Abstract
Proton radiotherapy is undergoing rapid expansion both within the UK and internationally, but significant challenges still need to be overcome if maximum benefit is to be realised from this technique. One major limitation is the persistent uncertainty in proton relative biological effectiveness (RBE). While RBE values are needed to link proton radiotherapy to our existing experience with photon radiotherapy, RBE remains poorly understood and is typically incorporated as a constant dose scaling factor of 1.1 in clinical plans. This is in contrast to extensive experimental evidence indicating that RBE is a function of dose, tissue type, and proton linear energy transfer, among other parameters. In this article, we discuss the challenges associated with obtaining clinically relevant values for proton RBE through commonly-used assays, and highlight the wide range of other experimental end points which can inform our understanding of RBE. We propose that accurate and robust optimization of proton radiotherapy ultimately requires a multiscale understanding of RBE, integrating subcellular, cellular, and patient-level processes.
introduction
Despite decades of investigation, uncertainties in relative biological effectiveness (RBE) remain a key Achilles’ heel for proton therapy. A fixed RBE of 1.1 was adopted as a pragmatic clinical standard1 but unease persists, particularly regarding end-of-range elevations in RBE. These end-of-range concerns influence decisions such as beam configuration for certain treatments, e.g. if a patient’s tumour is in close proximity to a critical organ such as the brainstem.2 Additional uncertainties in proton RBE according to tissue type/end point and dose level further limit our interpretation of results from both planning studies and clinical trials which seek to compare photons and protons.
Over the coming years, practical solutions to mitigate end-of-range RBE uncertainties are likely to be translated into clinical practice. It has been demonstrated that, under certain circumstances, intensity modulated proton therapy (IMPT) with multifield optimisation can be used to push end-of-range linear energy transfer (LET) hotspots away from organs at risk, at little cost to target dose distributions.3 However, LET (or other physics-based surrogates for RBE) have yet to be incorporated within broader treatment planning system (TPS) frameworks for robust optimisation. Beam number and beam angle selection will also be important considerations: wide “hinge-angles” between beams offer greater scope for LET optimisation and even proton arc-based solutions have been proposed.4 But ultimately, RBE is more than an end-of-range problem that can be easily mitigated by incorporating physics-based surrogates into treatment plan optimisation. Even after physics-based mitigation, RBE introduces considerable biological uncertainty into predictions of treatment response. Improving our understanding of RBE and photon vs proton radiobiology would undoubtedly enable us to improve patient outcomes in proton therapy.5
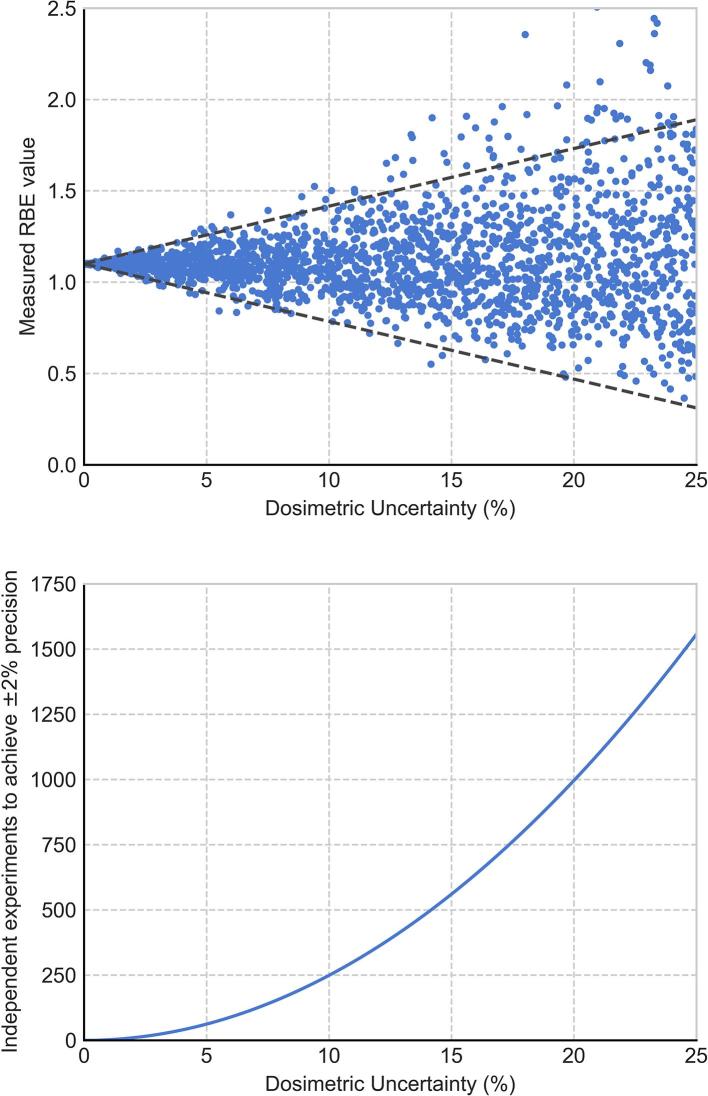
Traditionally, proton RBE has been estimated using repeated measures of clonogenic cell survival. In 2014, Paganetti performed a comprehensive analysis of experimental RBE values for clonogenic survival drawing upon 369 published data points, themselves the product of repeat measurements, from 76 studies.6 Even with the vast body of input data considered, and using relatively coarse binning in either α/β ratio or LET, the mean RBE values Paganetti reported were associated with 95% confidence intervals of up to ± 10%.6 While errors can be reduced by carrying out more simple survival comparisons, this approach is limited. As illustrated in Figure 1, with even modest assumptions of experimental error it could require several hundred independent experiments to provide ±2% confidence intervals for a single experimental condition, demands which are not feasible across the whole range of clinically relevant LET and α/β parameters. Consequently, the field needs to draw on a wider range of sources to better constrain and refine our models and predictions of RBE.
Figure 1.
Illustration of experimental RBE uncertainties. Top: “Measured” RBEs have been generated by sampling values for photon and proton radiosensitivity based on a “true” RBE of 1.1 with normally distributed uncertainties of different magnitudes (points). Even small experimental uncertainties can lead to large uncertainties in derived RBEs (95% CI, dotted line). Bottom: Number of independent experiments needed to achieve a 95% CI of ± 2% on RBE in this system. Even with modest experimental errors of 10%, over 250 experiments studying identical conditions are needed to achieve this target. CI,confidence interval; RBE, relative biologicaleffectiveness.
In this article, we outline the main sources of data which serve to improve our understanding of proton RBE at multiple scales, propose strategies to integrate such data into models and discuss the potential clinical impacts for proton therapy.
Multiscale sources of data
Nanoscopic and microscopic physics
Primary proton energy deposition patterns at the subcellular scale and secondary hadrons produced by nuclear interactions are key drivers of RBE variation. Survival following proton exposure is usually parameterised according to the amount of energy delivered to the system (dose) and the density or complexity of the delivery, which is typically described in terms of LET. However, while LET (averaged either by proton track or by dose) is a useful surrogate for the complexity of the damage resulting from the proton exposure, it provides an incomplete view of energy depositions on the nanoscale.
In reality, there is a change in not only the total amount of energy deposited by a proton as it passes through a cell, but also in the spatial distribution of energy depositions around a proton track and the energies of the secondary delta electrons produced. There is growing evidence that spatial effects may combine non-linearly to increase the complexity of damage in cells, making averaged LET at best an approximate surrogate for biological effect—particularly for the complex broad LET spectra which would be seen by cells during proton therapy treatment.
While these effects are complex, our capacity to model and quantify them is growing. A number of Monte Carlo track structure codes (such as Geant4-DNA7 and PARTRAC8) offer the potential to calculate individual energy deposition events on the scales relevant to biological damage. These physical predictions can increasingly be tested and validated using advances in nano- and microdosimetric techniques, such as tissue equivalent proportional counters9 to allow calculations of ionisation distributions, and fluorescent nuclear track detectors10, 11 which enable the co-visualisation of charged particle tracks and DNA damage in appropriately cultured cells.
Together, these observations can provide valuable insights into energy deposition on the nanoscale, and how this translates into the biophysical consequences of radiation, such as initial DNA damage and its complexity.
Cellular biology
Studies of differences in clonogenic cellular survival following exposure to different radiation types have been the mainstay of RBE studies for over five decades and have produced valuable insights into the biology underlying proton RBE, in spite of the above-mentioned statistical challenges. The overall trend of reduced survival with increasing LET has been well-understood for some time, being reviewed, e.g. by Jack Fowler in his 1981 book on particle therapy:12 as LET increases cells suffer more “single-hit cell death, reflected in an increase in the α linear–quadratic component and a steeper dose response curve, leading to greater sensitivity to a given dose.6 However, the mechanisms by which this translation from more complex energy deposition to increased cell killing occurs remains the subject of some disagreement. Changes in both the initial number13 and the complexity14 of double strand breaks (DSBs) have been proposed as potential mechanisms, as well as differences in the epigenetic effects of different radiation qualities.15
While tumour cell death is the key driver of tumour control probability, cellular responses are not limited to survival alone. Yields of DNA DSBs, mutations, and chromosome aberrations are all also known to vary with irradiation type and LET. Effects such as the perturbation of cell communication through the induction of inflammatory molecules should also be considered. These end points all have their own complexities and uncertainties, but they can be used to probe different aspects of the early stages of radiation response in both tumours and normal tissues, providing radiobiological insight far exceeding that offered by isolated study of cell survival.
Furthermore, by coupling these observations with the significant advances in molecular biology and our improved knowledge of how cells respond to and repair DNA damage, there is great potential to further refine our understanding of the key determinants of cellular fate. This is particularly significant in light of the growing interest in radiotherapy personalisation, and evidence that some DNA repair mutations which are commonly found in many cancers confer additional sensitivity to proton irradiation.16, 17
3D tissue and animal data
While in vitro cellular studies offer enormous potential for us to probe basic biological responses to proton and photon radiation, within clinical proton therapy each cell’s host environment at the micro-, tissue-, organ- and body-levels) will also prove important. Thus, there exists a clear requirement to determine how proton RBE effects observed in vitro translate to effects within more complete biological systems.
Developments in tissue engineering have led to the construction of realistic three-dimensional models of both normal tissues and tumours.18 These models (typically built upon fabricated scaffolds) can usefully mimic three-dimensional cellular arrangements, interactions and microenvironments found in vivo. To date, the application of such models to the study of proton RBE has been limited,19 but could provide extensive, reproducible data sets which bridge the gap between in vitro and in vivo RBE experiments.
In the early decades of proton therapy and indeed proton RBE (1950–2000), a variety of animal end points (such as skin reactions, organ weight loss or the dose at which half the laboratory animals died) were studied, with the majority returning RBE values broadly consistent with 1.1 (6). Over recent years, further experiments have been conducted to investigate how specific proton RBE effects observed in vitro—particularly, those associated with end-of-range LET elevation—translate within animals. For the early normal-tissue end point of skin reaction in mice, enhanced biological effects have been reported for the distal edge of the proton spread-out Bragg peak (SOBP) and the first part of the distal dose drop-off.20 Similarly, for another early end point of intestinal crypt regeneration in mice, proton irradiations at the distal edge of the SOBP were found to be statistically more effective than at the middle of the SOBP by a factor of 14% (1.05–1.23).21 For a late end point of mouse death due to pneumonitis after selective irradiation of the thorax, a statistically insignificant 6% increase in proton RBE from the middle to the end of the SOBP was reported.22 For both the mouse intestine and mouse thorax studies, however, the authors noted that in vivo RBE determination was difficult, in part because each biological system had a thickness of approximately 1.5 cm, such that the range of LETs considered across the system was broad and the RBE values yielded were not necessarily averages for the middle/distal portions of the SOBP.21, 22
The acquisition of further animal data to study clinically relevant RBE values in vivo will remain technically, ethically and financially challenging. However, in vivo demonstrations of effects predicted by multistage RBE models will likely prove necessary if such models are to be transferred to proton clinics. Animal models are likely to prove particularly helpful in validating the performance of RBE models in terms of tolerance doses and fractionation effects.23 Application of advanced imaging techniques may enable us to further probe RBE effects pre-clinically for relevant end points.
Follow-up imaging of variable RBE effects in patients
Within translational radiotherapy research, new emphases have been placed on the application of radiomics24 and data mining25 to learn from every patient treated”. These approaches are likely to prove especially valuable within proton therapy and the study of RBE, where patient numbers will remain small.
For ependymoma patients, a 2016 retrospective analysis found the incidence of voxelized image changes post-proton therapy (contoured hyperintensity on T2-FLAIR MR images) to be correlated to both proton dose and LET.26 The dose at which image changes occurred was found to be lower when combined with elevated LET values, indicating an increase in biological effectiveness with increased LET. This study offered the first clinical evidence for variable proton RBE and further proposed a method for developing clinically relevant, but again empirical, RBE models.23
Further evidence for variable proton biological effectiveness has recently been reported in a study of asymptomatic late-phase radiographic changes amongst chest wall patients27. For matched proton and photon cohorts, asymptomatic late-phase radiographic changes within the lung were found to be significantly more prominent amongst those treated with protons.27 An en-face proton beam arrangement was used, such that the authors report that the RBE elevation observed could be attributable to either (i) end-of-range proton LET elevation, (ii) the late, normal tissue end point considered, or a combination of these two.27 Regardless, follow-up imaging was used to demonstrate that clinically-relevant RBEs exceeded 1.1.
Particularly, as the field of quantitative imaging develops, there exists considerable potential for prospective clinical studies with harmonized follow-up imaging protocols to deliver additional insight into proton RBE and to play a role in RBE model development/validation. The anticipated opening of the UK’s first proton radiotherapy centres later this year may prove an ideal opportunity to build links between the clinical and research communities within the UK to further address these questions.
Clinical outcomes analyses
Prospective, randomized controlled trials remain the gold-standard for clinical evidence in radiation oncology and results from proton vs photon trials should enable us to directly compare the response of human tumours to the two radiation modalities. In particular, metrics such as TCD50, i.e. the dose required to achieve a local tumour control rate of 50%, may alert us to gross deficiencies in our assumed RBE value of 1.1 for tumour cell kill.6 Results from prostate trials might prove particularly insightful: RBE values substantially exceeding 1.1 are typically modelled across all SOBP positions (assuming standard fractionation), due to prostate’s low α/β ratio.28
For early- and late-phase normal tissue end points, investigation of the relationship between clinical outcome and proton RBE will prove more challenging as organs at risk (or parts, thereof) typically receive highly heterogeneous doses in proton treatments. Due to the photon “dose-bath”, entire organs at risk are typically irradiated to more homogeneous levels within photon treatments, such that separation of volume effects from RBE effects will not be easy. The collection and storage of both clinical and patient reported outcome data, plus voxelized dose and LET maps will prove important, if we are to work towards new voxel-by-voxel RBE and normal tissue complication probability models for healthy tissues. Further, rates of second cancer induction will be a key consideration for proton treatments,29 particularly for paediatric patients.
Approaches to integrate different data types
There are a variety of sources of RBE data which could be used to refine multiscale RBE models, but their integration is not trivial. Many of the most widely used RBE models are empirical modifications to linear-quadratic survival curves, based on fitting to large data sets of cell survival which are then extrapolated to clinical conditions.30–32 While the mathematical form of these modifications is motivated by our understanding of the underlying biology and physics, this empirical approach is poorly-suited to the integration of parameters from different end points, or making extrapolations to behaviours in complex in vivo systems. Instead, fully integrating all of these data to predict clinically-relevant RBEs requires more comprehensive models of radiation response. Ideally, each stage of the radiation response process should be described in a suitable series of interlinked models, allowing for communication of information between scales. For example, linking models of nanoscale energy deposition to DSB yield and complexity will enable predictions of damage resulting from novel exposures. These damage predictions can then be used as input into models of DNA repair, which in turn feed into models of cellular survival. Finally, these cellular models could be integrated into pre-clinical and clinical models of tumour and normal tissue response.
By rigorously validating these models at each stage of the radiation response process, they could be linked more fundamentally to our knowledge of the underlying biology, and given greater predictive power. A number of frameworks exist which seek to make more mechanistic predictions of radiation sensitivity in a variety of end points,33–36 but these models still lack the comprehensive coverage and validation required for clinical translation. A major challenge in this area is the availability of robust integrated validation data—while there are an abundance of experimental studies of changes in cell survival in terms of dose and LET, there are relatively few probing other intermediate end points such as DNA repair or chromosome aberrations. Fewer still incorporate all of these experimental end points in a single study. Thus, interexperiment comparisons are required, introducing a number of confounding factors, even as we attempt to build predictive models at the cellular level. Even more difficult is the validation of in vitro cellular models within pre-clinical systems and ultimately within patient outcome studies.
However, the multiscale approach offers the potential to develop more robust, translatable models of proton RBE which integrate all of the available data. To achieve this, closer collaboration is needed between the disciplines involved in proton therapy research, with greater links between modellers and physicists, chemists, biologists and clinical teams, to identify and design the experiments and clinical studies which can provide the greatest insights into this problem.
The potential impact of multiscale RBE modelling on clinical proton therapy
Within carbon ion radiotherapy, clinical dose optimisation has been performed based on empirical RBE models which draw upon tissue α/β values from photon irradiations and either voxelized LET distributions or microscopic energy deposition patterns.37 Proton clinics have been reluctant to follow suit and adopt dose optimisation based upon RBE modelling, because (i) despite decades of proton practice, there exists a paucity of clinical evidence that an assumed RBE of 1.1 results in significant over-/underdosage, and (ii) for protons, probable deviations from the fixed value of 1.1 within a patient are often perceived to be of the same order as uncertainties associated with empirical RBE modelling. These reasons, combined with hesitancy to change well-established clinical protocols and begin compromise target coverage in terms of physical dose, mean that, at present, even end-of-range proton RBE mitigation strategies (such as LET-based optimisation) are likely to gain rapid traction only if they can be implemented at little cost to standard proton physical dose distributions. Meanwhile, proton treatment planning studies which draw upon empirical RBE models are increasingly highlighting end-of-range RBE effects as a serious area for concern with regards to possible toxicities,38 and RBE uncertainties clearly impact upon both normal tissue complication probability and tumour control probability estimates for proton therapy.39 Improved understanding of proton RBE would bring increased precision to model-based selection of those patients who stand to benefit most from proton therapy,40 enhancing clinical trial design and interpretation. With sufficient validation, multiscale RBE models could also offer true “biological dose” optimisation within clinical treatment planning systems, dose-painting of radioresistant tumour regions and perhaps, even treatment individualisation based upon a specific patient’s tumour or normal tissue biology.
conclusion
Individual research groups have studied proton RBE at their specific scales for many decades, but thus far an overarching solution for clinical practice has remained elusive. Collaboration on proton RBE should be prioritised by our wider community: diverse expertise must be brought together as we seek to form consensus on proton RBE issues and ultimately work towards the validation of multi scale models. Over 40 years on, the words of Professor Jack Fowler are particularly pertinent41 :
“Radiobiology contributes to radiotherapy a framework of ideas, available to every thinking radiotherapist… First enough information has to be gathered for radiobiology to be able to explain the successes and failures of radiotherapy… Progress can be made by a continuing dialogue between radiotherapists and radiobiologists. At each stage the implications of radiobiological results should be reviewed for comparison with clinical experience”.41
Contributor Information
Tracy SA Underwood, Email: tsa.underwood@gmail.com.
Stephen J McMahon, Email: stephen.mcmahon@qub.ac.uk.
REFERENCES
- 1. ICRU report 78: prescribing, recording, and reporting proton-beam therapy. J Icru 2008; 7: 21–8. [Google Scholar]
- 2. Giantsoudi D, Adams J, MacDonald SM, Paganetti H. Proton treatment techniques for posterior fossa tumors: consequences for linear energy transfer and dose-volume parameters for the brainstem and organs at risk. Int J Radiat Oncol Biol Phys 2017; 97: 401–10. doi: 10.1016/j.ijrobp.2016.09.042 [DOI] [PubMed] [Google Scholar]
- 3. Unkelbach J, Botas P, Giantsoudi D, Gorissen BL, Paganetti H. Reoptimization of intensity modulated proton therapy plans based on linear energy transfer. Int J Radiat Oncol Biol Phys 2016; 96: 1097–106. doi: 10.1016/j.ijrobp.2016.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanchez-Parcerisa D, Kirk M, Fager M, Burgdorf B, Stowe M, Solberg T, et al. Range optimization for mono- and bi-energetic proton modulated arc therapy with pencil beam scanning. Phys Med Biol 2016; 61: N565–N574. doi: 10.1088/0031-9155/61/21/N565 [DOI] [PubMed] [Google Scholar]
- 5. Underwood T, Paganetti H. Variable proton relative biological effectiveness: how do we move forward? radiation oncology biology. Elsevier Inc 2016; 95: 56–8. [DOI] [PubMed] [Google Scholar]
- 6. Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014; 59: R419–R472. doi: 10.1088/0031-9155/59/22/R419 [DOI] [PubMed] [Google Scholar]
- 7. Bernal MA, Bordage MC, Brown JMC, Davídková M, Delage E, El Bitar Z, Bitar El Z, et al. Track structure modeling in liquid water: A review of the Geant4-DNA very low energy extension of the Geant4 Monte Carlo simulation toolkit. Phys Med 2015; 31: 861–74. doi: 10.1016/j.ejmp.2015.10.087 [DOI] [PubMed] [Google Scholar]
- 8. Friedland W, Dingfelder M, Kundrát P, Jacob P, structures T. DNA targets and radiation effects in the biophysical monte carlo simulation code PARTRAC. Mutation research - fundamental and molecular mechanisms of mutagenesis. Elsevier 2011; 711: 28–40. [DOI] [PubMed] [Google Scholar]
- 9. Nikjoo H, Khvostunov IK, Cucinotta FA. The response of tissue-equivalent proportional counters to heavy ions. Radiat Res 2002; 157: 435–45. doi: 10.1667/0033-7587(2002)157[0435:TROTEP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 10. Akselrod GM, Akselrod MS, Benton ER, Yasuda N. A novel Al2O3 fluorescent nuclear track detector for heavy charged particles and neutrons. Nucl Instrum Methods Phys Res 2006; 247: 295–306. doi: 10.1016/j.nimb.2006.01.056 [DOI] [Google Scholar]
- 11. Underwood TS, Sung W, McFadden CH, McMahon SJ, Hall DC, McNamara AL, et al. Comparing stochastic proton interactions simulated using TOPAS-nBio to experimental data from fluorescent nuclear track detectors. Phys Med Biol 2017; 62: 3237–49. doi: 10.1088/1361-6560/aa6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fowler JF. Nuclear particles in cancer treatment. medical physics handbooks; no. 8; 1981. [Google Scholar]
- 13. Semenenko VA, Stewart RD. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys Med Biol 2006; 51: 1693–1706. doi: 10.1088/0031-9155/51/7/004 [DOI] [PubMed] [Google Scholar]
- 14. Schipler A, Iliakis G. DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res 2013; 41: 7589–605. doi: 10.1093/nar/gkt556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durante M. New challenges in high-energy particle radiobiology. Br J Radiol 2014; 87: 20130626 p.. doi: 10.1259/bjr.20130626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grosse N, Fontana AO, Hug EB, Lomax A, Coray A, Augsburger M, et al. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys 2014; 88: 175–81. doi: 10.1016/j.ijrobp.2013.09.041 [DOI] [PubMed] [Google Scholar]
- 17. Liu Q, Ghosh P, Magpayo N, Testa M, Tang S, Gheorghiu L, et al. Lung cancer cell line screen links fanconi anemia/BRCA pathway defects to increased relative biological effectiveness of proton radiation. Int J Radiat Oncol Biol Phys 2015; 91: 1081–9. doi: 10.1016/j.ijrobp.2014.12.046 [DOI] [PubMed] [Google Scholar]
- 18. Rijal G, Li W. 3D scaffolds in breast cancer research. Biomaterials 2016; 81: 135–56. doi: 10.1016/j.biomaterials.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 19. Hamdi DH, Barbieri S, Chevalier F, Groetz JE, Legendre F, Demoor M, et al. In vitro engineering of human 3D chondrosarcoma: a preclinical model relevant for investigations of radiation quality impact. BMC Cancer 2015; 15: 20130626–14. doi: 10.1186/s12885-015-1590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sørensen BS, Bassler N, Nielsen S, Horsman MR, Grzanka L, Spejlborg H, et al. Relative biological effectiveness (RBE) and distal edge effects of proton radiation on early damage in vivo. Acta Oncol 2017; 56: 1387–91. doi: 10.1080/0284186X.2017.1351621 [DOI] [PubMed] [Google Scholar]
- 21. Gueulette J, Slabbert JP, Böhm L, De Coster BM, Rosier JF, Octave-Prignot M, et al. Proton RBE for early intestinal tolerance in mice after fractionated irradiation. Radiother Oncol 2001; 61: 177–84. doi: 10.1016/S0167-8140(01)00446-7 [DOI] [PubMed] [Google Scholar]
- 22. Gueulette J, Böhm L, Slabbert JP, De Coster BM, Rutherfoord GS, Ruifrok A, et al. Proton relative biological effectiveness (RBE) for survival in mice after thoracic irradiation with fractionated doses. Int J Radiat Oncol Biol Phys 2000; 47: 1051–8. doi: 10.1016/S0360-3016(00)00535-6 [DOI] [PubMed] [Google Scholar]
- 23. Jones B. Why RBE must be a variable and not a constant in proton therapy. Br J Radiol 2016; 89: 20160116–10. doi: 10.1259/bjr.20160116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moran A, Daly ME, Yip SSF, Yamamoto T. Radiomics-based assessment of radiation-induced lung injury after stereotactic body radiotherapy. Clin Lung Cancer 2017; 18: e425–e431. doi: 10.1016/j.cllc.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 25. Price G, van Herk M, Faivre-Finn C. Data mining in oncology: the ukCAT project and the practicalities of working with routine patient data. Clin Oncol 2017; 29: 814–7. doi: 10.1016/j.clon.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 26. Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol 2016; 121: 395–401. doi: 10.1016/j.radonc.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Underwood TSA, Grassberger C, Bass R, MacDonald SM, Meyersohn NM, Yeap BY, et al. Asymptomatic late-phase radiographic changes amongst chest wall patients are associated with a proton RBE exceeding 1.1. Int J Radiat Oncol Biol Phys 2018; 101: 809–19. doi: 10.1016/j.ijrobp.2018.03.037 [DOI] [PubMed] [Google Scholar]
- 28. Underwood T, Giantsoudi D, Moteabbed M, Zietman A, Efstathiou J, Paganetti H, et al. Can we advance proton therapy for prostate? considering alternative beam angles and relative biological effectiveness variations when comparing against intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2016; 95: 454–64. doi: 10.1016/j.ijrobp.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 29. Trott KR. Special radiobiological features of second cancer risk after particle radiotherapy. Phys Med 2017; 42: 221–7. doi: 10.1016/j.ejmp.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 30. McNamara AL, Schuemann J, Paganetti H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys Med Biol 2015; 60: 8399–416. doi: 10.1088/0031-9155/60/21/8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wedenberg M, Lind BK, Hårdemark B. A model for the relative biological effectiveness of protons: The tissue specific parameter α/β of photons is a predictor for the sensitivity to LET changes. Acta Oncol 2013; 52: 580–8. doi: 10.3109/0284186X.2012.705892 [DOI] [PubMed] [Google Scholar]
- 32. Carabe-Fernandez A, Dale RG, Jones B. The incorporation of the concept of minimum RBE ( RBE min ) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int J Radiat Biol 2007; 83: 27–39. doi: 10.1080/09553000601087176 [DOI] [PubMed] [Google Scholar]
- 33. McMahon SJ, McNamara AL, Schuemann J, Paganetti H, Prise KM. A general mechanistic model enables predictions of the biological effectiveness of different qualities of radiation. Sci Rep 2017; 7: 1–14. doi: 10.1038/s41598-017-10820-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McMahon SJ, Schuemann J, Paganetti H, Prise KM. Mechanistic modelling of DNA repair and cellular survival following radiation-induced DNA damage. Sci Rep 2016; 6: 1–14. doi: 10.1038/srep33290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frese MC, Yu VK, Stewart RD, Carlson DJ. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int J Radiat Oncol Biol Phys 2012; 83: 442–50. doi: 10.1016/j.ijrobp.2011.06.1983 [DOI] [PubMed] [Google Scholar]
- 36. Verkhovtsev A, Surdutovich E, Solov’yov AV. Multiscale approach predictions for biological outcomes in ion-beam cancer therapy. Sci Rep 2016; 6: 27654. doi: 10.1038/srep27654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karger CP, Peschke P. RBE and related modeling in carbon-ion therapy. Phys Med Biol 2017; 63: 01TR02–88. doi: 10.1088/1361-6560/aa9102 [DOI] [PubMed] [Google Scholar]
- 38. Underwood TS, Voog JC, Moteabbed M, Tang S, Soffen E, Cahlon O, et al. Hydrogel rectum-prostate spacers mitigate the uncertainties in proton relative biological effectiveness associated with anterior-oblique beams. Acta Oncol 2017; 56: 575–81. doi: 10.1080/0284186X.2016.1275781 [DOI] [PubMed] [Google Scholar]
- 39. Harald P. Relating the proton relative biological effectiveness to tumor control and normal tissue complication probabilities assuming interpatient variability in α/β. Acta Oncol 2017; 0: 1–8. [DOI] [PubMed] [Google Scholar]
- 40. Bijman RG, Breedveld S, Arts T, Astreinidou E, de Jong MA, Granton PV, et al. Impact of model and dose uncertainty on model-based selection of oropharyngeal cancer patients for proton therapy. Acta Oncol 2017; 56: 1444–50. doi: 10.1080/0284186X.2017.1355113 [DOI] [PubMed] [Google Scholar]
- 41. Fowler JF. Current aspects of radiobiology as applied to radiotherapy. Clin Radiol 1972; 23: 257–62. doi: 10.1016/S0009-9260(72)80042-4 [DOI] [PubMed] [Google Scholar]