Abstract
Context
Multiple autosomal recessive genes have been etiologically linked to primary adrenal insufficiency (PAI). Recently, sphingosine-1-phosphate lyase 1 (SGPL1) gene mutations were recognized as a cause of steroid-resistant nephrotic syndrome type 14 (NPHS14), a sphingolipidosis with multisystemic manifestations, including PAI.
Objective
To check if SGPL1 mutations are involved in the pathogenesis of PAI in patients who do not exhibit nephrotic syndrome.
Methods
Sequencing of the SGPL1 gene in 21 patients with familial glucocorticoid disease or triple A syndrome.
Results
We identified two missense SGPL1 variants in four patients, two of whom were first cousins. We describe in detail the proband, a boy born to Saudi Arabian consanguineous parents with a homozygous c.665G>A, p.R222Q SGPL1 variant. The patient presented with hypoglycemia and seizures at age 2 years and was ultimately diagnosed with PAI (isolated glucocorticoid deficiency). Brain MRI showed abnormalities in the basal ganglia consistent with a degenerative process albeit the patient had no neurologic symptoms.
Conclusions
New genetic causes of PAI continue to be identified. We suggest that screening for SGPL1 mutations should not be reserved only for patients with nephrotic syndrome but may also include patients with PAI who lack other clinical manifestations of NPHS14 because, in certain cases, kidney disease and accompanying features might develop. Timely diagnosis of this specific sphingolipidosis while the kidneys still function normally can lead to prompt initiation of therapy and improve outcome.
SPGL1 deficiency is a new cause of primary adrenal insufficiency. We report a family with SPGL1 defects and demonstrate the range of clinical phenotypes associated with it.
Primary adrenal insufficiency (PAI) is a life-threatening disorder that results from bilateral destruction or dysfunction of the adrenal cortex (1). It is clinically defined by the inability to produce sufficient adrenal glucocorticoids and/or mineralocorticoids and leads to the activation of the hypothalamic-pituitary axis and the renin-angiotensin system (2). In children, PAI is usually congenital, with a reported prevalence of 1/10,000 to 1/18,000 (3). PAI manifests with acute symptoms such as vomiting, electrolyte disturbances, hypoglycemia, and hypotension (Addisonian crisis) or more subtly as hyperpigmentation, failure to thrive, and a poor response to illness. Because adrenal insufficiency is fatal if undiagnosed, it is important to elucidate the underlying causative factor. PAI is a genetically heterogeneous disease, with some forms being syndromic. Specifically, PAI may be caused by defects in steroid synthesis, resistance to ACTH, defects in the synthesis of cholesterol, adrenal dysgenesis, and metabolic disorders that include peroxisomal and mitochondrial abnormalities (4, 5).
Familial glucocorticoid deficiency (FGD), a cause of PAI, refers to an underlying genetic disorder leading to ACTH resistance (i.e., failure of cortisol production under ACTH stimulation). Multiple genes have been implicated in FGD, including MC2R, MCM4, MRAP, NNT, and TXNRD2. Triple-A syndrome (AAAS) is a congenital disorder caused by mutations in the AAAS gene. Individuals affected by AAAS exhibit alacrima, achalasia, and adrenal insufficiency. Other genetic causes have been found in various forms of PAI, including NR0B1, CYP11A1, CYP11B1, and AIRE (2). Recently, mutations in the sphingosine-1-phosphate lyase 1 (SGPL1) gene were recognized as the cause of steroid-resistant nephrotic syndrome type 14 (NPHS14), a sphingolipidosis. Patients with NPHS14 have multisystemic manifestations, including PAI, neurologic defects, ichthyosis, primary hypothyroidism, immunodeficiency, cryptorchidism, and dyslipidemia (6–11).
Herein, we present data on the genetic analysis of SGPL1 in 21 patients with FGD or AAAS in an attempt to check if SGPL1 mutations are involved in the pathogenesis of PAI in patients who do not exhibit nephrotic syndrome.
Material and Methods
Clinical studies
The study cohort includes 21 patients with clinical features of FGD (n = 9) or AAAS (n = 12) obtained from clinical sites around the world. Fifteen patients are sporadic, and six are familial cases. Patients were referred by a pediatric endocrinologist who had made the diagnosis of PAI or by a pediatric subspecialist who suspected AAAS based on relevant cardinal features, such as alacrima and/or achalasia. Patients were studied under National Institutes of Health (NIH) clinical protocols 97CH0076, 97CH0127, and 00CH0180. Clinical characteristics are reported in Table 1, and family histories are reported in Table 2. For sporadic patients from reportedly nonconsanguineous families, the diagnosis of FGD was based on the presence of low cortisol levels, elevated ACTH levels, and a lack of other identifiable causes of adrenal insufficiency, such as adrenal autoantibodies or adrenoleukodystrophy. Clinical diagnosis of AAAS was based on the presence of one or more cardinal features (adrenal insufficiency, alacrima, or achalasia) in association with neurologic symptoms such as peripheral neuropathy, muscle weakness, mental retardation, or dysautonomia. These patients had previously been screened for all other causes of FGD and were found to carry no mutations in known genes (data not shown). For the genetic analyses, assent was obtained from the children who are included in this study, and informed consent was obtained from their parents and all the adult family members.
Table 1.
Clinical Phenotypes of the Patients Included in the Study
| Patient ID | Clinical Diagnosis a | Sex |
Age
|
Hormonal Studies
|
Other Findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| At Diagnosis | Current | Cortisol | ACTH | Response to ACTH | Aldosterone | Renin | ||||
| ACTHR01.03 | AAAS | M | 1 y | 14 y | Low | High | — | — | — | |
| ACTHR06.03 | AAAS | M | 11 mo | 28 y | Low | — | Suboptimal | — | — | |
| ACTHR09.03 | FGD | F | 3 mo | N/A | Low | High | — | Normal | Normal | |
| ALS17.03 | AAAS | F | 8 mo | 14 y | Normal | Normal | — | — | — | |
| ALS20.03 | FGD | F | 2 y | 16 y | Low | High | — | — | — | |
| ALS21.03 | AAAS | M | 4 y | 16 y | Low | Low | Yes | — | — | FGF10 mutation |
| ALS22.03 | FGD | F | 6 d | 18 y | Low | High | No | Normal | Low | |
| ALS23.03 | AAAS | F | 4 y | N/A | Low | High | No | Normal | Normal | |
| ALS25.03 | AAAS | M | 2 y | N/A | — | High | Yes | — | Normal | |
| ALS25.04 | AAAS | M | 5 wk | N/A | — | High | — | — | Normal | |
| ALS26.03 | FGD | M | 7 y | N/A | Low | High | Suboptimal | Normal | Normal | GHD |
| ALS27.03 | FGD | F | 8 y | N/A | Low | High | No | Normal | Normal | |
| ALS28.03 | AAAS | F | 2 y | N/A | Low | — | — | — | — | |
| ALS29.03 | AAAS | M | 1.5 y | N/A | Low | High | — | — | — | |
| ALS30.01 | AAAS | F | 3 y | N/A | Low | High | — | — | — | |
| ALS30.03 | AAAS | M | — | Undetectable | High | — | — | — | ||
| ALS32.05 | AAAS | M | 18 y | N/A | Low | Low | — | — | — | |
| ALS33.05 | FGD | M | 8 y | N/A | Low | Low | No | — | — | |
| ALS34.03 | FGD | F | 3 y | N/A | Low | High | No | — | Normal | |
| ALS35.03 | FGD | M | 3 y | 6 y | Low | High | No | — | Normal | |
| ALS35.08 | FGD | F | 15 y | 16 y | — | — | No | — | — | |
Abbreviation: N/A, not available.
Parameters for clinical diagnosis of AAAS and FGD as stated in Materials and Methods. Cortisol levels are presented in nmol/L (normal morning cortisol, 193–607); ACTH levels are presented in pmol/L (normal, 1.1 to 11.4). For aldosterone and renin, normal is reported based on the laboratory value or on a lack of symptoms in patients on glucocorticoid supplementation only.
Table 2.
Family History of the Patients Included in the Study
| Patient ID | Country of Origin | Familial or Sporadic | Consanguinity |
|---|---|---|---|
| ACTHR01.03 | USA | Sporadic | No |
| ACTHR06.03 | Canada | Sporadic | No |
| ACTHR09.03 | USA | Sporadic | No |
| ALS17.03 | USA | Familial | No |
| ALS20.03 | UK | Sporadic | No |
| ALS21.03 | USA | Familial | No |
| ALS22.03 | USA | Sporadic | No |
| ALS23.03 | USA | Sporadic | No |
| ALS25.03 | USA | Familial | No |
| ALS25.04 | USA | Familial | No |
| ALS26.03 | USA | Sporadic | No |
| ALS27.03 | USA | Sporadic | No |
| ALS28.03 | USA | Sporadic | No |
| ALS29.03 | USA | Sporadic | No |
| ALS30.01 | France | Sporadic | Yes |
| ALS30.03 | USA | Sporadic | No |
| ALS32.05 | USA | Sporadic | No |
| ALS33.05 | USA | Sporadic | No |
| ALS34.03 | Turkey/USA | Sporadic | No |
| ALS35.03 | Saudi Arabia | Familial | Yes |
| ALS35.08 | Saudi Arabia | Familial | N/A |
Abbreviation: N/A, not available.
DNA sequencing
Genomic DNA was isolated from peripheral leukocytes following the Maxwell 16 Blood DNA Purification Kit protocol in combination with the Maxwell 16 Instrument (Promega, Madison, WI). Specific amplification of the SGPL1 gene (10q22.1) was performed by PCR using 14 primer sets spanning the entire coding region that were designed using the Primer3Plus (http://primer3plus.com) software. PCR products were purified (ExoSAP-IT reagent; Affimetrix-USB, Cleveland, OH) and sequenced bidirectionally using the same primers (ABI3500; Applied Biosystems). The data collected from the bioanalyzer were further analyzed by Geneious version 11.1 (Biomatters Ltd., Newark, NJ). Primers are available upon request.
Statistical analysis
The statistical difference between the frequency of the variants identified in our patients and their prevalence in the ExAC database was calculated using the χ2 test.
Results
Genetic studies
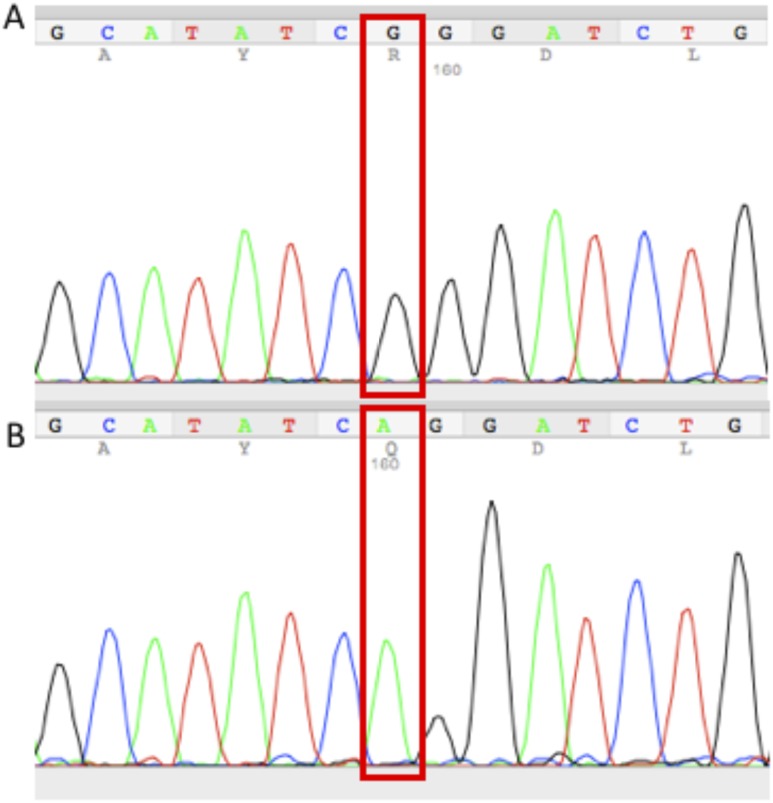
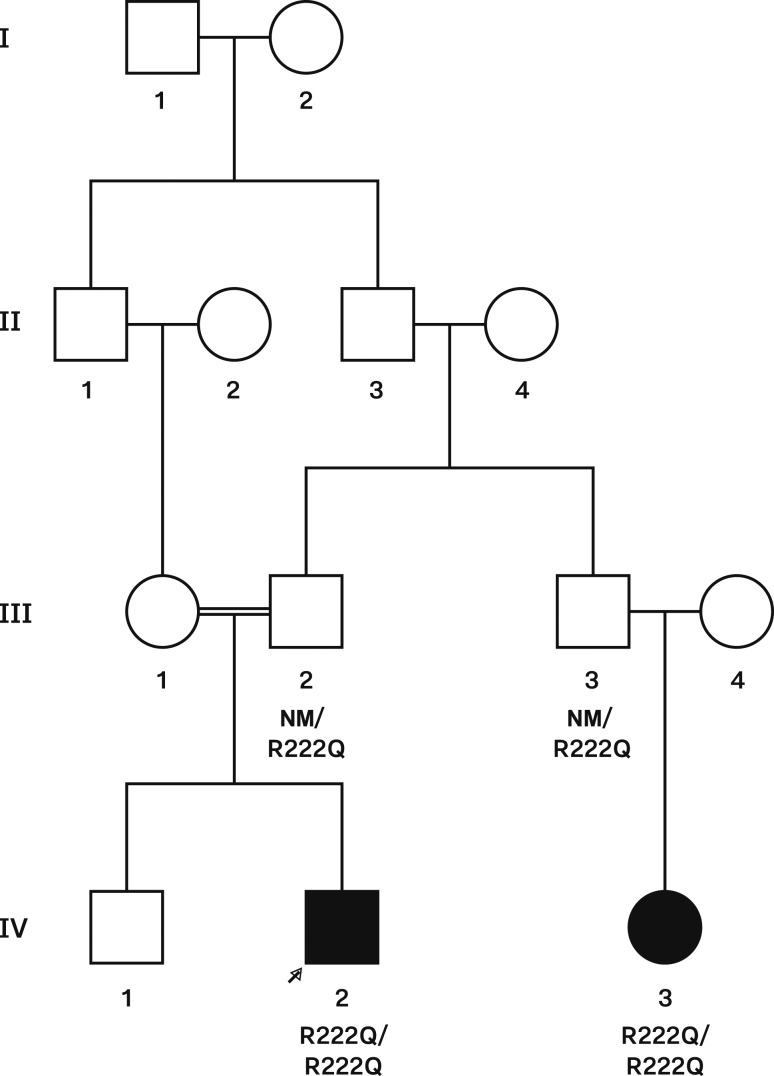
Of the 21 patients included in this study, patient ALS35.03 and his first cousin (patient ALS35.08) harbor a homozygous mutation (c.665 G>A, p.R222Q, rs769259446) in the SGPL1 gene (Figs. 1 and 2). This missense variant is included in ExAC but has a very rare allele frequency of 0.000008131. It has never been found in a homozygous state in the ExAC database, and six distinct in silico analysis tools predicted the pathogenicity of the p.R222Q alteration in the SGPL1 gene. The predictions are as follows (prediction in parentheses): MutationTaster (disease causing), Mutation Accessor (high), MetaSVM (damaging), Provean (damaging), Likelihood Ratio Test (deleterious), and SIFT (damaging).
Figure 1.
Sequencing chromatograms of the SGPL1 gene. (A) Sequence of a wild-type patient. (B) Sequence from patient ALS35.03 carrying the homozygous SGPL1 missense variant (c.665 G>A, p.R222Q).
Figure 2.
Pedigree of the consanguineous family ALS35 with the SGPL1 mutation described in the text. Squares indicate male family members, circles indicate female family members, black symbols indicate clinically affected family members, and arrow indicates the proband. The SGPL1 genotype is shown for family members whose DNA was available for genetic studies. NM, nonmutated allele.
Two additional patients (ALS25.03 and ALS34.03) were carriers of a missense variant (c.61G>T,p.V21L, rs12770335) with an allele frequency 0.09 in ExAC. The frequency of this variant in our cohort is not significantly different from the frequency in the general population using the χ2 test. Therefore, we hypothesize that this variant is not responsible for adrenal insufficiency, although we cannot rule out its participation in this phenotype. Herein, we focus on the detailed description of the clinical history of patient ALS35.03 and provide data on his first cousin ALS35.08.
Patient with SGPL1 deficiency
Patient ALS35.03 is a 5.5-year-old boy born to consanguineous Saudi Arabian parents (first cousins) who presented to the NIH with a history of seizures and hypoglycemia. He was born full term (birthweight, 3210 g) to a 20-year-old mother who experienced no complications during pregnancy, perinatally, or shortly thereafter. Since birth, his growth rate had been above the 50th percentile for height but recently was trending down toward the 50th percentile. His weight was initially on the 25th percentile but slowly increased to the 50th percentile by 5 years of age. At 2 years of age, seemingly pertinent symptoms were reported for the first time, when he experienced diarrhea and vomiting requiring hospitalization for IV fluids. No laboratory data are available from that time point. He was well until 8 months later when he experienced fever, vomiting, and diarrhea. He was brought to a hospital and was found to be hypoglycemic with a blood glucose of 30 mmol/L, which was attributed to vomiting and dehydration. He was treated again with IV fluids and discharged. The next illness occurred 7 months later, again starting with vomiting, diarrhea, and fever. This time body twitching was noticed, and he was brought to the emergency department for evaluation, where he experienced generalized seizures. Basic metabolic panel revealed the following: blood glucose 14 mmol/L, Na 136 mEq/L, K 4.5 mEq/L, bicarbonate 11 mEq/L, Cr 2.36 mg/dL, BUN of 39 mg/dL, liver function tests significantly elevated (aspartate aminotransferase 184 U/L and alanine aminotransferase 78 U/L), and complete blood count with WBC 17 × 103/μL (67% neutrophils, 29% lymphocytes); normal hemoglobin and platelets were noted. He was resuscitated with dextrose and IV fluids and admitted to the hospital for a few days. He was discharged with a glucose meter and instructions to check glucose levels daily.
Due to severe hypoglycemia, the patient was referred to a pediatric endocrinologist. Blood tests showed Na 136 mEq/L, K 4.3 mEq/L, bicarbonate 19 mEq/L, and creatinine 0.27 mg/dL. A metabolic work-up, including plasma carnitine, acylcarnitine profile, quantitative amino acids, very-long-chain fatty acids, and urine organic acids, revealed no abnormalities. Further work-up for adrenal insufficiency revealed significantly elevated ACTH and low cortisol levels at 8 am (5195 pg/mL and 1.3 μg/dL, respectively), normal renin activity level, and the absence of 21-α hydroxylase antibodies. Based on these results, he was diagnosed with primary adrenal insufficiency with isolated glucocorticoid deficiency (normal plasma renin activity and aldosterone level) and was promptly started on hydrocortisone replacement therapy. No mutations were found in the MC2R or DAX1 genes. An ultrasound of the adrenal glands showed a possible “triangular shaped” structure with soft tissue density that might correspond to the right adrenal; the left adrenal was not visualized.
After starting steroid replacement, the patient experienced only minor illnesses, for which he was treated with stress steroid doses, which prevented hospitalization in all but one case (i.e., at 4 years of age, he was hospitalized following another seizure after having received stress dose steroids).
At 5 years of age, the patient was referred to the NIH for further investigation of the underlying genetic disorder. There was no family history of PAI. One paternal cousin has been diagnosed with a form of congenital neurologic disorder. At the NIH, the patient underwent ACTH stimulation testing while off hydrocortisone; his cortisol levels remained <1 μg/dL throughout testing. Other important findings included elevated liver transaminases, with alanine aminotransferase 128 U/L and aspartate aminotransferase 61 U/L, and mildly elevated TSH (7.35 μIU/mL; normal range, 0.84 to 6.22), with normal free T4, total T4, and total T3 and negative thyroid autoantibodies. Complete blood showed a slightly low total white blood cell count of 4.62 K/μL (5.14 to 13.38) and a normal total lymphocyte count. Nevertheless, while reviewing previous laboratory tests, mildly low absolute lymphocyte counts were intermittently noticed (lowest 0.6 k/cumm). Further testing, including IgG, IgA, IgE, and IgM levels and T- and B-cell lymphocyte quantitation, revealed no abnormality according to our immunologists (data not shown). Our patient did not exhibit skin manifestations, including ichthyosis, which has been reported in patients with SGPL1 mutations (6, 12). However, he did have evidence of conjunctival hyperpigmentation, although his skin was no longer hyperpigmented.
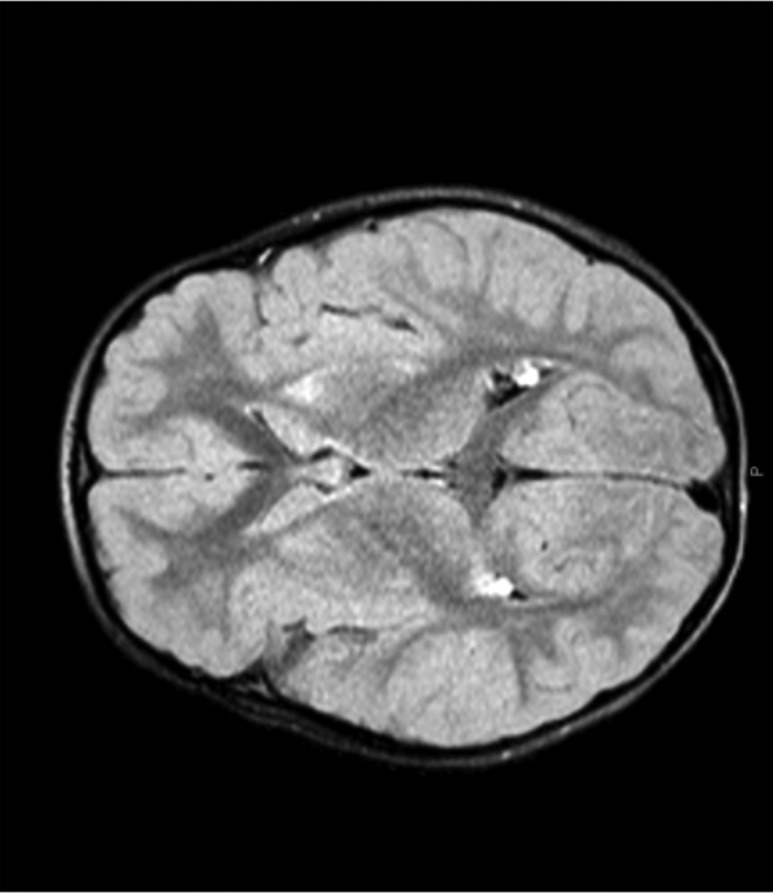
When this patient was found to have SGPL1 deficiency, a brain, pituitary, and orbit MRI was performed (Fig. 3). The pituitary gland and orbits were normal; however, the brain MRI had abnormal findings, including foci of T2/FLAIR hyperintensity noted bilaterally in the basal ganglia (most severely involving the caudate heads and anterior aspects of both putamen) with a decrease in the size of the caudate nuclei. The globus pallidus was completely spared bilaterally. The hyperintensity appears to be associated with subtle T1 hypointensity in the region of the caudate head. These findings may be suggestive of ischemic or degenerative process; inflammatory or infectious etiologies were not likely due to the lack of enhancement. However, differential considerations include hypoxic ischemic insult either associated with or independent of hypoglycemia, basal ganglia affecting disorders like mitochondrial diseases, and other degenerative processes. A thorough neurologic examination was normal at the time. Nevertheless, continued assessments and evaluation were warranted.
Figure 3.
MRI of patient ALS35.03 with findings of hyperintensity in the basal ganglia (caudate and putamen).
Because SGPL1 mutations are the underlying cause of steroid-resistant NPHS14, a kidney function evaluation was performed. Urinalysis was negative for proteinuria, and there were no other signs of nephrotic syndrome.
The first cousin (ALS35.08) of our patient, who harbored the same SGPL1 mutation, presented to Saudi Arabian physicians 2 years ago (at the age of 15 years) with lazy right eye and right hearing loss and later with right arm paralysis that progressed to hemiparesis. This patient also developed kidney failure and is under hemodialysis three times a week, but more information on his clinical status is unavailable.
Discussion
SGPL1 is an ER enzyme that is responsible for the final step of the sphingolipid breakdown pathway. Mutations in the SGPL1 gene result in the inefficient degradation of its substrate, sphingosine-1-phosphate (S1P), which is a bioactive sphingolipid. Because SGPL1 is a component of sphingolipid metabolism, the clinical disorder resulting from SGPL1 variants belongs to a group of inherited disorders of metabolism called “sphingolipidoses,” along with other well-known conditions such as Gaucher disease, Niemann–Pick disease, and Fabry disease. Homozygous loss-of-function mutations in the SGPL1 gene were recently identified as a cause of steroid-resistant nephrotic syndrome (SRNS), eventually leading to chronic kidney disease requiring renal transplant. Furthermore, the phenotype of patients bearing such mutations included a combination of ichthyosis/acanthosis, primary adrenal insufficiency, dyslipidemia, primary hypothyroidism, lymphopenia, and/or neuronal dysfunction. We screened a relatively large cohort of patients with various forms of adrenal insufficiency for SGPL1 gene mutations rather than patients with nephrotic syndrome.
The SGPL1 missense variant found in our patient (c.665 G>A, p.R222Q, rs769259446) has been previously identified in four families of Pakistani (n = 2), Turkish, and Saudi origin (6, 12). This SGPL1 missense variant (c.665 G>A, p.R222Q, rs769259446) is within highly conserved residues and is predicted to cause disruption to vital, highly conserved eukaryotic protein domains and results in protein with reduced lyase activity (6). Although detailed clinical data are not available for all patients, it seems that adrenal insufficiency had developed in some, but not all, with varying timing of diagnosis from ∼3 months to 6.5 years of age. Most affected individuals in the literature present in infancy or early childhood with progressive renal dysfunction (6). Nevertheless, the older patient reported with the p.R222Q that still had not developed adrenal insufficiency was 8 years of age. Subjects with SGPL1 gene mutations have varying degrees of adrenal insufficiency, some with just isolated glucocorticoid deficiency, like in the case of our patient, and others with both deficient mineralocorticoids and androgens. The exact mechanism of adrenal insufficiency remains unknown; however, we can hypothesize that accumulation of sphingolipid intermediates such as the bioactive S1P interfere with steroid biosynthesis. Regarding the immunodeficiency that has been reported in such patients, SGPL1 gene mutations may affect lymphocyte migration from the thymus to the peripheral lymphoid organs because the process is dependent on the S1P receptor and circulating S1P (13, 14). Regarding the neurologic aspect of the syndrome, it is presumably due to accumulated sphingolipids and seems to be progressive in cognitive and motor decline, with loss of previous attained developmental milestones, with some patients being diagnosed with ataxia and sensorineural loss (1).
The patient described herein may develop NPHS14, although he does not yet display any of the cardinal findings of the syndrome (e.g., proteinuria, hypoalbuminemia, etc). SRNS developed later in his cousin bearing the same mutation; he has already progressed to renal failure.
With respect to other frequently observed features of the SGPL1 syndrome, our patient did not display relevant skin manifestations such as ichthyosis or acanthosis, clinical neurologic dysfunction, dyslipidemia, evidence of immunodeficiency, and significant lymphopenia (although mildly low absolute lymphocyte counts were intermittently noticed). Nevertheless, mild subclinical hypothyroidism was revealed.
Brain MRI findings in our patient do not resemble those typically observed in other sphingolipidoses, which range from normal to predominantly white matter involvement. However, there have been reports of gray matter abnormalities as well as abnormalities in the basal ganglia, as seen in our patient, in various sphingolipidoses, such as Nieman Pick Type C and GM1 gangliosidosis (15–17). Subjects with SGPL1 mutations may exhibit normal brain MRIs or a variety of abnormalities, including contrast enhancement in globus pallidus, medial thalamic nuclei, and central pons or cerebellar enhancement (6). A recent case report of fetal hydrops, congenital nephrotic syndrome, and adrenal calcification in a patient harboring a SGPL1 mutation included a brain MRI that showed generalized cortical atrophy, simplified gyral pattern, hypoplastic temporal lobes, cerebellar hypoplasia, and hyperintensity in the pons (18). Therefore, there appears to be a variety of MRI findings associated with the SGPL1 syndrome. Despite the MRI findings, our patient does not currently present neurologic symptoms.
In summary, the frequency of SGPL1 genetic defects in patients presenting with FGD and/or AAAS seems to be low. Nevertheless, we were able to identify a patient with isolated glucocorticoid deficiency harboring the c.665 G>A, p.R222Q variant of SGPL (i.e., a cause of nephrotic syndrome type 14) prior to any clinical or laboratory findings of kidney disease. With respect to accompanying features of the syndrome, it is currently impossible to predict how our young patient is going to develop with respect to his renal and neurologic function. We strongly suggest that screening for SGPL1 gene mutations should not be reserved for patients with nephrotic syndrome but should also include patients with adrenal insufficiency particularly because the phenotype and timing of manifestations seem to vary extensively even among patients with the same mutation who belong to the same family tree. Hopefully, early molecular identification prior to renal damage may be therapeutically advantageous, especially if a targeted treatment option were to become available in the near future, as is the case with other sphingolipidoses.
Acknowledgments
Financial Support: This work was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health (project number Z1A HD008920) (to C.A.S.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AAAS
triple-A syndrome
- NIH
National Institutes of Health
- NPHS14
nephrotic syndrome type 14
- PAI
primary adrenal insufficiency
- SGPL1
sphingosine-1-phosphate lyase 1
- S1P
sphingosine-1-phosphate
- SRNS
steroid-resistant nephrotic syndrome
References
- 1. Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23(3):327–364. [DOI] [PubMed] [Google Scholar]
- 2. Flück CE. Mechanisms in endocrinology: update on pathogenesis of primary adrenal insufficiency: beyond steroid enzyme deficiency and autoimmune adrenal destruction. Eur J Endocrinol. 2017;177(3):R99–R111. [DOI] [PubMed] [Google Scholar]
- 3. Chabre O, Goichot B, Zenaty D, Bertherat J Epidemiology of primary and secondary adrenal insufficiency: Prevalence and incidence, acute adrenal insufficiency, long-term morbidity and mortality. Ann Endocrinol (Paris). 2017;78(6):490–494. [DOI] [PubMed] [Google Scholar]
- 4. Malikova J, Flück CE. Novel insight into etiology, diagnosis and management of primary adrenal insufficiency. Horm Res Paediatr. 2014;82(3):145–157. [DOI] [PubMed] [Google Scholar]
- 5. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152–2167. [DOI] [PubMed] [Google Scholar]
- 6. Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, Hurcombe J, Bierzynska A, Barbagelata E, Bergadá I, Cassinelli H, Das U, Krone R, Hacihamdioglu B, Sari E, Yesilkaya E, Storr HL, Clemente M, Fernandez-Cancio M, Camats N, Ram N, Achermann JC, Van Veldhoven PP, Guasti L, Braslavsky D, Guran T, Metherell LA. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J Clin Invest. 2017;127(3):942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nürnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJ, Metherell LA. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44(7):740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, Costigan C, Clark AJ, Metherell LA. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341(8843):461–462. [DOI] [PubMed] [Google Scholar]
- 10. Chan LF, Campbell DC, Novoselova TV, Clark AJ, Metherell LA. Whole-exome sequencing in the differential diagnosis of primary adrenal insufficiency in children. Front Endocrinol (Lausanne). 2015;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi YJ, Saba JD. Sphingosine phosphate lyase insufficiency syndrome (SPLIS): a novel inborn error of sphingolipid metabolism. Adv Biol Regul. 2018;S2212-4926(18)30139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, Shril S, Ashraf S, Tan W, Rao J, Airik M, Schapiro D, Braun DA, Sadowski CE, Widmeier E, Jobst-Schwan T, Schmidt JM, Girik V, Capitani G, Suh JH, Lachaussée N, Arrondel C, Patat J, Gribouval O, Furlano M, Boyer O, Schmitt A, Vuiblet V, Hashmi S, Wilcken R, Bernier FP, Innes AM, Parboosingh JS, Lamont RE, Midgley JP, Wright N, Majewski J, Zenker M, Schaefer F, Kuss N, Greil J, Giese T, Schwarz K, Catheline V, Schanze D, Franke I, Sznajer Y, Truant AS, Adams B, Désir J, Biemann R, Pei Y, Ars E, Lloberas N, Madrid A, Dharnidharka VR, Connolly AM, Willing MC, Cooper MA, Lifton RP, Simons M, Riezman H, Antignac C, Saba JD, Hildebrandt F. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest. 2017;127(3):912–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309(5741):1735–1739. [DOI] [PubMed] [Google Scholar]
- 14. Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulation. Neurology. 2011;76(8, Suppl 3)S3–S8. [DOI] [PubMed] [Google Scholar]
- 15. Josephs KA, Van Gerpen MW, Van Gerpen JA. Adult onset Niemann-Pick disease type C presenting with psychosis. J Neurol Neurosurg Psychiatry. 2003;74(4):528–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vieira JP, Conceição C, Scortenschi E. GM1 gangliosidosis, late infantile onset dystonia, and T2 hypointensity in the globus pallidus and substantia Nigra. Pediatr Neurol. 2013;49(3):195–197. [DOI] [PubMed] [Google Scholar]
- 17. Walterfang M, Fahey M, Desmond P, Wood A, Seal ML, Steward C, Adamson C, Kokkinos C, Fietz M, Velakoulis D. White and gray matter alterations in adults with Niemann-Pick disease type C: a cross-sectional study. Neurology. 2010;75(1):49–56. [DOI] [PubMed] [Google Scholar]
- 18. Bamborschke D, Pergande M, Becker K, Koerber F, Dötsch J, Vierzig A, Weber LT, Cirak S. A novel mutation in sphingosine-1-phosphate lyase causing congenital brain malformation. Brain Dev. 2018;40(6):480–483. [DOI] [PubMed] [Google Scholar]