Abstract
Background
Cigarette smoking is thought to increase the risk of Crohn’s disease (CD) and exacerbate the disease course, with opposite roles in ulcerative colitis (UC). However, these findings are from Western populations, and the association between smoking and inflammatory bowel disease (IBD) has not been well studied in Asia.
Aims
We aimed to compare the prevalence of smoking at diagnosis between IBD cases and controls recruited in China, India, and the USA, and to investigate the impact of smoking on disease outcomes.
Methods
We recruited IBD cases and controls between 2014 and 2018. All participants completed a questionnaire about demographic characteristics, environmental risk factors and IBD history.
Results
We recruited 337 participants from China, 194 from India, and 645 from the USA. In China, CD cases were less likely than controls to be current smokers (adjusted odds ratio [95% CI] 0.4 [0.2–0.9]). There was no association between current or former smoking and CD in the USA. In China and the USA, UC cases were more likely to be former smokers than controls (China 14.6 [3.3–64.8]; USA 1.8 [1.0–3.3]). In India, both CD and UC had similar current smoking status to controls at diagnosis. Current smoking at diagnosis was significantly associated with greater use of immunosuppressants (4.4 [1.1–18.1]) in CD cases in China.
Conclusions
We found heterogeneity in the associations of smoking and IBD risk and outcomes between China, India, and the USA. Further study with more adequate sample size and more uniform definition of smoking status is warranted.
Keywords: Smoking, Crohn’s disease, Ulcerative colitis, Asia, Environment
Introduction
The chronic intestinal disorder inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC) [1], which are characterized by alternating active and remission phases [2]. The incidence of CD and UC increased during the twentieth century. The highest incidence of IBD is in Western countries such as North America, Northern Europe, and Australia, with CD incidence ranging from 10.6 to 29.3 per 100,000 person-years and UC incidence ranging from 17.4 to 24.3 per 100,000 person-years in 1998–2010 [3]. In comparison, population-based incidence data were not available in Asia until the 1970s, reflecting an “emerging” disease pattern in Asia [4, 5]. In Singapore [4, 5], Hong Kong [6], Japan [7], and South Korea [8], where population-based statistics are available, the CD incidence rates per 100,000 person-years have increased from 0.04–0.6 in 1970–1992 to 1.0–1.3 in 1998–2006, and UC incidence rate increased from 0.1–2.0 in 1970–1990 to 3.1 in 2001–2005.
Differences in temporal trends in IBD incidence between Asian and Western populations might suggest potential differences in the etiology of IBD between these two populations. Previous studies found that differences in genetic presence or effect of genetic risk variants might have played a different role in the pathogenesis of IBD between the two populations. For example, NOD2/CARD15 loss of function mutations have been found in population-based studies to account for up to 27% of population-attributable risk for CD among Caucasians [9–11], whereas in Asians, established NOD2 risk alleles were absent in the largest study [12]. There is also a significant difference in the prevalence of IBD family history among IBD patients. The prevalence of having any family history of IBD was reported to be 8–40% among Western IBD patients [13–15], but only 0–4% among Asian IBD population [6, 15–17]. Environmental risk factors including smoking, appendectomy, hygiene, infections, antibiotics, other medications, diet, and other lifestyle factors have been studied in the Western population, but have been studied to a much more limited extent in Asian IBD patients [18]. The increasing incidence of IBD around the globe, especially in historically less prevalent regions, makes it important to compare the influence of environmental factors in different populations.
Cigarette smoking has been described as having discordant roles in CD and UC based on numerous observational studies since the late 1970s and early 1980s. In 1978, Mayberry et al. [19] reported that CD patients were more likely to smoke on or after diagnosis than controls. In contrast, UC patients were less likely to be current smokers and more likely to be former smokers on or after diagnosis compared with controls, as first reported by Harries et al. [20]. The 2014 United States Surgeon General’s Report on Smoking gave a causality assessment between smoking and IBD as suggestive but not sufficient [21]. This assessment was based on 53 studies of CD versus controls (RR = 1.6 [1.5–1.8]) and 61 studies of UC (RR = 0.54 [0.45–0.70]). Some inconsistency was seen when restricting to the 14 Asian studies where the researchers reported that both CD and UC cases were less likely to be current smokers than controls (CD: RR = 0.8 [0.6–1.1], N studies = 8; UC: RR = 0.4 [0.3–0.6], N studies = 13).
Our hypothesis was that differences might exist in the association of smoking and IBD between Asian and Western populations. To address this hypothesis, we targeted two specific goals. The first was to compare the prevalence of smoking at diagnosis between CD and UC cases and controls recruited in China, India, and the USA. The second was to investigate the impact of smoking on disease outcomes of CD and UC among the three populations, including medications and IBD-related surgeries.
Materials and Methods
Study Population
We conducted a case–control study among participants in China, India, and the USA. We recruited CD and UC cases and their friend or family controls from one source in China, one source in India, and three sources in the USA. In China, cases were diagnosed by gastroenterologists at the IBD clinic of the Sixth Affiliated Hospital of Sun Yat-sen University in Guangdong Province. The clinic was established in 2012. In India, cases were seen by gastroenterologists at the IBD clinic of Asian Institute of Gastroenterology in Hyderabad, established in 2004. In the USA, cases included those seen at The Johns Hopkins Hospital Meyerhoff IBD Center—affiliated gastroenterology clinics, at University of California, Irvine, and those who self-identified as CD or UC patients recruited through ResearchMatch. Research- Match is a disease-neutral, institution-neutral, online volunteer recruitment platform designed to match volunteers with researchers [22]. Individuals without IBD also participated in the study through ResearchMatch.
All participants completed a questionnaire administered by face-to-face interview with a healthcare professional or self-administered online (bit.ly/IBD-MIMAS) using REDCap [23]. The questionnaire inquired about demographic characteristics, smoking history, family history, IBD diagnosis, disease location, and treatment history. Participants were included in this analysis only if they completed the modules about smoking and IBD and were 18 years or older at the time of questionnaire completion. The questionnaire was presented in English for participants in India and the USA and in Mandarin for participants in China.
Assessment of Smoking Status and Quantity
Current/Former/Never
Both cases and controls self-reported the details of their active and passive smoking history. Our questionnaire asked about active smoking in several ways: a direct question “what was your smoking status at the time of CD/UC diagnosis”; start and stop year of smoking; and average packs smoked per week during different age periods (before 12 years old, 13–17, 18–25, 26–39, and 40 years or older). For cases, smoking status at IBD diagnosis was determined based on the relationship between start and stop year of smoking and the year of IBD diagnosis and categorized into current, former, and never smoker at diagnosis. In addition to start and stop year of smoking, the questionnaire directly asked cases their smoking status at the time of clinical diagnosis of IBD, at 6 months prior to diagnosis, and at the time of IBD symptom onset. For controls, smoking status was determined at the age equivalent to cases’ median age of diagnosis per country, in order to make the smoking status comparable between cases and controls. The questionnaire asked whether the participant ever spent a large portion of the day with someone who smoked or used tobacco products, which we used as the indicator for ever exposure to passive smoking.
Cumulative Packs
We calculated the cumulative packs of cigarettes smoked up to the age of IBD diagnosis for cases and up to the comparable age for controls (equivalent to cases’ median age of diagnosis). Based on the year of birth and the start and stop year of smoking, we were able to calculate the number of smoking years during each of the five age periods of interest, which we then multiplied by the average packs smoked per year (the reported average packs smoked per week times a factor of 52 weeks per year) during the corresponding age period to get the total number of packs smoked during that age period. We estimated the cumulative packs of cigarettes smoked by summing packs smoked during each age period up to the age of IBD diagnosis (and equivalent for controls) or the age when participant quit smoking, whichever came first. The cumulative packs were analyzed as a categorical variable containing five levels: < 0, > 0 and ≤ 200, > 200 and ≤ 400, > 400 and ≤ 1000, and > 1000 packs.
Other Tobacco-Containing Products
The questionnaire asked about ever use of tobacco products other than cigarettes, including shisha, hookah, beedi, cigars, electronic cigarette, vaping, and chewed tobacco.
Assessment of Disease Outcomes of Crohn’s Disease and Ulcerative Colitis
CD and UC cases were asked questions about their disease course and treatment history. The history of surgery and medication use was evaluated for all cases based on their self-recall. Surgical history was also categorized as a binary variable depending whether or not a CD/UC case ever had any surgery for CD/UC up to the time of questionnaire. Medication history of interest included ever use of immunosuppressants (including 6-mercaptopurine/azathioprine, methotrexate, cyclosporine, and tacrolimus) and biologic therapy (including infliximab, adalimumab, certolizumabpegol, golimumab, natalizumab, vedolizumab, or ustekinumab) for both CD and UC cases.
Potential Confounding Factors
Participants self-reported sex, date of birth, date of IBD diagnosis, and IBD family history. We defined the duration of disease as the time period between IBD diagnosis and questionnaire completion. Smoking history differs between men and women, and sex is hypothesized to be associated with IBD risk and outcomes. Thus, we considered sex as a potential confounder in the association of smoking with development of CD or UC and its outcomes. Other confounders for the IBD outcomes included age at diagnosis, family history of IBD, and disease duration.
Statistical Analysis
We analyzed the association of smoking and CD and UC by country of recruitment. In the primary analysis, we included all cases and controls, regardless of the matching status. CD and UC cases were compared with all controls. We used logistic regression models to calculate unadjusted and sex adjusted odds ratios (aOR) and 95% confidence intervals (CI) to examine the association of active smoking (current, former, vs. never smoking at diagnosis), passive smoking (ever vs. never), cumulative packs of cigarettes smoked (> 0 and ≤ 200, > 200 and ≤ 400, > 400 and ≤ 1000, and > 1000 vs. 0 packs), and other tobacco-containing products with CD and UC. We examined whether sex or age (≤ 25 vs. > 25 years old at diagnosis) modified the association of smoking and CD or UC. We conducted a secondary analysis including only matched case-control pairs (case matched to their friend or family control) using conditional logistic regression. We included only cases to analyze the effect of smoking at diagnosis on CD and UC disease outcomes. We examined the impact of smoking on the need for surgery and medications such as immunosuppressants and biologic therapy, adjusting for sex, age at diagnosis, family history of IBD, and disease duration at the time of questionnaire completion. To test the robustness of the results to the assumption that country of recruitment was the most important factor to stratify by, we stratified the primary analysis by country of birth, country of residence at diagnosis, and race and the results were not qualitatively different compared with analysis by country of recruitment (data not shown). We conducted all statistical analyses with SAS® 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethical Statement
This study is approved by the Institutional Review Boards of Sun Yat-sen University, Asian Institute of Gastroenterology, Johns Hopkins University, and University of California, Irvine. Participants’ completion of the questionnaire served as their consent to be in the research study.
Results
Participant Demographics
From April 2014 to March 2018, we recruited 337 participants (CD = 113, UC = 51, Control = 173) from the Sixth Affiliated Hospital of Sun Yat-sen University in China and 194 participants (CD = 25, UC = 65, Control = 104) from the Asian Institute of Gastroenterology in India. From May 2015 to March 2018, we recruited 645 participants (CD = 192, UC = 151, Control = 302) from Johns Hopkins, University of California, Irvine, and ResearchMatch Web site in the USA. US cases were more likely to be female, have a family history of IBD, and have a longer disease duration at the time of questionnaire than the China and India cases (Table 1). In China and the USA, CD cases were diagnosed at a younger age than UC, whereas in India CD and UC cases had a similar median age of diagnosis.
Table 1.
Demographic characteristics of inflammatory bowel disease (IBD) cases and controls by country of recruitment
| Crohn’s disease |
Ulcerative colitis |
Control |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| China n = 113 | India n = 25 | USA n = 192 | China n = 51 | India n = 65 | USA n = 151 | China n = 173 | India n = 104 | USA n = 302 | |
| Female | 38.1% | 24.0% | 62.5% | 60.8% | 44.6% | 67.6% | 49.1% | 23.1% | 71.5% |
| Age at questionnaire | 27.5 | 29.8 | 37.1 | 34.3 | 33.5 | 42.5 | 29.1 | 45.7 | 36.8 |
| Year, median, min–max | 18–50 | 18–65 | 18–82 | 19–60 | 18–65 | 20–76 | 18–67 | 20–74 | 18–76 |
| Duration of IBD at questionnaire | 1.9 | 1.5 | 11.0 | 2.0 | 1.9 | 8.7 | N/A | N/A | N/A |
| Year, median, min-max | 0–12.7 | 0–14.2 | 0–49.0 | 0–12.0 | 0–17.6 | 0–46.3 | |||
| Age at IBD diagnosis | 26.0 | 28.4 | 25.0 | 32.0 | 28.5 | 29.2 | N/A | N/A | N/A |
| Year, median, min–max | 13–77 | 17–51 | 5–73 | 16–56 | 15–64 | 10–70 | |||
| ≤12 | 0 | 0 | 5.2% | 0 | 0 | 3.3% | |||
| 13–17 | 9.7% | 4.0% | 15.6% | 2.0% | 4.6% | 5.3% | |||
| 18–25 | 40.7% | 24.0% | 33.3% | 29.4% | 36.9% | 31.1% | |||
| 26–39 | 40.7% | 64.0% | 28.7% | 37.2% | 33.9% | 37.8% | |||
| ≥40 | 8.9% | 8.0% | 17.2% | 31.4% | 24.6% | 22.5% | |||
| Calendar year of IBD diagnosis | 2015 | 2015 | 2005 | 2014 | 2014 | 2006 | N/A | N/A | N/A |
| Median, min–max | 2003–2016 | 2002–2017 | 1966–2016 | 2003–2017 | 1997–2017 | 1969–2016 | |||
| ≤1970 | 0 | 0 | 1.0% | 0 | 0 | 0.7% | |||
| 1971–1980 | 0 | 0 | 7.3% | 0 | 0 | 4.0% | |||
| 1981–1990 | 0 | 0 | 10.4% | 0 | 0 | 10.6% | |||
| 1991–2000 | 0 | 0 | 18.8% | 0 | 3.1% | 14.6% | |||
| 2001–2010 | 13.3% | 12.0% | 36.5% | 7.8% | 18.5% | 37.1% | |||
| ≥2011 | 86.7% | 88.0% | 26.0% | 92.2% | 78.4% | 33.1% | |||
| Any family history of IBD | 7.1% | 12.0% | 40.1% | 3.9% | 7.7% | 40.4% | N/Aa | N/A | N/A |
| Proportion matched (N matched/ total)b | 107/113 | 25/25 | 55/219 | 46/51 | 62/65 | 39/151 | 154/173 | 97/104 | 125/302 |
| Relationship to case | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Parent | 1.2% | 30.8% | 4.0% | ||||||
| Offspring | 2.3% | 5.8% | 4.3% | ||||||
| Sibling | 76.3% | 17.3% | 15.6% | ||||||
| Partner/spouse | 4.6% | 29.8% | 14.2% | ||||||
| Other relative | 1.7% | 6.7% | 0.7% | ||||||
| Friend | 2.9% | 2.9% | 2.6% | ||||||
| Independently recruited | 11.0% | 6.7% | 58.6% | ||||||
The prevalence of IBD family history for controls is not listed, as it would not reflect the prevalence of family history among individuals without inflammatory bowel disease in the source or target population, due to the recruiting mechanism of the present study where cases refer their family or friend without IBD to serve as controls
All participants contributed to the main analysis. The subset of matched case-control pairs contributed to the match case–control analysis
Smoking at Diagnosis (Fig. 1)
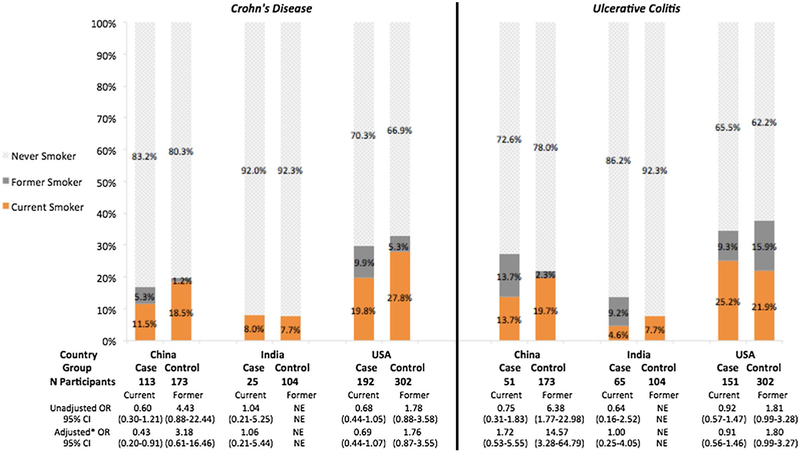
Fig. 1.
Prevalence of current, former, and never smoking at diagnosis and association of smoking status with Crohn’s disease and ulcerative colitis by country of recruitment. *Adjusted for sex. OR, odds ratio; NE, not estimable. The reference group of the unadjusted and adjusted ORs consisted of never smokers at diagnosis. The associations of former smoking and CD and UC were not estimable for India due to absolute absence of former smoking CD cases and controls
In China, CD cases were less likely relative to country-matched controls to be current smokers (aOR [95% CI]: 0.4 [0.2–0.9]). There was no association between current or former smoking and CD in the USA (current: 0.7 [0.4–1.1]; former: 1.8 [0.9–3.6]). In China and the USA, UC cases were more likely to be former smokers than controls (China 14.6 [3.3–64.8]; USA 1.8 [1.0–3.3]). In India, both CD and UC had similar current smoking status to controls at diagnosis (CD: 1.1 [0.2–5.4], UC: 1.0 [0.25–4.05]). There were very few former smokers in India (0 CD, 6 UC, 0 control). In the matched case-control analysis, the effect estimates for the association between smoking and CD and UC were similar, except for wider confidence intervals due to a decrease in sample size (data not shown). When we compared the direct query of smoking at diagnosis versus the calculation based on start and stop year of smoking the results were similar.
Passive Smoking (Appendix Table 1)
Table 2.
The prevalence of ever exposure to passive smoking and its association with Crohn’s disease and ulcerative colitis
| Passive smoking | Crohn’s disease |
Ulcerative colitis |
||||||
|---|---|---|---|---|---|---|---|---|
| Case (%) | Control (%) | Unadjusted OR, 95% CI |
Adjusteda OR, 95% CI |
Case (%) | Control | Unadjusted OR, 95% CI |
Adjusteda OR, 95% CI |
|
| China | ||||||||
| Ever | 62.0 | 54.3 | 1.37 (0.84–2.22) | 1.40 (0.85–2.29) | 62.8 | 54.3 | 1.42 (0.75–2.69) | 1.48 (0.75–2.90) |
| Never | 38.0 | 45.7 | 1 | 1 | 37.2 | 45.7 | 1 | 1 |
| India | ||||||||
| Ever | 12.0 | 8.6 | 1.44 (0.36–5.76) | 1.53 (0.33–7.09) | 7.7 | 8.6 | 0.88 (0.28–2.75) | 1.04 (0.29–3.66) |
| Never | 88.0 | 91.4 | 1 | 1 | 92.3 | 91.4 | 1 | 1 |
| USA | ||||||||
| Ever | 51.0 | 56.0 | 0.82 (0.57–1.18) | 0.87 (0.60–1.28) | 48.3 | 56.0 | 0.74 (0.50–1.09) | 0.69 (0.46–1.04) |
| Never | 49.0 | 44.0 | 1 | 1 | 51.7 | 44.0 | 1 | 1 |
Adjusted for active smoking status at IBD diagnosis and sex
Adjusting for active smoking status at diagnosis and sex, ever exposure to passive smoking was associated with lower odds of UC in the USA with borderline significance (0.7 [0.5–1.0]). There were no statistically significant associations between passive smoking and CD or UC in China or India.
Cumulative Packs of Cigarettes (Appendix Table 2)
Table 3.
The distribution of cumulative packs of cigarettes smoked at diagnosis and its association with Crohn’s disease and ulcerative colitis by country of recruitment
| Cumulative packs (CP) |
Crohn’s disease |
Ulcerative colitis |
||||||
|---|---|---|---|---|---|---|---|---|
| Case (N = 170) (%) |
Control (N = 277) (%) |
Unadjusted OR, 95% CI |
Adjusted* OR, 95% CI |
Case (N = 137) (%) |
Control (N = 264) (%) |
Unadjusted OR, 95% CI |
Adjusted* OR, 95% CI |
|
| USA | ||||||||
| CP = 0 | 79.4 | 72.9 | Ref | Ref | 68.6 | 75.4 | Ref | Ref |
| 0 < CP ≤ 200 | 1.2 | 2.5 | 0.43 (0.09–2.09) | 0.38 (0.08–1.90) | 5.8 | 4.6 | 1.41 (0.56–3.57) | 1.38 (0.54–3.50) |
| 200 < CP ≤ 400 | 7.7 | 10.1 | 0.70 (0.35–1.39) | 0.72 (0.36–1.44) | 6.6 | 3.4 | 2.12 (0.81–5.51) | 2.15 (0.83–5.60) |
| 400 < CP ≤ 1000 | 2.4 | 8.7 | 0.25 (0.09–0.74) | 0.27 (0.09–0.78) | 9.5 | 6.8 | 1.53 (0.72–3.25) | 1.54 (0.72–3.27) |
| CP > 1000 | 9.4 | 5.8 | 1.50 (0.72–3.09) | 1.56 (0.75–3.23) | 9.5 | 9.8 | 1.06 (0.52–2.15) | 1.06 (0.52–2.16) |
| Test for trend** | p = 0.03 | p = 0.01 | p = 0.45 | p = 0.55 | ||||
| Cumulative packs (CP) |
Crohn’s disease |
Ulcerative colitis |
||||||
| Case (N = 102) (%) |
Control (N = 148) (%) |
Unadjusted OR, 95% CI |
Adjusted* OR, 95% CI |
Case (N = 45) (%) |
Control (N = 146) (%) |
Unadjusted OR, 95% CI |
Adjusted* OR, 95% CI |
|
| China | ||||||||
| CP = 0 | 92.2 | 93.9 | Ref | Ref | 82.2 | 92.5 | Ref | Ref |
| 0 < CP ≤ 200 | 1.0 | 0.7 | 1.48 (0.09–23.93) | 1.06 (0.07–17.38) | 0 | 0.7 | NE | NE |
| 200 < CP ≤ 400 | 3.9 | 1.3 | 2.96 (0.53–16.47) | 2.12 (0.37–12.07) | 0 | 1.4 | NE | NE |
| 400 < CP ≤ 1000 | 0 | 0.7 | NE | NE | 2.2 | 1.4 | 1.82 (0.16–20.68) | 4.17 (0.33–53.12) |
| CP > 1000 | 2.9 | 3.4 | 0.89 (0.21–3.80) | 0.64 (0.14–2.80) | 15.6 | 4.1 | 4.26 (1.35–13.44) | 9.72 (2.44–38.67) |
| Test for trend** | p = 0.65 | p = 0.17 | p = 0.09 | p = 0.02 | ||||
| Cumulative packs (CP) |
Crohn’s disease |
Ulcerative colitis |
||||||
| Case (N = 24) (%) |
Control (N = 102) (%) |
Unadjusted OR, 95% CI |
Adjusted* OR, 95% CI |
Case (N = 63) (%) |
Control (N = 102) (%) |
Unadjusted OR, 95% CI |
Adjusted* OR, 95% CI |
|
| India | ||||||||
| CP = 0 | 95.8 | 94.1 | Ref | Ref | 88.9 | 94.1 | Ref | Ref |
| 0 < CP ≤ 200 | 0 | 1.0 | NE | NE | 4.8 | 1.0 | 5.14 (0.52–50.63) | 8.00 (0.80–80.27) |
| 200 < CP ≤ 400 | 0 | 3.9 | NE | NE | 3.2 | 3.9 | 0.86 (0.15–4.83) | 1.33 (0.23–7.70) |
| 400 < CP ≤ 1000 | 0 | 0 | NE | NE | 1.6 | 0 | NE | NE |
| CP > 1000 | 4.2 | 1.0 | 4.17 (0.25–69.25) | 4.23 (0.25–71.18) | 1.6 | 1.0 | 1.71 (0.11–27.95) | 2.67 (0.16–44.15) |
| Test for trend** | p = 0.49 | p = 0.66 | p = 0.38 | p = 0.01 | ||||
OR odds ratio, NE not estimable
Converting CPs to pack-year: 200 CPs ≈ 0.55 pack-year; 365 CP = 1 pack-year, 400 CPs ≈ 1.09 pack-years; 1000 CPs ≈ 2.74 pack-years
Adjusted for sex
P value for trend was estimated by the score of the global null hypothesis in the logistic models, equivalent to Cochran–Armitage trend test
Compared with controls, CD cases in the USA were less likely to have smoked 400–1000 packs of cigarettes before diagnosis (0.3 [0.1–0.8]), but more likely to have smoked over 1000 packs of cigarettes (1.6 [0.8–3.2]). UC cases in China were more likely to have smoked over 1000 packs than controls before diagnosis (9.7 [2.4–38.7]).
Use of Tobacco Products Other Than Cigarettes
In China, one control reported use of shredded tobacco for water pipes. In India, 40.0% (4/10) of ever-smoking controls and 5.3% (5/94) of never-smoking controls reported use of other tobaccos, and no CD or UC cases reported using other tobacco products. In the USA, the prevalence of other tobacco products was similar among ever smokers: 21.1% (12/57) for CD cases, 21.1% (12/57) for UC cases, and 23.0% (23/100) for controls. Use of other tobacco products was rare among never smokers: 5.2% (7/135) for CD cases, 4.3% (4/94) for UC cases, and 6.4% (10/202) for controls.
Smoking and IBD Outcomes (Fig. 2)
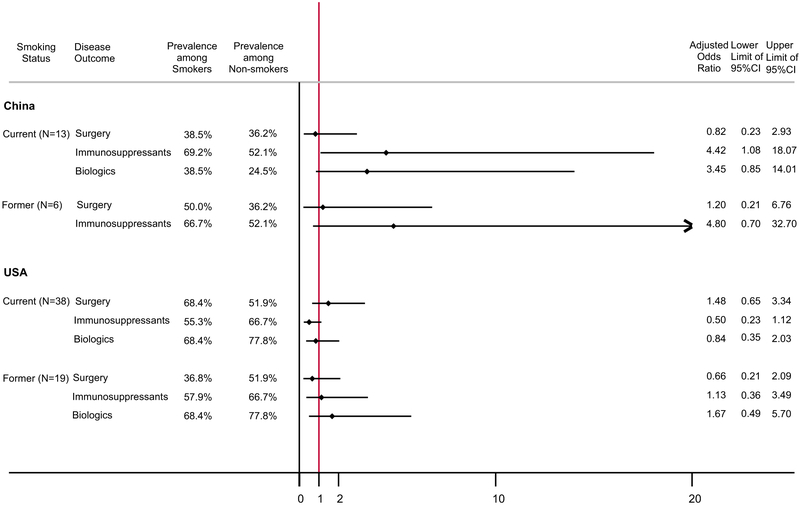
Fig. 2.
The prevalence of Crohn’s disease outcomes among current and former smokers at diagnosis compared with never smokers, by country of recruitment. The reference for the current and former smoker groups consisted of never-smoking Crohn’s disease cases (China: N = 94; USA: N = 135). Odds ratios were adjusted for sex, age at diagnosis, family history of IBD, and duration of disease at questionnaire. All outcomes were based on patient self-report at the time of the questionnaire. India not shown due to limited number of outcomes
Current smoking at diagnosis was significantly associated with greater use of immunosuppressants (4.4 [1.1–18.1]) in China among CD patients. Smoking was not significantly associated with other outcomes of CD or UC. The comparison of the association of smoking and UC outcomes between the three countries was not shown because of limited number of current and former smokers and lack of outcome events in the smoking groups in China and India. For UC cases in China, 1 of the 7 current smokers used immunosuppressants and none had surgery for IBD or biologics use; none of the 7 former smokers had surgery or immunosuppressant or biologics use.
Quitting Smoking and CD Outcomes
In China, 5 out of 13 CD cases were current smokers at the time of diagnosis quit smoking within 1 year after their diagnosis. In India and the USA, 2 out of 2 and 4 out of 38 current smokers quit smoking within 1 year after their diagnosis. In China, CD cases who quit smoking had lower rates of surgery and immunosuppressant use than those who did not quit within 1 year of diagnosis (surgery: 20 vs. 50%; immunosuppressant use: 60 vs. 75%; biologics use: 40 vs. 38%). In the USA, CD cases who quit smoking had lower rates of immunosuppressant and biologics use than those who did not quit within 1 year of diagnosis (surgery: 100 vs. 65%; immunosuppressant use: 50 vs. 56%; biologics use: 50 vs. 71%).
Discussion
There were differences in the association between smoking and IBD risk and outcomes in China, India, and the USA. In China, CD cases were less likely to be current smokers compared with controls. There was no association between current or former smoking and CD in the USA. In both China and the USA, UC cases were more likely to be former smokers than controls. In India, however, both CD and UC cases had similar smoking status at diagnosis to controls. Current smoking at diagnosis was significantly associated with greater use of immunosuppressant in CD cases in China but we did not find a significant association in the USA. For UC cases, smoking did not show any significant impact on the need for immunosuppressants, biologics, and surgery.
The impact of smoking on CD and UC has not been widely studied in Asia. Our findings in participants from China and India were partly similar to the association reported in the 2014 Surgeon General’s Report on the Health Consequences of Smoking [21]. That report identified five Asian studies that assessed smoking on or before diagnosis and adjusted for at least one potential confounding factor and found current smoking was associated with a decreased risk of both CD and UC in China, Iran, and Japan (CD: RR [95% CI] 0.5 [0.2–1.0], N studies = 2, total N cases = 71; UC: 0.3 [0.2–0.5], N studies = 5, total N cases = 1043) [21]. Our findings were also partly similar to those in a case-control study published in 2015, which studied 374 incident IBD cases and 789 neighborhood controls in 8 regions in Asia, which found that former smoking was associated with an increased risk of UC in Asians (aOR [95% CI] 2.0 [1.2–3.4]), while neither current nor former smoking was significantly associated with CD [24]. Two other Chinese studies also found former smoking to be a risk factor for UC [25]. Few Asian studies have examined the impact of smoking on IBD outcomes. A Korean study of 728 CD patients found that current and former smoking at diagnosis were associated with higher risk of the initial CD-related surgery (HR [95% CI]: current 1.9 [1.1–3.1]; former 1.8 [1.0–3.2]) [26]. A study of Jewish patients (261 CD, 273 UC and 430 controls) in Israel found no association between smoking and CD, as well as higher proportion of current smokers and lower proportion of ex-smokers in UC cases compared with controls [27]. Another study of 33 CD, 54 UC, and144 controls in Israel found the presence of current smokers was lower in CD than UC and no statically significant association between smoking and IBD [28].
We found that ever exposure to passive smoking was associated with lower odds of UC in the USA with borderline significance and no association between passive smoking and UC or CD in China and India. An Israeli study of 273 UC cases, 261 CD cases, and 430 controls also reported lack of association between passive smoking and IBD in Jewish population whereas UC patients quantitatively had less exposure to passive smoking than controls [29].
In our study, the associations between smoking and CD and UC outcomes were not statistically significant except for higher rate of immunosuppressant use in current smoking CD cases in China. This finding was consistent with a study that includes 330 IBD patients from eight Asian countries and 83 from Australia, which also found increased use of immunosuppressants in current smokers and no association between smoking and biologics use or surgery [30]. A recent study of 426 Indian CD patients found no association between smoking or oral tobacco use and disease outcomes [31]. A Japanese study of 520 CD cases found that current smoking was associated with greater need for surgical treatment compared with former and never smoking [32].
Many Western studies found current smoking to increase the risk and exacerbate the disease course of CD and to have the opposite impacts on UC [18, 21, 33]. However, we did not find statistically significant association between smoking and disease outcomes of CD and UC in the USA cases. There are studies that reported findings similar to ours in Western populations. For example, two studies found no statistically significant association between smoking and the risk of CD among 173 CD cases and 208 controls recruited from the Crohn’s and Colitis Foundation of America [34] and among 327 CD and 779 controls in Denmark [35]. Two large (408 and 1170 CD cases) European studies suggested a lack of association between smoking and disease location, the development of stricturing or penetrating behaviors, and the need for surgery [36, 37]. Another European study reported that smoking did not increase the risk of surgery and the prevalence of penetrating or stricturing behaviors among CD patients who were treated with immunosuppressive therapy [38, 39]. Early initiation and widespread use of immunosuppressants and biologics in our study might also obscure the impact of smoking on the need for surgery.
Differences in smoking definitions might explain the heterogeneity between our findings and some previous studies. The varying definitions of smoking have long been a concern for interpreting and comparing studies on smoking [40]. The majority of previous studies did not explicitly define controls’ smoking status “at diagnosis,” and instead they assessed controls’ smoking status at the time of survey completion or study enrollment [21], which were not biologically meaningful time points and lacked comparability to cases’ time at diagnosis. In our study, we asked detailed questions about smoking history, such as the start and stop year of smoking, and smoking in different age periods, and therefore we were able to assess controls’ smoking status at an age that was equivalent to cases’ median age of diagnosis. This increased the comparability of the smoking status of cases and controls, which was the basis for valid comparisons.
As the first study to compare the association of smoking and IBD between specific Asian and Western countries (China, India, and USA), our study contributes to the current knowledge about the impact of smoking on the occurrence and development of IBD, especially among Asian populations. Our study has a few limitations. The collection of information on environmental exposures and disease outcomes at the same time by way of questionnaire may render the study prone to recall bias. We tried to mitigate the recall bias by asking about smoking behavior in several different ways and verifying the answers to establish internal validity. Our cases were mostly recruited from tertiary referral centers, which might raise concerns of selection bias, although in China and India, almost all IBD patients, regardless of severity, are commonly seen at regional tertiary centers, given the rarity of IBD. IBD cases in the USA on average had longer duration of disease than those in China and India. We adjusted for disease duration when studying the association between smoking and disease outcomes because longer duration is associated with worse outcomes. Our study had limited sample size, especially of China and India, thus diminishing the power to detect influences of smaller magnitude. Power-hoc power calculation based on our sample size suggested that our study had a power of 0.35 and 0.52 to detect the observed effect estimates of current smoking on CD in China and the USA.
Some controls in our study, especially those in China and India, were family members or friends of cases. This might have caused our estimation of the association between smoking and IBD to be more conservative because family and friends were more likely to have resemblance in behavior and environmental exposures. About 4.7% of CD cases and 3.3% of UC cases in the USA were recruited from Research-Match.org. Self-reported information was collected for cases recruited from both online and clinic; therefore, they should be comparable.
There is heterogeneity in the associations of smoking and IBD risk and outcomes between China, India, and the USA. Further study with more adequate sample size and more uniform definition of smoking status is warranted.
Acknowledgments
We acknowledge ResearchMatch.org. Part of the recruitment for the study included was done via ResearchMatch, a national health volunteer registry that was created by several academic institutions and supported by the United States National Institutes of Health as part of the Clinical Translational Science Award (CTSA) program. ResearchMatch has a large population of volunteers who have consented to be contacted by researchers about health studies for which they may be eligible. We also acknowledge Girish Ganesh and Kavitha Medaboina for their help in recruiting participants at Asian Institute of Gastroenterology in Hyderabad, India. We acknowledge the grant support from Sun Yat-sen University Clinical Research 5010 Program (2014008), Ludwig-Bayless Science Award, and the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH.
Appendix
Footnotes
Conflict of interest No authors have conflict of interest with relation to this study.
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 4.Fung WP, Monteiro EH, Murugasu JJ, Ng KC, Kho KM, Lee SK. Non-specific ulcerative colitis in Chinese and Indians in Singapore. Med J Aust. 1971;2:361–365. [DOI] [PubMed] [Google Scholar]
- 5.Lee SK. Crohn’s disease in Singapore. Med J Aust. 1974;1:266–269. [DOI] [PubMed] [Google Scholar]
- 6.Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn’s disease in the Chinese population. Inflamm Bowel Dis. 2004;10:646–651. [DOI] [PubMed] [Google Scholar]
- 7.Morita N, Toki S, Hirohashi T, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995;30:1–4. [PubMed] [Google Scholar]
- 8.Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. [DOI] [PubMed] [Google Scholar]
- 9.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. [DOI] [PubMed] [Google Scholar]
- 11.Brant SR, Wang MH, Rawsthorne P, et al. A population-based case-control study of CARD15 and other risk factors in Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2007;102:313–323. [DOI] [PubMed] [Google Scholar]
- 12.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth MP, Petersen GM, McElree C, Vadheim CM, Panish JF, Rotter JI. Familial empiric risk estimates of inflammatory bowel disease in Ashkenazi Jews. Gastroenterology. 1989;96:1016–1020. [DOI] [PubMed] [Google Scholar]
- 14.Weterman IT, Pena AS. Familial incidence of Crohn’s disease in The Netherlands and a review of the literature. Gastroenterology. 1984;86:449–452. [PubMed] [Google Scholar]
- 15.Wang PQ, Hu J, Al Kazzi ES, et al. Family history and disease outcomes in patients with Crohn’s disease: a comparison between China and the United States. World J Gastrointest Pharmacol Ther. 2016;7:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XQ, Zhang Y, Xu CD, et al. Inflammatory bowel disease in Chinese children: a multicenter analysis over a decade from Shanghai. Inflamm Bowel Dis. 2013;19:423–428. [DOI] [PubMed] [Google Scholar]
- 17.Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e152. [DOI] [PubMed] [Google Scholar]
- 18.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. [DOI] [PubMed] [Google Scholar]
- 19.Mayberry J A review of environmental factors and Crohn’s disease. Tijdschrift voor gastro-enterologie. 1978;21:267–271. [PubMed] [Google Scholar]
- 20.Harries AD, Baird A, Rhodes J. Non-smoking: a feature of ulcerative colitis. Br Med J (Clinical research ed.). 1982;284:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the Surgeon General The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 22.Harris PA, Scott KW, Lebo L, Hassan N, Lightner C, Pulley J. ResearchMatch: a national registry to recruit volunteers for clinical research. Acad Med. 2012;87:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64:1063–1071. [DOI] [PubMed] [Google Scholar]
- 25.Niu J, Miao J, Tang Y, et al. Identification of environmental factors associated with inflammatory bowel disease in a southwestern highland region of China: a nested case-control study. PLoS ONE. 2016;11:e0153524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon CM, Park DI, Kim ER, et al. Clinical features and predictors of clinical outcomes in Korean patients with Crohn’s disease: a Korean association for the study of intestinal diseases multicenter study. J Gastroenterol Hepatol. 2014;29:74–82. [DOI] [PubMed] [Google Scholar]
- 27.Reif S, Lavy A, Keter D, et al. Lack of association between smoking and Crohn’s disease but the usual association with ulcerative colitis in Jewish patients in Israel: a multicenter study. Am J Gastroenterol. 2000;95:474–478. [DOI] [PubMed] [Google Scholar]
- 28.Reif S, Klein I, Arber N, Gilat T. Lack of association between smoking and inflammatory bowel disease in Jewish patients in Israel. Gastroenterology. 1995;108:1683–1687. [DOI] [PubMed] [Google Scholar]
- 29.Eliakim R, Reif S, Lavy A, et al. Passive smoking in patients with inflammatory bowel disease: an Israeli multicentre case-control study. Eur J Gastroenterol Hepatol. 2000;12:975–979. [DOI] [PubMed] [Google Scholar]
- 30.Ng SC, Zeng Z, Niewiadomski O, et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology. 2016;150:86–95.e83; quiz e13–84. [DOI] [PubMed] [Google Scholar]
- 31.Arora U, Ananthakrishnan AN, Kedia S, et al. Effect of oral tobacco use and smoking on outcomes of Crohn’s disease in India. J Gastroenterol Hepatol. 2018;33:134–140. [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Matsui T, Yano Y, et al. Long-term course of Crohn’s disease in Japan: incidence of complications, cumulative rate of initial surgery, and risk factors at diagnosis for initial surgery. J Gastroenterol Hepatol. 2015;30:1713–1719. [DOI] [PubMed] [Google Scholar]
- 33.Kuenzig ME, Lee SM, Eksteen B, et al. Smoking influences the need for surgery in patients with the inflammatory bowel diseases: a systematic review and meta-analysis incorporating disease duration. BMC Gastroenterol. 2016;16:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandler RS, Wurzelmann JI, Lyles CM. Oral contraceptive use and the risk of inflammatory bowel disease. Epidemiology (Cambridge, Mass.). 1992;3:374–378. [DOI] [PubMed] [Google Scholar]
- 35.Andersen V, Christensen J, Ernst A, et al. Polymorphisms in NF-kappaB, PXR, LXR, PPARgamma and risk of inflammatory bowel disease. World J Gastroenterol. 2011;17:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes T, Etchevers MJ, Merino O, et al. Does smoking influence Crohn’s disease in the biologic era? The TABACROHN study. Inflamm Bowel Dis. 2013;19:23–29. [DOI] [PubMed] [Google Scholar]
- 37.Aldhous MC, Drummond HE, Anderson N, Smith LA, Arnott ID, Satsangi J. Does cigarette smoking influence the phenotype of Crohn’s disease? Analysis using the Montreal classification. Am J Gastroenterol. 2007;102:577–588. [DOI] [PubMed] [Google Scholar]
- 38.Cosnes J, Carbonnel F, Beaugerie L, Le Quintrec Y, Gendre JP. Effects of cigarette smoking on the long-term course of Crohn’s disease. Gastroenterology. 1996;110:424–431. [DOI] [PubMed] [Google Scholar]
- 39.Domenech E, Carrion S, Garcia-Planella E, et al. Smoking status and response to thiopurines in steroid-dependent inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:971–975. [DOI] [PubMed] [Google Scholar]
- 40.Mahid SS, Minor KS, Stevens PL, Galandiuk S. The role of smoking in Crohn’s disease as defined by clinical variables. Dig Dis Sci. 2007;52:2897–2903. [DOI] [PubMed] [Google Scholar]