Abstract
DNA demethylating agents may increase the immunogenicity of malignant tumours and increase the efficacy of subsequent treatment with immune check point inhibitors. We investigated the safety of administrating the demethylating agent decitabine by hepatic arterial infusionin patients with unresectable liver meta stases from solid tumours in a dose escalation phase I clinical trial. A total of nine eligible patients were enrolled and initiated study treatment at three different dose levels (two patients at 10, four at 15 and six at a dose level of 20mg decitabine/m2/day) (per protocol there was no intent to escalate the dose above the median tolerated intravenous dose level). Decitabine was administered as a 1-hour hepatic arterial infusion on five consecutive days every 4 weeks. Intrapatient dose escalation was applied in five patients. Grades 1 and 2 haematological toxicity was the most frequent treatment-related adverse event. None of the patients experienced treatment-limiting adverse events. Expression analysis of 30 cancer test is antigens (CTA) in pretreatment and post-treatment biopsies from patients indicated an increased expression of 21 CTAs after treatment. There were no objective tumour responses on study treatment or during post study exposure to immune checkpoint therapy in four patients with uveal melanoma liver metastases. We conclude that the investigate d hepatic arterial administration regimen for decitabine can be safely applied, and a dose level of 20 mg/m2/day on five consecutive days every 4 weeks can be considered for further investigation in combinatorial immunotherapy regimens.
Trial registration number
Keywords: decitabine, liver metastases, hepatic arterial infusion
Key questions.
What is already known about this subject?
Decitabine is approved for the treatment of myelodysplastic syndromes and acute myeloid leukaemia.
Decitabine has demethylating effects at a lower dose.
DNA demethylating agents may increase the immunogenicity of malignant tumours.
What does this study add?
Hepatic arterial administration regimen for decitabine can be safely applied.
A dose level of 20 mg/m2/day is recommended on five consecutive days.
How might this impact on clinical practice?
Immunotherapy has improved the survival of patients with cancer; however, more research is needed in tumour types not sensitive to immunotherapy.
The combination of demethylating agents and immunotherapy could lead to more immune response.
Introduction
Genetic and epigenetic changes underlie the transformation of a normal to a malignant cell.1 Some tumours are characterised by genome-wide changes in the methylation status of their genomic DNA that includes demethylation of the promoter regions of genes encoding cancer testis antigens (CTAs) leading to aberrant expression of these CTAs in cancer cells.2 Expression of most CTAs in normal cells is restricted to the germ cells within the testis and placental tissue. New York esophageal cell carcinoma 1 (NY-ESO-1, is a member of the CTA family and is considered to be one of the most immunogenic.3 The immune system is capable of mounting spontaneous adaptive immune responses to epitopes encoded by CTAs.2 Consequently CTAs are considered attractive targets for immunotherapy.
Inhibiting the programmed cell death-1 (PD-1)/(programmed death-ligand 1) PD-L1 axis or the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) receptor has resulted in a paradigm shift in the treatment of certain cancer types such as melanoma and non-small-cell lung cancer.4–8 However, over half of all patients with any tumour type will not respond to these innovative immunotherapies. In a mouse model of mismatch repair proficient colorectal cancer, a notable and durable (>100 days) upregulation of the expression of the CTA member NY-ESO-1 could be achieved by exposing tumour cells to increasing doses of the demethylating agent 5-aza-20-deoxycytidine (decitabine). Subsequently, tumour cells could be eradicated using retrovirally transduced polyclonal peripheral blood T-cells from a patient with metastatic colorectal cancer expressing the T-cell receptor α-chain and β-chain genes encoding a human leucocyte antigen-A2-restricted, NY-ESO-1157–165-specific T-cell receptor.9
Decitabine is a deoxycytidine analogue that incorporates into the DNA and forms irreversible covalent bonds with methyltransferase at cytosine sites targeted for methylation; this leads to the inactivation of the methyltransferase, resulting in DNA hypomethylation and gene activation. Decitabine is metabolised by cytidine deaminase in the human liver and spleen and has a short plasma half-life of 20 min. The most frequent side effect is myelosuppression, which makes it difficult to combine with traditional cytotoxic agents. Decitabine is approved for the treatment of myelodysplastic syndromes and acute myeloid leukaemia.10 When administered for five consecutive days, the maximum tolerated daily dose is 20 mg/m2. It was hypothesised that the administration of decitabine by hepatic arterial infusion would lead to a potentially lower systemic exposure (depending on the first-pass clearance by the liver) while maximising exposure within liver metastases that preferentially derive their blood flow from this artery. At high doses decitabine has a direct antitumour effect, but the demethylating effects can already be present at lower dose levels.11–15 Decitabine is a cell cycle-dependent agent that only targets cells in S phase. Different dosing schedules exist for the treatment with decitabine, ranging from a continuous infusion over 72 hours to a repetitive 1-hour infusion every 5 days. Schedules with consecutive multiday administration of decitabine are expected to achieve a higher activity as more cells will be exposed when transiting through the S phase of the cell cycle.10 16–19 In this study decitabine was administered by a continuous hepatic arterial infusion over 1 hour repeated daily for five consecutive days in patients with pretreated liver metastases.
Materials and methods
Study design
This phase I study was designed as a dual-centre, open-label, single-arm, dose escalation study. The recruiting centres were the UZ Brussel and Hôpital Erasme, both located in Brussels, Belgium. There were three predefined dose levels: 10, 15 and 20 mg decitabine/m2/day by a 1-hour hepatic arterial infusion on five consecutive days every 4 weeks. A 3+3 phase I design was used to guide patient recruitment. Intrapatient dose escalating was allowed once a dose level was found to be sufficiently safe. Dose-limiting toxicity was defined as treatment-related ≥grade 3 toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V.4.0 not reversible to grade 2 or less within 96 hours. The protocol predefined maximum dose level to be investigated was 20 mg/m2/day for 5 days as this dose represents the maximum tolerated dose for intravenous administration with the 5-day regimen.
Study population
Patients with liver-predominant metastases from solid tumours who had experienced progression of their disease following standard of care were included. For the administration of decitabine, the placement of an arterial hepatic catheter was required. This could be done by a laparoscopic procedure or by an endovascular procedure according to published methodology.20
Key eligibility criteria verified during the screen procedures were age ≥18 years; WHO performance status of 0, 1 or 2; normal haematological, liver and renal function tests; and negative serological tests for HIV, syphilis, hepatitis B and hepatitis C. Treatment was administrated as an outpatient basis, but patients who preferred to be hospitalised during the study drug hospitalisation were allowed to do so.
Adverse events
Adverse events were graded according to the CTCAE V.4.0. Toxicity was assessed on each day of treatment and weekly in between treatments. A complete blood count with differential and platelets and metabolic panel were repeated daily during treatment and weekly in between treatments.
Study procedures
A pretreatment tumour biopsy from a liver metastasis was either obtained as archival tissue or a new biopsy was performed before the start of the treatment. A post-treatment biopsy of a liver metastasis was obtained 2 weeks after initiation of treatment. Before and on each day of study drug administration, blood values were analysed for liver set, renal blood value and blood cells.
Objective tumour responses were assessed every 3 weeks after initiation of study treatment and tumour response was assessed according to the Response evaluation criteria in solid tumors (RECIST) V.1.1 criteria.
Study objective
The primary objective was to evaluate the safety of escalating doses of decitabine by hepatic arterial infusion and establish the recommended dose of decitabine administered by hepatic arterial infusion. The secondary objectives were the best objective tumour response per RECIST and survival analysis, and to compare CTA expression in paired pretreatment and post-treatment biopsies.
mRNA expression analysis
All patients underwent a pretreatment biopsy of a liver metastasis. Three patients consented to a post-treatment biopsy 2 weeks after the start of decitabine treatment. Biopsies were formaldehyde-fixed and paraffin-embedded (FFPE), and only seven samples (five pretreatment and two post-treatment samples from five patients) were available and suitable for further analysis. Tumour cell enrichment was performed by macrodissection of four FFPE sections per sample (5 µm) prior to RNA extraction using the High Pure FFPET RNA Isolation Kit (Roche, Anderlecht, Belgium). The expression of 30 CTAs was analysed with the nCounter PanCancer Immune Profiling panel (NanoString Technologies, Seattle, Washington, USA) on a NanoString Analysis System (NanoString Technologies). The counts, generated per molecular ‘barcode’ (gene) by the nCounter system, were normalised using the nSolver V.3.0 software for negative and positive controls, as well as for the 40 housekeeping genes present in the panel (using the geometric mean).
Statistical analyses
All statistical analyses were performed using SPSS Statistics V.24 software. Kaplan-Meier curves were used to calculate probability curves for progression-free and overall survival. The expression analysis for the pretreatment and post-treatment samples was performed in R.
Results
Patients’ baseline characteristics
Between February 2014 and September 2016, a total of 10 patients were screened and 9 eligible patients initiated study treatment. One patient could not initiate treatment because of rapid progression of the disease with the development of hepatic failure. The median age was 59 (range 42–79). The primary tumour types included four uveal melanomas, one skin melanoma, four colorectal carcinomas and one epithelial ovarian cancer. All patients had progressed on standard of care. All four patients with colorectal carcinoma had been pretreated with FOLFOX (combination of 5-fluorouracil, leucovorin and oxaliplatin) and three patients with FOLFIRI (combination of 5-fluorouracil, leucovorin and irinotecan) and one patient with bevacizumab. All five patients with melanoma had been pretreated with ipilimumab and four with pembrolizumab (table 1).
Table 1.
Baseline characteristics of patients
| Variable | |
| Total (male/female) | 9 (5/5) |
| Median age (range), years | 59 (42–79) |
| Primary malignancy | |
| Uveal melanoma | 4 |
| Colorectal carcinoma | 4 |
| Melanoma | 1 |
| Performance status |
The hepatic artery catheter was placed by laparoscopy in four patients and percutaneous technique in five patients.
Treatment disposition, safety and tolerability within dose cohorts
A total of 15 treatment cycles were administered (two at the first, five at the second and eight at the third dose level; table 2). The median number of treatment cycles per patient was 2 (range 1–2).
Table 2.
Dose level of decitabine and number of patients
| Adverse event | Dose decitabine | Patients (n) |
| Dose level 1 | 10 | 2 |
| Dose level 2 | 15 | 5 (2+3) |
| Dose level 3 | 20 | 6 (3+3) |
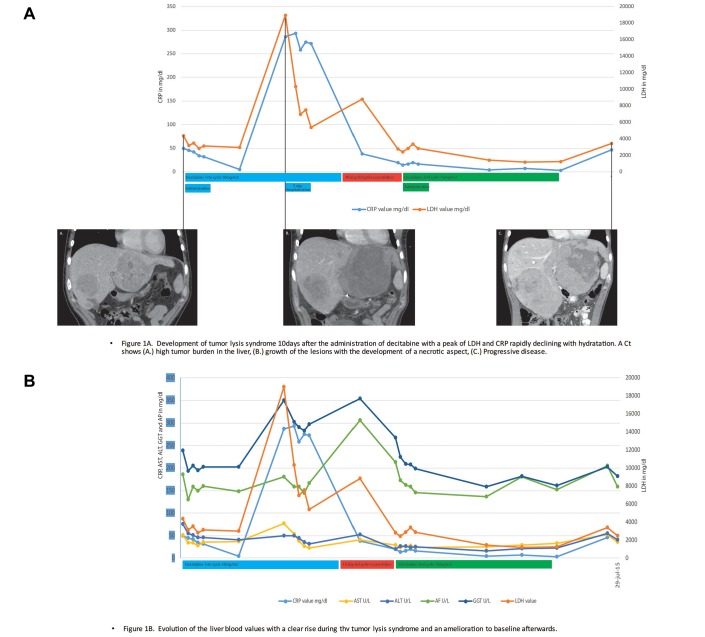
Across all three dose levels, treatment was generally well tolerated (table 3); six (66%) patients developed grade 1, seven (78%) patients grade 2 and 1 (11%) patient grade 3 adverse events (tumour lysis syndrome) (figure 1). All patients with colorectal cancer, who were more heavily pretreated with chemotherapy, experienced haematological toxicity as compared with only two out of five patients with non-colorectal cancer (40%). There was no indication of a higher incidence of adverse events with higher dose levels. Retreatment was delayed in two patients treated at the 10 mg/m2 dose level (by 1 week in a patient with colorectal cancer who experienced leucopaenia and by 3 weeks in a patient with uveal melanoma who developed a tumour lysis syndrome; figure 1A). All patients discontinued study treatment because of disease progression, and none because of adverse events.
Table 3.
Adverse events
| |
1 | 2 | 3 |
| CTCAE grade | |||
| n (%) | n (%) | n (%) | |
| Dose level: 10 mg/m²/day Number of treatment cycles=2 Number of patients exposed: 2 |
|||
| Anaemia | 1 (50) | ||
| Neutropaenia | 1 (50) | ||
| Leucopaenia | 1 | 1 (50) | |
| Tumour lysis syndrome | 1 (50) | ||
| Dose level: 10 mg/m²/day, ×5 days Number of treatment cycles=4 Number of patients exposed: 4 |
|||
| Anaemia | 2 (40) | 1 (20) | |
| Lymphopaenia | 1 (20) | ||
| Neutropaenia | 1 (20) | ||
| Leucopaenia | 1 (20) | ||
| Dose level: 20 mg/m²/day, ×5 days Number of treatment cycles=8 Number of patients exposed: 6 |
|||
| Anaemia | 1 (17) | ||
| Lymphopaenia | 1 (17) | ||
| Neutropaenia | 1 (17) | ||
| Leucopaenia | 1 (17) | ||
| Thrombocytopaenia | 1 (17) | ||
CTCAE, Common Terminology Criteria for Adverse Events.
Figure 1.
(A) Tumour lysis syndrome in a patient with uveal melanoma and high tumour burden in the liver. (B) evolution of CRP, LDH and liver blood values in a patient with tumour lysis syndrome. CRP, C-reactive protein; AST, Aspartate aminotransferase; ALT, Alanine aminotranferase; AP, alkaline phosphatase; GGT: gamma glutamyltransferase; LDH, Lactate dehydrogenase.
Noteworthy is the development of biliary cysts in one patient who was treated poststudy participation by hepatic arterial infusion of 5-fluorouracil. The patient died of septic shock following an endoscopic retrograde cholangiopancreatography.
Antitumour activity
All patients were evaluable for tumour response. No objective tumour responses were observed. The best response to treatment was a stable disease in one patient and progressive disease in eight patients. In three patients there was a meaningful improvement in liver function tests. In these three patients with elevated baseline gamma-glutamyltransferase (GGT) and alkaline phosphatase (AP) values, these values decreased by an average of 31% (range 13%–56%) during therapy. In one patient this decrease was limited in time to the treatment period of 5 days. In one patient the level of GGT and AP was still 30% lower after 3 months of treatment, without an objective tumour response on imaging (patient who developed a tumour lysis syndrome; figure 1B), and in one additional patient a clear decrease in elevated GGT, AP, lactate dehydrogenase (LDH) and C reactive protein (CRP) was observed during treatment. GGT and AP increased 4 weeks after the initiation of treatment. LDH and CRP never returned to baseline levels.
The median progression-free and overall survival for the whole study population were 5.4 weeks (95% CI 2.9 to 7.9) and 22.2 weeks (95% CI 11.4 to 33.1), respectively.
CTA mRNA expression analysis
Evaluation of the CTA mRNA expression levels pretreatment and post-treatment shows in 21 out of the 30 CTAs a trend towards an increased expression in the post-treatment samples compared with the pretreatment samples (table 4). When only focusing on the two patients from which presamples and postsamples were available, the trend towards an increased expression in the post-treatment samples is present for 4 out of the 21 CTAs: PAT1, CT45A1, DDX43 and MAGEC2.
Table 4.
Results of CTA mRNA expression analysis
| Pre > post | Post > pre |
| BAGE. | CT45A1. |
| CTAGE1. GAGE1. PBK. PRAME. PRM1. ROPN1. TMEFF2. TPTE. |
CTAG1B. CTCFL. DDX43. MAGE A1. MAGE A3. MAGE A4. MAGE B2. MAGE C1. |
|
MAGE C2. PASD1. PAT1. SEMG1. SPA17. SPACA3. SPANXB1. SPO11. SSX1. SSX4. SYCP1. TTK. |
The genes shown in bold are found in the two patients from which pre/post samples were available.
CTA, cancer testis antigen.
Discussion
In this phase I clinical trial, dose escalation of decitabine administered by hepatic arterial infusion up to a daily dose of 20 mg/m2 on five consecutive days was well tolerated and no treatment-limiting toxicity was encountered. Grades 1 and 2 haematological toxicity was observed across dose levels suggestive of a low first-pass clearance by the liver following hepatic arterial infusion. The incidence of haematotoxicity was low as compared with the incidence observed with this regimen when administered by the intravenous route. Also, patients with leukaemia and myelodysplastic syndromes treated on phase III studies may represent a more vulnerable population with respect to haematological toxicity. In this study the subpopulation of patients with colorectal cancer who were more heavily pretreated with chemotherapy were more prone to haematological toxicity than patients treated with immunotherapy. This is possibly related to the more limited reserve in bone marrow function. Of note is the observation that one patient with hepatic metastases of a uveal melanoma developed a tumour lysis syndrome.
The maximal tolerated dose was not established within the dose range explored in our phase I trial. According to the protocol it was not the intention to dose-escalate decitabine beyond the approved dose level that is approved for the 5-day administration level. In the absence of a meaningful indication for single-agent antitumour activity in this trial, further development of this regimen of decitabine by hepatic arterial infusion in combination with concomitantly administered immune checkpoint inhibitors is proposed. Within such combinatorial strategies, further dose escalation could be considered depending on the incidence and specificities of the encountered toxicities.
In this small pilot study, a tendency of increased CTA expression (for 21 of the 30 investigated CTAs) after decitabine therapy could be observed. Analysing only the two patients with pretreatment/post-treatment samples confirmed the trend for mainly four CTAs: PAT1, CT45A1, DDX43 and MAGEC2. These observations deserve confirmation in a larger sample set and whether anti-CTA immune responses can be induced.
Conclusion
In patients with pretreated hepatic metastases from solid tumours, decitabine can be safely administered by hepatic arterial infusion at a dose of 20 mg/m²/day on five consecutive days every 4 weeks. Preliminary data indicate upregulation of CTA expression following treatment, providing a basis for further study of this regimen in combination with other immunotherapies.
Acknowledgments
We would like to thank the patients for their participation in this study. We would also like to acknowledge Katrien Van den Bossche and Katrien Van Peteghem for their contribution to study data management.
Footnotes
Contributors: All authors contributed to generation of data, recruitment of patients and review of the article.
Funding: YJLJ was supported by a 1-year PhD scholarship grant from the Belgian Kom Op Tegen Kanker and the Willy Gepts funding (UZ Brussel). Decitabine was provided by Janssen-Cilag International NV free of charge.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: This trial was approved by the Institutional Ethics Committee of the UZ Brussel (ClinicalTrials.gov identifier: NCT02316028).
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002;3:415–28. 10.1038/nrg816 [DOI] [PubMed] [Google Scholar]
- 2. Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014;14:135–46. 10.1038/nrc3670 [DOI] [PubMed] [Google Scholar]
- 3. Gnjatic S, Nishikawa H, Jungbluth AA, et al. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 2006;95:1–30. 10.1016/S0065-230X(06)95001-5 [DOI] [PubMed] [Google Scholar]
- 4. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30. 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 7. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. The Lancet 2017;389:2492–502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chou J, Voong LN, Mortales CL, et al. Epigenetic modulation to enable antigen-specific T-cell therapy of colorectal cancer. J Immunother 2012;35:131–41. 10.1097/CJI.0b013e31824300c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004;103:1635–40. 10.1182/blood-2003-03-0687 [DOI] [PubMed] [Google Scholar]
- 11. Schrump DS, Fischette MR, Nguyen DM, et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res 2006;12:5777–85. 10.1158/1078-0432.CCR-06-0669 [DOI] [PubMed] [Google Scholar]
- 12. Derissen EJ, Beijnen JH, Schellens JH. Concise drug review: azacitidine and decitabine. Oncologist 2013;18:619–24. 10.1634/theoncologist.2012-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kopp LM, Ray A, Denman CJ, et al. Decitabine has a biphasic effect on natural killer cell viability, phenotype, and function under proliferative conditions. Mol Immunol 2013;54:296–301. 10.1016/j.molimm.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 14. Nie J, Liu L, Li X, et al. Decitabine, a new STaR in epigenetic therapy: the clinical application and biological mechanism in solid tumors. Cancer Lett 2014;354:12–20. 10.1016/j.canlet.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 15. Liu L, Chen L, Wu X, et al. Low-dose DNA-demethylating agent enhances the chemosensitivity of cancer cells by targeting cancer stem cells via the upregulation of microRNA-497. J Cancer Res Clin Oncol 2016;142:1431–9. 10.1007/s00432-016-2157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Appleton K, Mackay HJ, Judson I, et al. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J Clin Oncol 2007;25:4603–9. 10.1200/JCO.2007.10.8688 [DOI] [PubMed] [Google Scholar]
- 17. Cashen AF, Shah AK, Todt L, et al. Pharmacokinetics of decitabine administered as a 3-h infusion to patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Cancer Chemother Pharmacol 2008;61:759–66. 10.1007/s00280-007-0531-7 [DOI] [PubMed] [Google Scholar]
- 18. Garrido-Laguna I, McGregor KA, Wade M, et al. A phase I/II study of decitabine in combination with panitumumab in patients with wild-type (WT) KRAS metastatic colorectal cancer. Invest New Drugs 2013;31:1257–64. 10.1007/s10637-013-9947-6 [DOI] [PubMed] [Google Scholar]
- 19. Karahoca M, Momparler RL. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2'-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin Epigenetics 2013;5 10.1186/1868-7083-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Nieuwenhove Y, Aerts M, Neyns B, et al. Techniques for the placement of hepatic artery catheters for regional chemotherapy in unresectable liver metastases. Eur J Surg Oncol 2007;33:336–40. 10.1016/j.ejso.2006.09.025 [DOI] [PubMed] [Google Scholar]