Abstract
Background
It is unclear whether intensive surveillance protocols have resulted in a decreased incidence of colorectal cancer (CRC) in inflammatory bowel disease (IBD).
Aims
To determine the prevalence and characteristics of IBD associated high-grade dysplasia (HGD) or CRC that was undetected on prior colonoscopy.
Methods
This is a single-center, retrospective study from 1994 to 2013. All participants had a confirmed IBD diagnosis and underwent a colectomy with either HGD or CRC found in the colectomy specimen. The undetected group had no HGD or CRC on prior colonoscopies. The detected group had HGD or CRC identified on previous biopsies.
Results
Of 70 participants, with ulcerative colitis (UC) (n = 47), Crohn’s disease (CD) (n = 21), and indeterminate colitis (n = 2), 29% (n = 20) had undetected HGD/ CRC at colectomy (15 HGD and 5 CRC). In the undetected group, 75% had prior LGD, 15% had indefinite dysplasia, and 10% had no dysplasia (HGD was found in colonic strictures). Patients in the undetected group were more likely to have pancolitis (55 vs. 20%) and multifocal dysplasia (35 vs. 8%). The undetected group was less likely to have CRC at colectomy (25 vs. 62%). There was a trend toward right-sided HGD/CRC at colectomy (40 vs. 20%; p = 0.08). In addition, 84% of the lesions found in the rectum at colectomy were not seen on prior colonoscopy in the undetected group.
Conclusions
The prevalence of previously undetected HGD/CRC in IBD found at colectomy was 29%. The high proportion of undetected rectal and right-sided HGD/CRC suggests that these areas may need greater attention during surveillance.
Keywords: Inflammatory bowel diseases, Cancer screening, Colorectal neoplasms, Colonoscopy
Introduction
Patients with long-standing ulcerative colitis (UC) and Crohn’s colitis are at an increased risk of developing colorectal cancer (CRC) due to chronic colonic inflammation. The reported incidence of CRC has varied greatly with early estimates that were six times higher than that in the general population and accounting for 10–15% of deaths in IBD patients [1–3]. Several more recent studies have demonstrated a risk that is comparable to that of the general population [4, 5]. The variation in risk estimates is due to multiple factors that can bias results such as extent of disease, referral bias, duration of follow-up, and geography [6, 7].
Current clinical guidelines suggest colonoscopic surveillance for dysplasia every 1–3 years [8, 9]. However, it is unclear whether intensive surveillance protocols have resulted in a decrease in the incidence of CRC in IBD. Several studies have shown no change in the rate of CRC over time in IBD patients [4, 10, 11]. In those that have shown a decline in CRC, it is unclear whether this is related to improved quality of surveillance, improved medical therapeutics, or earlier surgical intervention [4, 11]. There are limited data describing the efficacy of colonoscopic surveillance as measured by missed or interval cancers in the setting of IBD. In one study of UC patients, more than half of the cases of CRC were interval cancers [12]. Another population-based investigation of older adults found that the risk of missed CRC was three times higher in IBD compared to the general population [13]. It is also unknown whether the incidence of missed or interval lesions has decreased over time.
The primary aim of this study was to investigate the prevalence of HGD or CRC lesions found at time of colectomy that were previously undetected on colonoscopy (undetected HGD/CRC), and to look at this trend over time. We hypothesized that the percent of undetected HGD/CRC at the time of colectomy should decrease with time. The secondary aim was to describe patient and lesion characteristics between patients with undetected HGD/ CRC compared with patients with previously detected HGD/CRC on prior colonoscopy.
Methods
Study Design, Study Sample, and Data Source
This is a retrospective study that included all patients with an established diagnosis of IBD who underwent a complete abdominal colectomy at the Johns Hopkins Hospital from 1994 to 2013 with either HGD or CRC in the colectomy specimen and had at least one pathology report from a previous colonoscopy prior to colectomy. The Johns Hopkins Pathology Data System (PDS) was searched for all pathology reports that included the word ‘‘colectomy’’ and ‘‘colitis.’’ Factors pertaining to the processing of the colectomy samples in the pathology laboratory, such as the number of cut sections, were dictated by a specific protocol that did not change between 1994 and 2013. All biopsies from the colonoscopies were reviewed by an expert gastrointestinal pathologist at Johns Hopkins, including cases for which the colonoscopy was performed at another institution.
Pathology reports were reviewed to identify patients with HGD or CRC in the colectomy specimens. Patients were excluded if they did not have an established history of IBD (ulcerative colitis, Crohn’s colitis or indeterminate colitis), had small bowel cancer, were missing pre-colectomy colonoscopy pathology reports, or had cancers that were not adenocarcinomas. Information on patient demographics, age at colectomy, disease duration, disease type and distribution (defined as the maximum extent of known disease), family history of IBD or CRC, and year of colectomy was abstracted. In those with prior dysplasia on colonoscopic biopsies, the date, severity of disease, and location of dysplasia were collected. Similar variables were obtained for HGD or CRC that was detected in the colectomy specimens.
Definitions
Patients in the undetected HGD/CRC group were defined as those who had no evidence of HGD or CRC on colonoscopies performed prior to their colectomies. Patients in the detected HGD/CRC group had HGD or CRC identified on a prior colonoscopy. The most recent colonoscopy prior to colectomy was used for dysplasia diagnosis in those with prior LGD, HGD, or CRC and was defined as the index colonoscopy. Any detected dysplasia on colonoscopy proximal to the splenic flexure was categorized as right sided, lesions distal to the splenic flexure up to the rectum were left sided, and those in the rectum were categorized as rectal dysplasia. HGD or CRC in more than one location was defined as multifocal. Patients with CRC were staged according to TNM staging.
Lesion Concordance
Concordance of location of dysplasia found on colonoscopy and in the colectomy specimens was evaluated by identifying each specific site of HGD/CRC in the colectomy specimen and determining if there was a corresponding site of dysplasia on the previous index colonoscopy. For this analysis, each specific site of dysplasia was isolated and accounted for inpatients with multifocal HGD/CRC.
Statistical Analysis
Patient characteristics and procedural factors were compared between the undetected and detected groups. The percent of undetected HGD/CRC per year was calculated between 1994 and 2013. Categorical variables were compared using Chi-square or Fisher exact test, where appropriate and continuous variables were compared using the Student’s t test. The percent of undetected HGD/CRC diagnosed within 1 year, 2 years, and more than 2 years of index colonoscopy was calculated to determine what proportion of these cases could be attributed to missed or interval lesions. All statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX). This study was approved by the Johns Hopkins institutional review board.
Results
Patient Characteristics
The initial search in the pathology database of ‘‘colitis’’ and ‘‘colectomy’’ yielded 1859 cases for patients of all ages. Out of these, 107 patients had HGD or CRC in the colectomy sample. After excluding patients without IBD (n = 22), those with small bowel cancer (n = 6), non-adenocarcinoma (n = 4), and no prior colonoscopies (n = 5), a total of 70 patients met criteria for the study. There were 47 patients with UC, 21 with CD, and 2 with indeterminate colitis. The mean age at diagnosis was 30 ± 15 years, and mean disease duration was 23 ± 12 years; 53 (76%) were men.
Prevalence and Characteristics of Undetected and Detected HGD/CRC
Out of the 70 patients, 20 patients (29%) had undetected HGD/CRC at the time of colectomy and 50 patients (71%) had detected HGD/CRC. Of the 20 patients with undetected HGD/CRC, 15 had HGD and 5 had CRC at the time of colectomy. There was no difference in patient characteristics between the two groups in terms of gender, age at diagnosis, disease type, time from disease onset to colectomy, and mean number of prior colonoscopies (Table 1). A greater proportion (55%) of the undetected HGD/CRC group had a history of pancolitis compared to only 20% in the detected HGD/CRC group, p < 0.01. Between the periods from 1994 to 2013, there was no clear trend toward a decline in the proportion of undetected HGD/CRC per year over time (Fig. 1).
Table 1.
Demographic and clinical characteristics of patient population
| Undetected HGD/CRCa (n = 20) | Detected HGD/CRC (n = 50) | p valueb | |
|---|---|---|---|
| Age at diagnosis (median years, IQR) | 26 (16–37) | 24 (20–36) | 0.55 |
| Sex (n, %) | 0.6 | ||
| Male | 16 (80) | 37 (74) | |
| Female | 4 (20) | 13 (26) | |
| Disease type (n, %) | 0.7 | ||
| Ulcerative colitis | 14 (70) | 33 (66) | |
| Crohn’s colitis | 5 (25) | 16 (32) | |
| Indeterminate | 1 (5) | 1 (2) | |
| Disease distribution (n, %) | <0.01 | ||
| Rectum | 3 (15) | 0 (0) | |
| Left sided | 2 (10) | 38 (76) | |
| Pancolitis | 11 (55) | 10 (20) | |
| Small bowel | 1 (5) | 0 (0) | |
| Ileocolonic | 3 (15) | 2 (4) | |
| Time from IBD diagnosis to colectomy (median years, IQR) | 17 (12–29) | 24 (15–29) | 0.67 |
| Family history of CRC (n, %) | 4 (17) | 3 (7) | 0.25 |
| History of dysplasia (n, %) | <0.01 | ||
| None | 2 (10) | 0 (0) | |
| Indefinite | 3 (15) | 0 (0) | |
| Low-grade dysplasia (LGD) | 15 (75) | 0 (0) | |
| High-grade dysplasia (HGD) | 0 (0) | 31 (62) | |
| Colorectal cancer (CRC) | 0 (0) | 19 (38) | |
| Dysplasia location on colonoscopy (n, %) | 0.03 | ||
| Rectum | 3 (18) | 18 (37) | |
| Left | 2 (12) | 13 (27) | |
| Right | 6 (35) | 14 (29) | |
| Multifocal | 6 (35) | 4 (8) | |
| Number of total colonoscopies (mean ± SD) | 2 (2–3) | 2 (1–2) | 0.14 |
Colorectal cancer
p values for significant difference in distribution of proportions and p value for difference in median age at diagnosis, number of colonoscopies, and number of biopsies
Fig. 1.
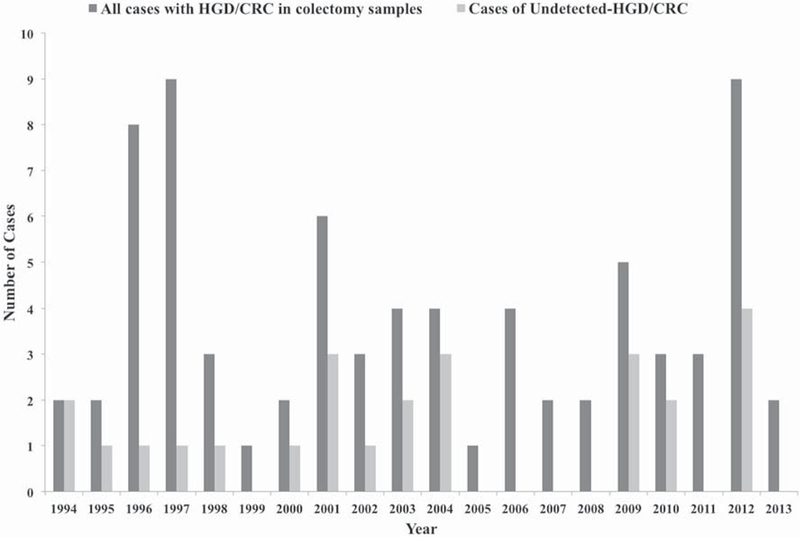
Proportion of undetected HGD/CRC out of total number of HGD/CRC cases found in colectomy specimens per year from 1994 to 2013 in patients with inflammatory bowel disease
All cases in the detected HGD/CRC group underwent a colectomy for prior HGD or CRC. In the undetected HGD/ CRC group, the indications for colectomy were LGD (71%), severe disease (10%), and complications (19%) such as bowel obstruction, perforation, and fistula. Those with undetected HGD/CRC had a median of 112 (IQR = 46–142) days between index colonoscopy and colectomy compared to 62 (IQR = 38–124) days in the detected group (Table 2), p = 0.22. In the undetected HGD/CRC group with prior LGD, 82% (n = 14) had a colectomy within one year of the index colonoscopy, 10% (n = 2) within 2 years, and 5% (n = 1) more than 2 years after dysplasia detection on colonoscopy. In the detected HGD/CRC group, 90% (n = 45) underwent a colectomy within 1 year and 10% (n = 5) within 2 years of diagnosis of dysplasia on colonoscopy.
Table 2.
Dysplasia characteristics in colectomy specimens and duration to colectomy
| Undetected HGD/CRC (n = 20) | Detected HGD/CRC (n = 50) | p valuea | |
|---|---|---|---|
| Time from colonoscopyb to colectomy (median days ± IQR) | 112 (46–142) | 62 (38–124) | 0.22 |
| Pathology at colectomy (n, %) | 0.02 | ||
| High-grade dysplasia (HGD) | 15 (75) | 19 (38) | |
| UC | 10 (67) | 14 (74) | |
| CD | 4 (27) | 4 (21) | |
| Indeterminate | 1 (7) | 1 (5) | |
| Colorectal cancer (CRC) | 5 (25) | 31 (62) | |
| UC | 4 (80) | 19 (61) | |
| CD | 1 (20) | 12 (39) | |
| Indeterminate | 0 (0) | 0 (0) | |
| T Categories of CRC (n/N, %) | |||
| Tx | 0 | 3 (10) | |
| Tis | 0 | 3 (10) | |
| T1 | 1 (20) | 6 (19) | |
| T2 | 1 (20) | 5 (16) | |
| T3 | 3 (60) | 7 (23) | |
| T4a | 0 | 1 (3) | |
| T4b | 0 | 6 (19) | |
| Location of HGD/CRC at colectomy (n, %) | |||
| Rectum | 5 (25) | 20 (40) | 0.24 |
| HGD | 3 (60) | 6 (30) | |
| CRC | 2 (40) | 14 (70) | |
| Left | 3 (15) | 13 (26) | 0.32 |
| HGD | 3 (100) | 5 (38) | |
| CRC | 0 (0) | 7 (62) | |
| Right | 8 (40) | 10 (20) | 0.08 |
| HGD | 5 (62) | 3 (30) | |
| CRC | 3 (38) | 7 (70) | |
| Multifocal | 4 (20) | 7 (14) | 0.53 |
| HGD | 4 (100) | 4 (57) | |
| CRC | 0 (0) | 3 (43) |
UC ulcerative colitis, CD Crohn’s disease
p values for significant difference in distribution of proportions and p value for difference in median time between colonoscopy and colectomy
Defined as the most recent colonoscopy prior to colectomy
Colonoscopy Dysplasia Characteristics
Among those with undetected HGD/CRC, 15 (75%) had prior LGD, 3 (15%) had indefinite dysplasia, and 2 (10%) had no dysplasia on colonoscopy. A majority of the undetected HGD/CRC group with prior LGD or indefinite dysplasia had a history of UC compared to CD (Supplementary Table). On the other hand, in the two patients with undetected HGD/CRC group and no prior dysplasia on colonoscopy, both patients had Crohn’s disease and were operated for obstructive symptoms and had HGD in colonic strictures at the time of colectomy. A higher percent of the undetected HGD/CRC group had multifocal dysplasia (35 vs. 8%) compared to the detected group, p = 0.03. Of the 5 patients in the undetected HGD/CRC group who had CRC at time of colectomy, 2 had history of rectosigmoid strictures with LGD found at colonoscopy, 2 cases had multifocal polypoid LGD, and one patient had one area of polypoid dysplasia that was not endoscopically resectable.
HGD/CRC Characteristics at Colectomy
At the time of colectomy, the undetected HGD/CRC group was more likely to have HGD (75%) than CRC (25%), p = 0.02 (Table 2). In the detected HGD/CRC group, 38% had HGD and 62% had CRC. Overall, the two groups did not differ in terms of the location of HGD/CRC found at the time of colectomy, although there was a trend toward a higher percent of right-sided HGD/CRC at colectomy in the undetected HGD/CRC group (40 vs. 20%; p = 0.08) (Table 2).
Out of the patients with detected HGD/CRC, a majority had either T1, T3, or T4b stage adenocarcinoma found in the colectomy specimens (Table 2). In the undetected HGD/CRC group with CRC, three cases were stage T3 and the other two were T1 and T2; none had T4 disease.
Correlation Between Dysplasia Location at Colonoscopy and CRC Location at Colectomy
The sites of dysplasia (LGD, HGD or CRC) on index colonoscopy were compared to the locations of HGD/CRC found in the colectomy specimens for each patient. In the undetected HGD/CRC group, 5 (84%) of the lesions found in the rectum at colectomy were not seen on prior colonoscopy, and these patients were noted as having right- or left-sided dysplasia on colonoscopy (Table 3). By contrast, in the detected HGD/CRC group, the rate of location discordance among rectal lesions was only 27%. For left- and right-sided lesions found at colectomy, location discordance was <30% for both undetected and detected HGD/ CRC groups.
Table 3.
Locationsa of dysplasia on colonoscopy and colectomy for the undetected and detected CRC groups
| CRC location at colectomy | Dysplasia location on most recent colonoscopy |
|
|||
|---|---|---|---|---|---|
| Leftb | Rightc | Rectum | Total | Discordance rate (n, %) | |
| Undetected CRC | |||||
| Left | 3 | 0 | 1 | 4 | 1 (25%) |
| Right | 1 | 7 | 0 | 8 | 1 (13%) |
| Rectum | 2 | 3 | 1 | 6 | 5 (84%) |
| Detected CRC | |||||
| Left | 14 | 2 | 0 | 16 | 2 (13%) |
| Right | 4 | 11 | 0 | 15 | 4 (27%) |
| Rectum | 4 | 3 | 19 | 26 | 7 (27%) |
Patients with multifocal dysplasia were categorized as having both left- and right-sided lesions
Dysplasia in the left colon
Dysplasia in the right colon including transverse colon
Discussion
In this study, among patients with IBD who underwent a colectomy at a single tertiary institution, the incidence of previously undetected HGD or CRC identified in colectomy specimens over the last 20 years was 29% (n = 20). We observed no decrease in the rate of undetected HGD/CRC cases per year over time. A higher percent in the undetected group had history of pancolitis and multifocal dysplasia on colonoscopy. As almost all patients had a colonoscopy within 2 years of colectomy, we believe that most of the HGD/CRC lesions were missed, and not interval lesions.
In our cohort, while 29% of the HGD/CRC found at colectomy were not detected on prior colonoscopy, only five of these cases were CRC. All these CRC cases had a prior colonoscopy, which showed either LGD within a rectosigmoid stricture, multifocal polypoid LGD, or an unresectable lesion found to have LGD. Therefore, none of the previously undetected cases of CRC arose from cases with a single flat LGD lesion on prior colonoscopy. Additionally, in contrast to the detected HGD/CRC group, none of the five patients had evidence of metastatic disease at colectomy.
A recent study by Krugliak Cleveland et al. [14] found no synchronous adenocarcinomas in patients who underwent colectomy for LGD at a single tertiary care center. In that study, only 1 out of 30 patients with LGD on colonoscopy was upstaged to HGD, and 3 additional patients were found to have one or more synchronous HGD lesions. The authors concluded that most dysplasia is visible with advanced colonoscopic imaging. Although we had 5 patients with LGD who were upstaged to CRC, these patients had evidence of a stricture or endoscopically visible lesions. Notably we had 15 patients with LGD who were found to have HGD at colectomy. While it is difficult to compare the results of our study to that of Krugliak Cleveland due to differences in study design (their starting point was patients with any dysplasia who went to colectomy, while our starting point was HGD or CRC at colectomy), it is possible that our high rate could be related to lack of standardization with advanced colonoscopy imaging.
In our cohort, we had 2 patients with LGD in rectosigmoid strictures on colonoscopy who had CRC at colectomy, and 2 patients with no dysplasia on biopsies from colonic strictures who had HGD at colectomy. The increased risk of neoplasia in colonic strictures in IBD has been documented to be as high as 40% in one study [15]. However, a more recent population-based study in France found a lower risk with 3.5% of patients with IBD and colonic strictures having dysplasia [16], indicating that closer surveillance may be needed in this group.
In terms of the location of the undetected HGD/CRC group, 40% were found to have right-sided lesions compared to 20% of the detected CRC group. This may be related to the higher rate of pancolitis in the undetected HGD/CRC group compared to the detected HGD/CRC group (52 vs. 20%). However, this right-sided predilection for missed or interval lesions may be similar to the pattern that is seen in sporadic CRC [17]. The reason for this right-sided predominance has been attributed to various factors such as inadequate bowel preparation, operator experience, or a different biologic characteristic of these tumors in the right colon [18].
When the location of HGD/CRC in the colectomy specimens was correlated with the location of previous dysplasia noted on colonoscopy, 5 of 6 (84%) of rectal lesions with HGD/CRC at colectomy were not identified on prior colonoscopy as having rectal LGD in the undetected HGD/CRC group. A predisposition for missed CRC in the rectum has been described previously in the setting of sporadic CRC [19–21]. While there were more HGD/CRC lesions found in the right colon in the undetected versus detected groups, there was good correlation between location of previous LGD on colonoscopy and location of HGD/CRC at colectomy. Thus, the right-sided lesions were detected on colonoscopy but were identified as being of lower grade (LGD instead of HGD or CRC), while most of the rectal cancers were not even identified on prior colonoscopies in the undetected group. The right colon and rectum are considered to be traditionally difficult areas to perform colonoscopy and surveillance practices, and our findings support this.
Some of the limitations of the study are the relatively small sample size (n = 70) and the lack of access to all the colonoscopy reports in the known HGD/CRC group. All characteristics of the dysplasia were based on the pathology reports, which were generated at Johns Hopkins. However, we were unable to comment accurately on procedural factors such as bowel prep, number of biopsies taken per colonoscopy, and use of high definition or chromoendoscopy. An additional limitation is that this is a descriptive study from one tertiary care center, such that there may be a component of referral bias and the results many not be widely generalizable. Also, because the study was conducted at a tertiary referral center, we do not have long-term follow-up data on these patients, which limits ability to assess factors including mortality. Finally, there is varied endoscopist experience in this sample, as many of the subjects had colonoscopies performed at another institution. However, most cases at our center were performed by gastroenterologists specializing in inflammatory bowel disease.
Despite these limitations, our study has numerous strengths. Currently, there are few studies on the prevalence of missed dysplastic lesions in IBD patients undergoing surveillance, and our study adds to the limited literature on this topic. Additionally, our study is one of few that uses cases of HGD/CRC found at colectomy as the denominator and then looks back to determine if lesions were missed. Some of the existing studies [13] used large administrative databases, which is limited by the amount of granular data that can be extracted on individual patient characteristics and risk factors. In our study, we illustrate a single tertiary care center experience during a 20-year period through detailed chart review that captures nuances of patient characteristics and features of dysplastic lesions including cancer stage and correlation with prior dysplasia location.
In conclusion, the prevalence of previously undetected HGD and CRC in IBD patients that was incidentally found at colectomy was 29% and did not appreciably change over the last 20 years. The 7% with undetected HGD/CRC did not occur in those with a single flat LGD lesion, and this should be reassuring. Our study reemphasizes the importance of aggressive surveillance in those with colonic strictures where HGD/CRC can be easily missed. Additionally, the high proportion of previously undetected rectal and right-sided HGD/CRC suggests that greater attention should be paid to adequately survey these areas.
Supplementary Material
Acknowledgments
This study was funded in part by the National Institute for Diabetes and Digestive and Kidney Diseases Grant T32DK07634 and DK190040.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10620-017-4652-5) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Munkholm P Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 2003;18:1–5. [DOI] [PubMed] [Google Scholar]
- 2.Lennard-Jones JE, Morson BC, Ritchie JK, Williams CB. Cancer surveillance in ulcerative colitis. Experience over 15 years. Lancet 1983;2:149–152. [DOI] [PubMed] [Google Scholar]
- 3.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Jess T, Simonsen J, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143:375.e371–381.e371. (quiz e313–374). [DOI] [PubMed] [Google Scholar]
- 5.Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol 2014;12:265.e261–273.e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakatos PL, Lakatos L. Challenges in calculating the risk for colorectal cancer in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2012;10:1179 author reply 1179–1180. [DOI] [PubMed] [Google Scholar]
- 7.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:746. e741–774.e741. (quiz e712–743). [DOI] [PubMed] [Google Scholar]
- 9.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–689. [DOI] [PubMed] [Google Scholar]
- 10.Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 2012;143:382–389. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 1994;343:71–74. [DOI] [PubMed] [Google Scholar]
- 12.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology 2006;130:1030–1038. [DOI] [PubMed] [Google Scholar]
- 13.Wang YR, Cangemi JR, Loftus EV Jr, Picco MF. Rate of early/ missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. Am J Gastroenterol 2013;108:444–449. [DOI] [PubMed] [Google Scholar]
- 14.Krugliak Cleveland N, Colman RJ, Rodriquez D, et al. Surveillance of IBD using high definition colonoscopes does not miss adenocarcinoma in patients with low-grade dysplasia inflamm. Bowel Dis 2016;22:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci 1990;35:349–352. [DOI] [PubMed] [Google Scholar]
- 16.Fumery M, de Chambrun GP, Stefanescu C, et al. Detection of dysplasia or cancer in 3.5% of patients with inflammatory bowel disease and colonic strictures. Clin Gastroenterol Hepatol 2015; 10:1770–1775. [DOI] [PubMed] [Google Scholar]
- 17.Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology 2014;146:950–960. [DOI] [PubMed] [Google Scholar]
- 18.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol 2006;101:2866–2877. [DOI] [PubMed] [Google Scholar]
- 20.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med 2004;141:352–359. [DOI] [PubMed] [Google Scholar]
- 21.Shergill AK, Conners EE, McQuaid KR, et al. Protective association of colonoscopy against proximal and distal colon cancer and patterns in interval cancer. Gastrointest Endosc 2015;82: 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


