Abstract
Purpose
The development of osteosarcoma therapeutics has been challenging, in part because of the lack of appropriate criteria to evaluate responses. We developed a novel criteria in a clinical trial of radium-223 dichloride (223RaCl2) for response assessment in osteosarcoma, NAFCIST (Na18F PET response Criteria in Solid Tumors).
Experimental design
Patients received one to six cycles of 223RaCl2, and cumulative doses varied from 6.84 MBq to 57.81 MBq. Molecular imaging with technetium-99m phosphonate scintigraphy, fluorine-18-fluorodeoxyglucose (18FDG) positron emission tomography (PET) or sodium fluoride-18 (Na18F) PET was used to characterise the disease. Correlation of biomarkers and survival was analysed with NAFCIST measure from Na18F PET.
Results
Of the 18 patients, 17 had bone lesions visible in at least one of the imaging studies. In four of seven patients with multiple skeletal lesions (>5), FDG PET and NaF PET studies could be compared. The skeletal tumour locations varied in our patient population: cranium=2, extremities=7, pelvis=10, spine=12 and thorax=9. The 18F-FDG PET and Na18F PET studies could be compared in all four patients who had multiple lung lesions (>5). Overall the Response Evaluation Criteria in Solid Tumors response was seen in one patient, but four patients experienced mixed responses better defined by Na18F PET. Changes in NAFCIST were correlated with changes in bone alkaline phosphatase levels (r=0.54) and negatively with cumulative dose of 223RaCl2 (r=− 0.53). NAFCIST correlated with overall survival (p value of 0.037) while the PERCIST (PET Response Criteria in Solid Tumors) did not (p value of 0.19).
Conclusions
Our results indicate that Na18F PET should be further studied in osteosarcoma staging. NAFCIST may be a promising criteria for high-risk osteosarcoma response evaluation and correlates with survival. Further validation studies are needed.
Keywords: osteosarcoma, 223RaCl2, Na18F PET, 18F-FDG PET, bone scintigraphy, RECIST, PERCIST, Imaging
Key questions.
What is already known about this subject?
The current Response Evaluation Criteria in Solid Tumors is suboptimal for use in osteosarcoma because even responding tumours do not shrink.
Response in osteosarcoma neoadjuvant therapy is instead evaluated by tumour necrosis from the resected specimens.
What does this study add?
Since fluoride is taken up avidly by the bone, sodium fluoride-18 (Na18F) positron emission tomography (PET)-CT scan can better image the qualitative bone response to a bone-targeted alpha particle therapy with radium-223.
How might this impact on clinical practice?
NAFCIST (Na18F PET response Criteria in Solid Tumors), a new way to evaluate treatment response in osteosarcoma, is presented.
This may help with better imaging of primary bone tumours qualitatively and quantitatively to bone-targeted therapy.
Introduction
Relapsed and refractory osteosarcoma has a poor prognosis.1,2 Patients with osteosarcoma recurrence or metastases after initial therapy have a 5-year overall survival rate of 16%.2–4 The median overall survival duration after first, second, third or fourth recurrence has been shown to be about 1 year. Although chemotherapy may prolong overall survival after first relapse, surgery is critical to prevent subsequent relapses.3 Thus, for patients with recurrent or metastatic osteosarcoma, more effective treatments are needed.5
The development of osteosarcoma therapeutics has been challenging, in part because of the lack of appropriate criteria to evaluate responses. The current Response Evaluation Criteria in Solid Tumors (RECIST) is suboptimal for use in osteosarcoma because even responding tumours do not shrink, hence leading to difficulties in assessing the clinical benefit of novel therapeutics in patients with osteosarcoma.2,4 Response in osteosarcoma neoadjuvant therapy is instead evaluated by tumour necrosis from the resected specimens.6
Diagnostic imaging provides information about the appearance, extent and radiographic characteristics of bone tumours, contributing substantially to the diagnosis and prognosis of the disease. Morphological imaging modalities such as CT and MRI are all commonly used to assess osteosarcoma. In addition, fluorine-18-fluorodeoxyglucose positron emission tomography (18F-FDG PET) can be used to quantify the physiological activity of osteosarcoma, which is characterised by increased glucose uptake that leads to biochemical changes before anatomical changes.7
Given the limitations of RECIST, criteria using PET/CT, the PET Response Criteria in Solid Tumors (PERCIST), were proposed, and these criteria mainly used 18F-FDG PET.7 Although 18F-FDG PET by itself has limitations in osteosarcoma, the use of CT-derived morphological information together with 18F-FDG PET has further improved the diagnostic performance of imaging techniques. The first meta-analysis reported a sensitivity of 91% and a specificity of 85% for 18F-FDG PET in the differentiation of bone and soft tissue sarcomas from benign lesions.8 A second meta-analysis based on 42 eligible trials reported that the pooled sensitivity of PET/CT to differentiate primary bone sarcomas from benign lesions was 96% (95% CI 93 to 98) and the pooled specificity was 79% (95% CI 63 to 90). For detecting recurrence, the pooled sensitivity was 92% (95% CI 85 to 97) and the specificity was 93% (95% CI 88 to 96), and for detecting distant metastasis the pooled sensitivity was 90% (95% CI 86 to 93) and the specificity was 85% (95% CI 81 to 87).9
Radium-223 dichloride (223RaCl2) is a low-toxicity and potentially high-efficacy targeted agent for osteosarcoma, as shown in preclinical and early clinical osteoblastic bone-forming tumour protocols.10,11 The use of 223RaCl2 in osteosarcoma could reduce the morbidity of therapy and mortality from metastases.12 We initiated a phase I study (ClinicalTrials.gov #NCT01833520), the primary objective of which was to determine the safety of escalating doses of monthly 223RaCl2 in patients with osteosarcoma with osteoblastic bone-forming metastases until a maximum tolerated dose or a dose of 100 kBq/kg was reached.13,14 The secondary objective was to compare the ability of quantitative imaging techniques (technetium-99m methylene diphosphonate [99mTc-MDP] scintigraphy, 18F-FDG PET/CT, sodium fluoride-18 [Na18F] PET/CT) to determine alkaline phosphatase reduction, indicating objective response to 223RaCl2. Molecular imaging with different isotopes was used to characterise the disease. To the best of our knowledge, there is no report in the literature of the role of Na18F PET/CT in the staging or restaging of metastatic osteosarcoma. An exploratory objective in this phase I study was to delineate the additional information provided by the different imaging modalities in the overall assessment of osteosarcoma. We hypothesised that NaF PET CT scan can better image the qualitative response in the bone to a bone-targeted alpha particle therapy. We analysed the qualitative and quantitative approaches to metabolic tumour response assessment with Na18F and 18F-FDG PET and developed a framework for Na 18 F PET response Criteria in Solid Tumors (NAFCIST), a new way to evaluate treatment response in osteosarcoma.
Patients and methods
Patients
Eighteen patients with high-risk relapsed bone-forming osteosarcoma participated in a phase I clinical trial of 223RaCl2 (www.clinicaltrials.gov #NCT01833520).14 After obtaining informed consent, the patients were enrolled per protocol. The following were the main inclusion criteria for this protocol: patients with progressive, locally recurrent or metastatic osteosarcoma (ie, high-risk only) with no standard curative options available with at least one indicator lesion avid on 99mTc-MDP scan or NaF bone PET scan. In addition, subjects with extremely rare bone-forming, osteosarcoma-like tumours that behave like osteosarcoma phenotypically and are clinically treated like osteosarcoma (eg, malignant fibrous histiocytoma of the bone or malignant transformation of giant cell tumour of the bone) were included if they satisfy all of the inclusion criteria.
Three patients had metastatic fibroblastic osteosarcoma, three had chondroblastic osteosarcoma, eight had osteoblastic osteosarcoma, one had a giant cell tumour, one had high-grade metastatic osteosarcoma, one had metastatic osteosarcoma and one had recurrent osteosarcoma. All of these patients had primary bone tumours (table 1). Patients received one to six cycles of 223RaCl2, with cumulative doses of 6.84–57.81 MBq. Two patients received all six planned doses, two received just one cycle, seven received three cycles and seven received two cycles.
Table 1.
Imaging results for each patient in the study
| Patient number/sex/age, years | Diagnosis | Changes in overall alkaline phosphatase levels/bone alkaline phosphatase levels, baseline→follow-up (IU/L) | Lesions (n), baseline→follow-up/location of lesions (new location(s) | Number of 223RaCl2cycles/cumulative activity |
||
| Bone scintigraphy | Na18F PET/CT (NAFCIST) | 18F-FDG PET/CT (PERCIST) | ||||
| 1/M/71 | Metastatic fibroblastic osteosarcoma | 102→120/9.8→10 | 3→3↑/bone (T, P, E) | 8→11 (42.4→66.5)/bone (+V)(soft tissue [P]) | 9→12 (30.4→32.4)/bone, soft tissue | 3/12.66 MBq |
| 2/M/15 | Metastatic osteoblastic osteosarcoma | 78→80/19→21 | 4→4↑/bone (V, T, P) | 1→2 (3.6→9.2)/bone | 3/13.71 MBq | |
| 3/M/20 | Metastatic chondroblastic osteosarcoma | 101→281/26→93 | 3→9↑/bone (V, T) | 9→14 (115.1→211.6)/bone (+C, P, E) (soft tissue [V]) | 6→>10↑ (24.0→46.6)/bone, soft tissue (lung) | 2/7.24 MBq |
| 4/M/29 | Metastatic osteoblastic osteosarcoma | 339→130/86→28 | 9→9↑/bone (C, P), soft tissue (T), lung | 12→14 (236.9→117.2)/bone, soft tissue, lung, brain | 10→10↑ (51.4→31.9)/bone, soft tissue, lung | 3/13.44 MBq |
| 5/M/18 | Recurrent osteosarcoma | 188 →258/46→69 | 3→5↑/bone (V, P) | 4→6 (235.4→135.5)/bone, soft tissue, lung | 6→8 (28.1→29.3)/bone, soft tissue, lung | 2/11.27 MBq |
| 6/F/46 | Giant cell tumour | 93→108/8.1→9.3 | >30→>30 NC/bone (C, V, T, P, E), soft tissue, lung | >50 (152.3)*/bone, soft tissue, lung, liver | >40→>60 (56.5→80.7)/bone, soft tissue, lung, liver | 3/19.00 MBq |
| 7/M/15 | Metastatic osteosarcoma | 185→282/70→110 | 5→8↑/bone (V, T, P, E) (soft tissue) | 5→7 (11.5→14.7)/bone, soft tissue | 2/10.64 MBq | |
| 8/M/18 | Metastatic osteoblastic osteosarcoma | 84→80/24→16 | 1→4↑/bone (V, T) | 6→7 (62.0→71.2)/bone (+C) | 2→5 (9.3→20.2)/bone (soft tissue) | 2/13.84 MBq |
| 9/M/16 | Metastatic osteoblastic osteosarcoma | 233→992/75→306 | 5→10↑/lung | >10→>50 (118.4→199.3)/lung (brain) | 2/15.30 MBq | |
| 10/M/24 | Metastatic osteoblastic osteosarcoma | 148/50 | 1→3↑/bone (C, V, P) | 5→16 (36.1→36.9)/bone, soft tissue, lung | 1/6.84 MBq | |
| 11/M/15 | Metastatic osteoblastic osteosarcoma | 256/92 | 2→2↑/bone (P, E), soft tissue | 2→2↑ (76.3→112.3)/bone, soft tissue | 1/7.14 MBq | |
| 12/M/58 | Metastatic osteoblastic osteosarcoma | 115→98/22→19 | 2→2NC→ 2↑†/bone (T), lung | 3→5↓→6↓† (381→302→429)/bone, soft tissue, lung | 3 (21.8)*/bone, soft tissue, lung | 6/57.81 MBq |
| 13/M/22 | Metastatic high-grade osteosarcoma | 1147→2098/348→599 | 6→6↑/lung, soft tissue (T) | 17→24 (120.4→125.6)/soft tissue, lung (bone [+T]) | 2/9.36 MBq | |
| 14/M/63 | Metastatic fibroblastic osteosarcoma | 185→75/43→8.7 | 3→3↓→3↓†/bone (V, P), soft tissue | 5→5→5† (247→99→81.5)/bone, soft tissue | 6/50.33 MBq | |
| 15/F/17 | Metastatic chondroblastic osteosarcoma | 192→149/66→53 | 1→4↑/bone (V, P) | 1→6 (15.3→39.7)/bone (soft tissue) | 3/25.21 MBq | |
| 16/M/26 | Metastatic chondroblastic osteosarcoma | 149→53/34→10 | 2→2 NC/bone (E), soft tissue | 3→2 (98.9→49.9)/bone, soft tissue | 3/16.93 MBq | |
| 17/M/15 | Metastatic osteoblastic osteosarcoma | 6971→770/>2000 | >10→>70↑/bone (C, V, T, P, E) (lung) | >10→>150 (152.8→302.9)/bone (soft tissue, lung) | 2/13.23 MBq | |
| 18/F/43 | Metastatic fibroblastic osteosarcoma | 116→80/32→18 | 5→5 NC/soft tissue (bone [C, T]) | 8→8↑ (259.7→215.9)/soft tissue (bone [+V]) | 6 (28.5)**/bone, soft tissue | 3/24.74 MBq |
*Study performed at baseline only.
†Studies were performed three times: baseline, after 3 cycles, and after 6 cycles.
↓, visual decrease (response); ↑, visual increase (progression); C, cranium; E, extremity; F, female; 18F-FDG, fluorine-18-fluorodeoxyglucose; M, male; NAFCIST, Na18F PET response Criteria in Solid Tumors; Na18F, sodium fluoride-18; P, pelvic; PERCIST, PET Response Criteria in Solid Tumors; PET, positron emission tomography; T, thoracic; V, vetebrae.
223RaCl2 administration
The primary goal of the phase I study was to determine the maximum tolerated or recommended dose of monthly 223RaCl2 in patients with recurrent or metastatic osteosarcoma. We compared the safety and toxicity of 50, 75 and 100 kBq/kg 223RaCl2. 223RaCl2 was given monthly for up to six cycles, and the effects of this bone-targeted therapy on monthly blood counts and alkaline phosphatase and other bone turnover markers were determined. The secondary objective of the study was to compare the ability of quantitative imaging techniques (99mTc-MDP, 18F-FDG PET/CT, Na18F PET/CT) to determine the reduction in alkaline phosphatase and other bone turnover markers, indicating objective response of osteosarcoma indicator lesions to 223RaCl2.
Imaging
Molecular imaging with 99mTc-MDP scintigraphy, 18F-FDG PET/CT or Na18F PET/CT was used to characterise the disease, as clinically indicated. Nuclear medicine studies were performed using commercially available 99mTc-MDP, 18F-FDG and Na18F to image indicator lesions. Changes in 99mTc-MDP uptake on single-photon emission CT/CT, 18F-FDG PET/CT standardised uptake value (SUV) or Na18F PET/CT SUV were assessed after the third and sixth doses of 223RaCl2 compared with baseline.
For all patients, anatomical imaging (CT or MRI) was performed for all sites of the disease, along with chest CT at baseline and restaging. RECIST was used to analyse CT images. In the analysis of lung metastases, up to three measurable (1 cm or larger) indicator lesions seen on the chest CT study were described at baseline and compared after dose 3 and dose 6. Per RECIST, a >20% increase in size was considered progression. Any change of <20% or analysis of a lesion <1 cm was used for ‘off study’ progression criteria. RECIST progression was considered progressive disease regardless of other imaging results.
Development of NAFCIST
For PET response evaluation, we used the PERCIST for 18F-FDG.7 In addition to PERCIST, we developed a new measure, NAFCIST (Na18 F PET response Criteria in Solid Tumors), for osteosarcoma evaluation using Na18F PET. The mean peak SUV in 1 cm3 was calculated for up to five lesions, up to two of which could belong to the same organ (bone, soft tissue [adjacent separable], lung, liver or brain). The PET images were analysed and measured separately by two experienced nuclear medicine physicians. A consensus was reached between the two physicians (KK, GR) considering information from normal clinical reports.
We also reviewed the literature for RECIST, RECIST 1.0, PERCIST and MD Anderson criteria for response evaluation. In RECIST, response rates are derived from unidimensional measurements of tumour lesions and the sum of diameters.15 The latest revision of the RECIST guidelines includes various updates, including the number of lesions that can be assessed (<10); how to apply RECIST in randomised phase III trials in which progression, not response, is the primary endpoint; how to use newer imaging technologies such as 18F-FDG PET and MRI; how to handle assessment of lymph nodes; whether response confirmation is truly needed; and, not least, the applicability of RECIST in trials of targeted non-cytotoxic drugs.16–18
In PERCIST, qualitative and quantitative approaches are used to assess the response of metabolic tumours using 18F-FDG PET, with a draft framework for using RECIST with PET.7 The MD Anderson criteria were developed as a practical approach for diagnosis and assessment of bone metastasis.19 Response is divided into four standard categories (complete response, partial response, stable disease and progressive disease), and the criteria include quantitative and qualitative assessments of the behaviour of bone metastases.
On the basis of our review of these criteria, we propose the ‘NAFCIST’ criteria, which takes into account the bone avid lesions in osteosarcoma. In NAFCIST, the primary outcome was determined by measuring the single most active lesion on each scan (not necessarily the same lesion). The secondary outcome was the summed activity of up to five most intense lesions (no more than two lesions per organ).
Statistics
All correlation analyses of PERCIST/NAFCIST versus baseline value of various markers (lactate dehydrogenase, alkaline phosphatase, C-terminal telopeptide, osteocalcin and bone alkaline phosphatase) were performed using Spearman rank correlation. Spearman rank correlation was also used for the assessment of associations between changes in NAFCIST/PERCIST and the number of cycles (1–2 cycles, 3 cycles and 6 cycles) as well as dose. We calculated the correlation between NAFCIST and PERCIST with overall survival using concordance index.
Results
Imaging summary
Imaging results, including the number of lesions, organs involved and distribution of lesion sites, are summarised in table 1. The number of lesions was based on a confluence of images; for example, a bone lesion with a soft tissue extension was considered one lesion. The lesion locations were categorised by skeletal components: cranium, thoracic girdle, vertebrae, pelvic girdle and extremities. All patients had multiple lesions at baseline according to at least one imaging modality. Bone scintigraphy and 18F-FDG PET studies could be compared in 10 patients at two time points and in two patients before 223RaCl2 administration. Bone scintigraphy and Na18F PET could be compared in 10 patients at two time points, in 2 patients at three time points and in 1 patient before 223RaCl2 administration (table 1).
In addition to the number of lesions, some of the lesion volumes were analysed on 18F-FDG and Na18F images. Table 2A presents the lung lesion volumes in patients with multiple lung lesions and bone lesions. Four patients had multiple lung lesions, and in two of these patients 18F-FDG and Na18F responses could be compared (table 2A). Seventeen patients had bone lesions detected by at least one of the imaging techniques. Seven patients had multiple (>5) skeletal lesions, and in four of these patients 18F-FDG and Na18F could be compared; the distributions of 18F-FDG and Na18F for each patient differed substantially. Overall, two patients had cranial lesions, 7 had lesions in the extremities, 10 had pelvic bone tumours, 12 had vertebral lesions and 9 had lesions in the thoracic region (table 1).
Table 2A.
Changes in SUVpeak, total glucose burden and glucose-identified tumour volume, and total Na18F burden and Na18F-identified tumour volume between baseline and 3-month follow-up according to 18F-FDG and Na18F PET studies of lung lesions in two patients
| Characteristics | Patient 4 | Patient 5 |
| SUVpeak | ||
| 18F-FDG | 9.8–10.9→5.1–9.0 | 1.1–2.3→1.8–8.5 |
| Na18F | 47.5–50.1→30.3–38.0 | 6.5→4.7–22.6 |
| Glycolysis | ||
| Total glucose, g | 936→746 | 5.9→288 |
| Volume, mL | 218→268 | 3.7→155 |
| Na18F burden | ||
| Total concentration, g | 4935→4100 | 4.8→68.7 |
| Volume, mL | 346→278 | 1.1→13.4 |
| Number of lesions | Two continuous regions | 18F-FDG: 3→10; Na18F: 1→6 |
Table 2B.
Correlation coefficients between changes in the proposed NAFCIST or PERCIST and various biomarkers
| Biomarkers | r | |
| NAFCIST | PERCIST | |
| Lactate dehydrogenase | 0.31 | −0.4 |
| Alkaline phosphatase | 0.54 | 0.1 |
| Bone alkaline phosphatase | 0.54 | 0.3 |
| C-terminal telopeptide | 0.08 | 0.1 |
| Osteocalcin | 0.25 | −0.05 |
| Cumulative activity | −0.53 | +0.41 |
18F-FDG, fluorine-18-fluorodeoxyglucose; NAFCIST, Na18F PET response Criteria in Solid Tumors; Na18F, sodium fluoride-18; PERCIST, PET Response Criteria in Solid Tumors; PET, positron emission tomography; SUVpeak, maximum standardised uptake value.
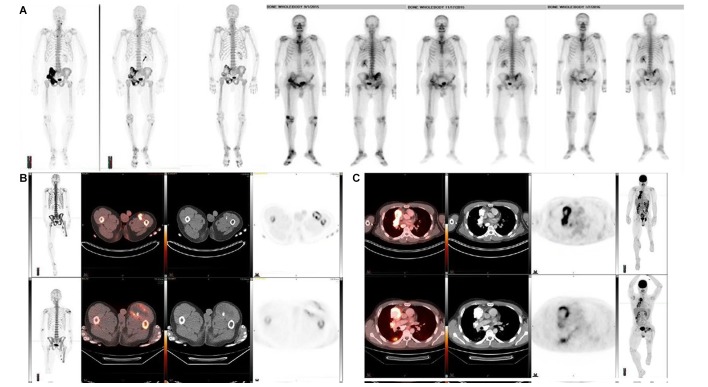
Overall, response using RECIST criteria was seen in one patient. Serial bone scans are shown in figure 1A, but the response was better visualised by Na18F, as shown in figure 1A. However, mixed responses were seen in many patients. Examples shown in the figures clearly demonstrate that some tumours shrank or disappeared, as shown by decreased tracer activity, whereas other tumours grew, as shown by increased activity. This was evident in a bone lesion with soft tissue extension (figure 1B) and in lung metastases (figure 1C).
Figure 1.
(A) Serial NaF and bone scans and images from patient 14. (A) Serial NaF PET scans at baseline, after three cycles of 223RaCl2 and after six cycles of 223RaCl2 (Maximum-intensity projectionss). (B) Serial Bone scan images at baseline, after three cycles of 223RaCl2 and after six cycles of 223RaCl2 (Anterior -Posterior / Posterior- Anterior projections). The response in the pelvic bones predominantly on the right was better visualised by Na18F than by serial bone scans. (B) Na18F PET images in patient 16 are shown at (top panel) baseline and (bottom panel) after three cycles of 223RaCl2. Left to right: maximum-intensity projections of the whole body, fusion PET/CT images, CT images and PET images from cross sections of the upper thighs. The mixed response in the upper thigh on the left was better visualised by evaluating Na18F concentration than by evaluating tumour size. (C) Na18F PET images for patient 4 are shown at baseline and after three cycles of 223RaCl2. Left to right: fusion PET/CT images, CT images, PET images from cross sections of the thorax at two levels and maximum-intensity projections of the whole body. The mixed response in the lung on the right was better visualised by evaluating Na18F concentration than by evaluating tumour size. 223RaCl2, radium-223 dichloride; Na18F, sodium fluoride-18; PET, positron emission tomography.
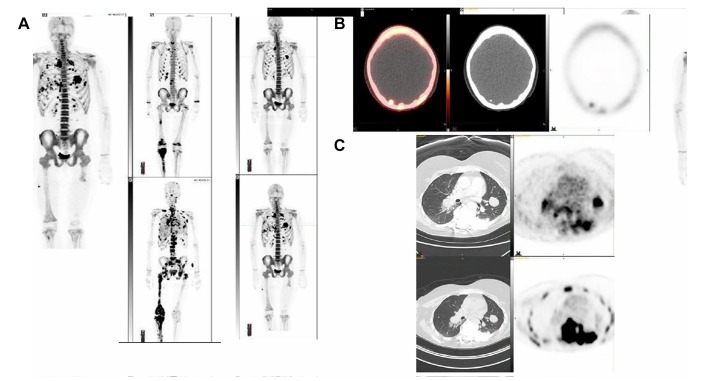
In addition, lung tumour uptake of Na18F and 18F-FDG varied (table 2A). Figure 2A shows an example of the varying distribution of 18F-FDG and Na18F in lung lesions. Most of the patients (14 of 18) had soft tissue metastases, and at least one of these lesions were calcified in every patient. In two patients, soft tissue lesions could be identified as lymph nodes. One patient had a liver metastasis. Two patients had brain metastases; one had them at the baseline and the other developed them prior to follow-up (figure 2B). In most of the patients, the soft tissue lesions were bone tumour extensions.
Figure 2.
(A) Na18F PET images (maximum-intensity projection). Examples of extensive and rapid progression after two cycles of radium-223 dichloride are shown for two patients. On the left, patient 17 had metastatic bone tumours at baseline (upper panel) and after two cycles (lower panel), with numerous boneand lung metastases (lower panel). On the right, patient 13 had metastatic lung tumours at baseline (upper panel) and rapid progression after two cycles (lower panel). (B) Na18F PET images in patient 9 after two cycles of radium-223 dichloride (left to right: fusion PET/CT image, CT image, PET image from across section of the brain and maximum-intensity projection of the whole body). New lesions can be observed in the occipital brain on the right, indicating progression. (C) Positron emission tomography 18F-FDG PET (upper row) and Na18F PET (lower row) images for patient are shown at baseline (images on the left and PET images from cross-sections of the thorax on the right). The tracer distributions in the lungs differed remarkably. 18F-FDG, fluorine-18-fluorodeoxyglucose; Na18F, sodium fluoride-18; PET, positron emission emission tomography.
Most of the patients did not respond to 223RaCl2, as demonstrated by the two examples in figure 2C. One patient rapidly developed numerous new bone and lung metastases.
NAFCIST outcomes correlate with changes in alkaline phosphatase
There was no correlation between NAFCIST and PERCIST. Also there was no correlation with PERCIST of outcomes and changes in biomarker levels (lactate dehydrogenase, alkaline phosphatase, bone alkaline phosphatase, C-terminal telopeptide or osteocalcin) between baseline and follow-up at 3 months or 6 months (r≤0.3). However, there was a correlation between the changes in NAFCIST and changes in alkaline phosphatase (r=0.54) and bone alkaline phosphatase (r=0.54), as seen in table 2B. PERCIST changes did not correlate with changes in bone alkaline phosphatase (r=0.30).
Correlation of cumulative 223RaCl2 dose with NAFCIST
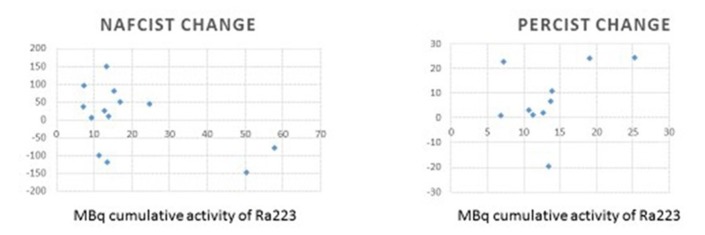
Additionally, cumulative 223RaCl2 dose (see table 1) was negatively correlated with changes in NAFCIST (r=−0.53); that is, the more 223RaCl2 administered, the more NAFCIST value decreased. PERCIST changes did not correlate with cumulative dose (r=0.41).
Figure 3 Changes in alkaline phosphatase did not correlate with cumulative dose (r=0.058), although changes in bone alkaline phosphatase correlated weakly with cumulative dose (r=−0.32).
Figure 3.
Correlation of changes in NAFCIST and PERCIST with cumulative activity with radium-223. 223RaCl2 dose was negatively correlated with changes in NAFCIST (r=−0.53); that is, the more 223RaCl2 administered, the more NAFCIST value decreased. PERCIST changes did not correlate with cumulative dose (r=0.41). 223RaCl2, radium-223 dichloride; NAFCIST, Na18 F PET response Criteria in Solid Tumors; PERCIST, PET Response Criteria in Solid Tumors; PET, positron emission tomography.
NAFCIST correlates with survival
We calculated the correlation of NAFCIST and PERCIST with survival. NAFCIST correlated with survival (p=0.037), versus PERCIST which did not (p=0.19). In addition the ‘concordance index’ for NAFCIST was 0.74 (CI 0.609 to 0.861) and for PERCIST was 0.44 (CI 0.32 to 0.56).
Discussion
Response evaluations in osteosarcoma clinical trials have been challenging. In this study we report the qualitative and quantitative aspects of imaging of osteosarcoma using several imaging modalities in this trial of 223RaCl2 and have developed a new response criteria using Na18F PET/CT for use in future clinical trials. This is the first study to evaluate three different modalities in patients with osteosarcoma. The imaging characteristics from our results demonstrate that osteosarcomas are very heterogenic and complex. This mirrors the complex molecular biology of osteosarcoma as well.1,3
Interestingly, the bone scans identified anywhere from 1 to more than 70 lesions. In the Na18F PET studies, up to more than 150 separate lesions could be identified (figure 2C), and in the 18F-FDG PET studies more than 60 lesions could be identified. These are innumerable metastases in clinical practice, but clearly the Na18F PET studied identified more lesions. The distribution of skeletal disease varied remarkably; 11 patients had pelvic lesions, 10 had lesions in the spine and 10 had lesions in the ribs. Cranial lesions were less common (five patients), and six patients had lesions in the extremities. In this trial, the three major types of osteosarcomas were osteoblastic, chondroblastic and fibroblastic. Also, the disease burden varied in these patients, as did the locations of the tumours, as shown in tables 1–2.
Other studies have demonstrated that 18F-FDG PET/CT is useful for follow-up imaging of castration-resistant prostate cancer with skeletal metastases. In these studies, lesions were considered abnormal when focal tracer accumulation was greater than background activity, usually if the SUV values were higher than 10. In addition, TLF10 has been developed.20,21 Additional methods for interpretation and quantification of bone lesions (benign or malignant) have also been developed. They take into account anatomical localisation, that is, five regions: cranium, vertebral column, thoracic girdle, pelvic girdle and extremities. As an analogue to the classic PERCIST analysis, in that method the two highest SUVmax values of skeletal uptakes from two regions were summed. In a small cohort, all patients with prostate cancer demonstrated at least moderate response in the skeleton.22
Osteosarcoma is known to be an aggressive malignancy. In our patient population, overall response was seen only in one patient per RECIST. However, mixed responses were seen in four patients, in whom some tumours clearly shrank, as indicated by decreased tracer activity on either Na18F PET or 18F-FDG PET, whereas other tumours grew, showing increased tracer activity. This mixed response occurred more often in the lung and soft tissue tumours in which no calcifications were present at baseline. The target of 223RaCl2 is calcium turnover in the skeleton, and in osteosarcomas this also includes calcified metastatic sites.
We observed the phenomenon of mixed response in partially calcified metastatic sites in only two patients with extensive lung disease, as shown in table 2A. In patient 4, there was a minor decrease in tracer activity evident on both Na18F PET and 18F-FDG PET; a minor decrease in total lesion glycolysis and a decrease in total Na18F burden were observed. However, the tumour volume measured by Na18F activity decreased, whereas the volume measured by 18F-FDG increased slightly, and Na18F demonstrated the lung disease present better than did 18F-FDG. In addition, alkaline phosphatase and bone alkaline phosphatase levels decreased in this patient. In patient 5, more extensive lung disease was demonstrated with 18F-FDG than with Na18F; the number of lesions increased according to both methods, and all other parameters increased as well: total lesion glycolysis increased more than 48-fold, tumour volume according to 18F-FDG increased more than 41-fold, total Na18F burden increased more than 14-fold, and tumour volume according to Na18F increased more than 12-fold. 18F-FDG was more sensitive to lung changes in this patient, but the tumours were also less calcified.
The cumulative dose of 223RaCl2 was low in most of the patients; only two patients received the planned six cycles, with a total dose of more than 50 MBq a piece. In addition, 12 patients received ≤15.3 MBq of cumulative activity. Despite the generally low 223RaCl2 doses in our study, we were able to demonstrate the correlation between cumulative dose and changes in NAFCIST and bone alkaline phosphatase levels, indicating that targeted bone destruction increases as the total dose of 223RaCl2 increases.
We have shown that Na18F PET/CT is better to image bone-forming component of osteosarcoma. We have shown that NAFCIST correlated with changes in bone alkaline phosphatase level, a tumour marker in osteosarcoma. More importantly we have shown that NAFCIST and not PERCIST correlates with overall survival. This is an important finding. Although this could be viewed as preliminary, this could be validated in future multicentre clinical trials. In addition some of the patients did not have all the studies performed, owing to the fact that some patients presented with a huge burden of disease and that some of the modalities cannot be performed in the patient given the radioactive nature of the imaging studies. This was a very specific cohort of patients who were on trial with 223RaCl2, and thus may not be used in general at this point.
On the basis of the preliminary data presented in the current study, we propose NAFCIST criteria (table 3). NAFCIST could supplant PET/CT functional response by adding metabolic response criteria for bone-forming disease. NAFCIST may represent a more accurate method of categorising osteosarcoma than RECIST, which mainly relies on unidimensional measurements of tumour lesions and the sum of diameters.
Table 3.
Na18F PET response criteria in primary bone tumours (NAFCIST)*
| Response category | Criteria |
| Complete metabolic response | Normalisation of all lesions (target and non-target) to SUV less than the mean skeletal SUV and equal to the normal surrounding tissue SUV; verification with follow-up study in 1 month if anatomical criteria indicate disease progression. |
| Partial metabolic response | >30% decrease in SUV peak*; verification with follow-up study if anatomical criteria indicate disease progression. |
| Progressive metabolic disease | >30% increase in SUV peak*; >75% increase in total Na18F burden of the five most active lesions; visible increase in the extent of Na18F uptake; new lesions; verification with follow-up study if anatomical criteria indicate complete or partial response. |
| Stable metabolic disease | Does not meet other criteria. |
*Primary outcome determination is measured on the single most active lesion on each scan (not necessarily the same lesion). Secondary outcome determination is the summed activity of up to the five most intense lesions (no more than two lesions per organ).
NAFCIST, Na18F PET response Criteria in Solid Tumors; Na18F, sodium fluoride-18; SUV, standardised uptake value.
In conclusion, our results indicate that Na18F PET is an essential part of osteosarcoma staging and restaging, and that Na18F PET and 18F-FDG PET are complementary. In addition, our newly developed NAFCIST outcomes were consistent with disease characteristics indicated by alkaline phosphatase levels and bone destruction. However, these are preliminary findings and hypothesis-generating. Further large-scale, prospective analysis through a cooperative group trial is warranted for the validation of NAFCIST in osteosarcoma.
Acknowledgments
The authors acknowledge Bayer for providing the radioactive isotopes.
Footnotes
Contributors: KK, VS: conception, study design, provision of study materials, wrote the first draft, analysed the data, revised reviewer comments. ER, PMA, GR, HAM: conception, study design, critical revision of paper. AR: biostatistical analysis and critical revision of the paper.
Funding: Funding was provided by the Shannon Wilkes osteosarcoma research programme, the High-Impact Clinical Research Support Program (HI-CRSP) at The University of Texas MD Anderson Cancer Center, and the National Institutes of Health Cancer Center Support Grant CA016672.
Competing interests: Vivek Subbiah receives research funding forclinical trials from Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprintmedicines, Loxo oncology, Takeda and Roche/ Genentech, National ComprehensiveCancer Network, NCI-CTEP and UT MD Anderson Cancer Center. Travel: Novartis, Pharmamar, AstraZeneca/Medimmune
Patient consent for publication: Not required.
Ethics approval: The UT MD Anderson Cancer Center Institutional Review Board approved the protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Subbiah V, Wagner MJ, McGuire MF, et al. Personalized comprehensive molecular profiling of high risk osteosarcoma: implications and limitations for precision medicine. Oncotarget 2015;6:40642–54. 10.18632/oncotarget.5841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lagmay JP, Krailo MD, Dang H, et al. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through children's cancer group, pediatric oncology group, and children's oncology group: learning from the past to move forward. JCO 2016;34:3031–8. 10.1200/JCO.2015.65.5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Egas-Bejar D, Anderson PM, Agarwal R, et al. Theranostic profiling for actionable aberrations in advanced high risk osteosarcoma with aggressive biology reveals high molecular diversity: the human fingerprint hypothesis. Oncoscience 2014;1:167–79. 10.18632/oncoscience.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006;106:1154–61. 10.1002/cncr.21724 [DOI] [PubMed] [Google Scholar]
- 5. Bacci G, Ferrari S, Mercuri M, et al. Predictive factors for local recurrence in osteosarcoma: 540 patients with extremity tumors followed for minimum 2.5 years after neoadjuvant chemotherapy. Acta Orthop Scand 1998;69:230–6. 10.3109/17453679809000921 [DOI] [PubMed] [Google Scholar]
- 6. Rosen G, Marcove RC, Caparros B, et al. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer 1979;43:2163–77. [DOI] [PubMed] [Google Scholar]
- 7. Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50(Suppl_1):122S–50. 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bastiaannet E, Groen H, Jager PL, et al. The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev 2004;30:83–101. 10.1016/j.ctrv.2003.07.004 [DOI] [PubMed] [Google Scholar]
- 9. Liu F, Zhang Q, Zhu D, et al. Performance of positron emission tomography and positron emission tomography/computed tomography using fluorine-18-fluorodeoxyglucose for the diagnosis, staging, and recurrence assessment of bone sarcoma: a systematic review and meta-analysis. Medicine 2015;94:e1462 10.1097/MD.0000000000001462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nilsson S, Larsen RH, Fosså SD, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005;11:4451–9. 10.1158/1078-0432.CCR-04-2244 [DOI] [PubMed] [Google Scholar]
- 11. Henriksen G, Fisher DR, Roeske JC, et al. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003;44:252–9. [PubMed] [Google Scholar]
- 12. Pandit-Taskar N, Larson SM, Carrasquillo JA. Bone-seeking radiopharmaceuticals for treatment of osseous metastases, part 1: α therapy with 223Ra-dichloride. J Nucl Med 2014;55:268–74. 10.2967/jnumed.112.112482 [DOI] [PubMed] [Google Scholar]
- 13. Subbiah V, Anderson PM, Kairemo K, et al. Alpha particle radium 223 dichloride in high-risk osteosarcoma: a phase I dose escalation trial. Clinical Cancer Research 2019. 10.1158/1078-0432.CCR-18-3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subbiah V, Anderson P, Rohren E. Alpha emitter radium 223 in high-risk osteosarcoma: first clinical evidence of response and blood-brain barrier penetration. JAMA oncology 2015;1:253–5. [DOI] [PubMed] [Google Scholar]
- 15. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 16. Subbiah V, Chuang H, Gambhire D, et al. Defining clinical response criteria and early response criteria for precision oncology: current state-of-the-art and future perspectives. Diagnostics 2017;7 10.3390/diagnostics7010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Litière S, Collette S, de Vries EG, et al. RECIST - learning from the past to build the future. Nat Rev Clin Oncol 2017;14:187–92. 10.1038/nrclinonc.2016.195 [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19. Hamaoka T, Costelloe CM, Madewell JE, et al. Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer 2010;102:651–7. 10.1038/sj.bjc.6605546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Etchebehere EC, Araujo JC, Fox PS, et al. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-Fluoride PET/CT in predicting overall survival. J Nucl Med 2015;56:1177–84. 10.2967/jnumed.115.158626 [DOI] [PubMed] [Google Scholar]
- 21. Rohren EM, Etchebehere EC, Araujo JC, et al. Determination of skeletal tumor burden on 18F-Fluoride PET/CT. J Nucl Med 2015;56:1507–12. 10.2967/jnumed.115.156026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kairemo K, Joensuu T. Radium-223-Dichloride in castration resistant metastatic prostate cancer-Preliminary results of the response evaluation using F-18-Fluoride PET/CT. Diagnostics 2015;5:413–27. 10.3390/diagnostics5040413 [DOI] [PMC free article] [PubMed] [Google Scholar]