Abstract
Studies in metastatic melanoma, non-small-cell lung carcinoma and renal cell carcinoma indicate certain bacteria within the gut microbiota enhance clinical responses to checkpoint blockade.
Cancer immunotherapy refers to a spectrum of strategies that empower the patient’s own immune system to attack their cancer. In particular, immunotherapeutic antibodies that block tumors from activating inhibitory pathways, also called checkpoints, involving T cells are now approved for use in nine different cancer types and in mismatch repair–deficient (MMRd) cancer of any histology. These therapies have enabled some patients with advanced cancer to move from near death to health; more often, cancer immunotherapy has a less dramatic but life-prolonging effect in patients with metastatic cancer. However, only 10–30% of treated patients respond to currently available immunotherapies1. Four recently published papers2–5 present tantalizing data showing that bacterial members of the colon microbiota can improve theoutcome of cancer immunotherapy for patients through impacting the immune system, and these may be actionable targets for therapy.
The human colon carries about 500–1,000 unique bacterial strains. This vast personal collection of bacteria is termed the microbiota (for the bacterial strains) or microbiome (for the genetic content of these bacteria). Although many details remain unknown, the available mouse and human data suggest that this complex community is vital for many aspects of health, including physiology, resistance to disease and digestion, among others. In parallel, we have come to understand that the colon microbiome of individuals with disease, such as inflammatory bowel disease, obesity or diabetes, is disrupted in comparison with that of unaffected individuals. Some mouse experiments suggest a causal role for the microbiota in disease acquisition, whereas there is meager data showing which members of the microbiota induce, slow or accelerate disease in humans and how they do so6. Importantly, translation of our current rather rudimentary knowledge into microbiota-based products that improve human health and prevent or treat disease is a large hole in our medical armamentarium.
These four recent studies build on prior proof-of-principle experiments, predominantly in mice, that suggested that colon microbiome composition impacts an individual’s response to cyclophosphamide, platinum drugs and immunotherapies, such as anti-CTLA4 or anti-PD-L1 antibodies, but substantive data in humans are lacking7–10. Three of the recent studies3–5 focused on metastatic melanoma; in total, about 110 patients who were mostly treated with anti-PD-1 (and a smaller subset with anti-CTLA4) were studied. The final report studied the microbiome of individuals with non-smallcell lung carcinoma (NSCLC; 60 patients) and renal cell carcinoma (40 patients) who were treated with anti-PD-1, and additional patients (including some with uroepithelial cancers) were analyzed to determine the impact of antibiotic exposure on outcomes to checkpoint blockade2.
In each study, the authors identified specific bacterial orders, families, genera or species in stool associated with improved outcomes in immunotherapy-treated patients, as well as bacteria genera that seemed to be associated with less favorable outcomes upon immunotherapy (Fig. 1). Specifically, both general microbiome features, such as higher alpha diversity or richness, which imply more types of bacteria were present, and members of each of the major bacterial phyla (or lineages) colonizing humans, namely Firmicutes, Actinobacteria, Proteobacteria, Bacteroidetes and Verrucomicrobia, were implicated in responses to immunotherapy. Gut bacteria associated with a negative response to immunotherapy seemed to be less diverse, with Bacteroides (phylum Bacteroidetes), Ruminococci and Roseburia (both Firmicutes) being prominent. Notably, sequencing and analytical approaches differed between papers, and even within a single disease, namely metastatic melanoma, the bacteria or communities that correlated with improved immunotherapeutic responses differed markedly between patients treated with anti-PD-1 antibodies with identical activity. Nonetheless, considering these data and others, Ruminococci, Bifidobacteria, Enterococci and Akkermansia may represent reproducible threads for improving immunotherapy responses, with Bacteroides possibly impeding responses.
Figure 1.
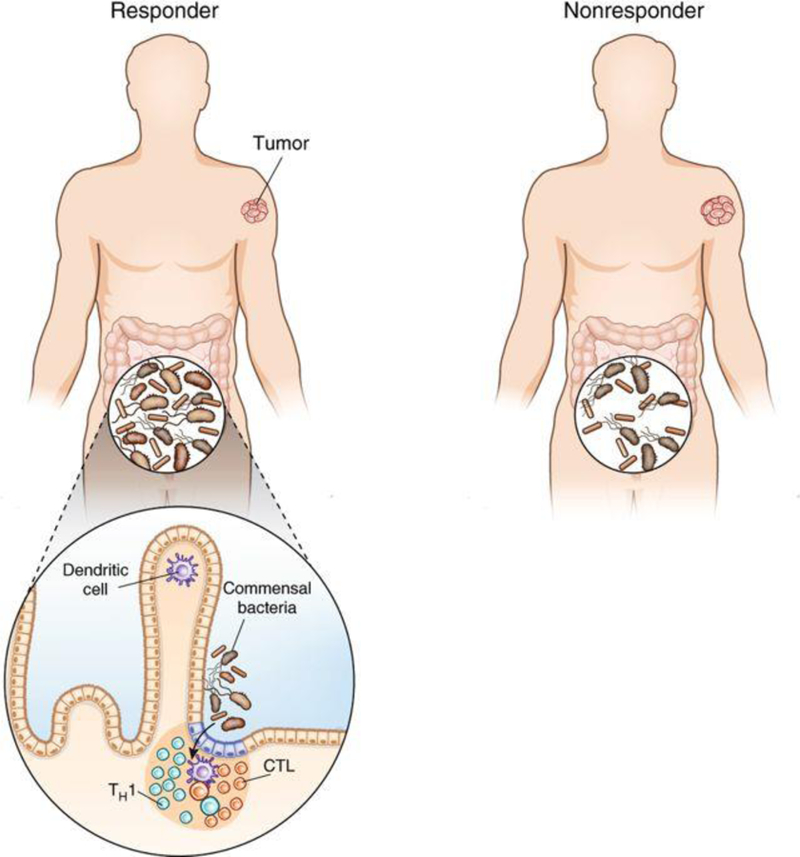
Colon microbiota may contribute to checkpoint-blockade response or nonresponse in patients with melanoma or epithelial cancers. Three studies2–4 show that increased gut microbiota diversity and the presence of certain bacterial genera are linked to an improved outcome following immunotherapy. One possible mechanism is that select mucosal and systemic immune responses, particularly TH1 (IFN-γ-producing CD4+ cells) and cytotoxic T lymphocyte (IFN-γ-producing CD8+ lymphocytes) responses, are induced by the microbiome across the gut barrier and hence may predict clinical response.
Proving causality beyond association in the human setting and further defining the quality of the microbial-induced systemic antitumor immune response is not easy, but the three studies by Routy et al.2, Gopaladrishnan et al.3 and Matson et al.4 make a good start. The fundamental approach taken by each group was to orally colonize germ-free mice or antibiotictreated mice either with human feces (fecal microbiome transfer, FMT) from responding (R) or nonresponding (NR) patients or with bacteria associated with a clinical response. In all three studies2–4, the response of mouse tumors to mouse anti-PD-1 was generally enhanced upon colonization with R microbiota but not with NR microbiota. A wealth of previously published data indicate that successful antitumor responses are promoted through TH1 (CD4+ cells producing interferon (IFN)-γ) and cytotoxic T lymphocyte (CTL; nonexhausted CD8+ cells producing IFN-γ and exhibiting killing activity) responses and are inhibited through regulatory T cell (Treg) and TH17 responses and certain inhibitory myeloid populations in the tumor microenvironment. All three studies were able to demonstrate some changes in these relevant immune responses upon inoculation with the R bacteria. Although further experiments are needed to confirm that colonization of the species implicated in therapeutic benefit was achieved, these data take the initial steps in establishment of a link between the human gut microbiome, modulation of the immune response and response to immunotherapy.
A key mechanistic question that remains to be answered is how gut colonization with certain microbial species can amplify an immunotherapy (i.e., anti-PD-1) that enhances antitumor T cell responses. One possibility is that antigen mimicry between microbial and tumor antigens enhances the antitumor immune response. In this case, it seems that each patient would require a different spectrum of bacteria that would mimic the tumor’s unique mutation-associated neoantigenic profile to correctly amplify the immune response. It is possible, however, that immune responses induced by certain microbes crossreact with shared tumor-associated antigens rather than patient-specific neoantigens. Alternatively, owing to the constant recirculation of lymphocytes through the gut and gut-associated lymphoid tissue, certain gut microbiota may nonspecifically ‘amp up’ systemic immunity in certain patients through mechanisms such as enhanced production of cytokines by gut dendritic cells. This mechanism might also enhance autoimmune effects of PD-1 blockade, which occurs in 10–15% of patients. Indeed, recent reports demonstrate that anti-PD-1-treated patients that experience autoimmune-related effects have a better antitumor response than those who do not11.
These data should and will spur much needed additional clinical and experimental studies. First, it is necessary to determine whether select bacterial communities or species or broad microbiota descriptors can serve as robust predictors of immunotherapy outcomes if used routinely before initiation of checkpoint-blockade therapy. Further, it is critical to define whether preimmunotherapy stool evaluation is a sufficient biomarker for guidance of clinical care and to determine whether more can be learned, for example, from individuals with durable or curative responses to immunotherapy. Although antibiotics taken close to the time of initiation of checkpoint-blockade therapy were reported to predict nonresponsiveness to therapy2, we need to better understand the relationship between timing, type and indication for antibiotic use and clinical immunotherapy outcomes. Importantly, analyses seeking to determine the predictors of therapy response in the microbiota should take antibiotic exposure into consideration given the profound effect of the most commonly used antibiotics on the gut microbiota. Perhaps most importantly, we do not know whether we can use these data in developing ‘immunotherapy probiotics’ to help patients, as we do not know whether the structure and function of the microbiota can be durably altered to promote immunotherapy efficacy.
Taken together, the results from these studies indicate that a broader analysis of more patients treated with immunotherapy and analysis of immune responses in recolonized germ-free mice will be a very fruitful endeavor and will provide a new dimension for expansion of the number of patients with cancer who can respond to immunotherapy.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Topalian SL, Drake CG & Pardoll DM Cancer Cell 27, 450–461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Routy B et al. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Gopalakrishnan V et al. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matson V et al. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaput N et al. Ann. Oncol 28, 1368–1379 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Lynch SV & Pedersen ON Engl. J. Med 375, 2369–2379 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Viaud S et al. Science 342, 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iida N, et al. Science 342, 967–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vétizou M et al. Science 350, 1079–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivan A et al. Science 350, 1084–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS et al. J. Clin. Oncol 35, 785–792 (2017). [DOI] [PubMed] [Google Scholar]


