Abstract
Purpose
Cancer treatment delay due to fertility preservation procedures is a barrier for patients with breast cancer who wish to preserve their fertility. This study aimed to describe the associations between fertility preservation and treatment delay in patients with breast cancer with reproductive concerns and assess the factors related to treatment delay.
Methods
Patients with primary breast cancer who visited the reproductive unit at our institution before cancer treatment between 2007 and 2015 were enrolled. The treatment delay cut-off was defined as follows: time to chemotherapy (TTC) >8 weeks for patients intending to receive neoadjuvant chemotherapy, TTC >12 weeks for patients intending to receive adjuvant chemotherapy, time to endocrine therapy (TTE) >12 weeks for patients intending to receive endocrine therapy without radiation therapy and TTE >20 weeks for patients intending to receive endocrine therapy after radiation therapy. Multivariable models were constructed to examine the factors of treatment delay.
Results
Overall, 212 patients met the inclusion criteria. Using the defined cut-offs, treatment delay was noted in 18% of the patients. Endocrine therapy was related to treatment delay (OR 4.49, 95% CI 1.02 to 19.7; p=0.05), but fertility preservation by artificial reproductive treatment (ART) was not. Pregnancy and delivery following treatment for breast cancer were achieved in 18 (19%) and 15 (16%) patients who underwent fertility preservation with ART.
Conclusion
Fertility preservation with ART was not associated with treatment delay in patients with breast cancer who were referred to reproductive specialists before cancer treatment.
Keywords: breast cancer, fertility, fertility preservation, treatment delay
Key questions.
What is already known about this subject?
Patients in the reproductive age group with breast cancer are interested in maintaining fertility and future reproductive function at the time of their cancer diagnosis.
One of the barriers to fertility preservation is the concern about cancer treatment delay.
What does this study add?
Treatment delay was noted in 18% of the patients who were referred to reproductive specialists.
Fertility preservation by artificial reproductive treatment (ART) was not related to treatment delay; endocrine therapy was the only factor related to treatment delay.
Pregnancy and delivery following treatment for breast cancer were achieved in 18 (19%) and 15 (16%) patients who underwent fertility preservation with ART, respectively.
How might this impact on clinical practice?
Our findings are informative for patients who have concerns regarding fertility preservation, especially those who are worried about treatment delay due to fertility preservation with ART.
Fertility preservation with ART before breast cancer treatment was a reliable method for future pregnancies and deliveries.
Introduction
Although cancer incidence is higher after the age of 50 years, thousands of younger people are diagnosed with cancer every year.1 2 Advancements in cancer treatments have led to a significant reduction in mortality.3 However, the prevalence of long-term side effects such as treatment-related infertility has increased.4 Many patients with cancer in the reproductive age group are interested in maintaining fertility at the time of their cancer diagnoses and future reproductive function.5 6 For women with newly diagnosed cancer, future fertility is one of the major concerns.7 A previous report showed that the risk or incidence of treatment-related infertility can lead to psychological and emotional distress, including moderate or severe depression.8 Moreover, the risk of infertility resulting from cancer therapy may adversely impact the treatment decisions.9–11
Although fertility preservation is an important issue, many barriers exist in this regard for women who choose to pursue fertility preservation treatment.7 12 One of the barriers to fertility preservation by artificial reproductive treatment (ART) is discussing fertility issues, and the other is cancer treatment delay due to a lack of accurate knowledge about fertility preservation with ART. With respect to discussing fertility issues, the European Society of Medical Oncology, American Society of Clinical Oncology (ASCO) and American Society of Reproductive Medicine recommend providing information about the potential risk of infertility and probability of fertility preservation for patients in the reproductive age group.13–15 Therefore, early referral to a fertility specialist and counselling women about their infertility risks before initiating cancer therapy are essential elements of comprehensive cancer care.16 17
Due to concerns of delay in cancer treatment, physicians lack knowledge and awareness on the safety of fertility preservation strategies in such situations.18 Furthermore, some patients give up fertility preservation because it may negatively impact their survival.13 19 Regarding optimal treatment timing of starting adjuvant chemotherapy, a large multi-institutional cohort study using National Comprehensive Cancer Network (NCCN) database reported that the mean time to chemotherapy (TTC) in major institutions in the USA was 12.0 weeks.20 Another retrospective study demonstrated that TTC influenced the survival outcome among high risk of breast cancer subtype.21 It reported that the risk of relapse and distant relapse was higher in patients who were treated after 61 days from the respective surgery than in those who were treated within 31–60 days.21 A recent population-based observational study with >20 000 subjects also demonstrated that patients treated after 91 or more days from the respective surgery experienced worse overall survival rates and had worse breast cancer–specific survival than patients treated within 90 days, especially in the triple-negative type.22
However, studies on the association between treatment delay and starting fertility preservation are lacking. Additionally, the factors associated with treatment delay remain unknown. This study aimed to describe the associations between fertility preservation and treatment delay in patients with breast cancer with reproductive concerns and assess the factors related to treatment delay using data from two high-volume hospitals with patients with breast cancer in Japan.
Methods
Design and subjects
This was a retrospective study using surveying of medical chart data of two institutions between June 2007 and November 2015. The subjects were patients with primary breast cancer who had visited the reproductive centre at St. Luke’s International Hospital after referral from either the division of breast surgery or medical oncology at the National Cancer Center Hospital or at St. Luke’s International Hospital. These two facilities are geographically close and have been engaged in treating the reproductive issues in patients with cancer since 2007.
Most of the patients who were referred to the reproductive specialists were planning for chemotherapy or endocrine therapy, with tamoxifen for at least 5 years. All the patients discussed their fertility issues with the specialists and the possibility of future pregnancy following treatment with or without fertility preservation techniques according to their age. The types of ART included embryo or oocyte cryopreservation during a natural cycle or with ovarian stimulation, and ovarian tissue cryopreservation.
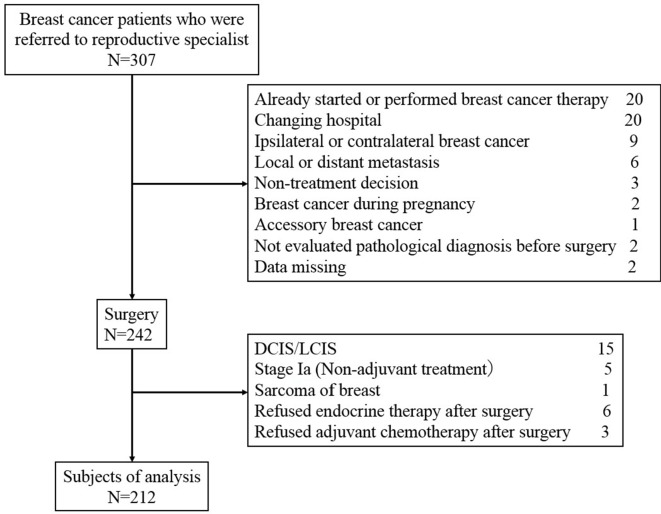
The exclusion criteria were patients (1) who had already started or completed breast cancer treatment including surgery, chemotherapy and endocrine therapy; (2) who did not receive either chemotherapy or endocrine therapy; (3) who had ductal carcinoma in situ and lobular carcinoma in situ; (4) whose cancer stage was Ia; (5) who could not continue cancer treatment in either institution and declined anticancer drug therapy; (6) who had locoregional recurrence, ipsilateral breast cancer, contralateral breast cancer or metastatic breast cancer; (7) who did not have confirmed pathological cancer diagnosis before initiating treatment; and (8) who had an uncommon type of breast tumour including accessory breast cancer, sarcoma of the breast and breast cancer during pregnancy. After exclusion, 212 patients were enrolled into the present study (figure 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram. DCIS, ductal carcinoma in situ; LCIS, loublar carcinoma in situ.
Data definition
In neoadjuvant chemotherapy (NACT) cases, TTC was defined as the period between the day of pathological diagnosis and the first day of administration of chemotherapy. In adjuvant chemotherapy cases, TTC was defined as the period between the day of the surgery and the first day of administration of adjuvant chemotherapy. In patients who were treated with endocrine therapy without chemotherapy, time to endocrine therapy (TTE) was defined as the period between the day of surgery and the first prescription of tamoxifen or aromatase inhibitor.
In the present study, optimal periods of TTC and TTE were defined. In adjuvant chemotherapy cases, optimal TTC was <12 weeks (84 days) from the surgery according to the evidence-based guidelines of the Japanese Society of Fertility Preservation (JSFP) and other previous reports. In NACT cases, the JSFP guideline states the optimal timing as “as soon as possible”; however, we predefined an arbitrary optimal TTC of <8 weeks (56 days) from diagnosis in this study. In TTE including endocrine therapy without radiation therapy, optimal TTE was defined as <12 weeks (84 days) from the surgery. In endocrine therapy after radiation therapy, optimal TTE was defined as <20 weeks (140 days) from the surgery. When TTC or TTE was longer than the optimal timing, we defined it as treatment delay.
Outcome
The primary outcome of this study was the frequency of treatment delay in patients who were referred to reproductive specialists. The secondary outcome was identification of the factors related to the treatment delay and evaluate TTC or TTE in the groups of patients who did and did not undergo fertility preservation with ART; the groups were labelled as ART+ and ART− groups, respectively. Reproductive outcomes, such as the number of pregnancies and/or delivery after breast cancer treatment, were assessed using descriptive analyses.
Statistical analysis
Wilcoxon rank-sum test was performed to evaluate TTC or TTE between the ART+ and ART− groups with regards to both chemotherapy and endocrine therapy. We calculated the treatment delay rate using the predefined cut-off criteria and compared between the two groups. To identify the factors related to treatment delay, either χ2 test or Fisher’s exact test was performed. Multivariate analysis was performed using the logistic regression model. The descriptive analysis was performed according to the number of pregnancies and deliveries after breast cancer treatment in ART+ group. All reported p values were two-sided, and statistical significance was set at p value <0.05 for the analyses. All statistical analyses were performed using JMP 1.2 software, V.12 (SAS Institute, Cary, North Carolina, USA).
Results
Of the 307 patients who were enrolled, 212 met the inclusion criteria. Table 1 shows the characteristics of the patients included in the analysis. There were 93 (43%) patients who underwent fertility preservation with ART (ART+) and 119 (57%) patients who did not (ART−). Most of the patient characteristics were similar between the two groups. Of the patients who underwent fertility preservation with ART, 26 (28%) patients were over 40 years of age and 30 (31%) were not suitable candidates for chemotherapy. Fertility preservation with ART was chosen by more patients who planned to receive adjuvant chemotherapy than by those who planned to receive NACT. Of the 93 patients who underwent fertility preservation with ART, 57 patients underwent embryo cryopreservation, 33 patients underwent oocyte cryopreservation, and one patient each underwent both embryo and oocyte cryopreservation, ovarian tissue cryopreservation, and both embryo cryopreservation and ovarian tissue cryopreservation, respectively. Egg collection was performed either during a natural cycle or following ovarian stimulation.
Table 1.
Patient characteristics
| All N=212 |
Fertility preservation with ART | P value | ||
| Present N=93 |
Absent N=119 |
|||
| Age at diagnosis, years | 0.15 | |||
| Median | 37 (23–49) | 37 (26–44) | 37 (23–49) | |
| <30 | 16 (7) | 8 (9) | 8 (7) | |
| 30–35 | 47 (22) | 13 (14) | 34 (28) | |
| 35–40 | 92 (43) | 46 (49) | 46 (39) | |
| 40–45 | 49 (23) | 22 (24) | 27 (23) | |
| >45 | 8 (6) | 4 (4) | 4 (3) | |
| Marriage status | 0.28 | |||
| Married | 98 (46) | 45 (48) | 53 (45) | |
| Not married | 111 (52) | 48 (52) | 63 (53) | |
| Divorced | 3 (2) | 0 | 3 (2) | |
| Have child/children | 0.16 | |||
| Yes | 15 (7) | 4 (4) | 11 (9) | |
| No | 197 (93) | 89 (96) | 108 (91) | |
| Stage | 0.99 | |||
| Ib or Ic | 65 (30) | 28 (30) | 37 (31) | |
| II | 120 (57) | 53 (56) | 67 (56) | |
| III | 27 (13) | 12 (13) | 15 (13) | |
| ER status | 0.97 | |||
| Positive | 178 (84) | 78 (84) | 100 (84) | |
| Negative | 34 (16) | 15 (16) | 19 (16) | |
| HER2 status | 0.06 | |||
| Positive | 42 (20) | 13 (14) | 29 (24) | |
| Negative | 170 (80) | 80 (86) | 90 (76) | |
| Surgery | NA | |||
| Yes | 212 (100) | 93 (100) | 119 (100) | |
| No | 0 (0) | 0 (0) | 0 (0) | |
| Chemotherapy | 0.9 | |||
| Neoadjuvant | 58 (27) | 20 (22) | 38 (31) | |
| Adjuvant | 87 (41) | 44 (47) | 43 (36) | |
| No | 67 (32) | 30 (31) | 37 (33) | |
| Endocrine therapy | 0.78 | |||
| Yes | 175 (83) | 76 (82) | 99 (83) | |
| No | 37 (17) | 17 (18) | 20 (17) | |
| Radiation therapy | 0.54 | |||
| Yes | 120 (44) | 51 (53) | 69 (59) | |
| No | 92 (56) | 43 (47) | 49 (41) | |
ART, artificial reproductive technique;ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; NA, not available.
TTC in patients who received NACT and adjuvant chemotherapy was 30.7 (SD 29.3) and 65.6 (29.5) days, respectively (table 2). TTE in patients who received endocrine therapy without and with radiation therapy was 74.0 (51.7) and 124.0 (67.5) days, respectively. TTC and TTE were not statistically different between the ART+ and ART− groups.
Table 2.
TTC and TTE between patients who underwent fertility preservation and patients who did not undergo
| N | All (days) |
Fertility preservation with ART | P value | ||
| Present (days) |
Absent (days) |
||||
| TTC in patients who received neoadjuvant chemotherapy | 58 | 30.7±27.3 | 39.0±29.3 | 26.4±25.5 | 0.07 |
| TTC in patients who received adjuvant chemotherapy | 87 | 65.6±50.6 | 64.1±25.5 | 67.2±67.1 | 0.08 |
| TTE in patients who received endocrine therapy patients without radiotherapy | 24 | 74.0±51.7 | 82.0±56.4 | 64.6±46.3 | 0.25 |
| TTE in patients who received endocrine therapy patients with radiotherapy | 42 | 124.0±67.5 | 143.8±96.3 | 114.1±38.7 | 0.28 |
Data are presented as mean±SD.
ART, artificial reproductive technique; TTC, time to chemotherapy; TTE, time to endocrine therapy.
Using this study’s definition of optimal treatment timing, the treatment delay according to the treatment type was 13%, 18%, 29% and 21% in patients who underwent NACT, adjuvant chemotherapy, endocrine therapy without radiation therapy and endocrine therapy after radiation therapy, respectively (table 3). Overall, treatment delay was observed in 18% of the patients. There was no treatment delay rate between the ART+ and ART− groups in each treatment arm.
Table 3.
Treatment delay rates according to the treatment arm
| Definition of treatment delay | Number of patients | Number of patients with delayed treatment N (%: treatment delay rate) |
OR (95% CI) | P value | |||
| Total | Fertility preservation with ART | ||||||
| Present | Absent | ||||||
| Patients who received neoadjuvant chemotherapy | TTC >8 weeks (56 days) | 58 | 7 (13) | 4 (7) | 3 (5) | 3.02 (0.60 to 15.2) | 0.21 |
| Patients who received adjuvant chemotherapy | TTC >12 weeks (84 days) | 87 | 16 (18) | 10 (11) | 6 (7) | 1.81 (0.60 to 5.50) | 0.41 |
| Patients who received endocrine therapy, RT(−) | TTE >12 weeks (84 days) | 24 | 7 (29) | 4 (17) | 3 (13) | 1.19 (0.20 to 6.99) | 1.00 |
| Patients who received endocrine therapy, RT(+) | TTE >20 weeks (140 days) | 42 | 9 (21) | 4 (10) | 5 (12) | 1.40 (0.31 to 6.23) | 0.71 |
| All patients | TTC or TTE >optimal treatment timing | 212 | 39 (18) | 23 (11) | 16 (8) | 1.86 (0.92 to 3.75) | 0.10 |
ART, artificial reproductive technique;RT(+), with radiotherapy;RT(−), without radiotherapy; TTC, time to chemotherapy;TTE, time to endocrine therapy.
Factors associated with treatment delay, which were candidates for multivariate analyses, were age, stage, oestrogen receptor (ER) expression, endocrine therapy and fertility preservation with ART. In the multivariate model, stage was excluded because none of the patients with stage III had treatment delay. ER was also excluded because ER expression and endocrine treatment undertaken were highly overlapping. Finally, age, endocrine therapy and fertility preservation with ART were included in the multivariate analysis model (table 4). The results indicated that endocrine therapy was a factor of treatment delay in patients who had concerns of fertility preservation (OR 4.49, 95% CI 1.02 to 19.7). Fertility preservation with ART was not related to treatment delay.
Table 4.
Factors associated with treatment delay
| Factors | Univariate | Multivariate | |||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 35 ≤ | 2.70 (1.07 to 6.82) | 0.03 | 2.33 (0.97 to 6.00) | 0.08 |
| Marriage status | Married | 0.87 (0.43 to 1.77) | 0.72 | ||
| Child | Not have | 3.35 (0.43 to 26.23) | 0.38 | ||
| Stage | Ib, Ic, or Ⅱ | Not calculated | 0.008 | ||
| ER | Positive | 4.20 (0.96 to 18.32) | 0.04 | ||
| HER2 | Positive | 1.27 (0.55 to 2.94) | 0.57 | ||
| Endocrine therapy | Yes | 4.69 (1.08 to 20.42) | 0.03 | 4.49 (1.02 to 19.7) | 0.05 |
| Chemotherapy | Yes | 0.60 (0.29 to 1.23) | 0.16 | ||
| Radiation therapy | Yes | 1.11 (0.62 to 1.98) | 0.69 | ||
| Fertility preservation with ART | Underwent | 1.86 (0.92 to 3.75) | 0.08 | 1.72 (0.84 to 3.56) | 0.14 |
HER2, human epidermal growth factor receptor 2ART, artificial reproductive technique;ER, oestrogen receptor.
The median observation time of the present study was 37 months. Of the 93 patients in the ART+ group, 18 (19%) patients achieved pregnancy and 15 (16%) delivered babies after breast cancer treatment. Of these 18 patients, 16 (17%) patients used materials cryopreserved before the initiation of the treatment (cryopreserved embryo in 15 patients and cryopreserved oocyte in one patient), and 2 (2%) patients conceived naturally (table 5). Of the 18 patients, 15 (16%) delivered babies (natural conception in one patient and cryopreserved materials in 14 patients). In contrast, of the 119 patients in the ART− group, 6 (5%) patients achieved pregnancy and 5 (4%) patients delivered babies. Of the six patients who achieved pregnancy, three (3%) conceived naturally and three (3%) used ART techniques after breast cancer treatment. Of those, five (4%) patients delivered babies, two (2%) had conceived naturally and three (3%) used ART techniques after breast cancer treatment.
Table 5.
Reproductive outcomes in the 93 patients in the ART+ group
| Total N (%) |
Type of pregnancy | ||
| Cryopreserved materials used N (%) |
Natural N (%) |
||
| Pregnancy after breast cancer treatment | 18 (19) | 16 (17) | 2 (2) |
| Delivery after breast cancer treatment | 15 (16) | 14 (15) | 1 (1) |
ART, artificial reproductive technique.
Discussion
In this study, the frequency of treatment delay and factors associated with it in patients who had concerns with fertility preservation were examined in the largest sample of cases assembled to date using data from two high-volume hospitals in Japan. In principle, fertility preservation with ART was not recommended to women older than 40 years as well as those who were not candidates for chemotherapy; however, some of them underwent fertility preservation with ART because of strong hopes for future pregnancies.
We noted that 18% of the patients experienced treatment delay, and receiving endocrine therapy was the only factor related to treatment delay. Undergoing fertility preservation with ART was not associated with treatment delay.
Our data also showed that TTC and TTE were not statistically different between the ART+ and ART− groups. Additionally, the treatment delay rate was not statistically different according to the treatment setting. However, in terms of absolute numbers, a strong conclusion on the lack of delay cannot be claimed, particularly in patients treated with neoadjuvant treatment (39 vs 26 days, p=0.07).
Several previous reports also found that the time to adjuvant chemotherapy was not different between the fertility preservation and no fertility preservation groups. A retrospective study that analysed 93 women with breast cancer undergoing fertility preservation reported no difference in the time from the initial diagnosis to adjuvant chemotherapy in women who underwent oocyte retrieval versus women who did not (71 vs 67 days, respectively, p<0.27).23 Similarly, another observational study showed that women referred to reproductive specialists preoperatively had a significantly shorter time interval from the initial diagnosis to initiation of ovarian stimulation (42.6 vs 71.9 days; p<0.001, respectively) and initiation of chemotherapy (83.9 vs 107.8 days; p=0.045) than women referred postoperatively.24
Concerning patients who received NACT, a retrospective study using data from the prospective ISPY2 trial, an ongoing phase II, multicentre, NACT-based clinical trial, also showed no treatment delay in patients who underwent ovarian stimulations before NACT.25 In this study, the mean time from diagnosis to initiation of NACT was 39.8 days in the group of patients who underwent ovarian stimulation and 40.0 days in the control group (p=0.75). Additionally, a cross-sectional study reported that fertility preservation with random start ovarian stimulation was not associated with treatment delay in NACT.26
With respect to TTC, mathematical models have suggested that a delay in the initiation of systemic chemotherapy could increase the risk of emerging drug-resistant micrometastatic disease.27 However, optimal timing between the breast cancer surgery and initiation of adjuvant chemotherapy is controversial.28–31 Several studies with large sample sizes showed a positive relationship between shorter TTC and survival.32 33 Similarly, a recent meta-analysis reported that for each 4-week delay in adjuvant chemotherapy initiation, there was a 6% increase in the risk of death.34
The optimal time for initiating NACT has not been clearly described in any breast cancer guidelines. A retrospective study that evaluated 3711 patients with stages I–III breast cancer receiving NACT at a single institution reported that the median time from diagnosis to NACT was 34 days.35 The authors also mentioned that no difference was observed in the survival between patients who received NACT 0–4, 4–8 and >8 weeks from the diagnosis, including triple-negative patients. Another retrospective study that also compared the impact of the time interval between breast cancer diagnosis and the initiation of NACT reported that the time interval between breast cancer diagnosis and the initiation of NACT did not impact the overall survival between the three groups (<30 days, 30–60 days and >60 days).36 Based on these findings, the definitions of optimal timing that we used in the present study were permissible.
Many studies have evaluated the association between TTC and breast cancer outcome, whereas only a few studies evaluated the association between TTE and the outcome. ASCO and NCCN recommend tamoxifen or aromatase inhibitor within 1 year of diagnosis because endocrine therapy generally follows completion of surgery, chemotherapy and radiation.37 They also mention that “the time window was fashioned based on the treatments patients had received because not all patients receive all modalities”.37 Therefore, the optimal time for starting endocrine therapy was defined as <12 weeks (86 days) for patients who did not receive radiation therapy and <20 weeks (140 days) for patients who received radiation therapy after surgery. Unexpectedly, our results demonstrated that endocrine therapy was the only factor associated with treatment delay. We suspect that it was because most patients who received neoadjuvant or adjuvant chemotherapy tried ovarian stimulation only once, while those who received hormone therapy tried it multiple times.
Safety of pregnancy after breast cancer is also a major concern for survivors. Recent data confirm the safety of pregnancy in women with a history of breast cancer diagnosis.38–40 In contrast, data regarding the safety of pregnancy using ART techniques after breast cancer treatment are limited. Our exploratory analysis revealed that the number of patients who became pregnant and delivered babies was statistically higher after use of fertility preservation techniques. Therefore, fertility preservation with ART is a more reliable method than natural conception for breast cancer survivors. However, long-term follow-up is required to confirm the impact of pregnancy with ART techniques on breast cancer recurrence or mortality.
Our findings are informative for patients who have concerns regarding fertility preservation, especially those who are worried about treatment delay due to fertility preservation with ART. However, the present study has several limitations. First, this study did not evaluate other factors that might potentially be related to the treatment delay, such as breast reconstructive surgery, multigene panel diagnosis, and physician’s attitude or knowledge of fertility issues. Second, this study did not consider the use of temporary ovarian suppression with gonadotropin-releasing hormone analogues because the evidence regarding its ovarian protective role was controversial at that time and it was not covered by the universal health coverage in Japan. Third, because our data were based on patients who lived in urban areas with well-established medical resources, the treatment delay rate of the present study may not be applicable to the medical care approach in rural areas. Fourth, regarding future pregnancies and deliveries, patients who underwent fertility preservation were more motivated to become pregnant than those who did not, while they could also undergo pregnancy and delivery. Fifth, because our study was a retrospective one, several analyses in this report lack statistical power.
Conclusions and future study
The present findings underscored that although 18% of patients experienced treatment delay, undergoing fertility preservation with ART was not related to treatment delay. Furthermore, it was suggested that fertility preservation with ART before breast cancer treatment was a reliable method for future pregnancy and delivery.
However, the long-term impact of fertility preservation on survival must be elucidated. Further investigations are required to reveal the long-term safety of fertility preservation in patients with breast cancer.
Footnotes
Contributors: AK designed the study and wrote the initial draft of the manuscript. KS and FA were involved in data collecting and critically reviewing the manuscript. SO contributed to analysis and interpretation of data, and CS assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Funding: This study was supported by Health and Labor Science Research Grant (H26-016) from the Ministry of Health, Labour and Welfare.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics approval was obtained by the review board from the National Cancer Center Hospital and St. Luke’s International Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. American Cancer Society Cancer Facts & Figures 2017, 2017. [Google Scholar]
- 2. National Cancer Institute , 2015. Cancer statistics review 1975–2012. Available: http://seer.cancer.gov/csr/1975_2012/browse_csr.php
- 3. Keegan THM, Ries LAG, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer 2016;122:1009–16. 10.1002/cncr.29869 [DOI] [PubMed] [Google Scholar]
- 4. Angarita AM, Johnson CA, Fader AN, et al. Fertility preservation: a key survivorship issue for young women with cancer. Front Oncol 2016;6 10.3389/fonc.2016.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psycho-Oncology 2012;21:134–43. 10.1002/pon.1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorman JR, Bailey S, Pierce JP, et al. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv 2012;6:200–9. 10.1007/s11764-011-0211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peddie VL, Porter MA, Barbour R, et al. Factors affecting decision making about fertility preservation after cancer diagnosis: a qualitative study. BJOG 2012;119:1049–57. 10.1111/j.1471-0528.2012.03368.x [DOI] [PubMed] [Google Scholar]
- 8. Rosen A, Rodriguez-Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Semin Oncol Nurs 2009;25:268–77. 10.1016/j.soncn.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 9. Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 2004;22:4174–83. 10.1200/JCO.2004.01.159 [DOI] [PubMed] [Google Scholar]
- 10. Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol 2014;32:1151–6. 10.1200/JCO.2013.52.8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Senkus E, Gomez H, Dirix L, et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 big 3-98. Psychooncology 2014;23:173–82. 10.1002/pon.3384 [DOI] [PubMed] [Google Scholar]
- 12. Gwendolyn P, Quinn STV. Fertility and cancer—patient, physician, and institutional barriers. US Oncol Hematol 2011;7:22–4. [Google Scholar]
- 13. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500–10. 10.1200/JCO.2013.49.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917–31. 10.1200/JCO.2006.06.5888 [DOI] [PubMed] [Google Scholar]
- 15. Peccatori FA, Azim HA, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. AnnOncol 2013;24(suppl 6):vi160–70. 10.1093/annonc/mdt199 [DOI] [PubMed] [Google Scholar]
- 16. Martínez F, Devesa M, Coroleu B, et al. Cancer and fertility preservation: Barcelona consensus meeting. Gynecol Endocrinol 2013;29:285–91. 10.3109/09513590.2012.743019 [DOI] [PubMed] [Google Scholar]
- 17. Deshpande NA, Braun IM, Meyer FL. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: a systematic review. Cancer 2015;121:3938–47. 10.1002/cncr.29637 [DOI] [PubMed] [Google Scholar]
- 18. Lambertini M, Di Maio M, Pagani O, et al. The BCY3/BCC 2017 survey on physicians' knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast 2018;42:41–9. 10.1016/j.breast.2018.08.099 [DOI] [PubMed] [Google Scholar]
- 19. Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril 2010;94:149–55. 10.1016/j.fertnstert.2009.03.028 [DOI] [PubMed] [Google Scholar]
- 20. Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in national comprehensive cancer network institutions. J Natl Cancer Inst 2013;105:104–12. 10.1093/jnci/djs506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagliato DdeM, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014;32:735–44. 10.1200/JCO.2013.49.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2016;2:322–9. 10.1001/jamaoncol.2015.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baynosa J, Westphal LM, Madrigrano A, et al. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg 2009;209:603–7. 10.1016/j.jamcollsurg.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 24. Lee S, Ozkavukcu S, Heytens E, et al. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol 2010;28:4683–6. 10.1200/JCO.2010.30.5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chien AJ, Chambers J, Mcauley F, et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II–III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat 2017;165:151–9. 10.1007/s10549-017-4288-3 [DOI] [PubMed] [Google Scholar]
- 26. Letourneau JM, Sinha N, Wald K, et al. Random start ovarian stimulation for fertility preservation appears unlikely to delay initiation of neoadjuvant chemotherapy for breast cancer. Hum Reprod 2017;32:2123–9. 10.1093/humrep/dex276 [DOI] [PubMed] [Google Scholar]
- 27. Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep 1979;63:1727–33. [PubMed] [Google Scholar]
- 28. Buzdar AU, Smith TL, Powell KC, et al. Effect of timing of initiation of adjuvant chemotherapy on disease-free survival in breast cancer. Breast Cancer Res Tr 1982;2:163–9. 10.1007/BF01806452 [DOI] [PubMed] [Google Scholar]
- 29. Cold S, Düring M, Ewertz M, et al. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG). Br J Cancer 2005;93:627–32. 10.1038/sj.bjc.6602734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jara Sánchez C, Ruiz A, Martín M, et al. Influence of timing of initiation of adjuvant chemotherapy over survival in breast cancer: a negative outcome study by the Spanish Breast Cancer Research Group (GEICAM). Breast Cancer Res Treat 2007;101:215–23. 10.1007/s10549-006-9282-0 [DOI] [PubMed] [Google Scholar]
- 31. Shannon C, Ashley S, Smith IE. Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol 2003;21:3792–7. 10.1200/JCO.2003.01.073 [DOI] [PubMed] [Google Scholar]
- 32. Colleoni M, Bonetti M, Coates AS, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. J Clin Oncol 2000;18:584–90. 10.1200/JCO.2000.18.3.584 [DOI] [PubMed] [Google Scholar]
- 33. Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 2006;24:4888–94. 10.1200/JCO.2005.01.6089 [DOI] [PubMed] [Google Scholar]
- 34. Raphael MJ, Biagi JJ, Kong W, et al. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2016;160:17–28. 10.1007/s10549-016-3960-3 [DOI] [PubMed] [Google Scholar]
- 35. Sanford RA, Lei X, Giordano SH, et al. Impact of delayed neoadjuvant systemic chemotherapy on survival outcomes in breast cancer patients. J Clin Oncol 2016;34(15_suppl):1038–38. 10.1200/JCO.2016.34.15_suppl.1038 [DOI] [Google Scholar]
- 36. Sebai ME, Psoter KJ, Gilmore RC, et al. Survival outcomes of neoadjuvant chemotherapy timing start in relation to date of diagnosis and surgery in cases of breast cancer. J Am Coll Surg 2017;225:S25–S26. 10.1016/j.jamcollsurg.2017.07.035 [DOI] [Google Scholar]
- 37. Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network Quality Measures. J Clin Oncol 2008;26:3631–7. 10.1200/JCO.2008.16.5068 [DOI] [PubMed] [Google Scholar]
- 38. Hartman EK, Eslick GD. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat 2016;160:347–60. 10.1007/s10549-016-3989-3 [DOI] [PubMed] [Google Scholar]
- 39. Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst 2018;110:426–9. 10.1093/jnci/djx206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambertini M, Martel S, Campbell C, et al. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2-positive early breast cancer: analysis from the NeoALTTO (big 1-06) and ALTTO (big 2-06) trials. Cancer 2019;125 10.1002/cncr.31784 [DOI] [PubMed] [Google Scholar]