Abstract
Objective
Transdermal, but not oral, estrogen replacement improves bone mineral density (BMD) in athletes with oligoamenorrhea (OA). Our objective was to determine mechanisms that may explain the impact of route of estrogen administration on bone outcomes.
Methods
Seventy-three participants with OA between 14 and 25 years old received (i) a 17β-estradiol transdermal patch continuously with cyclic oral micronized progesterone (PATCH), (ii) a combined ethinyl estradiol and desogestrel pill (PILL), or (iii) no estrogen/progesterone (NONE) for 12 months. We evaluated morning fasting levels of a marker of bone formation [N-terminal propeptide of type 1 procollagen (P1NP)], a marker of bone resorption (N-telopeptide), IGF-1, insulinlike growth factor binding protein 3, total testosterone, estradiol, SHBG, sclerostin, preadipocyte factor-1 (Pref-1), brain-derived neurotrophic factor (BDNF), calcium, 25(OH) vitamin D, and PTH levels at baseline and 12 months.
Results
Groups did not differ for age, weight, exercise activity, or markers of bone formation at baseline. Over 12 months, P1NP decreased the most in the PILL group (P = 0.03) associated with a decrease in IGF-1 levels (r = 0.37; P = 0.003). Sclerostin, Pref-1, and BDNF decreased in the PATCH group over 12 months. PATCH had the greatest increases in estradiol (P ≤ 0.0001), and estradiol increases were associated with increases in bone density.
Conclusion
Transdermal 17β-estradiol given over 12 months does not cause the decrease in IGF-1 observed with oral ethinyl estradiol. It also leads to decreases in sclerostin, Pref-1, and BDNF, which may mediate the beneficial effects of estrogen.
Treatment of hypogonadal athletes with transdermal 17β estradiol prevented the decline in bone formation markers and IGF-1 levels seen in the group given oral estrogen. We saw decreases in sclerostin.
Female athletes participating in endurance training are at an increased risk of the Female Athlete Triad of low energy availability, menstrual dysfunction, and low bone mineral density (BMD) (1). In amenorrheic athletes, estrogen deficiency is a strong predictor of low BMD, suggesting that estrogen replacement should be an effective strategy to increase BMD in females with the Female Athlete Triad. We have reported that physiologic estrogen replacement as the 17β-estradiol patch (with cyclic progesterone) (PATCH) results in significant increases in BMD at the lumbar spine and femoral neck in young female athletes with oligoamenorrhea (OA) compared with nonphysiologic estrogen replacement [as a combined oral contraceptive pill with 30 mcg of ethinyl estradiol and a progestogen (PILL)] or no estrogen replacement (NONE) (2), consistent with data from other conditions of estrogen deficiency (3, 4). Understanding the mechanisms underlying the efficacy of the PATCH (but not the PILL) in improving BMD in hypogonadal states is necessary for identification of future therapeutic targets.
Optimal bone accrual during adolescence and young adulthood is dependent on the bone trophic effects of rising levels of IGF-1, and the antiresorptive and also bone anabolic effects of rising sex steroids (5). Greater efficacy of the PATCH vs the PILL in increasing BMD in oligoamenorrheic athletes has been attributed (at least partly) to the IGF-1–suppressive effects of oral estrogen from hepatic first pass metabolism, which does not occur with the PATCH (2, 6, 7). Also, the PILL causes marked increases in SHBG levels in oligoamenorrheic athletes, an effect not observed with the PATCH (2). Higher SHBG would reduce bioavailability of sex hormones, reducing the efficacy of the PILL in improving BMD.
Estrogen replacement may also impact bone through its effect on factors that determine osteoblast differentiation and function, such as sclerostin, preadipoctye factor 1 (Pref-1), and brain-derived neurotrophic factor (BDNF). Sclerostin is a glycoprotein exclusively secreted by osteocytes that inhibits bone formation by blocking the osteogenic WNT/β-catenin signaling pathway (8). Although mechanical loading associated with physical activity decreases sclerostin (9), estrogen deficiency is associated with increased sclerostin (10). Pref-1 is a transmembrane protein expressed by preadipocytes that regulates the differentiation of mesenchymal stem cells (MSCs), the common progenitor cells of both osteoblasts and bone marrow adipocytes. BMD is influenced by relative rates of osteoblastogenesis vs adipogenesis (11, 12), and particularly in adolescents, an inverse relationship is described between BMD and marrow fat (13). Pref-1 levels are positively associated with marrow fat content and inversely with BMD (14, 15), and estrogen administration may downregulate Pref-1 (11). Lastly, BDNF is a protein expressed in the central and peripheral nervous systems, as well as by osteoblasts and chondrocytes, with negative effects on bone formation (16, 17). Rodents with central BNDF deletion have increased BMD (18, 19), and estrogen may reduce BDNF (20).
Data are lacking regarding effects of the PATCH vs the PILL on sclerostin, Pref-1, and BDNF in relation to bone outcomes in OA. Our objective was to compare the impact of the PATCH vs the PILL or no estrogen replacement on bone markers and BMD in young normal-weight OA in relation to changes in IGF-1, SHBG, sclerostin, Pref-1, and BDNF. We hypothesized that improvement in BMD in the PATCH (but not the PILL) group would be associated with a sparing of IGF-1 and SHBG levels, and greater reductions in sclerostin, Pref-1, and BDNF than observed in the PILL or no-estrogen groups.
Participants and Methods
Participant selection
Of 121 oligoamenorrheic female athletes ages 14 to 25 years old (19.9 ± 2.6 years) included in the parent study, 73 participants with data for bone turnover markers and other biochemical parameters at baseline and 12 months were included in the current study (2). OA was defined as the absence of menstrual periods for ≥3 months within a period of OA of ≥6 months (cycle length >6 weeks) or the absence of menarche at ≥16 years. Enrolled participants engaged in aerobic weight-bearing activity for 4 hours/week and/or ran ≥20 miles/week for ≥6 months preceding the study. Cyclists, swimmers, rowers, and gymnasts were excluded due to differences in the weight-bearing nature and impact of these activities.
Participants were required to have a bone age of ≥14 years, and a body mass index (BMI) between the 10th and 90th percentiles for age or >85% of the median BMI for age. A study psychologist screened participants to assess for current eating disorders or a prior history of eating disorders. Participants with active eating disorders were excluded from study participation. Sixteen participants had previously diagnosed or suspected eating disorders, but all met BMI criteria for participation and were deemed eligible for the study by the study psychologist and study investigators.
Exclusion criteria included conditions other that endurance training that may cause amenorrhea, including premature ovarian failure, polycystic ovary syndrome, other causes of hyperandrogenism, hyperprolactinemia, and thyroid dysfunction, and the use of medications affecting bone metabolism, such as estrogen, progesterone, anabolic steroids, glucocorticoids, phenytoin, phenobarbitone, bisphosphonates, and teriparatide, within 3 months of study participation. Medical conditions that may affect bone metabolism, such as diabetes, cancer, or pituitary, renal, or gastrointestinal disease, were also exclusion criteria. For participants randomized to receive estrogen and progesterone, additional exclusion criteria included a personal history of migraines or thromboembolism, a personal or family history of conditions that may increase risk for thromboembolism, or a first-degree relative with history of an estrogen-dependent cancer.
The Partners Health Care Institutional Review Board approved this study. Participants were recruited through mailings to pediatricians, nutritionists, therapists, and sports medicine specialists, as well as advertisements in local colleges, sporting events, and newspapers. Informed consent was obtained from participants ≥18 years and from parents of participants <18 years old. Informed assent was obtained from participants <18 years.
Experimental protocol
Subjects were seen at the Massachusetts General Hospital (MGH) Translational and Clinical Research Center. At the study screening visit, a study physician completed a detailed medical history and physical examination, and blood was drawn to rule out conditions other than low energy availability associated with exercise that may cause OA. Participants were weighed to the nearest 0.1 kg on an electronic scale in a hospital gown, and height was measured to the nearest millimeter in triplicate with a wall-mounted stadiometer. Participants provided a detailed recall of exercise history, including average hours per week of physical activity in the preceding 12 months. A hand and wrist x-ray were taken to determine bone age, and bone density was assessed using dual-energy x-ray absorptiometry (DXA). DXA assessment provided measures of areal bone mineral density (aBMD) as well as body composition.
At the baseline visit, participants completed a 4-day food diary to determine total caloric intake (Nutrient Data System for Research software version 2008; University of Minnesota Nutrition Coordinating Center, Minneapolis, MN), and resting energy expenditure was assessed using indirect calorimetry (VMAX Encore 29 metabolic cart; Viasys Health Care, Carefusion, San Diego, CA). Blood samples were drawn at least 2 hours postprandial for calcium, 25(OH) vitamin D [25(OH)D], phosphorus, PTH, a marker of bone formation [N-terminal propeptide of type 1 procollagen (P1NP)], and a marker of bone resorption [N-telopeptide (NTX)]. Blood samples were also drawn for IGF-1, insulinlike growth factor binding protein 3 (IGFBP-3), total testosterone, estradiol, SHBG, sclerostin, Pref-1, and BDNF. The free androgen index (FAI) was calculated to assess bioavailable testosterone as ([testosterone (ng/dL) × 3.47]/SHBG (nmol/L)) × 100 (21).
Oligoamenorrheic participants were randomized to receive (i) the transdermal 17β-estradiol (100 mcg patch applied twice weekly) for 12 months (PATCH) with 200 mg of micronized progesterone for 12 days of each month, or (ii) a combined estrogen-progesterone contraceptive pill (PILL) containing 30 mcg of ethinyl estradiol and 0.15 mg of desogestrel, or (iii) no estrogen (NONE) by the Massachusetts General Hospital Research Pharmacy based on a predetermined computer-generated randomization sequence (2). All participants took 1200 mg elemental calcium and 800 IU vitamin D daily. To monitor study medication compliance, participants completed medication calendars to record study medication intake and missed doses. Study investigators collected these calendars and any unused study medications at follow-up study visits every 3 months until the 12-month visit. Subjects with spontaneous menses in the NONE group were scheduled for visits within 10 days of menses onset for consistency, given variations in estradiol and bone turnover markers across the menstrual cycle.
Bone density and body composition assessment
DXA (Hologic QDR-Discovery A, Apex software version 13.3; Hologic Inc, Waltham, MA) was used to assess aBMD of the lumbar spine, total hip, and femoral neck, as well as measures of body composition (fat and lean mass). The coefficients of variation for BMD, fat mass, and lean mass for our institution are 0.8% to 1.1%, 2.1%, and 1.0%, respectively.
Biochemical analysis
A colorimetric assay was used to measure calcium [LabCorp Automated Chemistry, Raritan, NJ; sensitivity 0.8 mg/dL; intra-assay coefficient of variation (CV) 0.9% to 3.0%], an immunochemiluminometric assay to measure 25(OH)D (LabCorp Esoteric Testing, Burlington, NC; sensitivity 4.0 ng/mL; intra-assay CV 4.8% to 7.7%), and an electrochemiluminescence immunoassay to assess PTH (LabCorp Automated Chemistry, Burlington, NC; sensitivity 6.0 pg/mL; intra-assay CV 0.9% to 3.0%), 17β-estradiol (LabCorp Esoteric Testing, Burlington, NC, sensitivity 25.0 pg/mL; intra-assay CV 1.2% to 6.7%), SHBG (LabCorp Esoteric Testing, 2.00 nmol/L; intra-assay CV 1.1% to 1.7%), and testosterone (LabCorp Esoteric Testing, sensitivity 12.0 ng/dL; intra-assay CV 2.1% to 14.8%). Mass spectrometry was used to measure IGF-1 (Quest Diagnostics, Nichols Institute, San Juan Capistrano, CA; sensitivity 15.6 ng/mL; intra-assay CV 3.5% to 15%), and chemiluminescence to measure IGFBP-3 (Immunodiagnostic Systems, Inc., Scottsdale, AZ; sensitivity 50 ng/mL; intra-assay CV 1.92%). An enzyme-linked immunosorbent assay was used to measure NTX (LabCorp Esoteric Testing; sensitivity 3.2 nmol bone collagen equivalents (BCE)/L; intra-assay CV 11.9% to 14.0%), BDNF (R&D Systems; Minneapolis, MN; sensitivity 20 pg/mL; intra-assay CV 5%), sclerostin (R&D Systems, Minneapolis, MN; sensitivity 1.74 pg/mL; intra-assay CV 2%), and Pref-1 (R&D Systems; sensitivity 93.8 pg/mL). A radioimmunoassay was used to measure P1NP (Orion Diagnostics, Espoo, Finland; sensitivity 2.0 ng/mL; intra-assay CV 6.5% to 10.2%).
Statistical analysis
Analyses were conducted using JMP Statistical Discovery Software Version 12.0, and data are reported as the mean ± SEM (normally distributed data) or median and interquartile range (nonnormally distributed data). A completers-only analysis was performed for this mechanistic study for the 12-month completers (n = 26 for PATCH, n = 21 for PILL, and n = 26 for NONE). All data were first assessed for normality. To analyze baseline characteristics and changes in biochemical parameters over study course among treatment groups, data were analyzed with an ANOVA followed by the Tukey-Kramer test to control for multiple comparisons, and nonnormally distributed data were analyzed with a Wilcoxon Rank-Sum test followed by the Steel-Dwass test to control for multiple comparisons. Adjustments for (i) age and (ii) age, weight changes over time, and menstrual recovery are reported for completers using the generalized linear modeling (GLM) procedure (Table 1).
Table 1.
Comparison of Changes in Biochemical Parameters Over 12 Months in the NONE, PILL, and PATCH Groups (GLM Procedure) Adjusted for Age and After Adjusting for Age, Weight Changes Over Time, and Menstrual Recovery in the NONE Group Weight Changes Over Time, and Menstrual Recovery in the NONE Group
| Change Over 12 Months (Adjusted for Age) | Parameter Estimate ± SE |
Group × Visit P Value | PATCH vs NONE | PILL vs NONE | PILL vs PATCH | ||
|---|---|---|---|---|---|---|---|
| PATCH vs NONE | PILL vs NONE | PATCH vs PILL | |||||
| Δ PTH (pg/mL) | 1.8 ± 3.3 | −0.04 ± 3.5 | 2.2 ± 3.5 | 0.795 | 0.598 | 0.904 | 0.533 |
| Δ P1NP (ng/mL) | −4.81 ± 10.8 | −24.6 ± 11.1 | 19.8 ± 11.3 | 0.076 | 0.657 | 0.030 | 0.082 |
| Δ NTX (nM BCE/L) | −0.4 ± 1.83 | −1.2 ± 1.91 | 0.8 ± 1.9 | 0.813 | 0.827 | 0.527 | 0.670 |
| Δ IGF-1 (ng/mL) | −7.06 ± 22.5 | −54.56 ± 23.63 | 47.50 ± 24.09 | 0.06 | 0.755 | 0.024 | 0.053 |
| Δ IGF-1 z score | −0.11 ± 0.22 | −0.66 ± 0.24 | 0.55 ± 0.24 | 0.017 | 0.624 | 0.007 | 0.025 |
| Δ IGFBP-3 (ng/mL) | −170.08 ± 188.6 | 487.4 ± 193.5 | −657.5 ± 199.6 | 0.007 | 0.373 | 0.016 | 0.002 |
| Δ IGF-1/IGFBP-3 | 0.002 ± 0.004 | −0.017 ± 0.004 | 0.019 ± 0.004 | 0.0002 | 0.609 | 0.0003 | 0.0002 |
| Δ Testosterone (ng/dL) | −1.2 ± 3.7 | −0.4 ± 3.9 | −0.8 ± 3.9 | 0.948 | 0.748 | 0.915 | 0.841 |
| Δ Estradiol (pg/mL) | 40.3 ± 21.3 | −55.8 ± 23.09 | 96.1 ± 22.95 | 0.0005 | 0.063 | 0.020 | <0.0001 |
| Δ SHBG (nmol/L) | −7.4 ± 17.5 | 147.1 ± 18.2 | −154.5 ± 18.3 | <0.0001 | 0.673 | <0.0001 | <0.0001 |
| Δ FAI | −0.182 ± 0.4 | −1.22 ± 0.4 | 1.03 ± 0.4 | 0.004 | 0.610 | 0.002 | 0.007 |
| Δ Sclerostin (pg/mL) | −57.9 ± 22.4 | −53.8 ± 22.5 | −4.1 ± 23.1 | 0.022 | 0.013 | 0.022 | 0.860 |
| Δ Pref-1 (pg/mL) | −28.86 ± 37.5 | −22.5 ± 39.2 | −6.3 ± 40.2 | 0.722 | 0.446 | 0.569 | 0.876 |
| Δ BDNF (ng/mL) | −6363.5 ± 2904.8 | −2063.3 ± 2937.9 | −4300.1 ± 2980.7 | 0.100 | 0.034 | 0.486 | 0.157 |
| Change Over 12 Months (Adjusted for Age, Weight, and Menstrual Status) | PATCH vs NONE | PILL vs NONE | PATCH vs PILL | Group × Visit P Value | PATCH vs NONE | PILL vs NONE | PILL vs PATCH |
|---|---|---|---|---|---|---|---|
| Δ PTH (pg/mL) | 2.2 ± 4.14 | −0.4 ± 4.23 | 2.6 ± 3.4 | 0.719 | 0.599 | 0.922 | 0.443 |
| Δ P1NP (ng/mL) | −5.4 ± 14.05 | −24.6 ± 14.3 | 19.3 ± 11.5 | 0.139 | 0.705 | 0.090 | 0.099 |
| Δ NTX (nM BCE/L) | −1.3 ± 2.4 | −2.3 ± 2.5 | 0.95 ± 1.97 | 0.648 | 0.579 | 0.356 | 0.633 |
| Δ IGF-1 (ng/mL) | −0.528 ± 28.39 | −45.28 ± 29.2 | 44.75 ± 24.49 | 0.145 | 0.985 | 0.127 | 0.073 |
| Δ IGF-1 z score | −0.03 ± 0.29 | −0.54 ± 0.29 | 0.51 ± 0.24 | 0.078 | 0.907 | 0.073 | 0.042 |
| Δ IGFBP-3 (ng/mL) | −105.1 ± 254.9 | 582.2 ± 256.4 | −687.3 ± 213.2 | 0.008 | 0.683 | 0.030 | 0.003 |
| Δ IGF-1/IGFBP-3 | 0.0009 ± 0.005 | −0.017 ± 0.005 | 0.02 ± 0.004 | 0.0008 | 0.872 | 0.003 | 0.0005 |
| Δ Testosterone (ng/dL) | 0.4 ± 5 | 1.1 ± 5.1 | −0.7 ± 4.1 | 0.973 | 0.936 | 0.829 | 0.864 |
| Δ Estradiol (pg/mL) | 33.8 ± 23.9 | −62.2 ± 25.4 | 95.9 ± 21.4 | 0.0002 | 0.164 | 0.02 | <0.0001 |
| Δ SHBG (nmol/L) | 11.5 ± 22.5 | 165.8 ± 23.01 | −154.3 ± 18.7 | <0.0001 | 0.612 | <0.0001 | <0.0001 |
| Δ FAI | −0.28 ± 0.47 | −1.31 ± 0.48 | 1.03 ± 0.04 | 0.008 | 0.55 | 0.008 | 0.008 |
| Δ Sclerostin (pg/mL) | −79.1 ± 28.04 | −73.5 ± 27.9 | −5.6 ± 23.8 | 0.020 | 0.008 | 0.012 | 0.815 |
| Δ Pref-1 (pg/mL) | −52.4 ± 51.3 | −41.7 ± 52.3 | −10.7 ± 41.97 | 0.591 | 0.314 | 0.430 | 0.8 |
| Δ BDNF (ng/mL) | −8634.7 ± 3622.8 | −3388.6 ± 3619.2 | −5246.1 ± 2960.8 | 0.051 | 0.022 | 0.355 | 0.085 |
Bold type indicates statistically significant P value of <0.05.
For within-group comparisons, a paired-samples t test was used to compare biochemical parameters at baseline vs 12 months. Associations of changes in biochemical parameters over 12 months with changes in bone turnover markers and aBMD measures over the same duration were assessed using Pearson correlations for normally distributed data and Spearman correlations for nonnormally distributed data. A two-tailed P < 0.05 was considered significant.
Results
Baseline characteristics
These are presented in Table 2. At baseline, the groups did not differ for age, BMI, percent body fat, or duration of exercise per week. Biochemical parameters, including a marker of bone formation (P1NP), a marker of bone resorption (NTX), IGF-1, IGF-1/IGFBP-3 ratio, SHBG, estradiol, total testosterone, FAI, sclerostin, Pref-1, and BDNF did not differ among groups. Study completers did not differ from noncompleters for baseline characteristics (data not shown).
Table 2.
Baseline Characteristics of Oligoamenorrheic Athletes
| NONE (n = 26) | PILL (n = 21) | PATCH (n = 26) | P Value | PILL vs NONE | PATCH vs NONE | PATCH vs PILL | |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| Age (y) | 19.0 ± 0.5 | 20.5 ± 0.6 | 19.8 ± 0.6 | 0.189 | 0.186 | 0.411 | 0.906 |
| BMI (kg/m2) | 20.7 ± 0.3 | 20.6 ± 0.4 | 19.9 ± 0.4 | 0.285 | 0.960 | 0.291 | 0.459 |
| Percent of median BMI for age | 97.5 ± 1.5 | 98.7 ± 2.0 | 93.4 ± 1.6 | 0.106 | 0.967 | 0.191 | 0.132 |
| Hours/week exercise in past year | 9.3 (5.9–12.1) | 10.8 (6.9–17.0) | 8.2 (6.2–11.0) | 0.443 | 0.611 | 0.995 | 0.435 |
| Daily caloric intake (Cals) | 1883.0 (1575.2–2820.0) | 2041.0 (1688.8–2627.5) | 2274.0 (1793.5–2716.0) | 0.557 | 0.957 | 0.576 | 0.723 |
| Resting energy expenditure (Cals) | 1194.0 (1100.5–1321.0) | 1160.0 (1074.0–1326.5) | 1174.0 (1049.0–1248.0) | 0.857 | 0.999 | 0.845 | 0.931 |
| Percent body fat | 25.4 ± 0.7 | 25.3 ± 1.2 | 23.5 ± 0.8 | 0.237 | 0.911 | 0.176 | 0.622 |
| Lean body mass (kg) | 35.3 (38.0–42.9) | 38.7 (37.8–42.4) | 40.1 (36.3–45.3) | 0.903 | 0.942 | 0.844 | 0.985 |
| Biochemical parameters | |||||||
| 25(OH)D (ng/mL) | 33.8 (29.5–42.4) | 33.7 (31.3–50.5) | 36.7 (27.7–46.3) | 0.716 | 0.766 | 0.490 | 0.373 |
| Calcium (mg/dL) | 9.4 ± 0.1 | 9.4 ± 0.1 | 9.3 ± 0.1 | 0.230 | 0.337 | 0.440 | 0.376 |
| PTH (pg/mL) | 25.0 (22.5–36.8) | 26.0 (22.0 – 38.0) | 29.0 (22.0–36.0) | 0.938 | 0.995 | 0.957 | 0.949 |
| P1NP (ng/mL) | 93.5 (72.1–121.9) | 59.0 (52.5–97.5) | 72.5 (59.5–101.8) | 0.096 | 0.116 | 0.601 | 0.309 |
| NTX (nM BCE/L) | 14.9 (12.6–18.3) | 12.4 (10.3–19.0) | 16.3 (13.2–19.6) | 0.139 | 0.343 | 0.522 | 0.203 |
| IGF-1 (ng/mL) | 254.0 (191.5–332.0) | 208.0 (172.3–292.0) | 225.0 (176.0–287.0) | 0.252 | 0.291 | 0.437 | 0.874 |
| IGF-1 z score | −0.38 ± 0.12 | −0.42 ± 0.15 | −0.64 ± 0.17 | 0.452 | 0.981 | 0.461 | 0.596 |
| IGFBP-3 (ng/mL) | 4936.8 ± 120.6 | 4723.0 ± 215.7 | 5050.5 ± 191.1 | 0.306 | 0.312 | 0.934 | 0.457 |
| IGF-1/IGFBP-3 | 0.0535 ± 0.0033 | 0.0474 ± 0.0030 | 0.0475 ± 0.0031 | 0.309 | 0.408 | 0.371 | 0.999 |
| Testosterone (ng/dL) | 28.5 (17.0–40.3) | 30.0 (23.0–39.0) | 24.0 (17.0–35.0) | 0.369 | 0.930 | 0.500 | 0.431 |
| Estradiol (pg/mL) | 39.8 (26.0–56.8) | 31.0 (14.7–86.5) | 22.0 (11.2–32.0) | 0.201 | 0.618 | 0.144 | 0.816 |
| SHBG (nmol/L) | 55.0 (35.5–71.5) | 61.7 (48.1–75.3) | 54.5 (37.3–79.9) | 0.862 | 0.888 | 0.948 | 0.934 |
| FAI | 1.8 (1.0–3.5) | 1.8 (1.4–2.5) | 1.5 (0.9–2.6) | 0.422 | 0.846 | 0.737 | 0.395 |
| Sclerostin (pg/mL) | 111.0 ± 11.6 | 121.1 ± 10.4 | 136.2 ± 10.9 | 0.285 | 0.816 | 0.256 | 0.636 |
| Pref-1 (pg/mL) | 84.0 (34.7–136.7) | 113.4 (59.5–242.5) | 106.6 (77.4–151.8) | 0.385 | 0.645 | 0.355 | 0.996 |
| BDNF (ng/mL) | 21.39 (18.90–24.74) | 22.65 (17.74–26.00) | 22.66 (16.37–30.69) | 0.852 | 0.918 | 0.934 | 0.847 |
Mean ± SEM; median (first quartile to third quartile).
Changes in clinical characteristics over 12 months
Over 12 months, the randomization groups did not differ for changes in weight, activity level (amount or type), PTH, 25(OH)D, or calcium levels. Similarly, within-group analysis did not reveal significant changes in PTH, 25(OH)D, or calcium levels from baseline to 12 months in the three groups. About 40% (9 of 21) of athletes in the NONE group had menstrual recovery defined as three consecutive monthly menses in the last 6 months of the study. When the analysis was repeated after limiting the NONE group to those without menstrual recovery, our results did not change (data not shown).
Changes in areal BMD over 12 months
We have previously reported that lumbar spine, femoral neck, and total hip aBMD and BMD z scores improved in the PATCH group compared with the PILL and NONE groups over 12 months (2).
Changes in surrogate markers of bone turnover
Within-group and between-group differences in surrogate markers of bone turnover and other biochemical parameters are reported in Table 3. Table 1 shows differences between groups using the GLM method after controlling for age as well as age, weight changes over time, and menstrual recovery.
Table 3.
Comparison of Changes in Biochemical Parameters Over 12 Months in the NONE, PILL, and PATCH Groups (Within- and Between-Group Comparisons)
| Change Over 12 Months | NONE (n = 26) | P Valuea | PILL (n = 21) | P Valuea | PATCH (n = 26) | P Valuea | ANOVA P Valueb | P Values | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PILL vs NONE | PATCH vs NONE | PATCH vs PILL | ||||||||
| Δ 25(OH)D (ng/mL) | 1.55 ± 1.60 | 0.344 | 2.78 ± 2.21 | 0.224 | −0.17 ± 1.35 | 0.904 | 0.482 | 0.871 | 0.743 | 0.458 |
| Δ Calcium (mg/dL) | 0.02 ± 0.08 | 0.842 | 0.06 ± 0.12 | 0.624 | 0.05 ± 0.09 | 0.611 | 0.945 | 0.944 | 0.970 | 0.995 |
| Δ PTH (pg/mL) | 0.40 ± 2.42 | 0.870 | −0.27 ± 3.09 | 0.925 | 1.60 ± 1.94 | 0.418 | 0.857 | 0.979 | 0.931 | 0.849 |
| Δ P1NP (ng/mL) | −14.6 (–34.1 to 9.6) | 0.049 | −26.0 (–64.8 to –18.5) | 0.0003 | −11.5 (–38.8 to 4.6) | 0.031 | 0.033 | 0.070 | 0.978 | 0.053 |
| Δ NTX (nM BCE/L) | −0.9 (–5.0 to 1.6) | 0.035 | −1.4 (–4.1 to 1.4) | 0.146 | −2.0 (–5.0 to 0.7) | 0.128 | 0.907 | 0.997 | 0.952 | 0.907 |
| Δ IGF-1 (ng/mL) | −8.8 ± 15.8 | 0.584 | −65.2 ± 21.2 | 0.006 | −10.2 ± 13.2 | 0.448 | 0.037 | 0.052 | 0.998 | 0.066 |
| Δ IGF-1 z score | 0.11 ± 0.13 | 0.414 | −0.56 ± 0.22 | 0.018 | 0.05 ± 0.16 | 0.780 | 0.014 | 0.018 | 0.959 | 0.039 |
| Δ IGFBP-3 (ng/mL) | −236.6 ± 93.8 | 0.024 | 252.4 ± 153.7 | 0.129 | −409.5 ± 163.7 | 0.028 | 0.006 | 0.040 | 0.633 | 0.006 |
| Δ IGF-1/IGFBP-3 | −0.0006 ± 0.0029 | 0.831 | −0.0186 ± 0.0030 | <0.0001 | 0.0015 ± 0.0033 | 0.6464 | 0.001 | 0.003 | 0.887 | 0.002 |
| Δ Testosterone (ng/dL) | −1.12 ± 1.79 | 0.537 | −0.68 ± 4.16 | 0.866 | −1.84 ± 2.21 | 0.414 | 0.955 | 0.993 | 0.980 | 0.952 |
| Δ Estradiol (pg/mL) | −1.5 (–13.9 to 47.8) | 0.079 | −20.0 (–89.0 to 0.0) | 0.047 | 45.0 (11.5 to 73.8) | 0.0009 | <0.0001 | 0.076 | 0.014 | <0.0001 |
| Δ SHBG (nmol/L) | −5.3 (–14.0 to 12) | 0.359 | 152.4 (99.1 to 232.3) | <0.0001 | 4.6 (–10.5 to 16.1) | 0.814 | <0.0001 | <0.0001 | 0.819 | <0.0001 |
| Δ FAI | −0.1 (–0.8 to 0.7) | 0.613 | −1.1 (–2.0 to –0.6) | 0.0001 | −0.1 (–1.0 to 0.7) | 0.313 | 0.003 | 0.004 | 0.990 | 0.015 |
| Δ Sclerostin (pg/mL) | 12.4 ± 16.7 | 0.467 | −45.6 ± 13.8 | 0.005 | −58.2 ± 20.0 | 0.012 | 0.010 | 0.045 | 0.014 | 0.863 |
| Δ Pref–1 (pg/mL) | 2.0 (–16.4 to 41.5) | 0.691 | −8.3 (–36.7 to –1.2) | 0.055 | −33.5 (–64.6 to 5.1) | 0.014 | 0.838 | 0.943 | 0.825 | 0.970 |
| Δ BDNF (ng/mL) | 0.31 (–26.54 to 5.66) | 0.809 | 1.03 (–7.97 to 6.13) | 0.801 | −6.36 (–11.19 to 0.97) | 0.044 | 0.131 | 0.998 | 0.113 | 0.336 |
Bold type indicates statistically significant P value of <0.05.
Mean ± SEM; median (first quartile to third quartile).
P value for within-group comparison (paired t test or signed rank test).
ANOVA P value for between-group comparisons.
P1NP
All three groups showed a significant within-group decrease in P1NP levels over the 12 months, which may reflect the expected decrease in P1NP with increasing age (Table 3). However, reductions in P1NP in the PILL group were greater than the reductions observed in the NONE group and showed a trend with the PATCH group after controlling for age (Table 1). Results were overall similar, though attenuated, after controlling for age, weight changes, and menstrual recovery (Table 1).
NTX
Within-group analysis showed a significant decrease in NTX levels over 12 months in the NONE group, but not in the PILL or PATCH groups (Table 3). Between-group comparisons demonstrated no differences among groups for changes in NTX over the study duration (Table 3). After adjusting for age, mean NTX reductions were numerically highest in the PILL group, but this did not achieve statistical significance.
When we compared differences in these bone turnover markers among groups taking into consideration eating disorder status, we found that having a history of an eating disorder did not significantly alter our findings.
Changes in other biochemical parameters that may impact bone modeling
IGF-1 and IGFBP-3
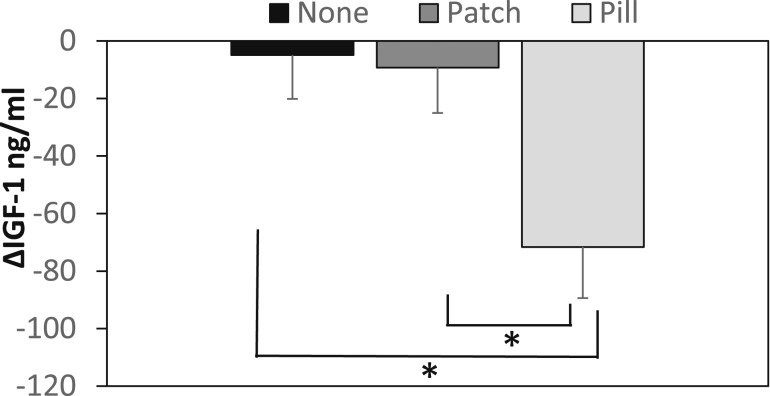
Over 12 months, within groups, IGF-1 levels decreased markedly in the PILL group, but not the other two groups. Because IGF-1 levels are age dependent, we controlled for age for across group comparisons, and the PILL group had significantly greater reductions in IGF-1 than the PATCH and NONE groups (Fig. 1). We also compared changes in IGF-1 z scores within and across groups. Like IGF-1, IGF-1 z scores (which are adjusted for age) decreased significantly over 12 months in the PILL group, but not in the PATCH or NONE groups. For across-group comparisons, the PILL group had significantly greater reductions in IGF-1 z scores compared with the PATCH and NONE groups. Results did not differ (but were attenuated) after controlling for weight changes and menstrual recovery. IGFBP-3 levels decreased over time within the PATCH and NONE groups, but not the PILL group, and reductions in IGFBP-3 were greater in the PATCH and NONE groups compared with the PILL group. Consequently, the ratio of IGF-1/IGFBP-3 (a surrogate for bioavailable IGF-1) decreased significantly over time in the PILL group (but not the PATCH or NONE groups), and the reduction in this ratio was greater in the PILL group than in the other two groups.
Figure 1.
Group differences in changes in IGF-1 levels over 12 mo adjusted for age. The PILL group had significant reductions in IGF-1 levels over 12 mo compared with the PATCH and NONE groups.
Sex steroids and SHBG
In within-group analysis, as expected, the PILL group (but not the PATCH or NONE groups) demonstrated significant increases in SHBG levels over 12 months, with greater increases in SHBG observed in this group compared with the other two groups on unadjusted and adjusted analysis. Estradiol levels increased significantly over the study duration in the PATCH group vs a reduction in the PILL group. Increases in estradiol levels were higher in the PATCH vs the other two groups on unadjusted analyses, and decreased in the PILL vs the NONE group on adjusted analysis. The FAI, reflective of bioavailable testosterone, declined significantly over 12 months in the PILL group (but not PATCH and NONE groups), likely from the rise in SHBG levels. Decreases in FAI in the PILL vs the other two groups persisted on adjusted analyses.
Sclerostin, Pref-1, and BDNF
Both the PILL and PATCH groups demonstrated significant reductions in sclerostin in within-group analyses, and these reductions were significantly greater than changes observed in the NONE group (differences persisted on adjusted analyses). Pref-1 levels decreased significantly in the PATCH group over the course of the study, with a similar trend observed for the PILL group. Changes in Pref-1 did not differ among groups. Within-group analysis showed a significant decrease in BDNF levels in the PATCH group, but not the other two groups. Changes in the BDNF levels did not differ across groups, but were lower in the PATCH vs NONE groups on adjusted analyses.
Associations of changes in bone turnover markers and aBMD with changes in IGF-1, SHBG, sclerostin, Pref-1, and BDNF
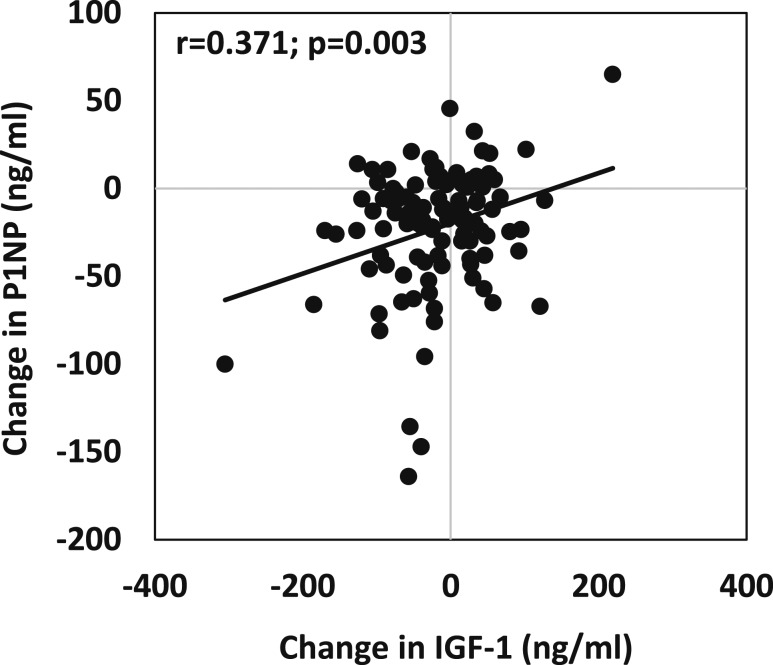
In the group, as a whole, 12-month changes in P1NP were associated positively with changes in NTX (r = 0.48, P ≤ 0.0001), and with 12-month changes in IGF-1 (r = 0.37, P = 0.003) (Fig. 2), IGF-1 z scores (r = 0.27, P = 0.03), and the IGF-1/IGFBP-3 ratio (r = 0.33, P = 0.05). Twelve-month changes in P1NP were also associated inversely with changes in SHBG (r = –0.28, P = 0.02). Similarly, changes in NTX over 12 months were positively associated with changes in IGF-1 (r = 0.27, P = 0.04) and IGF-1 z scores (r = 0.23, P = 0.02). Changes in bone turnover markers were not associated with changes in sex steroids, sclerostin, Pref-1, or BDNF. Twelve-month changes in femoral neck BMD and total hip BMD were associated positively with 12-month increases in estradiol (r = 0.27 and 0.34, P = 0.02 and 0.005, respectively). Changes in femoral neck BMD and total hip BMD were not associated with changes in IGF-1, sclerostin, Pref-1, or BDNF.
Figure 2.
Association of changes in IGF-1 levels with changes in P1NP levels over 12 mo for the entire group. Changes in IGF-1 levels were associated positively with changes in P1NP levels over 12 mo.
Discussion
This study demonstrates several potential mechanisms for the greater efficacy of the PATCH vs the PILL in improving bone outcomes in oligoamenorrheic athletes. PILL use is associated with a reduction in IGF-1 levels, IGF-1 z scores, and the IGF-1/IGFBP-3 ratio, and a decrease in estradiol and the FAI (from an increase in SHBG). Both the PATCH and the PILL groups showed a decrease in sclerostin levels over 12 months. The PATCH group also demonstrated increases in estradiol and reductions in Pref-1 and BDNF. The bone formation marker, P1NP, decreased in all groups over 12 months; however, the decrease was significantly greater in the PILL compared with the PATCH and NONE groups. Reductions in P1NP were associated positively with reductions in IGF-1 and IGF-1 z scores. Increases in femoral neck and total hip BMD measures were associated positively with increases in estradiol levels.
Overexercising athletes without a compensatory increase in caloric intake develop a chronic state of low energy availability. Low energy availability can suppress the hypothalamic-pituitary-gonadal axis, leading to an estrogen-deficient state associated with OA. During the adolescent and young adult years of increased bone accrual, low energy availability and estrogen deficiency result in suboptimal accrual rates, with implications for peak bone mass acquisition (22–24). Our studies and those of others have demonstrated significant compromise in skeletal integrity in OA compared with eumenorrheic athletes and nonathletes associated with a marked increase in risk for stress fractures (25, 26). It is thus essential to determine the appropriate therapeutic strategies to optimize bone accrual and outcomes in OA, particularly during the critical adolescent and young adult years of peak bone accrual. We have recently demonstrated increases in spine and femoral neck BMD and associated z scores in OA randomized to the PATCH compared with those randomized to the PILL or NONE groups (2), consistent with our findings in adolescent girls with anorexia nervosa, in whom transdermal estradiol replacement led to significant increases in BMD over 18 months compared with placebo (3). In this study, we explore the various mediators that may explain the beneficial effects of estrogen replacement via the transdermal vs the oral route on bone, and thus provide insight into future therapeutic targets for interventional studies. We specifically examine mediators of the differentiation of the MSC along the osteoblast vs the adipocyte lineage. Multiple hormones are now known to regulate MSC differentiation; for example, estradiol promotes osteoblastogenesis, glucocorticoids promote adipogenesis, and IGF-1 may induce both pathways (27–29). Sclerostin, Pref-1, and BDNF inhibit osteoblastogenesis.
Traditionally, the greater benefit associated with estrogen administration via the transdermal vs the oral route has been attributed to the IGF-1–suppressive effects of oral estrogen from hepatic first-pass effects (6). IGF-1 is an important bone trophic hormone, whose rising levels during puberty are critical for optimal adolescent bone accrual. Consistent with this, we report a decrease in IGF-1 levels, IGF-1 z scores, and the ratio of IGF-1/IGFBP-3 in the PILL group (not observed in the PATCH or NONE groups) associated with reductions in P1NP, which may explain the greater suppression in bone formation seen in this group compared with the other groups. However, changes in IGF-1 account for only a small proportion of the variance in BMD changes following transdermal estradiol. Thus, the beneficial effects of transdermal estradiol may be mediated via other mechanisms as well.
Hypogonadal states are associated with increased bone resorption given that estrogen is antiresorptive (although it may also have bone anabolic effects) (30, 31). In this study, we observed the greatest increases in estradiol levels in the PATCH group, whereas estradiol decreased in the PILL group. This is likely because ethinyl estradiol and progestogen in the PILL suppress the hypothalamic pituitary gonadotropin axis and therefore endogenous estradiol production. Of importance, the estradiol assay detects 17β-estradiol (released by the PATCH) but not ethinyl estradiol (present in the PILL). Thus, this assay does not consider ethinyl estradiol levels in blood, and it is unclear whether the efficacy of 17β-estradiol is greater than that of ethinyl estradiol in optimizing BMD in hypogonadal states. Regardless of the form of estrogen, the concomitant rise in SHBG levels in the PILL group would lead to lower bioavailable estrogen in the PILL vs the PATCH groups, and this may further impact bone outcomes. Of note, we found significant positive associations of increases in estradiol with increases in femoral neck and hip BMD in OA.
In addition to estradiol, testosterone may impact bone outcomes in OA. The PILL group had reductions in the FAI over the 12-month period (not observed in the PATCH group), indicative of lower levels of bioavailable testosterone in the PILL group from increased SHBG. This may also contribute to the decrease in bone formation seen with the PILL, as testosterone, in addition to being antiresorptive (following aromatization to estrogen), may have bone anabolic effects. However, we did not find any association of changes in FAI with changes in bone endpoints.
Sclerostin, a potent inhibitor of osteogenesis, decreased in both the PATCH and PILL groups, whereas no differences were noted in the NONE group after 12 months. This supports data in older pre- and postmenopausal women in whom estrogen replacement leads to reductions in sclerostin (32, 33). We have previously reported no changes in sclerostin after transdermal estrogen replacement in adolescents with anorexia nervosa (34), suggesting that nutritional repletion may be important to mediate the effect of estrogen on sclerostin. The lack of correlation between changes in sclerostin and changes in estradiol levels does not necessarily refute the concept that effects of estrogen may be partly mediated via sclerostin. Both groups showed a transient increase in bone formation after estrogen treatment followed by an eventual decline in bone resorption, which also leads to a decrease in bone formation from coupling of the two processes (33, 35). Further, we know from previous studies that changes in sclerostin correlate with estradiol replacement only acutely, while the hypogonadal state is being reversed (36). Once a steady state is achieved, systemic sclerostin does not correlate with estradiol, as evidenced by the lack of variability in sclerostin across the menstrual cycle (37). Of note, decreases in sclerostin may also mediate the antiresorptive effects of estrogen as sclerostin stimulates receptor activator of nuclear factor κ-B ligand (RANKL) production and inhibits osteoprotegerin, thereby increasing osteoclastogenesis (38). Data from the current study do not suggest a differential effect of PATCH vs PILL on systemic sclerostin levels, as both showed similar changes in sclerostin, which did not correlate with bone marker or BMD changes.
Pref-1 is a member of the epidermal growth factor family that inhibits MSC differentiation into both adipocytes and osteoblasts (39). We have shown that Pref-1 levels are higher in estrogen-deficient states such as anorexia nervosa and other states of hypothalamic amenorrhea vs controls (40, 41). Similar to studies of oral estrogen administration in postmenopausal women (42) and our studies of transdermal estrogen administration in adolescents with anorexia nervosa (11), we found that estrogen replacement led to decreases in Pref-1 in hypogonadal athletes. However, in this study, the effects were mostly seen in the PATCH group, and less so in the PILL group. Thus, regardless of nutritional status, adolescents with hypothalamic amenorrhea have a decrease in Pref-1 following estrogen replacement, and this is more marked with transdermal estradiol. The blunting of these effects in the PILL group may be explained by the lower systemic estradiol levels or other unknown mechanisms.
BDNF has recently been evaluated as a potential mediator for bone remodeling. Osteoblasts carry receptors for BDNF, and deletion of central BDNF expression resulted in increased bone mass (20, 43). Although we did not check central BDNF in our study, data indicate that BDNF crosses the blood-brain barrier, and serum BDNF correlates well with central BDNF (20). The decline in BDNF levels in the PATCH group may thus also contribute to improvements in BMD in this group.
Overall, our data indicate that the greater decline in P1NP, a bone formation marker, in the PILL group compared with the other two groups is likely a consequence of decreases in IGF-1 and the IGF-1/IGFBP-3 ratio (and possibly the FAI) in the PILL group. Thus, oral estrogen administration, at least as ethinyl estradiol, may prevent the beneficial effects of estrogen on aBMD, despite a concomitant decrease in sclerostin. Interestingly, bone formation markers decreased over time in all groups, though only minimally in the PATCH and NONE groups compared with the PILL group. The reduction in P1NP levels in the NONE group likely represents the physiologic reduction in bone turnover with increasing age during adolescence and young adulthood (44). In the PATCH group, maintenance of IGF-1 levels and the IGF-1/IGFBP-3 ratio, and reductions in sclerostin, Pref-1, and BDNF, may account for less marked changes in P1NP than observed in the PILL group. Changes in NTX, a bone resorption marker, though numerically higher in the PILL group, were not statistically different among groups.
A limitation of our study is that, in the absence of a reduction in bone resorption markers, and without an increase in bone formation markers, our bone turnover marker data do not elucidate the mechanism for improvements in BMD in the PATCH group. Of note, we assessed only one marker of bone formation and bone resorption in this study, and it is possible that other markers may have behaved differently. Further, bone formation and resorption markers usually change acutely and then achieve a steady state; thus, chronic changes in these markers, particularly during adolescence and adulthood, may not clearly reflect ongoing changes in BMD. A larger trial with closer follow-up visits is necessary to detect acute changes in bone turnover markers, and to determine the association of these acute changes with more chronic changes in BMD. An important consideration is that the PATCH group was administered 17-β estradiol and micronized progesterone, whereas the PILL group was administered ethinyl estradiol and desogestrel. Our study design does not allow us to determine whether effects of these products on bone turnover markers and other biochemical parameters were impacted by not just the route of administration, but also the specific estrogen or progestogen formulation and its metabolism, and/or the effective dose delivered locally to bone or cartilage. Another limitation is a relatively small sample size in each treatment group and a high attrition rate. However, the attrition rate approximates that in other similar studies, and importantly, our completers did not differ from noncompleters for baseline characteristics (3, 45). Also, we do not have follow-up data after treatment discontinuation to assess durability of estrogen effects.
To our knowledge, this is the first randomized controlled trial in adolescents with athletic amenorrhea exploring mechanisms whereby a specific route of administration of estrogen may be more beneficial to bone than another. In conclusion, our study suggests that the beneficial effects of transdermal 17β-estradiol on BMD in adolescents and young adults with athletic OA are mediated by increases in estradiol and decreases in sclerostin, Pref-1, and BDNF, while maintaining levels of IGF-1, the IGF-1/IGF BP-3 ratio, and the FAI. In contrast, oral ethinyl estradiol causes a marked decrease in bone formation secondary to decreases in the IGF-1/IGFBP-3 ratio, and the decrease in bone formation, along with reductions in the FAI, may further explain the lack of efficacy of oral ethinyl estradiol in improving bone outcomes in youth with hypothalamic amenorrhea. Further studies are needed to better dissect the biologic effects of estrogen administered by transdermal and oral routes.
Acknowledgments
Financial Support: This work was supported by grants R01HD060827 (to M.M.), K24HD071843 (to M.M.), K23DK110419 (to V.S.), UL1TR001102 (to M.M.), and P30DK040561 (to V.S.) from the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov no. NCT00946192 (registered 24 July 2009).
Author Contributions: M.M. conceived the project and designed the study. M.M., V.S., and K.E.A. contributed to study protocol and key data interpretation. K.E.A., M.M., L.P.T.F., and V.S. were involved in patient assessments, study visits, and data coordination. H.L., A.B., L.P.T.F., and M.M. performed the statistical analysis. V.S. wrote the initial draft of the paper. M.M., A.B., L.P.T.F., and K.E.A. critically edited and revised the paper.
Disclosure Summary: M.M. has served on the scientific advisory board of Novo Nordisk, is a co-investigator on an investigator-initiated grant from Novo Nordisk, and is a consultant for Sanofi Pharmaceuticals. None of these conflicts are relevant to the current study. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 25(OH)D
25(OH) vitamin D
- aBMD
areal bone mineral density
- BCE
bone collagen equivalent
- BDNF
brain-derived neurotrophic factor
- BMD
bone mineral density
- BMI
body mass index
- CV
coefficient of variation
- DXA
dual-energy x-ray absorptiometry
- FAI
free androgen index
- GLM
generalized linear models
- IGFBP-3
insulinlike growth factor binding protein 3
- MSC
mesenchymal stem cell
- NTX
N-telopeptide
- OA
oligoamenorrhea
- P1NP
N-terminal propeptide of type 1 procollagen
- Pref-1
preadipocyte factor-1
References
- 1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine . American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882. [DOI] [PubMed] [Google Scholar]
- 2. Ackerman K, Singhal V, Baskaran C, Slattery M, Campoverde Reyes K, Toth A, Eddy K, Bouxsein M, Lee H, Klibanski A, Madhusmita M. Estrogen replacement improves bone mineral density in oligo-amenorrheic athletes: a randomized clinical trial. Br J Sports Med. 2018;bjsports-2018-099723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, Goldstein MA, Ebrahimi S, Clauss L, Weigel T, Mickley D, Schoenfeld DA, Herzog DB, Klibanski A. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26(10):2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cetinkaya MB, Kökçü A, Yanik FF, Başoğlu T, Malatyalioglu E, Alper T. Comparison of the effects of transdermal estrogen, oral estrogen, and oral estrogen-progestogen therapy on bone mineral density in postmenopausal women. J Bone Miner Metab. 2002;20(1):44–48. [DOI] [PubMed] [Google Scholar]
- 5. Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85(11):3951–3963. [DOI] [PubMed] [Google Scholar]
- 6. Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72(2):374–381. [DOI] [PubMed] [Google Scholar]
- 7. Ho KK, Weissberger AJ. Impact of short-term estrogen administration on growth hormone secretion and action: distinct route-dependent effects on connective and bone tissue metabolism. J Bone Miner Res. 1992;7(7):821–827. [DOI] [PubMed] [Google Scholar]
- 8. Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. [DOI] [PubMed] [Google Scholar]
- 9. Pietrzyk B, Smertka M, Chudek J. Sclerostin: intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med. 2017;26(8):1283–1291. [DOI] [PubMed] [Google Scholar]
- 10. Kim RY, Yang HJ, Song YM, Kim IS, Hwang SJ. Estrogen modulates bone morphogenetic protein-induced sclerostin expression through the Wnt signaling pathway. Tissue Eng Part A. 2015;21(13-14):2076–2088. [DOI] [PubMed] [Google Scholar]
- 11. Faje AT, Fazeli PK, Katzman D, Miller KK, Breggia A, Rosen CJ, Mendes N, Misra M, Klibanski A. Inhibition of Pref-1 (preadipocyte factor 1) by oestradiol in adolescent girls with anorexia nervosa is associated with improvement in lumbar bone mineral density. Clin Endocrinol (Oxf). 2013;79(3):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 13. Singhal V, Maffazioli GD, Cano Sokoloff N, Ackerman KE, Lee H, Gupta N, Clarke H, Slattery M, Bredella MA, Misra M. Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone. 2015;77:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27(9):1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Z, Zhang Y, Zhou Z, Shi H, Qiu X, Xiong J, Chen Y. BDNF regulates the expression and secretion of VEGF from osteoblasts via the TrkB/ERK1/2 signaling pathway during fracture healing. Mol Med Rep. 2017;15(3):1362–1367. [DOI] [PubMed] [Google Scholar]
- 17. Ai LS, Sun CY, Zhang L, Zhou SC, Chu ZB, Qin Y, Wang YD, Zeng W, Yan H, Guo T, Chen L, Yang D, Hu Y. Inhibition of BDNF in multiple myeloma blocks osteoclastogenesis via down-regulated stroma-derived RANKL expression both in vitro and in vivo. PLoS One. 2012;7(10):e46287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. [DOI] [PubMed] [Google Scholar]
- 19. Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–353. [DOI] [PubMed] [Google Scholar]
- 20. Camerino C, Zayzafoon M, Rymaszewski M, Heiny J, Rios M, Hauschka PV. Central depletion of brain-derived neurotrophic factor in mice results in high bone mass and metabolic phenotype. Endocrinology. 2012;153(11):5394–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405–413. [DOI] [PubMed] [Google Scholar]
- 22. Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. [DOI] [PubMed] [Google Scholar]
- 23. Weaver CM. Adolescence: the period of dramatic bone growth. Endocrine. 2002;17(1):43–48. [DOI] [PubMed] [Google Scholar]
- 24. Brown KA, Dewoolkar AV, Baker N, Dodich C. The female athlete triad: special considerations for adolescent female athletes. Transl Pediatr. 2017;6(3):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, Klibanski A, Bouxsein M, Misra M. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012;51(4):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ackerman KE, Cano Sokoloff N, DE Nardo Maffazioli G, Clarke HM, Lee H, Misra M. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc. 2015;47(8):1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao JW, Gao ZL, Mei H, Li YL, Wang Y. Differentiation of human mesenchymal stem cells: the potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. Am J Med Sci. 2011;341(6):460–468. [DOI] [PubMed] [Google Scholar]
- 28. Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66(2):236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17(4):144–149. [DOI] [PubMed] [Google Scholar]
- 30. Khastgir G, Studd J, Holland N, Alaghband-Zadeh J, Sims TJ, Bailey AJ. Anabolic effect of long-term estrogen replacement on bone collagen in elderly postmenopausal women with osteoporosis. Osteoporos Int. 2001;12(6):465–470. [DOI] [PubMed] [Google Scholar]
- 31. Riggs BL, Khosla S, Melton LJ III. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. [DOI] [PubMed] [Google Scholar]
- 32. Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011;26(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mödder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone. 2011;49(2):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faje AT, Fazeli PK, Katzman DK, Miller KK, Breggia A, Rosen CJ, Mendes N, Klibanski A, Misra M. Sclerostin levels and bone turnover markers in adolescents with anorexia nervosa and healthy adolescent girls. Bone. 2012;51(3):474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hannon R, Blumsohn A, Naylor K, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res. 1998;13(7):1124–1133. [DOI] [PubMed] [Google Scholar]
- 36. Farr JN, Roforth MM, Fujita K, Nicks KM, Cunningham JM, Atkinson EJ, Therneau TM, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S. Effects of age and estrogen on skeletal gene expression in humans as assessed by RNA sequencing. PLoS One. 2015;10(9):e0138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liakou CG, Mastorakos G, Makris K, Fatouros IG, Avloniti A, Marketos H, Antoniou JD, Galanos A, Dontas I, Rizos D, Tournis S. Changes of serum sclerostin and Dickkopf-1 levels during the menstrual cycle. A pilot study. Endocrine. 2016;54(2):543–551. [DOI] [PubMed] [Google Scholar]
- 38. Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci USA. 2015;112(5):E478–E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73(4):725–734. [DOI] [PubMed] [Google Scholar]
- 40. Aronis KN, Kilim H, Chamberland JP, Breggia A, Rosen C, Mantzoros CS. Preadipocyte factor-1 levels are higher in women with hypothalamic amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. J Clin Endocrinol Metab. 2011;96(10):E1634–E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, Herzog DB, Misra M, Klibanski A. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(11):4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abdallah BM, Bay-Jensen AC, Srinivasan B, Tabassi NC, Garnero P, Delaisse JM, Khosla S, Kassem M. Estrogen inhibits Dlk1/FA1 production: a potential mechanism for estrogen effects on bone turnover. J Bone Miner Res. 2011;26(10):2548–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang M, Lu BJ, Duan YY, Chen XF, Ma JG, Guo Y. Genetics association study and functional analysis on osteoporosis susceptibility gene BDNF. Yi Chuan. 2017;39(8):726–736. [DOI] [PubMed] [Google Scholar]
- 44. Gracia-Marco L, Ortega FB, Jiménez-Pavón D, Rodríguez G, Valtueña J, Díaz-Martínez AE, González-Gross M, Castillo MJ, Vicente-Rodríguez G, Moreno LA. Contribution of bone turnover markers to bone mass in pubertal boys and girls. J Pediatr Endocrinol Metab. 2011;24(11-12):971–974. [DOI] [PubMed] [Google Scholar]
- 45. DiVasta AD, Feldman HA, Beck TJ, LeBoff MS, Gordon CM. Does hormone replacement normalize bone geometry in adolescents with anorexia nervosa? J Bone Miner Res. 2014;29(1):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]