Abstract
Background
Steroids are frequently used in patients with metastatic non-small cell lung cancer (mNSCLC), but they could be detrimental for patients treated with immune checkpoint inhibitors (ICIs). Here, we assessed the association between early use of steroids, clinical outcomes and peripheral immune blood cells modulation in patients with mNSCLC treated with ICIs.
Methods
We reviewed patients with mNSCLC treated at our institution between April 2013 and December 2017. Early use of steroids was defined as the use of a daily prednisone-equivalent dose ≥10 mg for at least 1 day within 28 days after ICI initiation. Peripheral immune blood cell counts were retrieved at baseline and at 4 and 6 weeks after ICI initiation.
Results
Out of 151 patients included, 35 (23%) made early use of steroids that was associated with poor disease control (OR 0.32, p=0.006), progression-free survival (HR 1.80, p=0.003) and overall survival (HR 2.60, p<0.001). Early use of steroids significantly correlated with higher median absolute neutrophil count, neutrophil to lymphocyte ratio (NLR) and derived NLR, and lower median absolute and relative eosinophil count, both at 4 and 6 weeks after ICI initiation.
Conclusions
In patients with mNSCLC treated with ICIs, early use of steroids was associated with worse clinical outcomes and remarkable modulation of peripheral blood immune cells, which could contribute to restraining the activation of antitumour immunity. If confirmed in prospective studies, these findings would highlight the importance of carefully evaluating and, whenever possible, avoiding steroids during early phases of ICI treatment.
Keywords: non-small cell lung cancer, immune checkpoint inhibitors, steroids
Key questions.
What is already known about this subject?
Earlier this year, the deleterious impact of baseline early steroids on the efficacy of PD-1 (Programmed cell Death protein-1)/PD-L1 (Programmed cell Death-Ligand 1) blockade in patients with non-small-cell lung cancer (NSCLC) was reported.
Similarly, a brief report showed the scant overall survival associated with the early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab.
However, the major critic to the postulation that the baseline/early use of steroids can directly impair immune checkpoint inhibitor (ICI) efficacy is that a precocious requirement of steroidal therapy in patients with advanced NSCLC could be due to a rapid clinical worsening/progression, being the early use of steroids the consequence, instead of the cause, of worse clinical outcomes.
What does this study add?
Our data indicate that an early use of steroids can directly impact on ICI efficacy by modulating peripheral blood immune cells profile, and even if further studies are required to validate the role of neutrophils, lymphocytes and eosinophils modulation, our study suggests the biological basis behind the interaction between early use of steroids and ICI efficacy.
How might this impact on clinical practice?
Our findings discourage the use of steroids during the first 4 weeks of ICI treatment in patients with advanced NSCLC.
Since early initiation of steroids, or its chronic use, cannot be avoided in some patients, chemotherapy could be a preferred treatment option in these cases.
Background
Metastatic non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide.1 In recent years, the advent of immunotherapy has deeply modified the treatment paradigm of metastatic NSCLC without a genetic driver.2 Indeed, several clinical trials have shown superior efficacy of immune checkpoint inhibitors (ICIs) over chemotherapy, with the result of improving disease control, progression-free survival (PFS) and overall survival (OS). Consequently, anti-PD-1/PD-L1 agents have entered clinical practice in first and more advanced lines of therapy.3–6
Despite the efficacy of ICIs, only a limited number of patients actually benefit from these agents. Different predictive factors have been investigated in an effort to explain the heterogeneous response to ICIs, with high PD-L1 expression and high tumour mutational burden having emerged as predictive of clinical benefit and improved patient survival.7 8 More recently, the neutrophil to lymphocyte ratio (NLR), which reflects systemic inflammation and immune system activation, demonstrated a prognostic and predictive role in patients with advanced NSCLC treated with ICIs.9–14 Therefore, it is highly likely that a complex interaction between tumour genetic/epigenetic and host factors crucially contributes to determine the efficacy of immunotherapy in individual patients.15 16 In this view, a critical role could be played by concomitant therapies, especially those that affect the immune cell populations (eg, immune-suppressive agents), which could prevent ICI-induced enhancement of antitumour immune response.17 In particular, the use of steroids, prescribed to patients with cancer for several medical conditions (eg, dyspnoea, pain, brain metastases, spinal cord compression, treatment-related adverse events),18 is controversial. Recent reports from ‘real-world’ practice have shown that use of steroids at the initiation of treatment with ICIs is associated with lower response rate and shorter survival in patients with advanced NSCLC.19 20 Conversely, retrospective data showed that the use of steroids for immune-related adverse events (irAEs) during anti PD-1 treatment did not significantly affect patients’ survival.21 Therefore, the impact of steroids use on ICI efficacy in patients with metastatic NSCLC remains controversial.
In this study, we performed a retrospective cohort study to investigate the impact of early steroid use on the prognosis of patients with metastatic NSCLC treated with ICIs at our institution. Since corticosteroids can modulate peripheral immune blood cells,22 we explored the association between early use of steroids and modification in peripheral immune blood cells profile.
Methods
Patients’ population
We reviewed all patients with metastatic NSCLC treated with ICIs at the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano between April 2013 and December 2017 and included in the APOLLO prospective observational registry. Patients evaluable for tumour response according to the RECIST (Response Evaluation Criteria In Solid Tumours) V.1.1 criteria23 were evaluated, and data on demographics, clinical and pathological characteristics, as well as data on ICI treatment, clinical outcomes, use of steroids, and absolute and differential peripheral white blood cell counts, at baseline and at 4 weeks and 6 weeks after ICI initiations were retrieved. We also calculated the NLR by dividing the absolute neutrophil count (ANC) by the absolute lymphocyte count (ALC), and the derived NLR (dNLR) by dividing the ANC by the difference of white blood count (WBC) minus the ANC. Based on available literature reporting on the association between peripheral blood immune cells-based indexes and clinical outcomes of patients with cancer treated with immunotherapy,13 24–27 we used a cut-off value of 5 for NLR, 3 for dNLR and 1.5 for the relative eosinophil count (REC).
Early use of steroids was defined as the use of a daily prednisone-equivalent dose ≥10 mg for at least 1 day within 28 days after ICI initiation. Patients who made early use of steroids were included in the exposed cohort, while the remaining patients were included in the control cohort.
Statistical analyses
Descriptive statistics were used to summarise patients’ characteristics. χ2 test or Fisher’s exact test, as appropriate, was used to analyse the association between early use of steroids and relevant clinical, pathological and laboratory features. PFS was defined as the time between ICI treatment initiation and radiological documentation of disease progression or patient death from any cause, whichever came first. OS was defined as the time between ICI treatment initiation and patient death from any cause. PFS and OS probabilities were calculated with the Kaplan-Meier method, and survival curves were compared with the log-rank test. The reverse Kaplan-Meier method was used for follow-up quantification.28 We used Cox proportional hazard model to assess the impact of individual variables on PFS and OS (univariable analysis); variables significantly associated with clinical outcomes were then included in a multivariable model. Results of Cox proportional hazard model analyses were reported as HR and 95% CIs. Peripheral blood immune cell counts and indexes in the control and exposed cohorts were visually described by means of waterfall plots and box plots. Two-sample Wilcoxon test was used to compare the median values of peripheral white blood cells counts and indexes between the control and exposed cohorts at different time points. Statistical analyses were performed using R (V.3.5.0) and RStudio (V.1.1.456) software. Statistical significance threshold for all statistical tests and survival analyses was set at p<0.05.
Results
Patients’ characteristics
Out of 151 patients included, 83 (55%) received steroids at any time during ICI treatment, while 35 (23%) made early use of steroids. Patients’ characteristics in the entire population or according to the early use of steroids are summarised in table 1. Six patients (4%) received combinatorial PD-L1 and CTLA-4 blockade, while 145 (96%) received single-agent anti PD-1/PD-L1 treatment. Early use of steroids was associated with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥2 (OR 4.57, 95% CI 1.10 to 20.37, p=0.03), the presence of brain metastases (OR 4.83, 95% CI 2.07 to 11.41, p<0.001) and number of metastatic sites >2 (OR 3.08, 95% CI 1.33 to 7.89, p=0.01), while no association was observed with patients’ age, gender, smoking status, tumour histology, PD-L1 status, liver or bone metastases, line of ICI treatment, or ICI regimen. In 19 out of 35 patients in the exposed cohort (54%), treatment with steroids was already ongoing at the time of ICI initiation with the purpose of supportive care medication (baseline use of steroids), while the remaining 16 patients (46%) started steroids during the first 28 days after ICI initiation (baseline steroids-naive patients). In the latter case, only four patients received steroids because of irAEs. The median number of days on steroids within the first 4 weeks of ICI treatment was 28 (range, 1–28). The median total prednisone-equivalent dose of steroids within the first 4 weeks of ICI treatment was 280 mg (range, 20–875 mg).
Table 1.
Characteristics of the patients of the entire study population and according to the early use of steroids
| Characteristics | Total (N=151) n (%) |
Control cohort (n=116) n (%) |
Exposed cohort (n=35) n (%) |
OR (95% CI) | P value | |
| Age (years) | <65 | 64 (42) | 45 (39) | 19 (54) | – | 0.12 |
| ≥65 | 87 (58) | 71 (61) | 16 (46) | |||
| Gender | Male | 89 (59) | 73 (63) | 16 (46) | – | 0.08 |
| Female | 62 (41) | 43 (37) | 19 (54) | |||
| Smoking | Never or former | 103 (71) | 83 (75) | 20 (57) | – | 0.06 |
| Current | 43 (29) | 28 (25) | 15 (43) | |||
| NA | 5 | 5 | 0 | |||
| ECOG PS | 0–1 | 142 (94) | 112 (97) | 30 (86) | 4.57 (1.10 to 20.37) | 0.03 |
| ≥2 | 9 (6) | 4 (3) | 5 (14) | |||
| Histology | Squamous | 33 (22) | 29 (25) | 4 (11) | – | 0.11 |
| Non-squamous | 118 (78) | 87 (75) | 31 (89) | |||
| PD-L1 status | Negative | 46 (51) | 37 (51) | 9 (47) | – | 0.80 |
| Positive | 45 (49) | 35 (49) | 10 (53) | |||
| NA | 60 | 44 | 16 | |||
| Metastatic sites (N) | 1–2 | 65 (43) | 57 (49) | 8 (23) | 3.20 (1.38 to 8.16) | 0.006 |
| >2 | 86 (57) | 59 (51) | 27 (77) | |||
| Brain mets | No | 118 (78) | 99 (85) | 19 (54) | 4.83 (2.07 to 11.41) | <0.001 |
| Yes | 33 (22) | 17 (15) | 16 (46) | |||
| Liver mets | No | 117 (77) | 89 (77) | 28 (80) | – | 0.68 |
| Yes | 34 (23) | 27 (23) | 7 (20) | |||
| Bone mets | No | 87 (58) | 72 (62) | 15 (43) | – | 0.05 |
| Yes | 64 (42) | 44 (38) | 20 (57) | |||
| Line of treatment | 1–2 | 85 (56) | 65 (56) | 20 (57) | – | 1 |
| >2 | 66 (44) | 51 (44) | 15 (43) | |||
| ICI regimen | Mono | 145 (96) | 110 (95) | 35 (100) | – | 0.34 |
| Dual | 6 (4) | 6 (5) | 0 (0) | |||
P values below the significance threshold are reported in bold.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor; NA, not available.
Impact of early steroids use on clinical outcomes
In the exposed cohort, we observed one complete response (CR), 5 partial responses (PR), 6 disease stabilisations (SD) and 23 disease progressions as best response, while 28 PR, 44 stabilisations and 44 progressions occurred in the control group. Overall, patients in the exposed cohort had a 68% lower probability to achieve disease control (CR+PR+SD) than patients in the control cohort, with a disease control rate of 34% vs 62%, respectively (OR 0.32, 95% CI 0.14 to 0.71, p=0.006). No significant differences in the objective response rate were observed (p=0.39).
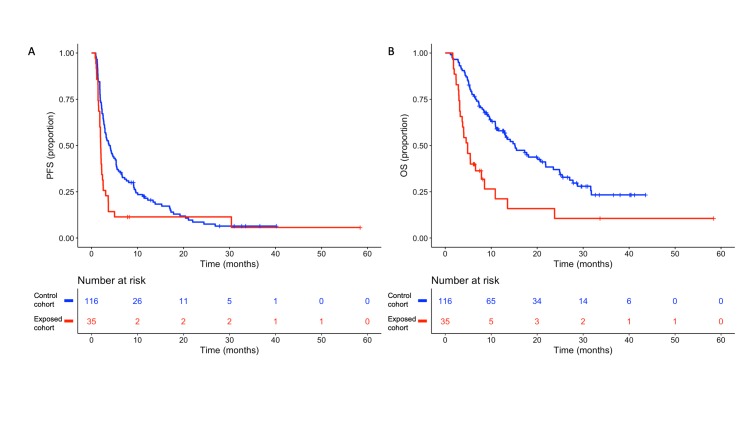
With a median follow-up time of 28.6 months, the median PFS for patients in the exposed and control cohorts was 1.98 months and 3.94 months, respectively (HR 1.80, 95% CI 1.20 to 2.80, p=0.003) (figure 1A). In the multivariable model including other covariates significantly associated with PFS (ie, ECOG PS and tumour PD-L1 expression), early use of steroids was independently associated with poorer PFS (HR 1.88, 95% CI 1.08 to 3.28, p=0.03) (table 2). Patients in the exposed cohort also had a lower median OS when compared with patients in the control cohort (4.86 vs 15.14 months, respectively; HR 2.60, 95% CI 1.70 to 4.10, p<0.001) (figure 1B). The impact of early use of steroids on OS remained significant after adjusting for other covariates associated with OS (HR 2.38, 95% CI 1.49 to 3.81, p<0.001) (table 3). No differences in PFS or OS were observed in the entire study population, between patients who received steroids at any time during ICI treatment and patients who did not (median OS 5.4 months vs 4.7 months, respectively; p=0.65) (online supplementary figure 1). Notably, among patients in the control cohort, a better PFS (HR 0.62, 95% CI 0.42 to 0.93, p=0.02) and a trend towards a better OS (HR 0.80, 95% CI 0.50 to 1.28, p=0.35) were observed in patients who received steroids after 28 days from ICI initiation with respect to patients who did not receive steroids at any time during the ICI treatment (online supplementary figure 2). Furthermore, when we restricted the analysis to the exposed cohort, we found no differences between patients with baseline use of steroids and patients who were steroids-naive at baseline (median PFS 1.9 months vs 2.1 months, respectively, log-rank p=0.22; median OS 4.7 months vs 5.4 months, respectively, log-rank p=0.70).
Figure 1.
Kaplan-Meier curves for (A) progression-free survival (PFS) and (B) overall survival (OS) according to the early use of steroids. Blue line indicates patients in the control cohort, while red line indicates patients in the exposed cohort.
Table 2.
Cox proportional hazards models for progression-free survival
| Characteristics | Univariate analyses | Multivariable model | |||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | ≥65 vs <65 | – | 0.74 | – | – |
| Gender | Female vs male | – | 0.80 | – | – |
| Smoking | Current vs never/former | – | 0.62 | – | – |
| ECOG PS | 2 vs 0–1 | 2.10 (1.00 to 4.10) | 0.04 | 4.83 (1.42 to 16.40) | 0.01 |
| Histology | Squamous vs non-squamous | – | 0.51 | – | – |
| PD-L1 status | Positive vs negative | 0.56 (0.36 to 0.88) | 0.01 | 0.48 (0.30 to 0.77) | 0.002 |
| Metastatic sites (N) | >2 vs 1–2 | – | 0.13 | – | – |
| Brain mets | Yes vs no | – | 0.22 | – | – |
| Liver mets | Yes vs no | – | 0.09 | – | – |
| Bone mets | Yes vs no | – | 0.05 | – | – |
| Line of treatment | >2 vs 1–2 | – | 0.84 | – | – |
| ICI regimen | Mono vs dual | – | 0.08 | – | – |
| Early use of steroids | Yes vs no | 1.80 (1.20 to 2.80) | 0.003 | 1.88 (1.08 to 3.28) | 0.03 |
P values below the significance threshold are reported in bold.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor.
Table 3.
Cox proportional hazards models for overall survival
| Characteristics | Univariate analyses | Multivariable model | |||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age | ≥65 vs <65 | – | 0.97 | – | – |
| Gender | Female vs male | – | 0.81 | – | – |
| Smoking | Current vs never/former | – | 0.93 | – | – |
| ECOG PS | 2 vs 0–1 | 3.60 (1.70 to 7.60) | <0.001 | 2.24 (1.01 to 4.95) | 0.04 |
| Histology | Non-squamous vs squamous | – | 0.13 | – | – |
| PD-L1 status | Positive vs negative | – | 0.20 | – | – |
| Metastatic sites (N) | >2 vs 1–2 | – | 0.22 | – | – |
| Brain mets | Yes vs no | – | 0.37 | ||
| Liver mets | Yes vs no | – | 0.05 | ||
| Bone mets | Yes vs no | – | 0.03 | – | 0.09 |
| Line of treatment | >2 vs 1–2 | – | 0.85 | – | – |
| ICI regimen | Dual vs mono | – | 0.93 | – | – |
| Early use of steroids | Yes vs no | 2.60 (1.70 to 4.10) | <0.001 | 2.38 (1.48 to 3.83) | <0.001 |
P values below the significance threshold are reported in bold.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICI, immune checkpoint inhibitor.
esmoopen-2018-000457supp001.pdf (411.2KB, pdf)
esmoopen-2018-000457supp002.pdf (413.5KB, pdf)
Association between early use of steroids and modulation of peripheral blood immune cells
Peripheral blood immune cell counts were available at baseline for 147 patients, at 4 weeks after ICI initiations for 146 patients and at 6 weeks after ICI initiation for 142 patients.
Patients taking steroids at the time of ICI initiation had higher baseline median WBC (11 280 vs 7200; p<0.001), ANC (8800 vs 5000; p<0.001), absolute monocyte count (AMC) (600 vs 500; p=0.01), NLR (6.9 vs 3.4; p<0.001) and dNLR (3.4 vs 2.1), and lower median absolute eosinophil count (AEC) (100 vs 200; p=0.01) and REC (1.0 vs 2.3; p<0.001), with respect to all other patients (table 4). Furthermore, patients with baseline use of steroids had a higher chance of having an NLR ≥5 (OR 5.40, 95% CI 1.95 to 16.70, p<0.001) and a dNLR ≥3 (OR 10.32, 95% CI 3.43 to 39.43, p<0.001), and a lower chance of having an REC ≥1.5 (OR 0.14, 95% CI 0.04 to 0.41, p<0.001), with respect to all other patients (online supplementary table 1). No differences in baseline peripheral blood cells were observed between baseline steroids-naive patients in the exposed cohort and patients in the control cohort (online supplementary table 2 and online supplementary table 3).
Table 4.
Median peripheral blood immune cell counts and indexes at baseline according to the baseline use of steroids
| No baseline steroids | Baseline steroids | P value* | |||
| Median | IQR | Median | IQR | ||
| WBC† | 7.20 | 6.05–9.09 | 11.28 | 9.40–13.24 | <0.001 |
| ANC† | 5.00 | 3.95–6.45 | 8.80 | 7.13–10.53 | <0.001 |
| ALC† | 1.40 | 1.00–1.90 | 1.30 | 0.95–1.65 | 0.48 |
| AMC† | 0.50 | 0.40–0.60 | 0.60 | 0.43–080 | 0.01 |
| AEC† | 0.20 | 0.10–0.30 | 0.10 | 0.00–0.18 | 0.01 |
| REC‡ | 2.30 | 1.40–3.82 | 1.00 | 0.00–1.45 | <0.001 |
| ABC† | 0.00 | 0.00–0.00 | 0.00 | 0.00–0.00 | 0.84 |
| NLR | 3.40 | 2.50–5.40 | 6.90 | 4.20–9.20 | <0.001 |
| dNLR | 2.10 | 1.70–3.00 | 3.40 | 3.00–4.70 | <0.001 |
P values below the significance threshold are reported in bold.
*Wilcoxon test
†×103.
‡%.
ABC, absolute basophil count; AEC, absolute eosinophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; dNLR, derived neutrophil to lymphocyte ratio. NLR, neutrophil to lymphocyte ratio; REC, relative eosinophil count; WBC, white blood count;
esmoopen-2018-000457supp005.pdf (483.3KB, pdf)
esmoopen-2018-000457supp006.pdf (410.7KB, pdf)
esmoopen-2018-000457supp007.pdf (483.5KB, pdf)
At 4 weeks after ICI initiation, patients in the exposed cohort had a higher median WBC (8760 vs 7520; p<0.001), ANC (7000 vs 5000; p<0.001), NLR (6.9 vs 3.4; p<0.001) and dNLR (3.6 vs 2.0; p<0.001), and a lower median AEC (100 vs 200; p=0.009) and REC (1.2 vs 2.6; p<0.001), when compared with patients in the control cohort (table 5). Conversely, no differences were observed in median ALC, AMC and absolute basophil count. Similar findings were observed at 6 weeks after ICI initiation, with the exception of median ALC which was lower for patients in the exposed cohort (1000 vs 1500; p=0.004). We confirmed these data when peripheral blood immune cell counts/indexes were treated as dichotomous variables by using established thresholds. In particular, at 4 weeks after ICI initiation, early use of steroids was positively associated with an NLR ≥5 (OR 4.01, 95% CI 1.79 to 9.28, p<0.001) and a dNLR ≥3 (OR 5.60, 95% CI 2.46 to 13.24, p<0.001), and negatively associated with an REC ≥1.5 (OR 0.15, 95% CI 0.06 to 0.33, p<0.001) (online supplementary table 4). Of note, at 4 and 6 weeks NLR ≥5, dNLR ≥3 and REC <1.5 were associated with worse clinical outcomes in our study population (online supplementary figure 3 and online supplementary figure 4).
Table 5.
Median peripheral blood immune cell counts and indexes at 4 and 6 weeks after ICI initiation according to the early use of steroids
| 4 weeks | 6 weeks | |||||||||
| Control cohort | Exposed cohort | P value* | Control cohort | Exposed cohort | P value* | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| WBC† | 7.52 | 6.22–8.94 | 8.76 | 8.08–12.20 | <0.001 | 7.75 | 6.19–9.73 | 8.67 | 7.35–11.22 | 0.01 |
| ANC† | 5.00 | 3.80–6.20 | 7.00 | 5.60–9.70 | <0.001 | 5.15 | 3.70–6.90 | 7.45 | 5.78–8.73 | <0.001 |
| ALC† | 1.50 | 1.00–1.90 | 1.10 | 0.80–1.70 | 0.07 | 1.50 | 1.10–1.90 | 1.0 | 0.70–1.70 | 0.004 |
| AMC† | 0.50 | 0.40–0.60 | 0.60 | 0.50–0.60 | 0.08 | 0.50 | 0.40–0.60 | 0.50 | 0.40–0.70 | 0.82 |
| AEC† | 0.20 | 0.10–0.30 | 0.10 | 0.10–0.20 | 0.009 | 0.20 | 0.10–0.30 | 0.10 | 0.00–0.20 | <0.001 |
| REC‡ | 2.60 | 1.50–3.80 | 1.20 | 0.70–3.30 | <0.001 | 2.35 | 1.30–3.90 | 1.20 | 0.00–2.40 | <0.001 |
| ABC† | 0.00 | 0.00–0.10 | 0.00 | 0.00–0.00 | 0.46 | 0.00 | 0.00–0.10 | 0.00 | 0.00–0.10 | 0.61 |
| NLR | 3.4 | 2.4–5.2 | 6.9 | 3.5–10.6 | <0.001 | 3.6 | 2.4–5.4 | 8.1 | 3.2–14.6 | <0.001 |
| dNLR | 2.0 | 1.6–2.8 | 3.6 | 2.6–4.9 | <0.001 | 2.2 | 1.6–3.0 | 4.0 | 2.3–5.8 | <0.001 |
P values below the significance threshold are reported in bold.
*Wilcoxon test.
†×103.
‡%.
ABC, absolute basophil count; AEC, absolute eosinophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; dNLR, derived neutrophil to lymphocyte ratio.ICI, immune checkpoint inhibitor; NLR, neutrophil to lymphocyte ratio; REC, relative eosinophil count; WBC, white blood count;
esmoopen-2018-000457supp008.pdf (490.5KB, pdf)
esmoopen-2018-000457supp003.pdf (543.6KB, pdf)
esmoopen-2018-000457supp004.pdf (540.3KB, pdf)
Discussion
Steroids are frequently used in patients with advanced NSCLC, especially with the aim of palliating symptoms, or for the management of adverse events related to different treatments (eg, radiotherapy, chemotherapy or immunotherapy). However, patients receiving steroids have typically been excluded from clinical trials investigating ICIs.
In the present study, we showed that, in patients with metastatic NSCLC treated with ICIs, the early use of steroids was associated with poor disease control (OR 0.32, 95% CI 0.14 to 0.71, p=0.006), low PFS (HR 1.80, 95% CI 1.20 to 2.80, p=0.003) and low OS (HR 2.60, 95% CI 1.70 to 4.10, p<0.001). Multivariable analyses confirmed these data after adjusting the impact of early use of steroids for other clinically relevant variables.
Our findings are consistent with the data of a recently published brief report which showed a negative impact of steroids use during the first 30 days of nivolumab treatment on the OS of patients with advanced NSCLC,29 and with other three independent studies in which use of steroids at the time of ICI initiation was associated with worse clinical outcomes in the same clinical setting.19 20 30 There are several reasons why steroids can affect the outcomes of anti PD-1/PD-L1 therapy, including impairment of T-cell activation31 and inflammation,32 microbiome modifications,33 Th1 to Th2 phenotype skewing,34 35 and T regulatory cells recruitment.36 All these events, along with the capacity of corticosteroids to promote M2 macrophage polarisation,37 may restrain the efficacy of antitumour immunotherapy and lead to primary or adaptive resistance to ICIs.38
Notably, assessing the overall impact of steroids use at any time during ICI treatment, we found no differences in patient outcomes, similar to what was previously reported by Leighl and colleagues.21 Different hypotheses can help explain these results, suggesting that steroids are unable to halt an effective antitumour immune response once they have been effectively induced by ICIs. First, immunotherapy is more efficacious in the presence of an intact immune system.39 For instance, Chen and colleagues40 demonstrated that early on-treatment modifications in the efficiency of antigen presentation, T-cell activation and T-cell homing predict response to ICIs. Steroids could affect these important processes in the immune cascade, thus preventing the activation of an effective antitumour immune response if given precociously during ICI treatment. As well as being dose-dependent, the ability of corticosteroids in influencing T-cell survival is also cell cycle-dependent and time-dependent.41 Therefore, after immunotherapy-mediated T-cell-boosted activation, effector cells may be protected from steroid-induced apoptosis. We also found that the late-only use of steroids (after 28 days from ICI initiation) was associated with a better PFS and a trend towards a better OS with respect to no use of steroids. Of note, the main reason for the late use of steroids during the ICI treatment is the management of irAEs, and several evidence linked the incidence of irAEs with better outcomes in patients with cancer treated with immunotherapy,42 43 explaining, at least in part, our observation.
Although steroid-induced increase in NLR and ANC has already been observed in patients with castration-resistant prostate cancer,44 this is the first study reporting on the association between early use of steroids and modulation in peripheral blood immune cells at both 4 and 6 weeks after ICI initiation. Since a high NLR/dNLR and a low REC at 4 and 6 weeks were consistently associated with reduced benefit from ICIs, our results suggest that early use of steroids could impair patient prognosis by modulating peripheral blood cells. In particular, the high number of blood neutrophils following steroids treatment may reflect the presence of myeloid-derived suppressive cells in tumour microenvironment. Corticosteroids are also able to upregulate the proportion of circulating CD4+/interleukin (IL)-17+ T cells,45 and IL-17 can mediate an increase in tumour-associated neutrophils and resistance to PD-1 blockade.46 Steroids are known to induce apoptosis in eosinophils.47 We showed for the first time that, in patients with metastatic NSCLC treated with immunotherapy, an REC ≥1.5 at 4 and 6 weeks after ICI initiation has a positive impact on both PFS and OS, and is negatively associated with early use of steroids. These findings indicate that steroids can interfere with eosinophil-mediated immune homeostasis regulation, which in turn leads to a modulation of ICI efficacy.
The major critic to the postulation that the early use of steroids can impair ICI efficacy is that a precocious requirement of steroidal therapy as supportive care medication in patients with metastatic NSCLC could be due to a rapid clinical worsening/progression, being the early use of steroids the consequence, instead of the cause, of worse clinical outcomes. Our data about the modulation of peripheral blood immune cells by the early use of steroids suggest an alternative hypothesis. Even if further studies (including appropriate preclinical investigation) are required to validate the role of neutrophils, lymphocytes and eosinophils modulation, our study suggests the biological basis behind the interaction between early use of steroids and ICI efficacy.
Although our study relies on a prospective registry, its main limitation consists in its retrospective nature. Prospective studies are therefore needed to definitely validate the negative impact of early steroid use in patients treated with immunotherapy, as well as to assess its implication in patients treated with the combination of chemotherapy and ICIs.
In conclusion, we showed that the early use of steroids is associated with modulation in peripheral blood cells and significantly worse disease control, PFS and OS in patients with advanced NSCLC treated with ICIs. If confirmed by future studies, our findings should discourage the use of steroids during the first 4 weeks of ICI treatment in patients with advanced NSCLC. Since early initiation of steroids, or its chronic use, cannot be avoided in some patients, chemotherapy could be a preferred treatment option in these cases.
Footnotes
Contributors: Study concept and design: GF, MCG, DS. Data acquisition: GF, GG, MP, GLR, CP, MI, RF, MG. Data analysis: GF, VT. Data interpretation: GF, CV, RF, NZ, MPC, AS, AB, FdB, MCG, DS. Manuscript preparation: GF, GG, CV, AS, MPC, AB, MCG, MG. Manuscript review: GF, GG, GLR, CP, MI, RF, NZ, MG, AS, AB, VT, MPC, DS, MCG, FdB.
Funding: This research was supported by the Italian Ministry of Health with the research grant 5x1000/2014 (CUP: B43C17000350001) to MCG.
Competing interests: GLR declares personal fees from Eli Lilly, BMS and AstraZeneca, outside the submitted work. CP declares personal fees from BMS and MSD, outside the submitted work. MCG declares personal fees from MSD, AstraZeneca, Eli Lilly and BMS, outside the submitted work. DS declares personal fees from AstraZeneca, Boehringer Ingelheim and BMS, outside the submitted work.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the local Institutional Review Board (INT 22_15) and conducted according to the ethical principles for medical research involving human subjects adopted in the Declaration of Helsinki. All patients signed a written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2. Fucà G, de Braud F, Di Nicola M. Immunotherapy-based combinations: an update. Curr Opin Oncol 2018;30:345–51. 10.1097/CCO.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced Nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 7. Aguiar PN, De Mello RA, Hall P, et al. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy 2017;9:499–506. 10.2217/imt-2016-0150 [DOI] [PubMed] [Google Scholar]
- 8. Rizvi NA, Hellmann MD, Snyder A. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;48:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Putzu C, Cortinovis DL, Colonese F, et al. Blood cell count indexes as predictors of outcomes in advanced non-small-cell lung cancer patients treated with nivolumab. Cancer Immunol Immunother 2018;67:1349–53. 10.1007/s00262-018-2182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zer A, Sung MR, Walia P, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer 2018;19:426–34. 10.1016/j.cllc.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 11. Park W, Kwon D, Saravia D, et al. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer 2018;19:280–8. 10.1016/j.cllc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 12. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176–81. 10.1016/j.lungcan.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 13. Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 2018;67:459–70. 10.1007/s00262-017-2092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1–7. 10.1016/j.lungcan.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 15. Shi T, Ma Y, Yu L, et al. Cancer immunotherapy: a focus on the regulation of immune checkpoints. IJMS 2018;19:1389 10.3390/ijms19051389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fucà G, Galli G, Poggi M, et al. Low baseline serum sodium concentration is associated with poor clinical outcomes in metastatic non-small cell lung cancer patients treated with immunotherapy. Target Oncol 2018;13:795–800. 10.1007/s11523-018-0599-5 [DOI] [PubMed] [Google Scholar]
- 17. Remon J, Vilariño N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): approaches on special subgroups and unresolved burning questions. Cancer Treat Rev 2018;64:21–9. 10.1016/j.ctrv.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 18. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed Death–1 pathway inhibitors among patients with Non–Small-Cell lung cancer and preexisting autoimmune disorders. JCO 2018;36:1905–12. 10.1200/JCO.2017.77.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez-Bernal G, Mezquita L, Auclin E, et al. Baseline corticosteroids (CS) could be associated with absence of benefit to immune checkpoint inhibitors (ICI) in advanced non-small cell lung cancer (NSCLC) patients. Ann Oncol 2017;28:1323P. [Google Scholar]
- 20. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed Death-Ligand 1 blockade in patients with Non–Small-Cell lung cancer. JCO 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 21. Leighl N, Gandhi L, Hellmann MD. Pembrolizumab for NSCLC: immune-mediated adverse events and corticosteroid use. J Thorac Oncol 2015;10:S66–S890.26710300 [Google Scholar]
- 22. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 2011;335:2–13. 10.1016/j.mce.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 2018;4:351–7. 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heppt MV, Heinzerling L, Kähler KC, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer 2017;82:56–65. 10.1016/j.ejca.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 26. Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016;22:5487–96. 10.1158/1078-0432.CCR-16-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Russo A, Franchina T, Ricciardi GRR, et al. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with nivolumab or docetaxel. J Cell Physiol 2018;233:6337–43. 10.1002/jcp.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–6. 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 29. Scott SC, Pennell NA. Brief report: early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. [DOI] [PubMed] [Google Scholar]
- 30. Dumenil C, Massiani MA, Dumoulin J, et al. Clinical factors associated with early progression and grade 3-4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS One 2018;13:e0195945 10.1371/journal.pone.0195945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell. Mol. Life Sci. 2006;63:60–72. 10.1007/s00018-005-5390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colotta F, Re F, Muzio M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 1993;261:472–5. 10.1126/science.8332913 [DOI] [PubMed] [Google Scholar]
- 33. Tetel MJ, de Vries GJ, Melcangi RC. Steroids, stress and the gut microbiome-brain axis. J Neuroendocrinol 2018;30:e12548 10.1111/jne.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Libert C, Dejager L. How steroids steer T cells. Cell Rep 2014;7:938–9. 10.1016/j.celrep.2014.04.041 [DOI] [PubMed] [Google Scholar]
- 35. Franchimont D, Louis E, Dewe W, et al. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul Pept 1998;73:59–65. 10.1016/S0167-0115(97)01063-X [DOI] [PubMed] [Google Scholar]
- 36. Edward JA, Sanyal M, Le W, et al. Selective expansion of human regulatory T cells in nasal polyps, and not adjacent tissue microenvironments, in individual patients exposed to steroids. Clin Immunol 2017;179:66–76. 10.1016/j.clim.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 37. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–95. 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–23. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shore ND. Advances in the understanding of cancer immunotherapy. BJU Int 2015;116:321–9. 10.1111/bju.12692 [DOI] [PubMed] [Google Scholar]
- 40. Chen PL, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016;6:827–37. 10.1158/2159-8290.CD-15-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lanza L, Scudeletti M, Puppo F, et al. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol 1996;103:482–90. 10.1111/j.1365-2249.1996.tb08306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374–8. 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD anderson cancer center experience. Invest New Drugs 2018;36:638–46. 10.1007/s10637-017-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mehra N, Sharp A, Lorente D, et al. Neutrophil to lymphocyte ratio in castration-resistant prostate cancer patients treated with daily oral corticosteroids. Clin Genitourin Cancer 2017;15:678–84. 10.1016/j.clgc.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 45. Hajkova M, Hermankova B, Javorkova E, et al. Mesenchymal stem cells attenuate the adverse effects of immunosuppressive drugs on distinct T cell Subopulations. Stem Cell Rev and Rep 2017;13:104–15. 10.1007/s12015-016-9703-3 [DOI] [PubMed] [Google Scholar]
- 46. Akbay EA, Koyama S, Liu Y, et al. Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thorac Oncol 2017;12:1268–79. 10.1016/j.jtho.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meagher LC, Cousin JM, Seckl JR, et al. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol 1996;156:4422–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000457supp001.pdf (411.2KB, pdf)
esmoopen-2018-000457supp002.pdf (413.5KB, pdf)
esmoopen-2018-000457supp005.pdf (483.3KB, pdf)
esmoopen-2018-000457supp006.pdf (410.7KB, pdf)
esmoopen-2018-000457supp007.pdf (483.5KB, pdf)
esmoopen-2018-000457supp008.pdf (490.5KB, pdf)
esmoopen-2018-000457supp003.pdf (543.6KB, pdf)
esmoopen-2018-000457supp004.pdf (540.3KB, pdf)