Abstract
Context
There is limited information on the influence of vitamin D on physical performance in black Americans.
Objective
To determine if maintenance of serum 25(OH)D >75 nmol/L prevents a decline in physical performance.
Design
The Physical Performance, Osteoporosis and Vitamin D in African American Women (PODA) trial had a prospective, randomized, placebo controlled, double-dummy design with two arms: one of which is placebo vitamin D3 adjusted to maintain serum 25(OH)D >75 nmol/L.
Patients
The target population was healthy elderly black women with serum 25(OH)D between 20 and 65 nmol/L. The trial was 3 years in duration with measurement of physical performance every 6 months: grip strength, Short Physical Performance Battery (SPPB), 10 chair rises, and 6-minute walk distance. A total of 260 women entered the study and 184 completed 3 years. Mean age was 68.2 years. Baseline 25(OH)D was 53 nmol/L; total SPPB was 11 (10 to 12).
Setting
Research center in an academic health center.
Main Outcomes Measure
Prevention of decline in physical performance measures.
Intervention
Participants were randomly assigned to placebo or active vitamin D. Vitamin D3 dose was adjusted to maintain serum 25(OH)D >75 nmol/L.
Results
There was a decline with time in grip strength and the 6-minute walk test. The SPBB increased with time. There were no substantial differences between the placebo and active vitamin D3 groups with respect to the temporal patterns observed for any of the performance measures.
Conclusion
There is no benefit of maintaining serum 25(OH)D >75 nmol/L in preventing the decline in physical performance in healthy black American women.
Maintenance of serum 25(OH)D >75 nmol/L in elderly black women did not prevent decline in physical performance compared with placebo group with serum 25(OH)D approximating the recommended daily allowance of 50 nmol/L.
The decline in physical performance with age is associated with increased frailty, a loss of autonomy, and an increase in falls and fractures (1, 2). This decline is associated with low vitamin D status [low serum 25(OH)D levels] (3–5). Evidence supports the role of vitamin D in maintaining physical performance and strength in older adults (6–8). Some studies suggest a direct effect of vitamin D on muscle function and muscle mass (9, 10). There is an association between vitamin D deficiency and muscle weakness, poor balance, and falls. Higher serum concentrations of 25(OH)D have been associated with greater muscle mass, increased grip strength, stronger quadriceps, improved lower extremity functioning, and better functional performance (7, 11–14).
Besides aging, there are differences in physical performance and strength in sex and race. With increasing age, racial differences increase. Black Americans have low 25(OH)D partially as a result of decreased absorption of UV rays in the skin (15). They have, therefore, been considered a “vulnerable” population. However, despite their lower 25(OH)D levels, black Americans have a superior skeletal mass; this is the “vitamin D paradox.” (16). They also have a superior muscle mass and fewer falls and fractures (16–19). The Institute of Medicine (IOM) set the recommended daily allowance (RDA)-associated 25(OH)D at 50 nmol/L for all sexes and ethnic groups. The Endocrine Society, on the other hand, suggested an optimal serum level of 25(OH)D >75 nmol/L, and stated that blacks comprise an “at risk” population for vitamin D deficiency. There is, however, insufficient evidence from randomized clinical trials to establish the role of vitamin D in preventing a decline in physical performance in black women.
In an exploratory short-term prospective study, we observed an increase in muscle strength in response to vitamin D3 supplementation in black American women (20). As a result, we hypothesized that vitamin D supplementation will prevent the aging decline in physical performance and muscle strength in older black American women. To test our hypothesis, we initiated the Physical Performance, Osteoporosis and Vitamin D in African American Women trial (PODA). We report here on this study, which posed the following question: Does maintaining serum 25(OH)D >75 nmol/L (as suggested by the Endocrine Society guidelines) prevent the decline in physical performance in elderly black women?
Methods
Study design
The PODA study is a prospective, randomized, double-dummy, placebo-controlled, 3-year clinical trial of vitamin D3 supplementation in black women older than 60 years of age. The trial was approved by the institutional review board of Winthrop University Hospital. Details of the study population and design have been reported previously, as have the responses on bone mineral density (21, 22). At least three of four grandparents were self-reported to be black as an entry criterion. Participants with 25(OH)D ≤20 nmol/L and >65 nmol/L at baseline were excluded from the study.
A total of 260 healthy participants were randomized to vitamin D3 or placebo. The vitamin D3 was adjusted at 3-month intervals to maintain the serum 25(OH)D level between 75 and 172 nmol/L. The blind was maintained by the research pharmacist adjusting the placebo dose to match the distribution of dose changes in the active group (a double-dummy design).
Physical performance and measures of strength
Neuromuscular function was assessed by the Hawaii modification of the Short Physical Performance Battery (SPPB), grip strength, and 6-minute walk test (6MWT) at baseline and every 6 months thereafter (23). The Hawaii modification expands the original SPPB battery to make it more demanding to avoid a “ceiling effect.” In addition to producing its own score, the modified battery also allows for the calculation of a score for the traditional SPPB. Under this modification, participants completed 10 repeated chair stand rises.
Grip strength, an indicator of upper extremity muscle strength, was measured using a handgrip dynamometer (Jamar Dynamometer; Alimed Inc., Dedham, MA). Grip strength was measured in the dominant hand and the mean of three measurements was recorded. The outcome is force-generated (lb/in2), with higher values indicative of greater grip strength. Participants also completed a 6MWT to assess walking endurance. The outcome was total distance traveled in meters. The mean distance covered in the 6MWT by healthy older adults was >500 m (24). Physical activity was assessed by the Paffenbarger index. There was no difference in physical activity between groups through the study.
Laboratory
Fasting blood samples were collected at baseline and visits every 3 months. Serum samples for measurement of vitamin D metabolites were analyzed at baseline and annually by the Department of Laboratory Medicine at the University of Washington (Seattle, WA) using liquid chromatography-tandem mass spectrometry with deuterated internal standards for each analyte (25). Concentrations of 25(OH)D2, 25(OH)D3, and 24,25(OH)2D3 were standardized to National Institute of Standards and Technology Standard Reference Material 972a. Serum 25(OH)D was measured for purposes of inclusion and of dose adjustment by Labcorp (Burlington, NC) using a DiaSorin platform. Intact parathyroid hormone levels were measured in serum at baseline and at 6-month intervals using the Immulite 2000 Analyzer assay (Diagnostic Products Corporation, Los Angeles, CA). Study design, recruitment strategies, compliance, and participant safety were monitored semiannually by a Data Safety and Monitoring Board.
Statistical analysis
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). For all participants, absolute values are expressed as mean (standard deviation) if considered to be normally distributed, median (interquartile range) if nonnormal, or count (%) if categorical. Baseline differences in measurements were formally tested via t or Wilcoxon test, as appropriate for the assumed distribution. Percent change from baseline for all performance outcomes is presented graphically as the average % change ± 1 SE. To incorporate data from all study visits, we examined absolute performance values as a function of treatment group, study visit, and the interaction between study visit and group (to assess whether there are treatment group differences in the temporal patterns observed). Model estimates were generated using generalized estimating equations to account for the longitudinal nature of the data. For any model with a statistically insignificant interaction effect (P > 0.10), a more parsimonious model was estimated as the final model, constraining the effect of time to be the same for both treatment groups. Statistical contrasts were estimated from the model to formally test the change in performance at 36 months from baseline. P values based on these contrasts are presented. To assess statistical significance with respect to group differences over time, we accounted for differences in baseline levels in these measurements by adding a group effect to the model. In secondary models and to assess the robustness of our a priori approach, we performed a longitudinal analysis of covariance, explicitly adjusting for baseline outcome levels in the model and performing a joint test of the group and interaction of time and group effects. Correlations (r) between measurements and changes in measurements were estimated using Spearman correlation coefficients.
Results
Demographics and baseline
The predominant ethnicity of participants was black. Table 1 shows some differences at baseline between groups. Small, statistically significant differences were noted between groups in five chair stand scores, total SPPB score, and 6MWT. These were all slightly superior in the active treatment group, suggesting higher performance in this group. There were no substantial differences in the numbers in each group with slow gait speed (<0.8 m/s) (10% overall) or weakness (grip strength <16 kg) (2.7% overall). Baseline serum 25(OH)D did not differ between groups.
Table 1.
Demographics and Clinical Characteristics
| Active (N = 130) | Placebo (N = 130) | Overall (N = 260) | P a | |
|---|---|---|---|---|
| Demographics and behavioral | ||||
| Age, yb | 67.8 (65.1-71.5) | 69.0 (65.4-73.4) | 68.2 (65.4-72.5) | 0.251 |
| BMI, kg/m2b | 30.1 (26.4-34.6) | 29.9 (26.8-33.9) | 30.0 (26.5-34.1) | 0.923 |
| Calcium intake, mgb | 842.0 (600-1142) | 826.5 (628.0-1185) | 828.0 (614.0-1164) | 0.857 |
| Physical performance and activity | ||||
| SPPB Balance Scoreb | 4 (4-4) | 4 (4-4) | 4 (4-4) | 0.948 |
| SPPB Gait Speed Scoreb | 4 (3-4) | 4 (3-4) | 4 (3-4) | 0.312 |
| SPPB 5 Chair Stand Scoreb | 4 (3-4) | 3 (2-4) | 4 (3-4) | 0.002 |
| SPPB total scoreb | 12 (10-12) | 11 (10-12) | 11 (10-12) | 0.009 |
| 10 Chair stand time, sb | 23.0 (18.6-27.7) | 24.2 (19.4-28.5) | 23.5 (19.1-28.2) | 0.191 |
| Grip strength, lb/in2 | 64.7 ± 13.4 | 61.9 ± 13.9 | 63.3 ± 13.7 | 0.100 |
| Caloric expenditure, kcal/wkb | 3637 (2352-5166) | 3092 (1912-4908) | 3392 (2064-4963) | 0.202 |
| 6MWT, mb | 407.0 (357.0-453.0) | 387.0 (324.0-432.0) | 396.0 (347.0-444.0) | 0.015 |
| Bone density | ||||
| Hip total BMD, g/cm2 | 0.919 ± 0.130 | 0.935 ± 0.134 | 0.927 ± 0.132 | 0.329 |
| T-score total femur | −0.185 ± 1.065 | −0.054 ± 1.096 | −0.120 ± 1.080 | 0.329 |
| Femoral neck BMD, g/cm2b | 0.767 (0.694-0.9) | 0.805 (0.718-0.9) | 0.785 (0.707-0.9) | 0.109 |
| Wrist 1/3 BMD, g/cm2 | 0.689 ± 0.067 | 0.692 ± 0.075 | 0.691 ± 0.071 | 0.684 |
| Spine BMD, g/cm2 | 1.005 ± 0.162 | 1.023 ± 0.171 | 1.014 ± 0.167 | 0.396 |
| Whole body total BMD, g/cm2b | 1.127 (1.076-1.2) | 1.156 (1.070-1.2) | 1.138 (1.070-1.2) | 0.222 |
| Whole body muscle mass, gb | 44,885 (40,629-50,305) | 44,129 (40,412-49,323) | 44,549 (40,546-49,560) | 0.502 |
| Appendicular muscle mass/height2, kg/m2b | 7.8 (7.1-8.5) | 7.7 (7.2-8.4) | 7.8 (7.1-8.5) | 0.696 |
| Total body fat, % | 40.4 ± 5.0 | 41.3 ± 5.0 | 40.8 ± 5.0 | 0.151 |
| Laboratory | ||||
| Free 25(OH)D, pg/mL | 4.7 ± 1.2 | 4.8 ± 1.3 | 4.7 ± 1.3 | 0.565 |
| 25(OH)D3, ng/mL | 21.5 ± 6.5 | 22.2 ± 6.9 | 21.8 ± 6.7 | 0.352 |
| 1,25(OH)2D3, pg/mL | 52.4 ± 13.7 | 52.6 ± 15.4 | 52.5 ± 14.6 | 0.926 |
| 24,25(OH)2D3, ng/mL | 1.4 ± 0.6 | 1.5 ± 0.7 | 1.4 ± 0.6 | 0.107 |
| PTH, pg/mLb | 56.1 (41.0-73.6) | 56.4 (39.5-73.8) | 56.2 (39.8-73.8) | 0.977 |
| Serum Ca, mg/dLb | 9.5 (9.3-9.8) | 9.5 (9.3-9.8) | 9.5 (9.3-9.8) | 0.943 |
| Serum Cr, mg/dLv | 0.8 (0.7-0.9) | 0.7 (0.6-0.9) | 0.8 (0.6-0.9) | 0.472 |
| Serum P, mg/dLb | 3.5 (3.2-3.8) | 3.5 (3.2-3.8) | 3.5 (3.2-3.8) | 0.732 |
Normally distributed variables were presented as mean ± SD; not normally distributed variables presented as median (IQR).
Abbreviations: BMD, bone mineral density; BMI, body mass index.
For continuous data, P values are from Wilcoxon rank-sum test for nonnormally distributed variables and two independent samples t test for normally distributed variables. For categorical variables, P values are from Fisher exact test.
Not normally distributed.
Changes from baseline and interaction with time
The % change in selected performance measures over time is given in Table 2. A longitudinal model for each performance measure was estimated to examine the effects of treatment group, study visit, and the interaction between study visit and treatment group. There were no statistically significant interaction effects observed in any of the performance measures presented (all P > 0.50); therefore, the effect of time on performance was considered the same regardless of treatment group. Estimated effects of time and the interaction between time and treatment group on physical performance are presented in Table 3. The upper bound of the 95% confidence interval for the interaction effect represents the maximum estimated improvement resulting from vitamin D supplementation.
Table 2.
Average Percent Change in Physical Performance From Baseline During the Study Period
| Time, Mo | Measurement | Active | Placebo | Overall |
|---|---|---|---|---|
| 12 | Grip strength | 9.7 (38.8) | 8.4 (19.0) | 9.1 (30.5) |
| Grip strength/BMI | 10.2 (39.9) | 8.0 (20.4) | 9.1 (31.7) | |
| 6MWT | −1.8 (16.3) | −2.9 (20.8) | −2.3 (18.6) | |
| SPPB | 2.8 (14.9) | 2.7 (27.1) | 2.7 (21.8) | |
| 24 | Grip strength | −2.7 (31.4) | −3.5 (25.9) | −3.1 (28.7) |
| Grip strength/BMI | −1.9 (32.1) | −2.6 (28.2) | −2.3 (30.2) | |
| 6MWT | −0.5 (14.6) | −1.7 (12.9) | −1.1 (13.8) | |
| SPPB | 1.7 (18.5) | 5.1 (21.1) | 3.3 (19.8) | |
| 36 | Grip strength | −14.3 (28.0) | −17.1 (22.0) | −15.6 (25.2) |
| Grip strength/BMI | −13.0 (28.5) | −16.1 (23.2) | −14.5 (26.0) | |
| 6MWT | −3.5 (18.3) | −5.0 (13.6) | −4.3 (16.2) | |
| SPPB | 3.7 (16.5) | 6.2 (23.0) | 4.9 (19.9) |
Data are presented as average percent change from baseline (SE).
Abbreviation: BMI, body mass index.
Table 3.
SPPB Classification at Baseline
|
|
SPPB Class
|
|||
|---|---|---|---|---|
| Minimal Limitations SPPB: 10-12 | Mild Limitations SPPB: 7-9 | Moderate Limitations SPPB: 4-6 | Total | |
| Placebo group, n (%) | 99 | 26 | 5 | 130 |
| 76.15 | 20.00 | 3.85 | ||
| Active treatment group, n (%) | 111 | 15 | 4 | 130 |
| 85.38 | 11.54 | 3.08 | ||
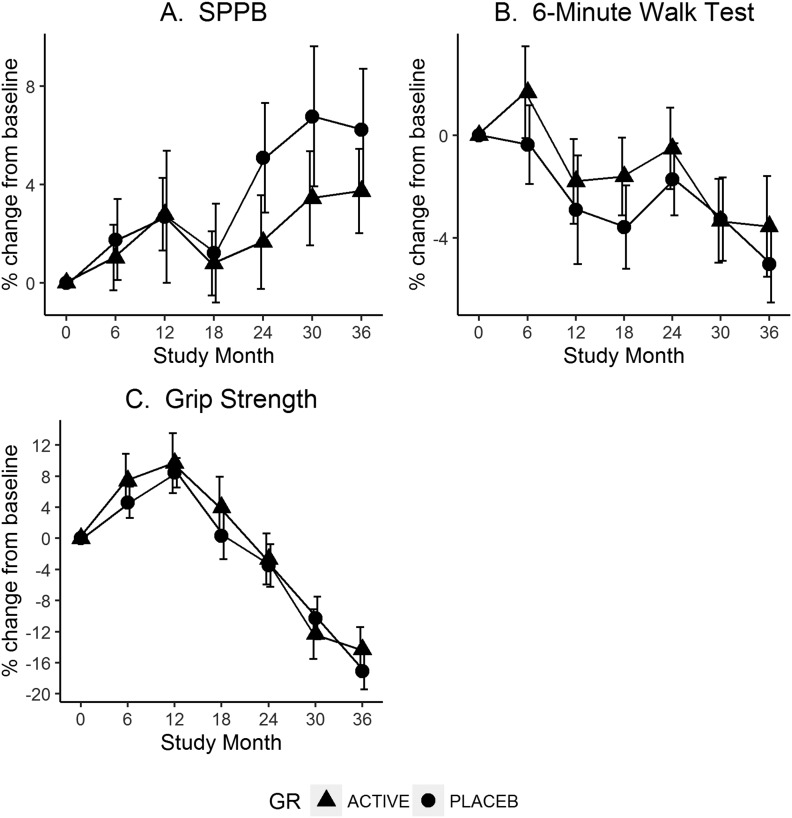
Reduced models with the main effects of group and time were then estimated to summarize changes in performance during the study period. The primary contrast estimated from each model compared 36-month performance with baseline levels. We observed a statistically significant improvement in performance over time for SPPB (P = 0.003) and declines in performance for grip strength (P < 0.001) and grip strength adjusted for body mass index (P < 0.001). A change in performance in the 6MWT was observed such that there was an overall average decline of 4% in meters covered by the end of the study period, but was not statistically significant in the primary model contrast (P = 0.15). Changes in performance variables are depicted in Fig. 1.
Figure 1.
Change in physical performance measures by group over 36 months. (A) SPPB. (B) 6MWT. (C) Grip strength. GR, group; placeb, placebo.
Classification by limitations at baseline in the SPPB scores are given in Table 4. In the minimal limitations category at baseline group, a total of 149 participants had 36-month data available. Of these, 91% (136/149) remained stable and 8% (13/149) declined to have mild limitations. Nobody declined past the mild category. In the mild/moderate limitations category at baseline group, a total of 31 participants had 36-month data available. Of these, 61% (19/31) improved (to minimal limitations category) at 36 months, 35% (11/31) remained stable, and 1 participant declined. Of the 19 who improved, the average change in SPPB score was 3 points (for example, an SPPB of 7 at baseline compared with an SPPB of 10 at the 36-month visit). Each component of the SPPB was also analyzed separately. Because an improvement was observed for all components regardless of treatment arm, no single component or study time period explained why the SPPB increased over time. There was a statistically significant improvement in both the total gait speed score (overall 7% average improvement at 36 months; P = 0.001) and the chair stand score (16%; P = 0.02), whereas the observed improvement in balance score was not statistically significant (2%; P = 0.80). We examined tests of the global null hypothesis across all measures of physical performance and could not conclude that there was a statistically significant difference in the trajectory over time between treatment groups.
Table 4.
Estimated Effect of Vitamin D Supplementation on Physical Performance
| Outcome | Estimated Yearly Change (95% CI) | Direction of Change | Effect of Vitamin D Supplementation on Yearly Change (95% CI) a | P b |
|---|---|---|---|---|
| Grip strength | −1.68 (−2.1 to −1.3) | Decline | −0.048 (−0.56 to 0.46) | 0.85 |
| Grip strength/BMI | −0.05 (−0.06 to −0.04) | Decline | −0.004 (−0.02 to 0.02) | 0.71 |
| SPPB | 0.13 (0.02 to 0.24) | Increase | −0.05 (−0.19 to 0.09) | 0.51 |
| 6MWT | −0.005 (−0.02 to 0.01) | Stable | −0.005 (−0.02 to 0.01) | 0.60 |
Abbreviation: BMI, body mass index.
Based on the interaction between treatment group and time in a repeated measures linear model containing treatment group, time, and the interaction between treatment group and time.
Z test for the estimated interaction effect between treatment group and time.
A subgroup analysis for participants with a serum 25(OH)D <50 nmol/L showed no statistically significant difference with the average change in performance measures between groups. Similar findings were found in a subgroup analysis including those with a baseline serum 25(OH)D <40 nmol/L. We could not find evidence for a detrimental effect at high vitamin D intake with high 25(OH)D levels. We examined those participants who were treated (at some point or consistently) with a dose of 3600 IU or greater during the first year of the study (n = 77) and their outcomes at 3 years. We found changes in performance that were very similar to the original active group. For example, grip strength declined by 12.2%, 6MWT declined by 4.6%, and SPPB increased by 1.3%. There is no evidence that this group of high-dose individuals experienced any detrimental effects. Similar results occur if we examine participants with higher levels of 25(OH)D within 1 year postrandomization.
Consistent with our performance measurements, activity levels decreased slightly but significantly in both treatment groups over the course of the study (P = .02). However, there was no difference in this rate of decline between study groups (P = .90).
Vitamin D and parathyroid hormone response
Baseline values for serum 25(OH)D3 are given in Table 1. Values (mean ± SE) for 12, 24, and 36 months in the active group were 108 ± 2.3, 113.8 ± 2.9, and 117 ± 2.9 nmol/L, respectively. Corresponding values for the placebo group were 48 ± 2.0, 49 ± 2.1 and 51 ± 2.7 nmol/L. Ninety percent of the active group maintained serum 25(OH)D >75 nmol/L. The mean dose of vitamin D3 in the active arm was 3490 (± 1465) IU/d. Serum calcitriol increased by 10% at 36 months in the treatment group.
Changes in parathyroid hormone (PTH) were not significantly correlated (P > 0.15) with changes in grip strength (r = 0.09) or in 6MWT (r = −0.10) or SPPB (r = −0.01). PTH did decline over time significantly (P < 0.001) for both treatment groups, with an average decline of 18.5 pg/mL and percent change of −19.9% at 36 months.
Discussion
Although we noted a decline with age in some physical performance measures, there was no difference between the placebo and active vitamin D groups. The placebo group had a mean serum 25(OH)D level of ∼50 nmol/L, the value recommended as equivalent to the RDA by the IOM (26). Thus, we conclude that there is no benefit in elderly healthy black women in raising serum 25(OH)D above the RDA-associated level recommended by the IOM of 50 nmol/L.
Most previous studies of the influence of vitamin D did not exclude individuals with vitamin D deficiency [i.e., very low 25(OH)D <30 nmol/L]. Evidence reviews on fall and fracture prediction recently prepared for the US Preventive Services Task Force examined studies that excluded deficiency in community-dwelling asymptomatic postmenopausal women and men. In this population (which is similar to ours), there was no benefit to vitamin D supplementation in prevention of falls (or fractures) (27, 28). This is a change in the US Preventive Services Task Force recommendation, and is primarily the result of the population included (i.e., community dwelling and not osteoporotic or having vitamin D deficiency).
Our study did not have sufficient numbers to determine if very low (deficient) levels of 25(OH)D are associated with decreased physical performance or its improvement with vitamin D supplementation. Indeed, we excluded those with serum 25(OH)D <20 nmol/L from our study because of ethical concerns about allowing those in the deficiency range to remain untreated for 3 years. We did examine the subpopulation with 25(OH)D <50 nmol/L (lower than that of the RDA) and <40 nmol/L (the estimated average recommendation and found no difference in change in physical performance when compared with those with 25(OH)D levels >75 nmol/L.
Nonetheless, there is very good evidence from other studies that those with vitamin D deficiency [very low 25(OH)D] have impaired physical performance that responds to vitamin D replacement. Several meta-analyses show the benefits of vitamin D supplementation on physical performance in deficiency states. In 10 of 12 trials of participants with <50 nmol/L serum 25(OH)D, there were benefits observed on postural sway and the Timed-Up and Go Test (29). In a systematic review of 29 trials, a positive effect of vitamin D was shown on muscle strength (30). Stockton (31) reported no effect on muscle strength with baseline 25(OH)D >25 but did find a benefit when baseline 25(OH)D was <25 nmol/L.
Recently, there has been concern expressed about U-shaped curves in the distribution of vitamin D with benefits of 25(OH)D levels >30 nmol/L and harms with high bolus doses of vitamin D (32). The medical community was put on notice that very high doses of vitamin D may actually result in increased falls and fractures (33). Gallagher (33) calculated that serum 25(OH)D levels >112 nmol/L may be of concern. In the current study, however, we found no evidence for a decline in physical performance with high doses of vitamin D. The mean values for 25(OH)D in our treatment group approached this threshold.
A strength of this study is its duration. Examination of Fig. 1 shows that a 6-month or 1-year study duration could lead to distinctly different conclusions compared with this 3-year study. We suggest that short-term studies should be viewed with caution. Another strength was our ability to maintain a selected serum 25(OH)D level (>75 nmol/L). Weaknesses of the study are that we did not include individuals deficient in vitamin D; in addition, our study population was high functioning, so there may have been a ceiling effect. As a result, our findings may not be applicable to other races, sex, age groups, or institutionalized black women.
It was surprising to observe an increase in the SPPB in both groups. There is no clear explanation for this finding and it is contrary to what is expected from multiple other studies (34, 35). The increase in the SPPB was consistent in each of its components. Our only speculation concerning this increase is that it reflects a training effect in a highly motivated and healthy, high-functioning study group.
In conclusion, we found no benefit on physical performance of maintaining serum 25(OH)D >75 nmol in this highly performing community-dwelling group of elderly black women. Raising serum 25(OH)D above the RDA (800 IU) to improve physical performance cannot be recommended for this population.
Acknowledgments
We thank Sharon Sprintz for her expertise as a dual-energy X-ray absorptiometry technician; Jane Greensher for her expertise as the nurse coordinator; and our fellow researchers Albert Shieh, Gianina Usera, Ruban Dhaliwal, Alexandra Stolberg, and Subhashini Katumuluwa who were responsible for clinical care, data gathering, data presentation, and analysis; the National Institutes of Health and Office of Dietary Supplements; and DSMB committee members Munro Peacock, Bess Dawson-Hughes, Lynette Smith, Judy Hannah, Rebecca Costello, Christopher Sempos, and Andy Hoofnagle, Division of Clinical Chemistry, University of Washington.
Financial Support: This work was supported by National Institutes of Health and Office of Dietary Supplements grant R01-AG032440-01A2 (J.F.A.).
Clinical Trial Information: ClinicalTrials.gov no. NCT01153568 (registered 30 June 2010).
Author Contributions: J.F.A. designed and supervised and wrote the manuscript. M.M. was responsible for medical supervision of the study participants. M.F. and S.I., the study statisticians, were responsible for the data and statistical analyses and contributed to the writing of the manuscript. J.G. participated in the planning, conduct, and writing of the manuscript. L.R., the laboratory director. was responsible for the biochemical assays.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 6MWT
6-minute walk text
- IOM
Institute of Medicine
- PTH
parathyroid hormone
- RDA
recommended daily allowance
- SPPB
Short Physical Performance Battery
References
- 1. Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45(1):92–100. [DOI] [PubMed] [Google Scholar]
- 2. Aloia JF, Vaswani A, Ma R, Flaster E. Comparison of body composition in black and white premenopausal women. J Lab Clin Med. 1997;129(3):294–299. [DOI] [PubMed] [Google Scholar]
- 3. Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13(3):187–194. [DOI] [PubMed] [Google Scholar]
- 4. Rinaldi I, Setiati S, Oemardi M, Aries W, Tamin TZ. Correlation between serum vitamin D (25(OH)D) concentration and quadriceps femoris muscle strength in Indonesian elderly women living in three nursing homes. Acta Med Indones. 2007;39(3):107–111. [PubMed] [Google Scholar]
- 5. Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1(7960):626–629. [DOI] [PubMed] [Google Scholar]
- 6. Visser M, Deeg DJ, Puts MT, Seidell JC, Lips P. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr. 2006;84(3):616–622. [DOI] [PubMed] [Google Scholar]
- 7. Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Mineral Res. 2002;17(5):891–897. [DOI] [PubMed] [Google Scholar]
- 8. Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzalmann M.. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Mineral Res. 2003;18(2):343–351. [DOI] [PubMed] [Google Scholar]
- 9. Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91(8):2980–2985. [DOI] [PubMed] [Google Scholar]
- 10. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 11. Kritchevsky SB, Tooze JA, Neiberg RH, Schwartz GG, Hausman DB, Johnson MA, Bauer DC, Cauley JA, Shea MK, Cawthon PM, Harris TB, Rubin SM, Tylavsky FA, Houston DK; Health ABC Study . 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: the health ABC study. J Clin Endocrinol Metab. 2012;97(11):4156–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paik JM, Farwell WR, Taylor EN. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2012;23(6):1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53(2):183–189. [DOI] [PubMed] [Google Scholar]
- 14. Tracy JK, Meyer WA, Grigoryan M, Fan B, Flores RH, Genant HK, Resnik C, Hochberg MC. Racial differences in the prevalence of vertebral fractures in older men: the Baltimore Men’s Osteoporosis Study. Osteoporos Int. 2006;17(1):99–104. [DOI] [PubMed] [Google Scholar]
- 15. Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–593. [DOI] [PubMed] [Google Scholar]
- 16. Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88(2):545S–550S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–2108. [DOI] [PubMed] [Google Scholar]
- 18. Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Mineral Res. 2005;20(2):185–194. [DOI] [PubMed] [Google Scholar]
- 19. Hanlon JT, Landerman LR, Fillenbaum GG, Studenski S. Falls in African American and white community-dwelling elderly residents. J Gerontol A Biol Sci Med Sci. 2002;57(7):M473–M478. [DOI] [PubMed] [Google Scholar]
- 20. Kyriakidou-Himonas M, Aloia JF, Yeh JK. Vitamin D supplementation in postmenopausal black women. J Clin Endocrinol Metab. 1999;84(11):3988–3990. [DOI] [PubMed] [Google Scholar]
- 21. Dhaliwal R, Mikhail M, Usera G, Stolberg A, Islam S, Ragolia L, Aloia JF. The relationship of physical performance and osteoporosis prevention with vitamin D in older African Americans (PODA). Contemp Clin Trials. 2018;65:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aloia J, Fazzari M, Islam S, Mikhail M, Shieh A, Katumuluwa S, Dhaliwal R, Stolberg A, Usera G, Ragolia L. Vitamin D supplementation in elderly black women does not prevent bone loss: a randomized controlled trial. J Bone Mineral Res. 2018;33(11):1916–1922. [DOI] [PubMed] [Google Scholar]
- 23. Guralnik JM. Successful aging: is it in our future? Arch Intern Med. 2008;168(2):131–132. [DOI] [PubMed] [Google Scholar]
- 24. Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 25. Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography-tandem mass spectrometry. Clin Chem. 2012;58(12):1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Institute of Medicine Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 27. Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(16):1705–1716. [DOI] [PubMed] [Google Scholar]
- 28. Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, Viswanathan M. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(15):1600–1612. [DOI] [PubMed] [Google Scholar]
- 29. Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59(12):2291–2300. [DOI] [PubMed] [Google Scholar]
- 30. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–4345. [DOI] [PubMed] [Google Scholar]
- 31. Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–871. [DOI] [PubMed] [Google Scholar]
- 32. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–183. [DOI] [PubMed] [Google Scholar]
- 33. Gallagher JC. Vitamin D and falls - the dosage conundrum. Nat Rev Endocrinol. 2016;12(11):680–684. [DOI] [PubMed] [Google Scholar]
- 34. Fielding RA, Guralnik JM, King AC, Pahor M, McDermott MM, Tudor-Locke C, Manini TM, Glynn NW, Marsh AP, Axtell RS, Hsu FC, Rejeski WJ; LIFE study group . Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PLoS One. 2017;12(8):e0182155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez-Gomez D, Bandinelli S, Del-Panta V, Patel KV, Guralnik JM, Ferrucci L. Three-year changes in physical activity and decline in physical performance over 9 years of follow-up in older adults: the Invecchiare in Chianti Study. J Am Geriatr Soc. 2017;65(6):1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]