Abstract
Objective
To investigate whether volumetric enhancement on baseline MRI and volumetric oil deposition on unenhanced CT would predict HCC necrosis and response post-TACE.
Method
Of 115 retrospective HCC patients (173 lesions) who underwent cTACE, a subset of 53 HCC patients underwent liver transplant (LT). Semiautomatic volumetric segmentation of target lesions was performed on dual imaging to assess the accuracy of predicting tumour necrosis after TACE in the whole cohort and at pathology in the LT group. Predicted percentage tumour necrosis is defined as 100 % - (%baseline MRI enhancement - %CT oil deposition).
Results
Mean predicted tumour necrosis by dual imaging modalities was 61.5 % ± 31.6%; mean percentage tumour necrosis on follow-up MRI was 63.8 % ± 31.5 %. In the LT group, mean predicted tumour necrosis by dual imaging modalities was 77.6 % ± 27.2. %; mean percentage necrosis at pathology was 78.7 % ± 31.5 %. There was a strong significant correlation between predicted tumour necrosis and volumetric necrosis on MRI follow-up (r = 0.889, p<0.001) and between predicted tumour necrosis and pathological necrosis (r = 0.871, p<0.001).
Conclusion
Volumetric pre-TACE enhancement on MRI and post-TACE oil deposition in CT may accurately predict necrosis in treated HCC lesions.
Keywords: Carcinoma, hepatocellular/pathology; Carcinoma, hepatocellular/therapy; Imaging, three-dimensional; Chemoembolization, therapeutic; Liver transplant
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequently diagnosed cancer and the third leading cause of cancer-related death worldwide [1]. Loco-regional therapy such as transarterial chemoembolization (TACE) has been proposed for patients with large size or multifocal, un-resectable tumour restricted to the liver while awaiting liver transplant (LT) [2].
Conventional TACE typically involves the injection of chemotherapeutic agents mixed with ethiodized oil and embolic particles into the tumour-feeding artery.
While overall survival is the ultimate goal in clinical oncological research, other surrogate endpoints, such as imaging-based tumour response rates, have become indispensable for clinical trials and for everyday therapeutic decisions [3].
Conventional approaches for treatment response assessment, such as Response Evaluation Criteria in Solid Tumors (RECIST), which is based on tumour size reduction, are suboptimal in predicting HCC patients’ outcome treated with TACE [4]. Since anticancer efficacy of TACE is assessed by viable rather than global tumour size reduction, the European Association for the Study of Liver (EASL) and modified RECIST (mRECIST), which quantify the viable portion of tumour by contrast-enhanced MRI, have been proposed [5]. Although mRECIST and EASL are better predictors than RECIST for tumour response, in tumours with patchy and irregular necrosis, linear determination of diameter is problematic, inaccurate and susceptible to observer-based erroneous measurements [6]. Therefore, computer-assisted semiautomated segmentation and volumetric techniques have been developed and may improve the current accuracy and reproducibility, specifically in cases where tumour necrosis is patchy [7].
Previous studies have proposed that the extent of accumulated intratumoral iodized oil, termed ‘lipiodol retention’, serves as a biomarker of tumour necrosis [8, 9]. In addition, it has been shown that the enhancing portion of the tumour reflects the viable tumour and is used as a marker for differentiating post-treatment residual viable tumour from necrosis [10].
Evaluation of enhancement on contrast-enhanced CT in tumours with oil deposition is challenging due to artifacts produced by the high attenuation of iodized oil. Therefore contrast-enhanced MRI is utilized for detection of residual viable tumour after cTACE [11].
By considering contrast-enhancement on MRI and oil deposition on CTscan as markers of tumour viability and tumour necrosis, respectively, the purpose of our study was to investigate whether volumetric enhancement on baseline MRI and volumetric oil deposition on unenhanced CT would predict HCC necrosis and response post-TACE.
Materials and methods
Patient selection
This retrospective study was approved by the Institutional Review Board and requirement for patient informed consent was waived. The medical records of 210 HCC patients who had received TACE at our institution from July 2001 until October 2016 were reviewed.
A total of 210 patients were identified. Exclusion criteria were patients who had drug-eluting bead-TACE (DEB-TACE) (n = 68), did not undergo MR imaging within 3 months after and before cTACE (n = 15), or had low image quality (n = 12). The study cohort included 115 cases, 173 lesions, with a total of 153 cTACE treatments.
A subset of the whole study cohort underwent cTACE as a bridge to liver transplantation (LT). In this subset of patients (53 patients with 57 target lesions), we assessed the accuracy of predicting tumour necrosis using combined imaging modalities with the degree of necrosis at pathology. In cases with multiple cTACE treatments in the whole cohort and in liver transplant groups, target lesions were measured after all TACEs were completed.
cTACE technique
Conventional TACE was performed according to our standard institutional protocol for HCC patients placed on the liver transplantation list, and all procedures were performed by interventional radiologists expert in cTACE procedures using a standard approach reported elsewhere [12]. Briefly, for cTACE, a mixture of ethiodized oil (Lipiodol; Guerbet, Aulney-sous-Bois, France) and 50 mg doxorubicin (Adriamycin; Pharmicia& Upjohn, Kalamazoo, MI, USA) was injected in the hepatic arterial vasculature through a selectively to super-selectively advanced microcatheter using continuous fluoroscopic monitoring fluoroscopic guidance. This was followed by injection of up to 4ml of 100- to 300-μm diameter microsphere particles (Embosphere; Biosphere Medical, Boston, MA, USA). All patients had dynamic contrast MRI within 3 months before and after cTACE and non-contrast abdominal CT scan within 24 h of cTACE to evaluate the iodized oil accumulation in tumour.
Quantification of volumetric tumour enhancement on MRI before and after cTACE and volumetric oil deposition after cTACE
MR imaging technique
All 115 HCC cases had two MR imaging studies each within 3 months before and after cTACE. Both studies were performed using a 1.5-T magnet (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) with a phased-array torso coil, using our standard clinical protocol including (1) T2-weighted turbo spin echo sequence (matrix size, 256 × 256; slice thickness, 8 mm; inter-slice gap, 2 mm; 25–37 slices; repetition time/echo time 4,500/92 ms and receiver band-width, 32 kHz), (2) breath-hold unenhanced and contrast-enhanced (after injection of 0.1 mmol gadopentetate dimeglumine (Magnevist; Bayer, Wayne, NJ, USA) per kilogram of body weight) T1-weighted three-dimensional fat-suppressed spoiled gradient-echo images (field of view, 320–400 mm; matrix, 192 × 160; slice thickness, 2.5 mm; 96–112 slices per phase; repetition time/echo time 5.77/2.77 ms, receiver bandwidth 64 kHz and flip angle 10°) in the hepatic arterial phase (HAP; 20 s), portal venous phase (PVP; 70 s) and delayed phase (3 min), and (3) a breath-hold diffusion-weighted echo-planar sequence (matrix size, 128 × 128; section thickness, 8 mm; intersection gap, 2 mm; 48 sections; b value = 0 and 750 s/mm2; repetition time/echo time 3,000/69 ms and receiver bandwidth 64 kHz).
CT technique
Unenhanced abdominal CT scan was performed 24 h after cTACE with a multi-slice CT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany) using a standard abdominal scan protocol as described previously [13]. The scanning parameters were the following: 120 kVp, 545 mA; scan speed, 0.33 s/revolution; detector collimation, 0.6 mm/row; helical pitch factor, 0.575/revolution. Images were then reconstructed using body kernel B30f, with a 400 × 400 × 220 mm field of view (matrix size 512 × 512 × 300) with a voxel size of 0.78 mm3.
Lesion selection
A radiologist with 20 years of experience identified each target lesion. The selection criteria of the treated target lesion included lipiodol retention within the tumour and that the tumour margin was visible on imaging. Baseline patient demographics are summarized in Table 1.
Table 1.
Baseline characteristics of patients with hepatocellular carcinoma
| Parameter | Value |
|---|---|
| Study population | |
| No.of patients | 115 |
| Age (y) | 60.5±9 |
| Sex | |
| Male | 90 (78 %) |
| Female | 25 (22 %) |
| Aetiology | |
| HCV | 63 (54.7 %) |
| HBV | 14 (12 %) |
| Alcoholic | 12 (10 %) |
| NASH | 6 (5 %) |
| HIV | 2 (1.7 %) |
| Unknown | 18 (15.6 %) |
| Disease pattern | |
| Unifocal | 48 (41.7 %) |
| Bi/multi-focal | 54 (46.9 %) |
| Infiltrative | 13 (11.3 %) |
| LT group | |
| No. of patients | 53 |
| No. of target lesions | 57 |
HCV hepatitis C virus, HBV hepatitis B virus, HIV human immunodeficiency virus, NASH non-alcoholic fatty liver, LT group patients who underwent liver transplant
Image post-processing
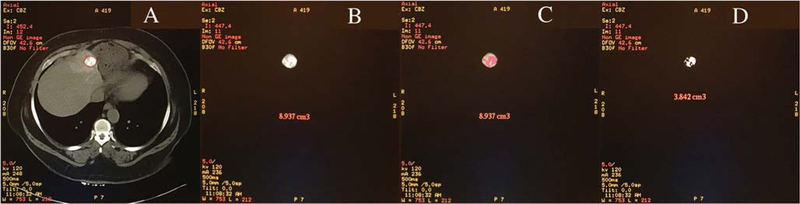
Contrast MRI and CT scan images were uploaded onto the Volumetric Advantage Workstation (GE Healthcare). Image processing was performed by a single observer. The observer was blinded to the pathology report (Fig. 1).
Fig. 1.
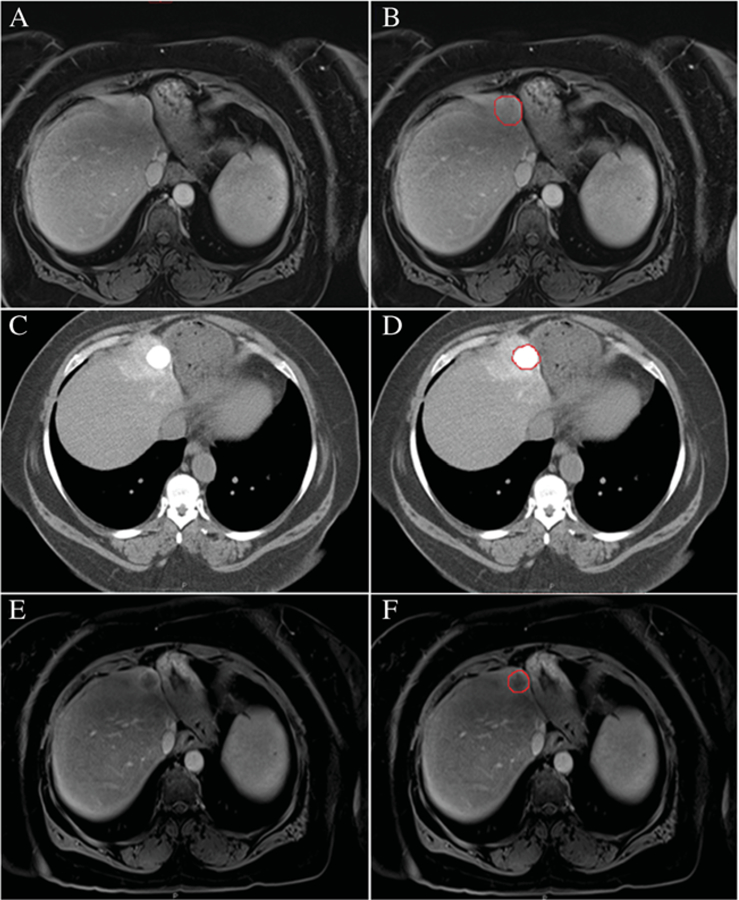
A small hepatocellular carcinoma on an axial T1contrast-enhanced image on baseline MRI (A-B), axial CT image 24 h post-cTACE (C-D) and MRI follow-up post-cTACE (E-F)
Portal venous phase images were utilized to segment the entire tumour by hand tracing and generate a total tumour volume semi-automatically by software (Figs. 2 and 3). Tumour volumetric measurement for each case lasted approximately 5–10 min. Then a threshold signal intensity was optimized to highlight the enhancing, presumably viable, component of the tumour, while carefully excluding non-enhancing presumable necrotic regions. Threshold value ranged between 250 and 800. Viable tumour volume was reported as a percentage of total tumour volume. The reason for using venous phase for segmentation is that heterogeneous early volumetric enhancement of some tumours in the hepatic arterial phase would be a limiting factor in assessing treatment response. Increasing enhancement in the entire tumour volume in the portal venous phase may help distinguish viable from necrotic zones of the tumour.
Fig. 2.
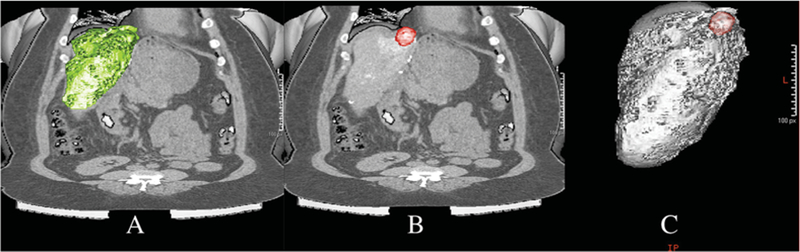
Whole liver volume segmentation on post-cTACE CT obtained 24 h after therapy (A). Total segmented tumour volume (B). Liver and hepatocellular carcinoma lesion in 3D image (C)
Fig. 3.
Segmented hepatocellular carcinoma lesion on CT image post-cTACE uploaded onto the Advantage Workstation (A), entire tumour volume (B), optimization of threshold signal intensity to highlight the oil deposition portion (C) and viable tumour volume (D)
For measuring oil deposition on unenhanced CT after cTACE, the same process was performed (Fig. 3). The tumour was segmented on CT and threshold attenuation was selected to identify the volume of oil deposition within the segmented tumour. Threshold values ranged between 200 and 1,000 Hounsfield units. Oil deposition was reported as a percentage of total tumour volume. Predicted percentage tumour necrosis by dual imaging modalities was calculated as 100 % - (%baseline MRI enhancement - %CT oil deposition).
Volumetric necrosis on follow-up MRI after TACE was calculated as (100 % - %enhancement in MRI).
Predicted tumour necrosis was compared to percentage tumour necrosis on post-cTACE MRI as well as to pathological degree of necrosis if patients underwent LT.
Pathological standard
Explanted livers were processed in surgical pathology according to routine clinical protocol. Briefly the explanted livers were serially sliced at 5- to 10-mm intervals. Targeted lesions were samples (approximately 1 section for every 1 cm of tumour size), formalin fixed, paraffin embedded, H&E stained and examined with light microscopy.
Percentage necrosis was defined as the surface area of necrotic tissue as a total of sampled tumour surface area and it was estimated at 10 % increments [14] (Fig. 4).
Fig. 4.
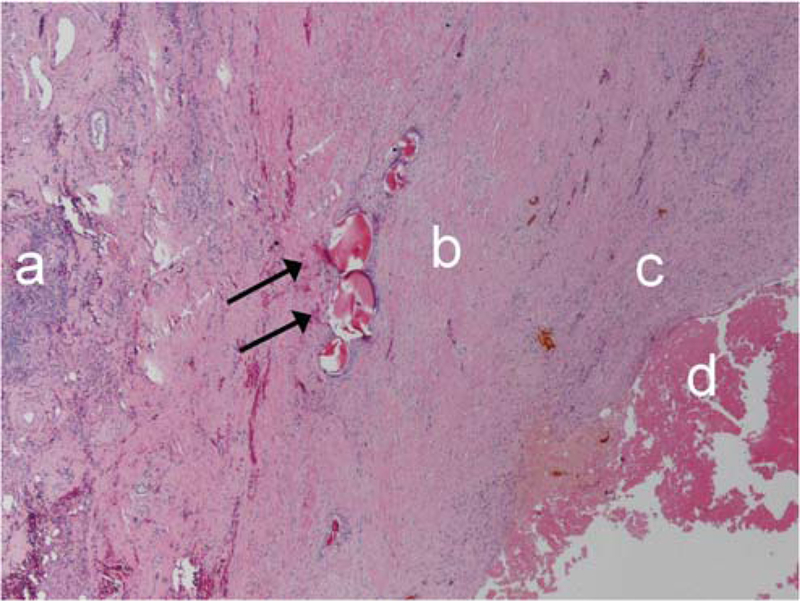
Inflammation (a), dense collagen (b), granulation (c), tumour necrosis (d) and embolic material (black arrows)
One observer who was blinded to the imaging findings reviewed the target lesions and recorded the histopathological type of viable tumour and quantified percentage of necrosis. In cases with multiple tumours, the target lesions were in different segments to the other lesions. The pathologist was blinded to the imaging features of necrosis but not to the location of the targeted lesion. In the current study, the pathologist also compared pathology necrosis with the pathology report, which revealed excellent intra-observer reproducibility of 97 % agreement.
Statistical analysis
Stata statistical software package, version 14 (Stata Corp. College Station, TX, USA) and MedCalc for Windows, version 17.2 (MedCalc Software, Ostend, Belgium) were used to perform all the statistical analyses. Quantitative data in normal distribution were represented as means and standard deviations and compared using Anova. Pearson’s correlation test was used to test the correlation between predicted tumour necrosis as 100 % - (%MRI enhancement - %CT oil deposition) with the two reference standards: percentage of tumour necrosis at pathology in 57 lesions and percentage of necrosis on MRI follow-up in all 115 cases. The correlation coefficients (r) from these comparisons were interpreted as follows: < 0.20, no agreement; 0.21–0.40, weak agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; > 0.80 and almost strong agreement [15]. Inter-observer reproducibility was assessed using the intra-class correlation coefficient (ICC). The Kruskal-Wallis test and Mann-Whitney test were performed as appropriate. All p-values were two-sided and considered statistically significant at p < 0.05.
Results
Characteristics of the patients and HCC lesions included in this study are summarized in Table 1. Complete pre-TACE MRI, post-TACE CT imaging and MRI follow-up data were available for 115 patients (173 lesions) (mean age 61±9 years; male/female: 90/25).
Completed data on pre-TACE MRI, post-TACE CT imaging and pathology report of lesion necrosis following liver transplantation were available for 53 patients with 57 HCC lesions named as the LT group (mean age:59±7 years; male/female: 47/6).
The intervals between pre-TACE MRI and cTACE and cTACE and follow-up MRI were 22±19 days and 51±46 days, respectively. In the LT group, the interval between follow-up MRI and liver transplant was 76±72 days and the interval between cTACE and liver transplant was 111±89.
The mean predicted necrosis percentage by combination imaging modalities in the whole study population was 61.5 % ± 31.6 % (range, 0–100 %) whereas mean percentage tumour necrosis on follow-up MRI was 63.8 % ± 31.5 % (range, 0–100 %).
In the LT group, mean predicted necrosis percentage by combining the two imaging modalities was 77.6 % ± 27.2 % (range, 0–100 %) whereas mean percentage necrosis at pathology was 78.7 % ± 31.5 % (range, 0–100 %). There was a significant positive correlation between predicted tumour necrosis and volumetric necrosis on follow-up MRI (r = 0.889, p < 0.001) and a significant positive correlation between predicted tumour necrosis and pathological percentage necrosis (r = 0.871, p < 0.001) (Fig. 5). In addition, variability of two measurements was assessed by Bland-Altman plot analysis (Fig. 6).
Fig. 5.
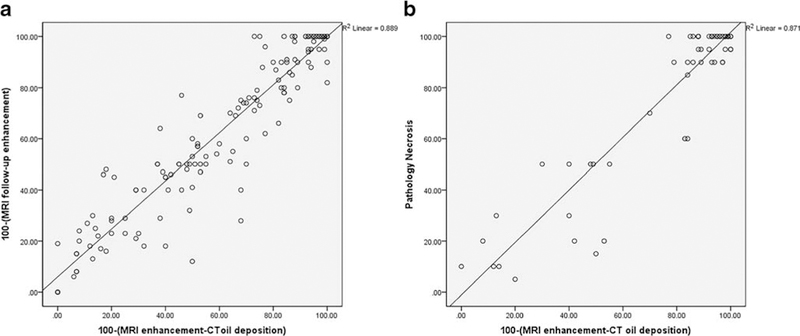
Correlation of MRI follow-up necrosis as (100 % - MRI follow-up enhancement %) and predicted volumetric percentage enhancement post treatment as 100 % - (Volumetric baseline MRI enhancement % – Volumetric CT lipiodol deposition %) (A), correlation of percentage necrosis at pathology and predicted volumetric percentage enhancement post-treatment as 100 % - (Volumetric baseline MRI enhancement % – Volumetric CT lipiodol deposition %) (B)
Fig. 6.
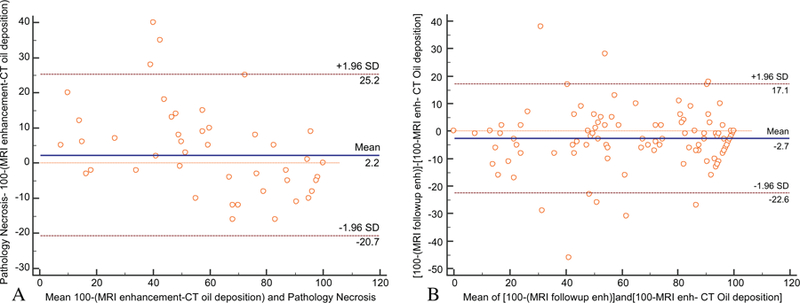
Bland-Altman plots for the two measurements for tumour necrosis with the representation of the 95 % limits of agreement (dashed brown lines), in the whole cohort (A) and in transplanted patients (B). The blue line shows the mean of difference between two measurements
For the whole cohort, there was no significant correlation between necrosis on follow-up MRI and tumour size (r = - 0.06). Regarding the pattern of the disease in the whole cohort, 48 cases were unifocal, 54 cases were bi/multifocal and 13 cases were infiltrative. There was no significant association between pattern of the disease and tumour necrosis on follow-up MRI (p-value = 0.194). A multivariable linear regression model for predicting tumour necrosis in the whole cohort using volumetric enhancement on MRI and volumetric oil deposition on CT was significant and independent of the tumour size and pattern of the disease (regression coefficient = 0.94, p-value < 0.001).
In the transplant group patients, there was no significant correlation between pathology necrosis and tumour size (r = 0.4). In 53 transplant cases, 37 cases were unifocal and 16 cases were bi/multifocal. There was no significant association between disease pattern and pathology necrosis (p-value = 0.23). Regarding the stage of the disease, among patients with liver transplant, 33 cases were stage 1, 18 cases were stage 2 and two cases were stage 3. There was no significant association between pattern of the disease and percentage of necrosis on pathology (p = 0.86). Multivariable linear regression model for predicting tumour necrosis in the LT group using volumetric enhancement on MRI and volumetric oil deposition on CT was significant and independent of the tumour size and pattern of the disease (regression coefficient = 0.759, p-value < 0.001).
In order to evaluate intra-observer agreement, the reader repeated measurements using all the techniques on a subset of 20 cases. This was performed 6 weeks after the initial readings to minimize recall bias and the reader was not provided with the previously recorded measurements. Intra-observer reproducibility and variability was assessed. Excellent intra-observer reproducibility was noted (ICC, 0.986; 95 % CI, 0.966–0.99).
Discussion
TACE is a widely used loco-regional palliative modality, and it plays a pivotal role in the management of patients with HCC due to the tumour’s unique vasculature [16, 17]. The goal of TACE is to cause tumour necrosis and tumour growth control; however, the ultimate goal of therapy is to prolong patient survival [18]. Precise assessment of tumour response after TACE and determining tumour viability is essential for further management of HCC [19]. Current well-established methods for assessing tumour response after treatment including EASL and mRECIST have several limitations, such as presence of asymmetrical residual rim enhancement that may be difficult to measure, resulting in poor measurement reproducibility.
Since necrotic tumours may have patchy enhancement and ill-defined borders, three-dimensional (3D) viable tumour measurement could be applied as a substitute for the bidimensional ones used by mRECIST and EASL in the accurate stratification of tumour response.
Lipiodol as a fundamental element in the treatment protocol of cTACE has the function of a drug-carrying embolizing agent. Previous studies reported that retained iodized lipiodol in HCCs after cTACE can be a marker of necrotic zones of the tumour [20]. Theoretically, the preserved embolization potency of a lipiodol agent allows it to embolize portal venules effectively in viable parts of the tumour leading to tumour necrosis by oil deposition [20]. A previous study also showed that addition of nitroglycerin to cTACE enhances permeability and deposition of oil, which could be reliably assessed by dual-energy CT scan [21].
Volumetric measurement of oil deposition in unenhanced CT following cTACE cannot be used individually as tumour response criteria since it depends on the percentage of tumour viability prior to TACE. Therefore, assessment of tumour necrosis after cTACE could potentially be performed by both MRI before cTACE to determine the tumour viability and CT following cTACE to determine the additional necrosis in the viable portion of the tumour.
We observed a significant positive correlation between predicted tumour necrosis by combination of imaging modalities and residual necrosis calculated on follow-up MRI and on pathology.
The mean prediction of tumour necrosis in a subset of the LT group was 77.6 %, which is much higher than the mean prediction of tumour in the whole population, which was 61.5 %. The reason for such a difference could be related to multiple TACE treatments before LT, which may have led to more tumour necrosis. Volumetric analyses have also been shown to be reproducible and have minimal inter-observer and intra-observer variability both in volumetric tumour segmentation and in pathology necrosis report [22–24].
The role of post-treatment MRI enhancement in predicting the extent of necrosis in pathology in HCC patients following LT was previously reported with ≥ 85 % specificity [25]. Chapiro et al. also showed a high level of correlation between radiological methods, 3D quantitative contrast-enhanced and diffusion-weighted MR, and histopathological findings in HCC patients after cTACE [14]. The degree of intratumoral oil deposition has been found to be associated with tumour necrosis and prognosis in HCC patients following cTACE [6, 20]. Takayasu et al. also reported intratumoral lipiodol retention as a significant factor affecting local recurrence and survival rate [20]. A previous study on 490 HCC patients treated with cTACE showed a strong correlation between lipiodol deposition as a relevant therapeutic target and overall patient survival [26]. Despite using 2D quantitative parameters in all previously mentioned studies for comparing lipiodol retention with survival or tumour necrosis, a recent study by Wang et al. has measured 3D oil deposition on cone beam CT and multi-detector CT with semiautomatic software after cTACE in HCC patients to predict volumetric tumour necrosis [27]. In addition, a previous study in 41 HCC patients treated with DEB TACE has proposed the value of volumetric apparent diffusion coefficient (ADC) and enhancement in MRI as a diagnostic tool for predicting tumour response [28]. In the current study, all cases, with/without tumour oil deposition, have been included, since we wanted to predict tumour response in whole cases following cTACE. In those HCC nodules without dense deposit oil deposition, the enhancement of tumour after cTACE did not change significantly with enhancement before cTACE, which showed that cTACE was not effective since there was not good deposition of oil.
To our knowledge, our study represents the first attempt using a volumetric technique defined as 100 % - (%enhancement on MRI - %oil deposition on CT) to predict the percentage of necrosis and to better stratify tumour response following cTACE. The results of our study help to determine necrosis early after cTACE, which was confirmed by follow-up MRI. The combined measurements of enhancement on MRI before TACE and oil deposition on CT post-TACE may accurately predict tumour necrosis and accurately determine the need for subsequent therapy without the need to obtain MRI post-treatment.
Despite the advantage of volumetric assessment in tumour response, which quantifies the entire tumour volume rather than axial flat measurements, clinicians have been reluctant to use volume measurements because they are time-consuming and laborious, and are more prone to technical errors than linear measurement.
Limitations
This study has limitations, including its retrospective design, which lead to potential patient selection bias. In the LT group, the interval between follow-up MRI and liver transplant was quite long and tumour growth or spontaneous necrosis can occur; however, this would be less likely after cTACE treatment. In addition, the accuracy of volumetric tumour measurement is limited by technical factors and is more dependent on precise contouring and accurately distinguishing the borders between lesion and normal tissue; however, prior studies reported relatively high reproducibility in assessment of tumour volume. Our results need to be further validated with a larger sample size and better designed studies as cohort prospective studies to reduce heterogeneity between diverse MRI scanners.
In addition, since in the subgroup of study population that was assessed to predict tumour necrosis by pathology only patients who were successfully bridged to LT were included, our findings may be affected by several confounders and reflect a selected subgroup whose tumours were less aggressive and more responsive to cTACE.
Conclusion
In conclusion, a combination of two imaging modalities, volumetric pre-cTACE enhancement in MRI and post-cTACE oil deposition in unenhanced CT, have the potential to accurately predict necrosis and tumour response in treated HCC lesions.
This method will facilitate further therapeutic decision in HCC patients undergoing cTACE and will potentially obviate the need for immediate post-treatment MRI in patients with HCC.
Key Points.
Imaging-based tumour response can assist in therapeutic decisions.
Lipiodol retention as carrier agent in cTACE is a tumour necrosis biomarker.
Predicting tumour necrosis with dual imaging potentially obviates immediate post-treatment MRI.
Predicting tumour necrosis would facilitate further therapeutic decisions in HCC post-cTACE.
Pre-TACE MRI and post-TACE CT predict necrosis in treated HCC.
Methodology.
retrospective
diagnostic study
performed at one institution
Acknowledgments
Funding
The authors state that this work has not received any funding.
Abbreviations
- ADC
Apparent diffusion coefficient
- CI
Confidence interval
- CT
Computed tomography
- cTACE
Conventional TACE
- DEB-TACE
Drug-eluting bead-TACE
- EASL
European Association for Study of Liver Disease
- HAP
Hepatic arterial phase
- HCC
Hepatocellular carcinoma
- ICC
Intra-class Correlation Coefficient
- LT
Liver transplant
- mRECIST
modified RECIST
- MRI
Magnetic resonance imaging
- PVP
Portal venous phase
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
Standard deviation
- TACE
Transarterial chemoembolization
Footnotes
Compliance with ethical standards
Guarantor The scientific guarantor of this publication is Ihab R Kamel.
Conflict of interest The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry No complex statistical methods were necessary for this paper.
Informed consent Written informed consent was waived by the Institutional Review Board.
Ethical approval Institutional Review Board approval was obtained.
References
- 1.Bray F, Ferlay J, Laversanne M et al. (2015) Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer 137:2060–2071 [DOI] [PubMed] [Google Scholar]
- 2.Lanza E, Donadon M, Poretti D et al. (2016) Transarterial therapies for hepatocellular carcinoma. Liver Cancer 6:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsurusaki M, Murakami T (2015) Surgical and locoregional therapy of HCC: TACE. Liver Cancer 4(3):165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forner A, Ayuso C, Varela M et al. (2009) Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 115:616–623 [DOI] [PubMed] [Google Scholar]
- 5.Hayano K, Lee SH, Sahani DV (2015) Imaging for assessment of treatment response in hepatocellular carcinoma: current update. Ind J Radiol Imaging 25:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monsky WL, Kim I, Loh S et al. (2010) Semiautomated segmentation for volumetric analysis of intratumoral ethiodol uptake and subsequent tumor necrosis after chemoembolization. AJR Am J Roentgenol 195:1220–1230 [DOI] [PubMed] [Google Scholar]
- 7.Ray S, Hagge R, Gillen M et al. (2008) Comparison of two-dimensional and three-dimensional iterative watershed segmentation methods in hepatic tumor volumetrics. Med Phys 35:5869–5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan SW, Fidelman N, Ma E, Kerlan RK Jr, Yao FY (2012) Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl: Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc 18:727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu SC, Hui JW, Hui EP et al. (2014) Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology 270:607–620 [DOI] [PubMed] [Google Scholar]
- 10.Agnello F, Salvaggio G, Cabibbo G et al. (2013) Imaging appearance of treated hepatocellular carcinoma. World J Hepatol 5(8): 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, Honjo K, Fujita T et al. (1995) Therapeutic efficacy of trans-catheter arterial chemoembolization for hepatocellular carcinoma: MRI and pathology. J Comput Assist Tomogr 19:198–203 [DOI] [PubMed] [Google Scholar]
- 12.Chapiro J, Duran R, Lin M et al. (2015) Identifying staging markers for hepatocellular carc inoma before transarterial chemoembolization: comparison of three-dimensional quantitative versus non-three-dimensional imaging markers. Radiology 275: 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultana S, Awai K, Nakayama Y et al. (2007) Hypervascular hepatocellular carcinomas: bolus tracking with a 40-detector CT scanner to time arterial phase imaging. Radiology 243:140–147 [DOI] [PubMed] [Google Scholar]
- 14.Chapiro J, Wood LD, Lin M et al. (2014) Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology 273(3):746–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med 22(3):276–282 [PMC free article] [PubMed] [Google Scholar]
- 16.Paul SB, Sharma H (2014) Role of transcatheter intra-arterial therapies for hepatocellular carcinoma. J Clin Exp Hepatol 4:S112–S121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW (2013) Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol 19:7515–7530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo CM, Ngan H, Tso WK et al. (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35(5):1164–1171 [DOI] [PubMed] [Google Scholar]
- 19.Sato Y, Watanabe H, Sone M et al. (2013) Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci 118:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayasu K, Arii S, Matsuo N et al. (2000) Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol 175:699–704 [DOI] [PubMed] [Google Scholar]
- 21.Liu YS, Chuang MT, Tsai YS, Tsai HM, Lin XZ (2012) Nitroglycerine use in transcatheter arterial (chemo)embolization in patients with hepatocellular carcinoma and dual-energy CT assessment of Lipiodol retention. Eur Radiol 22:2193–2200 [DOI] [PubMed] [Google Scholar]
- 22.Bonekamp D, Bonekamp S, Halappa VG et al. (2014) Interobserver agreement of semiautomated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol 83:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valero V 3rd, Amini N, Spolverato G et al. (2015) Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg 19:272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samrao D, Wang D, Ough F et al. (2012) Histologic parameters predictive of disease outcome in women with advanced stage ovarian carcinoma treated with neoadjuvant chemotherapy. Transl Oncol 5:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riaz A, Lewandowski RJ, Kulik L et al. (2010) Radiologic-pathologic correlation of hepatocellular carcinoma treated with chemoembolization. Cardiovasc Intervent Radiol 33:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DY, Ryu HJ, Choi JY et al. (2012) Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther 35(11):1343–1350 [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Lin M, Lesage D et al. (2014) Three-dimensional evaluation of lipiodol retention in HCC after chemoembolization: a quantitative comparison between CBCT and MDCT. Acad Radiol 21: 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corona-Villalobos CP, Halappa VG, Geschwind JF et al. (2015) Volumetric assessment of tumour response using functional MR imaging in patients with hepatocellular carcinoma treated with a combination of doxorubicin-eluting Rbeads and sorafenib. Eur Radiol 25:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]