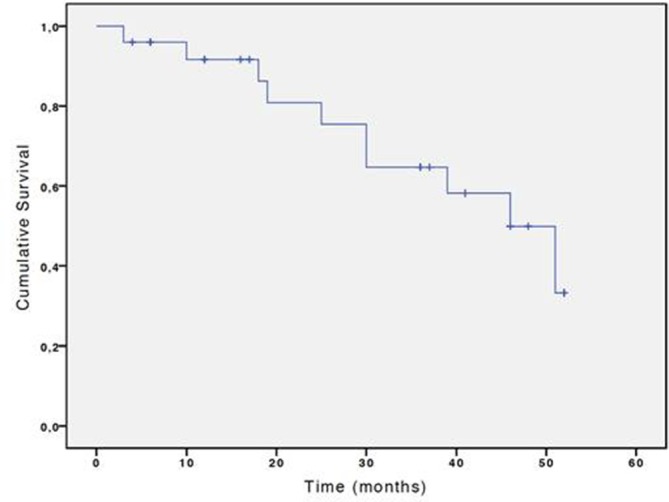
Cumulative overall survival probability after RFA. RFA, radio frequency ablation.
Discussion
Although RFA is considered a very effective tool to destroy small HCC nodules in situ,1,2 its technical effectiveness is greatly limited when the nodule size exceeds 3.0 cm.3 It is well known that the complete ablation rate progressively declines with increasing tumour size4: the complete ablation rate in 3.0–5.0 cm HCC nodules has been previously found to be 60%, but as low as 24% in large HCCs > 5.0 cm. RFA has been shown to have the worst performance in infiltrating tumours (only 35% completely ablated) irrespective of the tumour size.4
In addition, a high rate of LTP in lesions larger than 3.0 cm has been previously demonstrated even after an initial complete post-treatment response.5,23 To treat medium-large tumours by RFA as a sole therapy, the overlapping ablations technique has been proposed although with conflicting clinical results: whereas Livraghi et al4 obtained the poor above mentioned results, Chen et al24 utilized a mathematical model to contribute towards a very high rate of complete ablation (87%) in a mixed population of primary and secondary liver tumours. Several shortcomings of this type of approach should be highlighted:4,25 (1) needle repositioning after each ablation may be technically challenging since the target tumour may be obscured by bubbles provoked by previous heating, and without CEUS it may very difficult to recognize non-ablated areas; (2) perfusion mediated cooling (which can reduce the effectiveness of subsequent ablation) occurs due to the increased time between ablations; (3) the areas of ablation may not necessarily be coalescent.
Several experimental works20–22 have demonstrated that multiple heat sources (both RF and MW) spaced at predetermined distance allowed more heat to be contained to the target area, resulting in more confluent zones of ablation. Additional factors favouring this kind of approach should be taken into account: (1) local ischemia caused by adjacent ablations may reduce perfusion-mediated cooling in each ablation zone; (2) placement of multiple applicators prior to any tissue heating may be simpler than repositioning after each ablation since the tumour is not obscured by previous ablations; (3) simultaneous ablations are inherently faster than sequential ablations when sufficient power is available. Manufacturers have licensed RF and MW machines enabling operators to translate into clinical practice the experimental studies.
Although preliminary clinical reports on feasibility and efficacy of such approach have been reported since 2007 by Laeseke et al15 this method has not gained widespread adoption perhaps due to challenging technical difficulties in positioning multiple needles and increasing costs of the ablative procedure. However, over the last few years a number of papers have strengthened the idea that this technique may be considered as a valid tool to treat unresectable medium-large HCC tumours.16–19 Some authors adopted a switching monopolar RF system similar16 or identical17 to the one which we used in the present study and reported high rates of 1-year survival (99.4 and 98.8% respectively). However, more than two thirds of the entire population studied were comprised of HCC tumours < 3.0 cm. Neither paper reported OS or LTP according to size, and only in the paper by Tan et al17 did tumour size >3.0 cm turn out to be an independent predictor of worse LTP and OS rates.
On the other hand, the so called “no-touch technique” has been proposed by French authors to treat large HCC nodules (>5.0 cm)18 and small HCC up to 5.0 cm19 by means of multi-bipolar RF. These authors obtained high rates of technical effectiveness (100%), and primary effectiveness (81–100%) with a very low rate of LTP (7.2–14%). It is worth mentioning that Hocquelet et al19 showed that the subgroup with medium HCC nodules (3.0–5.0 cm) had lower primary RFA failure and better LTP rates in comparison to matched patients treated with a monopolar single RF needle. Of note, in the above mentioned studies patients with large >5.0 cm and medium (3–5 cm) were 21 patients18 and 32 patients,19 numbers not so different from ours.
Our experience with a switching RF system demonstrated a high rate of technical feasibility and effectiveness (100%) and a LTP rate not different (less than 20%) from other series dealing with smaller tumours26–28 and better than older series dealing with medium-large HCC tumours.4 Our patient population was highly selected: although considered not eligible for surgery, they all had preserved functional reserve, and all but one had only a single HCC. Recently, Saviano et al13 compared liver resection and a single-step combination approach (RFA plus TACE) in solitary medium-large HCC tumours, a clinical scenario similar to that of our study. A cumulative LTP 3 year rate of 58.1% was observed in the 29 patients of RFA plus TACE group, significantly higher than that found in matched resected patients (21.8%). Although in Saviano’s series 20% of tumours were larger than 5 cm in contrast to 11% of the present series, cumulative LTP rate at 3 years (29.3%) of our study appears to be closer to their resection group results. Similarly, 3-year OS in our series (64.7%) turned out to be better than that (48.2%) reported by Saviano et al..13
Although statistically not significant size ≥4.0 cm was associated with neoplastic regrowth after successful treatment. In particular, 2 out of 5 of the relapsing tumors (40%) had baseline size of 4.0 cm. This observation has prompted a change in our protocol in order to further abate LTP: three needles are now employed if nodule size exceeds 3.5 cm.
The multiple needle technique in our hands was safe since no deaths or major complications occurred, in keeping with similar studies.16–18
Our results are not directly comparable with series dealing with sequential combined therapy (TACE plus RFA)12,14 since patients with multinodular disease and a wide range of tumour size were enrolled. However, it is conceivable that our approach consisting of a quick, safe and effective treatment modality in a single session may be advantageous over sequential combined therapy in terms of costs and quality of life by reducing number of hospital accesses and patient discomfort.
Differently from our technique some Microwave equipment may activate simultaneously multiple antennas (up to three): ablation areas obtained in experimental settings are larger and more circular than those created with sequential power delivery.20 However, studies specifically focused on clinical results obtained with simultaneously activated antennas in medium-large HCC nodules are lacking.
Limitations of our study should be considered: this series represents a single centre experience and is relatively small (even if the follow-up was acceptably long), and the technique is challenging since less experienced operators might find it difficult to precisely insert multiple needles in a predetermined spatial configuration
Conclusion
In unresectable patients, deployment of multiple energy sources may provide an useful thermal ablation option and deliver safe and effective first-line therapy for medium-large HCC tumours (especially if solitary), with good outcomes in terms of LTP and OS. Larger studies are needed to confirm these results and to compare this approach to other treatment modalities such as combined therapy and multiple MW antennas
Contributor Information
Giampiero Francica, Email: giampierofrancica@gmail.com.
Michele Altiero, Email: michele.altiero@pinetagrande.it.
Ettore Laccetti, Email: ettore.laccetti@pinetagrande.it.
Filomena Pezzullo, Email: filomena.pezzullo@pinetagrande.it.
Michela Tanga, Email: michela.tanga@pinetagrande.it.
Giuseppe Avitabile, Email: giuseppe.avitabile@pinetagrande.it.
Mathew Elameer, Email: mselameer@googlemail.com.
Mariano Scaglione, Email: mscaglione@tiscali.it.
REFERENCES
- 1. European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 2. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–80. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 3. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013; 59: 300–7. doi: 10.1016/j.jhep.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 4. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000; 214: 761–8. doi: 10.1148/radiology.214.3.r00mr02761 [DOI] [PubMed] [Google Scholar]
- 5. Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 2009; 115: 1914–23. doi: 10.1002/cncr.24196 [DOI] [PubMed] [Google Scholar]
- 6. Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013; 257: 929–37. doi: 10.1097/SLA.0b013e31828329b8 [DOI] [PubMed] [Google Scholar]
- 7. Cucchetti A, Djulbegovic B, Tsalatsanis A, Vitale A, Hozo I, Piscaglia F, et al. When to perform hepatic resection for intermediate-stage hepatocellular carcinoma. Hepatology 2015; 61: 905–14. doi: 10.1002/hep.27321 [DOI] [PubMed] [Google Scholar]
- 8. Medhat E, Abdel Aziz A, Nabeel M, Elbaz T, Zakaria Z, Shousha H, et al. Value of microwave ablation in treatment of large lesions of hepatocellular carcinoma. J Dig Dis 2015; 16: 456–63. doi: 10.1111/1751-2980.12259 [DOI] [PubMed] [Google Scholar]
- 9. Xu Y, Shen Q, Wang N, Liu P, Wu P, Peng Z, et al. Percutaneous microwave ablation of 5-6 cm unresectable hepatocellular carcinoma: local efficacy and long-term outcomes. Int J Hyperthermia 2016; 201: 1–8. doi: 10.1080/02656736.2016.1239842 [DOI] [PubMed] [Google Scholar]
- 10. Sun X, Li RU, Zhang B, Yang Y, Cui Z. Treatment of liver cancer of middle and advanced stages using ultrasound-guided percutaneous ethanol injection combined with radiofrequency ablation: A clinical analysis. Oncol Lett 2016; 11: 2096–100. doi: 10.3892/ol.2016.4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol 2011; 18: 1624–9. doi: 10.1245/s10434-011-1673-8 [DOI] [PubMed] [Google Scholar]
- 12. Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Kayahara T, Makino Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology 2013; 269: 927–37. doi: 10.1148/radiol.13130387 [DOI] [PubMed] [Google Scholar]
- 13. Saviano A, Iezzi R, Giuliante F, Salvatore L, Mele C, Posa A, et al. Liver resection versus radiofrequency ablation plus transcatheter arterial chemoembolization in cirrhotic patients with solitary large hepatocellular carcinoma. J Vasc Interv Radiol 2017; 28: 1512–9. doi: 10.1016/j.jvir.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 14. Hirooka M, Hiraoka A, Ochi H, Kisaka Y, Joko K, Michitaka K, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation: outcomes in patients with Barcelona clinic liver sancer stage B hepatocellular carcinoma. AJR Am J Roentgenol 2018; 210: 891–8. doi: 10.2214/AJR.17.18177 [DOI] [PubMed] [Google Scholar]
- 15. Laeseke PF, Frey TM, Brace CL, Sampson LA, Winter TC, Ketzler JR, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol 2007; 188: 1485–94. doi: 10.2214/AJR.06.1004 [DOI] [PubMed] [Google Scholar]
- 16. Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology 2013; 268: 589–600. doi: 10.1148/radiol.13121736 [DOI] [PubMed] [Google Scholar]
- 17. Tan Y, Jiang J, Wang Q, Guo S, Ma K, Bie P. Radiofrequency ablation using a multiple-electrode switching system for hepatocellular carcinoma within the Milan criteria: long-term results. Int J Hyperthermia 2018; 34: 298–305. doi: 10.1080/02656736.2017.1330495 [DOI] [PubMed] [Google Scholar]
- 18. Seror O, N'Kontchou G, Nault JC, Rabahi Y, Nahon P, Ganne-Carrié N, et al. Hepatocellular Carcinoma within Milan Criteria: No-Touch Multibipolar Radiofrequency Ablation for Treatment-Long-term Results. Radiology 2016; 280: 611–21. doi: 10.1148/radiol.2016150743 [DOI] [PubMed] [Google Scholar]
- 19. Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol 2017; 66: 67–74. doi: 10.1016/j.jhep.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 20. Harari CM, Magagna M, Bedoya M, Lee FT, Lubner MG, Hinshaw JL, et al. Microwave ablation: comparison of simutaneous and sequential activation of multiple antennas in liver model systems. Radiology 2016; 278: 95–103. doi: 10.1148/radiol.2015142151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brace CL, Sampson LA, Hinshaw JL, Sandhu N, Lee FT. Radiofrequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol 2009; 20: 118–24. doi: 10.1016/j.jvir.2008.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clasen S, Rempp H, Schmidt D, Schraml C, Hoffmann R, Claussen CD, et al. Multipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: correlation between volume of coagulation and amount of applied energy. Eur J Radiol 2012; 81: 111–3. doi: 10.1016/j.ejrad.2010.10.031 [DOI] [PubMed] [Google Scholar]
- 23. Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013; 31: 426–32. doi: 10.1200/JCO.2012.42.9936 [DOI] [PubMed] [Google Scholar]
- 24. Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology 2004; 232: 260–71. doi: 10.1148/radiol.2321030821 [DOI] [PubMed] [Google Scholar]
- 25. Dodd GD 3, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol 2001; 177: 777–82. [DOI] [PubMed] [Google Scholar]
- 26. Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008; 43: 727–35. doi: 10.1080/00365520701885481 [DOI] [PubMed] [Google Scholar]
- 27. Francica G, Saviano A, De Sio I, De Matthaeis N, Brunello F, Cantamessa A, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis 2013; 45: 336–41. doi: 10.1016/j.dld.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 28. Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014; 270: 900–9. doi: 10.1148/radiol.13130940 [DOI] [PubMed] [Google Scholar]
- 29. Seror O, N’Kontchou G, Van Nhieu JT, Rabahi Y, Nahon P, Laurent A, et al. Histopathologic comparison of monopolar versus no-touch multipolar radiofrequency ablation to treat hepatocellular carcinoma within Milan criteria. J Vasc Interv Radiol 2014; 25: 599–607. doi: 10.1016/j.jvir.2013.11.025 [DOI] [PubMed] [Google Scholar]