Abstract
Fetal glucocorticoid exposure is a key mechanism proposed to underlie prenatal “programming” of adult cardiometabolic and neuropsychiatric disorders. Regulation of fetal glucocorticoid exposure is achieved by the placental glucocorticoid “barrier,” which involves glucocorticoid inactivation within the labyrinth zone of the murine placenta by 11 ß -hydroxysteroid dehydrogenase 2 (11ß-HSD2). Thus, the absence of placental 11ß -HSD2 may impact on fetal and placental development. The current study investigated transport of amino acids and glucose, key factors required or fetal growth, and vascular development in placentas from 11ß-HSD2+/+, +/-, and -/- fetuses derived from 11ß-HSD2+/- matings. At embryonic day 15 (E15) (term E19), 11ß-HSD2-/- fetal weight was maintained in comparison to 11ß-HSD2+/+ fetuses. The maintenance of 11ß-HSD2-/- fetal weight occurred despite a reduction in placental weight, suggesting that compensatory changes occur in the placenta to maintain function. However, by E18, 11ß-HSD2-/- fetal and placental weights were both reduced. Transport studies revealed up-regulation of placental amino acid transport to 11ß-HSD2-/- offspring at E15, coinciding with an increase in the expression of the amino acid transporters. Furthermore, at E18, placental glucose transport to 11ß-HSD2-/- offspring was markedly reduced, correlating with lower fetal weight and a decrease in glucose transporter 3 expression. Stereological analyses of the labyrinth zone of the placenta revealed that the reduction in placental weight at E18 was associated with restriction of the normal increase in fetal vessel density over the final third of pregnancy. Our data suggest that restriction of fetal growth in 11ß-HSD2-/- mice is mediated, at least in part, via altered placental transport of nutrients and reduction in placental vascularization.
Keywords: 11ß-HSD2, Glucocorticoid, Placenta, Developmental Programming
Introduction
Although glucocorticoids are essential for fetal maturation in late gestation, excessive fetal glucocorticoid exposure reduces fetal growth and is associated with susceptibility to disorders such as hypertension, insulin resistance and anxiety related disorders (1–4). Impairment of fetal growth has predominantly been attributed to direct effects of glucocorticoids on the fetus, prematurely shifting tissue development from a proliferative to a more functionally mature state (5). However, fetal growth is dependent on a complex interplay of maternal, placental and fetal endocrine signals, and glucocorticoid-mediated fetal growth retardation is likely also to relate to disturbances in placental growth and function (6, 7).
Transfer of maternal glucocorticoids to the fetus is controlled by the placental glucocorticoid ‘barrier’, 11β-hydroxysteroid dehydrogenase 2 (11ß-HSD2), which catalyses rapid inactivation of physiological glucocorticoids (corticosterone to 11-dehydrocorticosterone in rodents) within the placenta. In the mouse, 11ß-HSD2 is present in the labyrinthine trophoblast (8), the fetal portion of the placenta and the key site of maternal-fetal exchange (9). Labyrinthine 11ß-HSD2 mRNA is highly expressed, peaking at E15.5 and turning off by E16.5 (8). Crucially, activity of 11ß-HSD2 within the placenta in rats and humans correlates with birth weight (1, 10, 11), suggesting that normal variation in fetal exposure to maternal glucocorticoids impact on fetal growth. Indeed, maternal treatment during pregnancy with dexamethasone, a poor substrate for 11ß-HSD2, reduces placental and birth weight with “adverse” outcomes for adult cardiometabolic, neuroendocrine, and behavioural function. Furthermore, 11ß-HSD2 knockout mice (11β-HSD2-/-) have a lower birth weight than congenic littermate controls (11ß-HSD2+/+) of 11β-HSD2+/- crosses (2). Of course, disruption of 11ß-HSD2 in the fetally-derived labyrinth zone of 11ß-HSD2-/- fetuses exposes not only the fetus but also the placenta to increased local corticosterone levels, thus potentially altering placental function.
Treatment of rats with glucocorticoids such as dexamethasone, which are poor substrates for 11β-HSD2, restricts placental vascular development, via inhibition of the endothelial cell-specific mitogen, vascular endothelial growth factor (VEGF-A) and peroxisome proliferators-activated receptors PPARγ, a regulator of VEGF-A expression (6, 12). Impaired vascular arborisation within key areas of the placenta which are involved in nutrient exchange between the maternal and fetal circulations are likely to have effects on placental function. However, glucocorticoid effects on placental function have been discordant. Thus, chronic restraint stress during late gestation in rats reduces placental 11ß-HSD2 expression and expression of glucose transporter (GLUT) 1, with an associated reduction in fetal plasma glucose (7), whereas late gestation dexamethasone increases placental GLUT1 and 3 expression (13), and another synthetic glucocorticoid, triamcinolone, down-regulates placental GLUT1 and 3 protein and mRNA (14). Any physiological relevance of these manipulations is unresolved. Furthermore, whereas system A amino acid transporter (SNAT) activity and expression are upregulated by cortisol exposure in BeWo cells (15), they are unaltered in human placental villous fragments exposed to cortisol (16, 17), and any effects of glucocorticoids on placental amino acid transport in rodent pregnancy are presently unexplored.
The purpose of this current study was to elucidate the importance of 11ß-HSD2 in placental function. This was achieved via characterisation of placental function and morphology of 11ß-HSD2+/+, +/- and -/- fetuses derived from 11ß-HSD2+/- matings, thus eliminating maternal pathophysiology as a contributing factor.
Materials and Methods
Animals
Male and female 11ß-HSD2+/- mice congenic on the C57BL/6J background were mated overnight, and the morning on which a vaginal plug was present was designated day 1 (E1) of pregnancy. Offspring consisting of 11ß-HSD2+/+, +/-, -/- mice were compared within the same litter. Genotyping was performed by PCR as described previously (2). Animals were given standard chow and water ad libitum, lights were on between 7 A.M. and 7 P.M., and all studies were performed to the highest standards of humane animal care under the aegis of the United Kingdom Animals Scientific Procedures Act, 1986.
At E15 and E18 (term = E19), pregnant females were decapitated and fetuses and placentas were removed and weighed. Fetal tails were collected for genotyping and at E18, fetal trunk blood was collected for plasma glucose measurements. Placental zones were separated by blunt dissection and frozen for real-time quantitative RT-PCR or fixed in formaldehyde and processed for paraffin histology for subsequent stereological analyses. Placental dry weights were obtained by drying the placentas in an oven at 37°C until a constant weight was obtained. The water content of each placenta was calculated by subtracting the dry from the wet weights and expressing it as a percentage of wet weight.
Placental Transport of Radiolabelled Glucose and Amino Acids
Placental transport of radiolabelled glucose and amino acids were established using modified methods (18). Briefly, pregnant mice at E15 and E18 were anesthetised. 100 μl of PBS containing 3.5 μCi 14C-MeAIB (NEC671, PerkinElmer, Beaconsfield, UK) or 3.5 μCi 14C-methyl-D-glucose (NEC377050UC, PerkinElmer, Beaconsfield, UK) was injected i.v. and 4 min after injection, animals were killed by cervical dislocation and fetuses and placentas were removed and weighed. Fetal tails were collected for genotyping and the remainder of the fetus was lysed overnight at 55°C in Biosol (National Diagnostics, Hessle Hull, UK). Fractions of fetal samples were then added to scintillation tubes for β counting (Tri-Carb 2100TR, Packard, Pangbourne, UK). Radioactive counts in each fetus were then used to calculate the amount of radioisotope transferred per gram of placenta (to give a relative measure of placental transfer of the solute), or per gram of fetus (to give a relative measure of the amount of solute received by the fetus). Average values for wild-type, heterozygote and knockout fetuses within a litter were then calculated. These values were then used to calculate a mean for all litters at E15 and E19.
Glucose Assay
Plasma glucose was measured by hexokinase assay (Infinity Glucose Kit, ThermoScientific, Bucks, UK).
Immunohistochemistry
Placental sections were exhaustively sectioned at 7 μm thickness, depariffinized and rehydrated, then incubated for 20 min in 3% H2O2 to block endogenous peroxidases. Proteinase K solution (Dakocytomation, Ely, UK) was applied to assist epitope retrieval. Polyclonal rabbit primary antibody to von Willebrand factor (to distinguish fetal capillary endothelium, Dakocytomation, Ely, UK) was used at a dilution of 1:500 for 1 h at room temp followed by a 30 min room temperature incubation in goat anti-rabbit secondary (Vector Laboratories, Ltd., Peterborough, UK). After washing, slides were incubated with ABC solution (Vector Laboratories, Ltd., Peterborough, UK) and specific binding was detected using the DAB system (Vector Laboratories, Ltd., Peterborough, UK). Slides were counterstained with haemotoxylin before dehydrating in graded alcohols and mounting in Di-N-Butyle Phthalate in Xylene (VWR International Ltd, Poole, UK).
Placental stereological assessment
Placental volume, placental component volume and detailed labyrinth zone analyses were all assessed as detailed previously (19). Briefly, absolute placental volume was assessed by point counting at x1.25 magnification, to enable a complete view of the section. Volumes of placental components were then assessed at x10 magnification, by random sampling of 15 fields of view and point counting was conducted to estimate labyrinth zone, spongiotrophoblast and decidual volumes. The labyrinth zone was assessed at x40 magnification and volumes of fetal capillaries, maternal blood spaces and trophoblasts were obtained by point counting. Surface area measurements for these labyrinth zone compartments were determined by overlaying a grid of cycloid arcs. Estimation of capillary length, diameter and density was obtained by using a counting frame with two contiguous forbidden lines (20). Measures were adjusted for tissue shrinkage by measurement of average maternal erythrocyte diameter before and after tissue processing (19). All measurements were performed blind and intra-observer error was <5%.
Measurement of mRNA expression by quantitative RT-PCR analysis
Total RNA was extracted from tissue samples using Trizol reagent (Life Technologies Inc., Paisley, UK) and extracted RNA was treated with DNase I (Invitrogen, Paisley, UK) to remove contaminating genomic DNA. RNA (1 µg) was then reverse transcribed at 55ºC for 50 min using MMLV RT (Promega, Southampton, UK) according to manufacturer’s instructions. Real-time PCR was performed using the TaqMan ABI Prism 7900 sequence detector (Applied Biosystems, Chester, UK). Expression levels were quantified using Lightcycler 480 Probes Master (Roche Diagnostics, Burgess Hill, UK) with primer-probe sets (Applied Biosystems) for the following genes; 18S (lot no. Hs99999901_s1), Pparγ (Mm00440945_m1), Slc2a1 (Mm00441473_m1), Slc2a3 (Mm00441483_m1), Slc38a1 (Mm00506391_m1), Slc38a2 (Mm00628416_m1), Slc38a4 (Mm00459056_m1), Vegf-a (Mm00437304_m1). Data acquisition used Sequence Detector 1.6.3. software (Applied Biosystems). A standard curve for each primer probe set was generated in triplicate by serial dilution of pooled cDNA. Each sample was run in triplicate and the mean values of the triplicates were used to calculate transcript level from the standard curve. The results are expressed as a ratio to 18S ribosomal RNA to normalize the transcript levels. RT negative controls and intron spanning primers were used where possible to prevent genomic DNA amplification.
Statistical analysis
All data are expressed as mean ± SEM, with each litter representing an n of one. For fetal and placental weights, there were an n of 35-43. Each experimental group for stereological and real time RT-PCR and plasma glucose measures had an n of 6-8, whilst for transport studies there were an n of 8-15. Two-way ANOVAs were used to assess variation in placental and fetal weights, placental transport, stereology and plasma glucose measures for gestational age and genotype. When significant interaction terms (P<0.05) were found in these ANOVAs, analyses of subsets of data were made by one-way ANOVA. Where the F test for the ANOVA reached statistical significance (P<0.05), differences were followed by Tukey’s post hoc test.
Results
Fetal and placental weights
All fetuses regardless of 11ß-HSD2 genotype increased in weight between E15 and E18 (4 fold, P<0.001; Table 1). Placental weight remained stable with time, but the placentas from 11ß- HSD2-/- mice were consistently smaller than either 11ß-HSD2+/+ or +/- littermates. Therefore, at E15 11ß-HSD2-/- fetal weight was maintained despite a reduction (11%, P<0.05, Table 1) in placental weight. Consequently, fetal/placental weight ratios were higher in the 11ß-HSD2-/- than wild-type fetuses. However, by E18, 11ß-HSD2-/- fetal and placental weights were both reduced in comparison to 11ß-HSD2+/+ littermates (fetus: 12%, P<0.01; placenta: 6%, P<0.05, Table 1) and this was accompanied by a reduction in the fetal/placental ratio. The water content of 11ß-HSD2-/- placentas did not differ from their wild-type or heterozygous littermates showing that the reduction in weight is not due to altered fluid/water content (Table 1).
Table 1.
Fetal and placental wet weights, fetal/placental ratio and placental dry weight of 11ß-HSD2+/+, +/- and -/- fetuses at E15 and E18.
| E15 | E18 | |||||
|---|---|---|---|---|---|---|
| +/+ | +/- | -/- | +/+ | +/- | -/- | |
| Placental weight (mg) | 101±4 | 98±3 | 90±5* | 98±2* | 98±2 | 92±2* |
| Fetal weight (mg) | 203±5 | 198±3 | 193±7 | 863±21✤ | 820±31 | 757±13* |
| Fetal/Placental ratio | 2.06±0.1 | 2.09±0.06 | 2.52±15* | 8.89±0.3✤ | 8.48±0.3 | 8.41±0.2* |
| Placental content water(%) | 84.2±0.6 | 83.6±1.1 | 84.9±0.8 | 83.5±0.9 | 83.6±0.6 | 82.2±1.1 |
Values are the mean ± SEM (n = 35 to 43 per group).
denotes an overall effect of gestational age (two-way ANOVA, P<0.05)
denotes differences from 11ß-HSD2+/+ within the time point (one-way ANOVA, P<0.05).
Placental transport of amino acids and glucose
To determine if the impaired growth of the placentas from 11ß-HSD2-/- mice had an impact on the transport of nutrients to the fetus, placental transport of amino acids and glucose were determined.
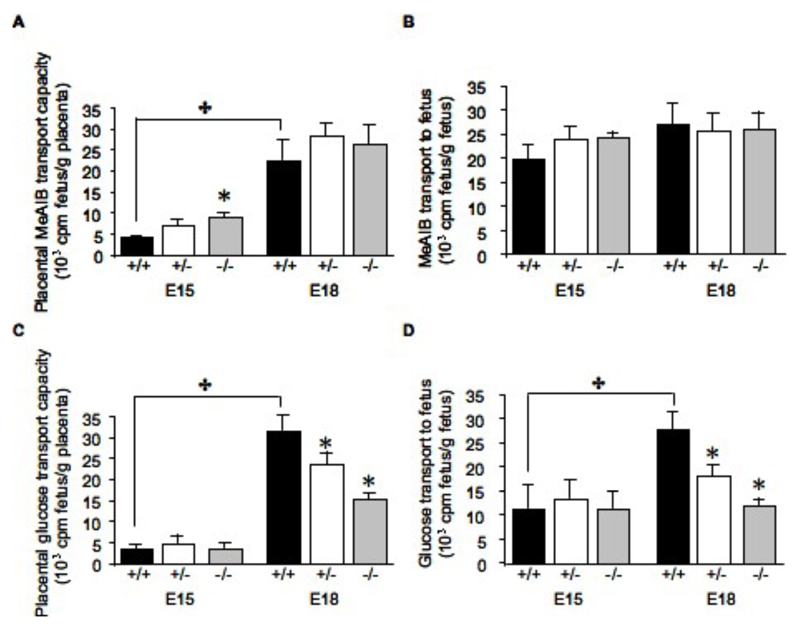
Amino acid transport: System A placental transport capacity of 14C-MeAIB increased in all genotypes from E15 to E18 (Figure 1A & B). Interestingly, at E15 the transport capacity of placentas from 11ß-HSD2-/- was significantly higher than from +/+ or +/- littermates (Figure 1A), but this did not result in a significant change in amino acid transport to the fetus (Figure 1B). No effect of genotype was observed on amino acid transport at E18.
Figure 1.
Changes in placental transport of 14C-MeAIB and 14C-glucose in 11ß-HSD2+/+, +/- and -/- fetuses at E15 and E18 expressed per gram of placenta (A and C respectively) or per gram of fetus (B and D respectively). Values are the mean + SEM. ✤ denotes an overall effect of gestational age (two-way ANOVA, P<.05), * denotes differences from 11ß-HSD2+/+ within the time point (one-way ANOVA, P<0.05).
Glucose transport: Placental transport capacity of 14C-glucose was significantly increased at E18 in comparison to E15 in placentas from fetuses of all genotypes (Figures C). At E15, levels of glucose transport across the placenta were independent of genotype, but at E18 the glucose transport capacity of the placenta was significantly reduced in both 11ß-HSD2+/- and -/- fetuses (53 and 25%, respectively, in comparison to wild-type; P<0.01; Figure 1C); in both -/- and +/+, this resulted in the fetus receiving less glucocse per gram fetal weight than wild-type littermates (57 and 35% less, respectively; P<0.01; Fig1D).
Fetal plasma glucose measurements
Levels of fetal plasma glucose at E18 reflected the placental transport findings with 11ß-HSD2-/- and +/- fetuses having lower plasma glucose than wild-type (11ß-HSD2+/+: 83.6 ± 4.4 mg/dl, 11ß-HSD2+/-: 64.5 ± 9.1 mg/dl, 11ß-HSD2-/-: 68.9 ± 5.7 mg/dl; P<0.05).
Placental stereological measures
Stereological measurements were performed to determine which regions of the placenta from 11ß-HSD2-/- mice contribute to the reduced placental size. All placentas exhibited a significant expansion in the labyrinth zone between E15 and E18 (43% in the wild-type placenta, P<0.01; Table 2), however the normal gestational increase in labyrinthine fraction was compromised in placentas from 11ß-HSD2-/- fetuses, with an expansion of only 18% between E15 and E18 (P<0.05, Table 2). In contrast, the spongiotrophoblast fraction decreased (P<0.05) between E15 (48.5% of total placental volume) and E18 (29% of total placental volume), whilst there was no significant change in the decidual volume between those two time points (see Table 2). Neither of the spongiotrophoblast fraction nor the decidual volume was modified by genotype (Table 2).
Table 2. Stereological measures of placentas from 11ß-HSD2+/+, +/- and -/- fetuses at E15 and E18.
| E15 | E18 | |||||
|---|---|---|---|---|---|---|
| +/+ | +/- | -/- | +/+ | +/- | -/- | |
| Placental fractions (% of total placental volume) | ||||||
| Decidua basalis | 25.4±3.11 | 31.3±2.14 | 19.6±2.67 | 18.5±1.50 | 17.7±1.61 | 21.9±1.66 |
| Spongiotrophoblast | 38.5±2.6 | 32.6±3.08 | 40.85±3.04 | 18.4±3.07✤ | 18.7±3.56 | 28.7±2.95 |
| Labyrinth zone | 33.9±3.16 | 33.7±2.37 | 37.2±1.29 | 59.5±2.75✤ | 59.6±3.05 | 45.6±2.44* |
| Chorion | 2.16±0.03 | 2.37±0.02 | 2.3±0.03 | 3.6±0.03✤ | 3.9±0.03 | 3.7±0.04 |
| Absolute MBS volume (10-3 cm3) | 3.8±0.3 | 3.3±0.1 | 2.8±0.6 | 9.1±1✤ | 9.4±1 | 6.4±2 |
| Absolute FC volume (10-3 cm3) | 4.3±0.1 | 4.1±0.3 | 3.4±0.2 | 14.8±2✤ | 14.5±1 | 6.3±1* |
| MBS surface area density (cm2/cm3) | 270.56±30.21 | 276.70±40.26 | 194.42±35.94 | 431.09±46.34✤ | 398.13±20.09 | 498.65±66.49 |
| FC surface area density (cm2/cm3) | 370.29±27.33 | 373.02±28.55 | 285.96±38.2 | 473.39±24.93✤ | 450.26±16.77 | 314.11±45.87* |
| FC length (m) | 25.34±5.12 | 24.98±3.45 | 25.21±4.61 | 152.9±9.33✤ | 153.2±11.0 | 134.6±8.48* |
| FC diameter (μm) | 14.3±1.4 | 14.0±0.6 | 14.2±0.8 | 10.7±0. ✤ | 10.6±0.3 | 9.0±0.4* |
Values are the mean ± SEM (n = 6-8 per group).
denotes an overall effect of gestational age (two-way ANOVA, P>0.05)
denotes differences from 11ß-HSD2+/+ within the time point (one-way ANOVA, P<0.05). MBS, maternal blood space; FC, fetal capillaries.
As the labyrinth zone is the site of 11ß-HSD2 expression and is also crucial for nutrient transfer from mother to fetus, this zone was investigated in greater detail. The gestational increase in the labyrinth zone of all placentas was found to be attributable to increased volume and surface area of both maternal blood spaces and fetal capillaries, the latter reflecting a concomitant increase in fetal capillary length (Table 2). While the maternal blood space and fetal capillary volume were not significantly altered in 11ß-HSD2-/- placentas compared to wild-type littermates at E15 (Table 2), by E18 there was a dramatic decrease in fetal capillary volume (58% less than wild-type at E18, P<0.01), surface area density (34% less than wild-type at E18, P<0.01) length (12% less than wild-type at E18, P<0.01, Table 2) and diameter (Table 2). However, maternal blood space volume remained unaltered in placentas of 11ß-HSD2-/- fetuses in comparison to wild-type littermates. Thus, the vascular neogenesis normally observed in placental fetal capillaries over the final third of gestation was impaired in 11ß-HSD2-/- fetuses.
Quantification of placental gene expression
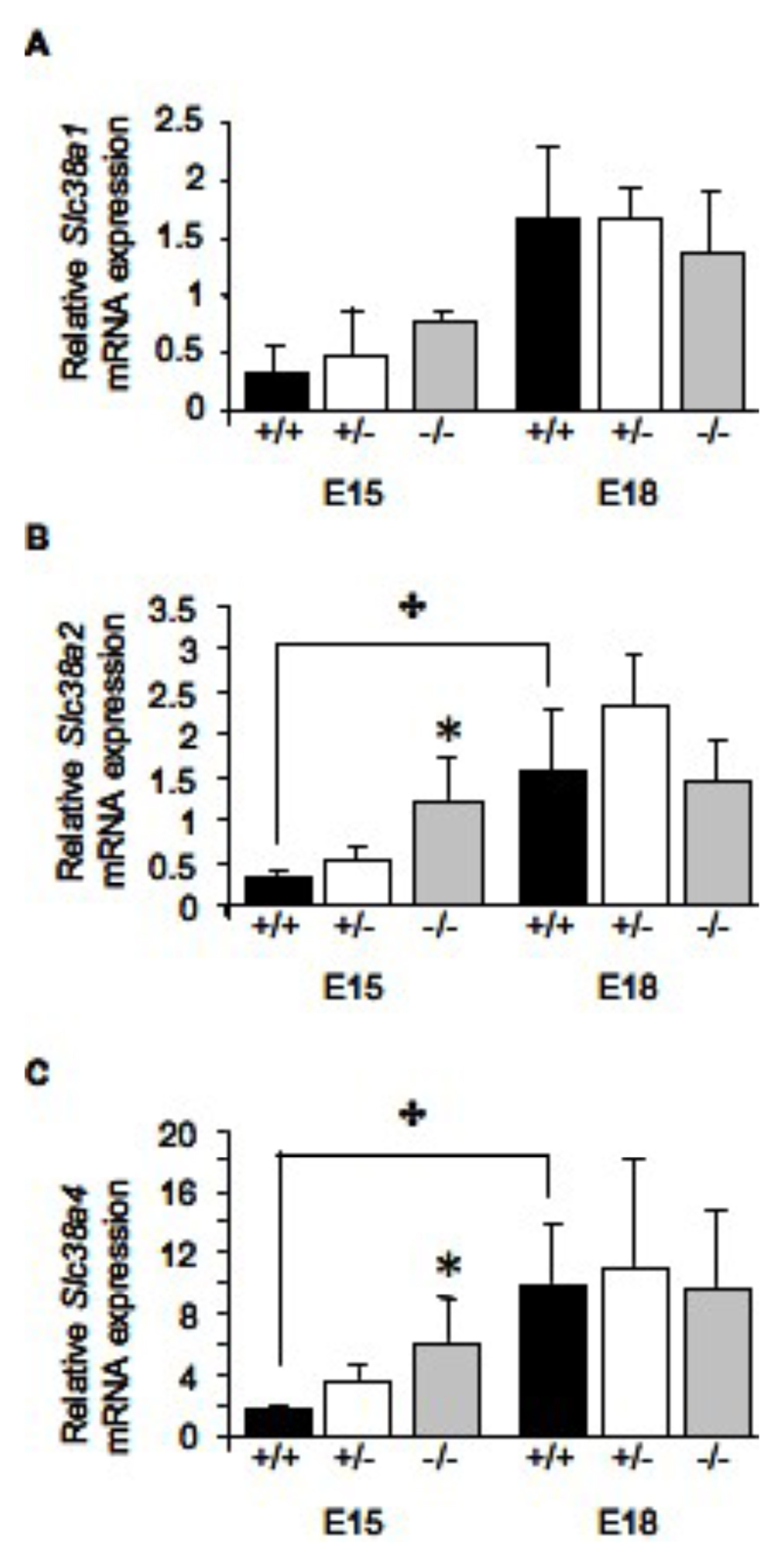
As there were significant functional and structural changes observed in placentas from HSD2-/- mice, we wished to determine if there is a concomitant change in expression of nutrient transporters and growth factors within the zones of the placenta which may impact on placental function. At E15, real-time quantitative PCR revealed that expression of Slc38a2 and Slc38a4 was upregulated 4-fold (P<0.05, Figure 2B and C) in placentas from 11ß-HSD2-/- fetuses in comparison to wild-type littermates. The gestational increase in expression of SNATs Slc38a2 (SNAT 2) and Slc38a4 (SNAT 4) in wild-type and 11ß-HSD2+/- placentas was absent in 11ß-HSD2-/- placentas (Fig 2), such that there were no differences in expression lveles across genotypes at E18. The expression of Slc38a1 was not affected by genotype. Interestingly, the upregulation of the amino acid transporters at E15 parallels the observed increased System A transporter function at this time point in 11ß-HSD2-/- placentas (Figure 1A).
Figure 2.
Relative mRNA expression of the System A amino acid transporters Slc38a1 (A), Slc38a2 (B) and Slc38a4 (C) in the labyrinth zone of placentas from 11ß-HSD2+/+, +/- and -/- fetuses at E15 and E18. Values are the mean + SEM. ✤ denotes an overall effect of gestational age (two-way ANOVA, P<0.05), * denotes differences from 11ß-HSD2+/+ within the time point (one-way ANOVA, P<0.05).
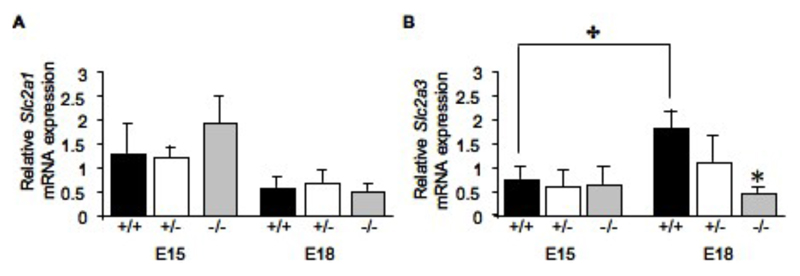
The expression profiles of GLUTs exhibited a different profile. There was no significant change in Slc2a1 (GLUT1) attributable to fetal genotype, though there was a trend for developmental decline in expression from E15 to E18 (Figure 3A). The expression of the glucose transporter Slc2a3 (GLUT3) was increased by 52% (P<0.05) between E15 and E18 in the wild-type placentas (Figure 3B). Importantly, at E18 Slc2a3 expression was reduced by 67% (P<0.05) in 11ß-HSD2-/- than in wild-type littermate placentas, abolishing the gestational increase observed in the wild-type placentas (Figure 3B). These results are consistent with the impaired glucose transport observed across the placenta of E18 11ß-HSD2-/- mice.
Figure 3.
Relative mRNA expression of the glucose transporters Slc2a1 (A) and Slc2a3 (B) in the labyrinth zone of placentas from 11ß-HSD2+/+, +/- and -/- fetuses at E15 and E18. Values are the mean + SEM. ✤ denotes an overall effect of gestational age (two-way ANOVA, P<0.05), * denotes differences from 11ß-HSD2+/+ within the time point (one-way ANOVA, P<0.05).
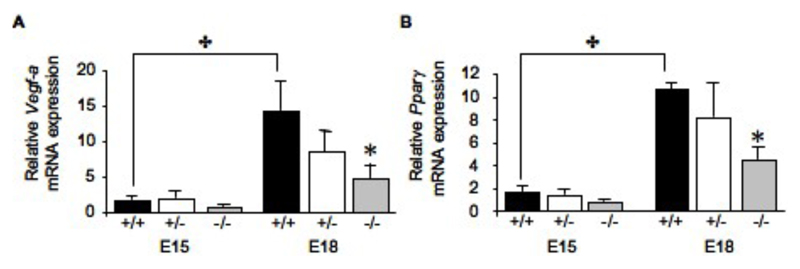
Vegf-a mRNA expression, a marker for angiogenesis, was found to be increased over gestation in the labyrinth zone of placentas from wild-type fetuses (8-fold, P<0.01, Figure 4A), but levels were decreased 3-fold (P<0.05) in 11ß-HSD2-/- littermates in comparison to wild-types at E18, abolishing the normal gestational rise in Vegf-a expression. A similar pattern of expression was revealed for a major regular of VEGF expression, Pparγ mRNA, with a halving of the normal gestational increase in expression (P<0.05, Figure 4B). Together the low expression levels of these genes, which are key for angiogenesis, may be responsible, at least in part, for the inadequate fetal capillary growth observed in the 11ß-HSD2-/- placentas.
Figure 4.
Relative expression of Vegf-a (A) and Pparγ (B) mRNAs in the labyrinth zone of placentas from 11ß-HSD2+/+, +/- and -/- fetuses at E15 and E18. Values are the mean + SEM. ✤ denotes an overall effect of gestational age (two-way ANOVA, P<0.05), * denotes differences from 11ß-HSD2+/+ within the time point (one-way ANOVA, P<0.05).
Discussion
Here we show the absence of 11ß-HSD2 compromised not only fetal growth but also placental growth. 11ß-HSD2-/- placentas had elevated amino acid transport at E15 in conjunction with increased expression of Slc38a2, whereas expression of Slc2a3 and glucose transport was diminished at E18. Thus, 11ß-HSD2-/- placentas had significantly reduced fetal capillary development within the labyrinth zone, the zone regulating nutrient exchange, accompanied by a decline in Vegf-a and Pparγ mRNA expression, factors known to regulate angiogenesis. These changes in placental function and morphology are likely to have ramifications for fetal development with consequences for later adult health.
At E15, despite a reduction in placental size, fetal weight is maintained, generating an increase in fetal to placental ratio that is indicative of enhanced placental function. Indeed, placental amino acid transport of 11ß-HSD2-/- fetuses was up-regulated at E15, alongside increased expression of Slc38a2 and Slc38a4. These observations likely contribute to the maintenance of fetal weight because amino acids contribute a large proportion of the carbon and nitrogen required for fetal growth (21). Increase in amino acid system A transport activity after glucocorticoid exposure has been previously demonstrated in vitro (15), whereas the data here suggest that this also occurs in vivo. Further studies are required to characterize the specific mechanisms by which excess placental glucocorticoid exposure up-regulates amino acid transport.
Later in pregnancy, at E18, the smaller placenta of the 11ß-HSD2-/- fetus appears unable to maintain normal fetal growth, and fetal weight falls behind control littermates. At this time the transplacental transfer of glucose and plasma glucose levels was reduced in 11ß-HSD2-/- fetuses. Glucose is a primary nutrient required for fetal development, and is transported across the placenta by facilitated diffusion primarily via GLUT1 and GLUT3 (22). The expression of GLUT1 has been localized to basal placental membranes and blood vessels, suggestive of a role for glucose transport to placental tissue (23, 24). In contrast, GLUT3 is present on the maternal-facing side of the labyrinth trophoblasts and, therefore, may be responsible for fetal glucose delivery. Thus, our observations of reduced Slc2a3 expression in the labyrinth zone of placentas from 11ß-HSD2-/- fetuses most likely accounts for the reduction in the transplacental transfer of glucose. Regulation of placental GLUT expression by glucocorticoids has been previously demonstrated in rat pregnancy and human villous extracts (7, 13, 14). Thus, it appears that the supply of glucose to the fetus is a critical feature of models of glucocorticoid excess in pregnancy. This may have important ramifications for “setting” fetal metabolism and adult health in later life, but further research is required to establish the significance of decreased placental transfer of glucose.
Interestingly, the 11ß-HSD2+/- fetuses, which exhibit approximately 50% of wild-type 11ß- HSD2 activity, also exhibited increased transplacental transfer of MeAIB at E15 and decreased transplacental glucose transfer at E18 alongside corresponding changes in glucose and amino acid transporter expression. This suggests that 11ß-HSD2+/- fetuses may also be ‘programmed’ and exhibit an intermediate phenotype between 11ß-HSD2+/+ and 11ß-HSD2-/- fetuses. However, previous work conducted on 11ß-HSD2+/- mice from heterozygous matings has not revealed any alteration in behaviour (2) or metabolic phenotype (Abrahamsen, 2007, unpublished observations) to date, despite an intermediate birth weight (2).
At E15 there was no significant alteration in fetal capillary density in 11ß-HSD2-/- placentas compared with wild-type littermates. This time point is just after the initial quiescent phase of labyrinthine fetal capillary development, which ceases at around E14.5 (19). Therefore, impairment of fetal capillary development in placentas from 11ß-HSD2-/- fetuses as a consequence of excess placental glucocorticoid exposure must occur after E14.5. This is supported by our observations that reveal that at E15, whereas not significant, there is a trend for reduced fetal capillary volume, surface area, and length. However, by E18, fetal capillary development was markedly impaired in placentas from 11ß-HSD2-/- fetuses, and this corresponded to a reduction in Vegf-a and Pparγ mRNAs in the labyrinth zone. These results are consistent with recent studies of rat placentas from dexamethasone-treated pregnancies (6, 12). Thus, it appears that glucocorticoid excess during rodent pregnancy retards fetal vessel growth within the labyrinth zone, at least in part, by direct effects of glucocorticoids on the expression of VEGF-A. Previous studies have implicated VEGF-A as a key factor for placental vascular remodeling (25, 26). Furthermore, glucocorticoids inhibit VEGF-A expression (27–30), perhaps via changes in PPAR_, which positively regulates VEGF-A expression (31, 32), and is suppressed by dexamethasone (12). Importantly, it is unknown whether the previous observations of altered placental function in dexamethasone-treated pregnant rats are a direct effect of glucocorticoids on the placenta or via indirect effects on the dam. In contrast, our model of 11ß-HSD2 heterozygous matings, whereby 11ß -HSD2+/+, +/-, and -/- fetuses are generated by the same mother, clearly demonstrates a direct effect of increased glucocorticoid exposure on placental function. Indeed, the placenta may be key to the effects of glucocorticoids on fetal growth because studies in sheep reveal that administration of glucocorticoids directly to the fetus does not retard fetal growth (33).
Although 11ß -HSD2 is absent in the labyrinth zone of placenta from 11ß -HSD2-/- fetuses, it is also absent in the fetal tissues, including kidney, gut, lung, and brain (8). Therefore, we cannot eliminate the possibility that our observed changes in placental function may be a result of altered fetoplacental cross talk. Until a placenta-specific knockout of 11ß -HSD2 is developed, the differential significance of placental 11ß -HSD2 for fetal and placental development cannot be comprehensively elucidated. Furthermore, there is evidence for a fetal gender-specific effect on placental glucocorticoid sensitivity (34), although it is uncertain if this is also the case in the present study. Further studies need to be conducted on a much larger scale to verify if the effects of placental 11ß -HSD2 absence on placental function are sex specific.
In conclusion, deletion of 11ß -HSD2 and, therefore, overexposure of the fetal-placental unit to high maternal glucocorticoids result in reduced fetal and placental growth. Placentas from 11ß -HSD2_/_ fetuses have impaired labyrinth zone capillary development and altered transport of nutrients. These alterations in placental phenotype are likely a consequence of increased placental glucocorticoid exposure. The initial consequence of placental 11ß-HSD2 absence is an up-regulation of amino acid transport that coincides with maintained fetal weight. However, as gestation progresses, normal fetal capillary development is compromised, and placental glucose transport is diminished that may contribute to the observed reduction in fetal weight. These data suggest that placental 11ß -HSD2 is crucial for optimal fetal development, and that the deleterious effect of excess glucocorticoids on fetal development and subsequent adult health is mediated, at least in part, by altered placental function.
Acknowledgements
Address all correspondence and requests for reprints to: C. S. Wyrwoll, Endocrinology Unit, Queen’s Medical Research Institute, Centre for Cardiovascular Science, 47 Little France Crescent, EdinburghEH164TJ, United Kingdom. E-mail: cwyrwoll@staffmail.ed.ac.uk.
Grants: This work was supported by a Wellcome Trust project grant (to M.C.H. and J.R.S.).
Footnotes
Disclosure Statement: The authors have nothing to declare.
References
- 1.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 2.Holmes MC, Abrahamsen CT, French KL, Paterson JM, Mullins JJ, Seckl JR. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. J Neurosci. 2006;26:3840–3844. doi: 10.1523/JNEUROSCI.4464-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 5.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147:5568–5574. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 7.Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- 8.Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 9.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 10.Murphy VE, Zakar T, Smith R, Giles WB, Gibson PG, Clifton VL. Reduced 11-hydroxysteroid dehydrogenase type 2 activity is associated with decreased birth weight centile in pregnancies complicated by asthma. J Clin Endocrinol Metab. 2002;87:1660–1668. doi: 10.1210/jcem.87.4.8377. [DOI] [PubMed] [Google Scholar]
- 11.Stewart PM, Rogerson FM, Mason JI. Type 2 11_-hydroxysteroid dehydrogenase messenger ribonucleic acid and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal adrenal steroidogenesis. J Clin Endocrinol Metab. 1995;80:885–890. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt DP, Mark PJ, Waddell BJ. Placental expression of peroxisome proliferator-activated receptors in rat pregnancy and the effect of increased glucocorticoid exposure. Biol Reprod. 2006;74:23–28. doi: 10.1095/biolreprod.105.045914. [DOI] [PubMed] [Google Scholar]
- 13.Langdown ML, Sugden MC. Enhanced placental GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation. Mol Cell Endocrinol. 2001;185:109–117. doi: 10.1016/s0303-7207(01)00629-3. [DOI] [PubMed] [Google Scholar]
- 14.Hahn T, Barth S, Graf R, Engelmann M, Beslagic D, Reul JM, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- 15.Jones HN, Ashworth CJ, Page KR, McArdle HJ. Cortisol stimulates system A amino acid transport and SNAT2 expression in a human placental cell line (BeWo) Am J Physiol Endocrinol Metab. 2006;291:E596–E603. doi: 10.1152/ajpendo.00359.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. AmJ Physiol Regul Integr Comp Physiol. 2005;288:R656–R662. doi: 10.1152/ajpregu.00407.2004. [DOI] [PubMed] [Google Scholar]
- 17.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88:1205–1211. doi: 10.1210/jc.2002-021332. [DOI] [PubMed] [Google Scholar]
- 18.Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci USA. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- 20.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 21.Lemons JA, Adcock EW, 3rd, Jones MD, Jr, Naughton MA, Meschia G, Battaglia FC. Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest. 1976;58:1428–1434. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- 23.Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology. 1997;138:3997–4004. doi: 10.1210/endo.138.9.5369. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Bondy CA. Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest. 1993;91:845–852. doi: 10.1172/JCI116305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 27.Alagappan VK, McKay S, Widyastuti A, Garrelds IM, Bogers AJ, Hoogsteden HC, Hirst SJ, Sharma HS. Proinflammatory cytokines upregulatemRNA expression and secretion of vascular endothelial growth factor in cultured human airway smooth muscle cells. Cell Biochem Biophys. 2005;43:119–129. doi: 10.1385/CBB:43:1:119. [DOI] [PubMed] [Google Scholar]
- 28.Alonso G, Galibert E, Duvoid-Guillou A, Vincent A Hyperosmotic stimulus induces reversible angiogenesis within the hypothalamic magnocellular nuclei of the adult rat: a potential role for neuronal vascular endothelial growth factor. BMC Neurosci. 2005;6:20. doi: 10.1186/1471-2202-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, Edwards NA, Oldfield EH. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest. 1996;98:1400–1408. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koedam JA, Smink JJ, van Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol Cell Endocrinol. 2002;197:35–44. doi: 10.1016/s0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 31.Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, Nishizawa Y. Peroxisome proliferator-activated receptor-_ agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;271:571–574. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda E, Tokuda H, Ishisaki A, Hirade K, Kanno Y, Hanai Y, Nakamura N, Noda T, Katagiri Y, Kozawa O. PPAR-_ ligands up-regulate basic fibroblast growth factor-induced VEGF release through amplifying SAPK/JNK activation in osteoblasts. Biochem Biophys Res Commun. 2005;328:137–143. doi: 10.1016/j.bbrc.2004.12.163. [DOI] [PubMed] [Google Scholar]
- 33.Newnham JP, Evans SF, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med. 1999;8:81–87. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<81::AID-MFM3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]