Abstract
Protein digestion is critical for infants. Dissimilarities between infants and adults in food intake and digestive physiology lead to distinct patterns of proteolysis between individuals. However, such differences are not well represented in many studies on protein digestion of baby foods. The complex biological structures of baby foods and the physiology of the infant digestive system are key factors affecting proteolysis during the first two years of life. Well-controlled in vitro studies have demonstrated that varying digestion conditions alter the specificity, rate, and extent of proteolysis of baby foods. Nonetheless, these models do not completely replicate in vivo proteolysis or the complex biogeography of the gastrointestinal tract. Animal and clinical studies have revealed the fate of dietary proteins along the digestive tract and the overall health impact on subjects. Building comprehensive and annotated datasets from human infants will require innovative and standardized measurement. Now, more systematic evaluations of digestion are emerging to advance the knowledge and its translation as food design for effective diet and health management in infants.
Keywords: digestion model, food breakdown, infant, nutrition, proteolysis
1. Introduction
Protein intake early in life is essential for infant health, affecting growth, body composition, neurodevelopment, appetite and hormonal regulation [1]. Problems with protein digestion cause short-term and long-term adverse consequences, such as pain, diarrhea, intolerance, allergy, malabsorption, and constipation [2]. After ingestion, proteins in baby foods undergo proteolysis within the infant digestive tract before being absorbed or excreted. During this process, some of the proteins are partially digested. These proteins and their peptide fragments can interact with other dietary molecules and cellular components in infants, exerting beneficial functions including nutrient delivery and antimicrobial activity [3], or leading to deleterious effects such as inflammation and allergic responses [4, 5]. Therefore, understanding how dietary proteins are chemically and biologically processed during infant digestion has profound implications for food and health.
Due to their immature state, infants require baby foods that are suited to their development. Properties of baby foods are critical for protein breakdown during digestion. Based on physical and chemical characteristics, baby foods can be classified into three categories: breast milk, human milk substitute, and complementary food. Breast milk has evolved under selective pressure to be the initial complete diet for newborns, providing nutrition and protection. As such, exclusive breastfeeding for 6 months followed by continued breastfeeding up to 1 or 2 years is recommended [6, 7]. Human milk substitute as infant formula is given to infants when breast milk is unavailable [8]. Despite substantial efforts invested to make human milk substitute similar to breast milk, the chemical and structural, much less the biological properties of breast milk, are still impossible to accurately reconstruct [7, 9]. Complementary foods are nutrient-containing foods other than breast milk that are introduced to infants generally between 6 and 24 months of age, as a complement to breast milk or breast milk substitute [10]. The composition, structure, and texture of complementary foods, such as cereal and meat puree, are distinct from breast milk in many ways [8, 11], resulting in different patterns of protein digestion.
Besides varying food intake, physiological dissimilarity between infants and adults contributes to divergent patterns of food digestion. However, conditions of infant digestion have not been well represented in many in vitro studies. The growing concern about building in vitro models based on specific infant physiology has been highlighted in recent reviews. Bourlieu et al. [12] reviewed studies that characterized gastroduodenal conditions in full-term and preterm infants from birth to six months. Poquet and Wooster [13] analyzed characteristics of infant physiology that are related to lipid digestion. In terms of protein digestion, the immaturity of the infant digestive system affects the efficacy of protein digestion and absorption, leading to unique issues in this population. For example, cow’s milk allergy primarily affects infants under two years old, and its incidence declines among children older than three years old [14].
Regarding the relationship of diet to infant health, infant-specific research models are needed to understand protein digestion in more detail. This review will identify key features that affect the hydrolysis of proteins in baby foods, highlight approaches that are adapted for conditions of infant protein digestion, and discuss challenges and directions of future work in this field.
2. Properties of baby foods that affect protein digestibility
2.1. Protein molecular structure
The sequence of amino acids in a protein is an intrinsic feature that affects its digestibility. For example, gluten, which is present in baby foods sourced from wheat and rye, contains gliadin peptides that are resistant to gastrointestinal breakdown. The abundance and location of proline residues in gliadin confers its resistance to gastric and intestinal proteases [15]. Moreover, the folded structure of a protein influences its digestibility [16]. Hydrogen bonds stabilize proteins and can create a compactly folded tertiary structure, making it difficult for enzymes to access cleavable peptide bonds. Post-translational modifications might block proteases from binding and inhibit the enzymatic activity; thus, dephosphorylation of bovine milk protein concentrate increases protein digestibility in an infant in vitro model [17]. Nevertheless, effect of modifications on digestibility is negligible in some cases, for example, glycosylation of codfish parvalbumin [18]. Disulfide bonds or non-disulfide crosslinking can adversely affect protein digestibility, and breaking disulfide bonds of proteins in soybean and sorghum increases in vitro digestibility [19, 20].
Resistance to digestion is a characteristic of food allergens [21]. This molecular property allows the intact proteins or their large fragments containing epitopes to reach the gut and trigger adverse immune responses in high risk populations [4, 22]. Cow’s milk protein is the most common protein source for infant formulas, and cow’s milk protein allergy is the most prevalent food allergy in early childhood [23]. Partially or extensively hydrolyzed infant formulas have been developed to reduce allergenicity and recommeneded for high risk infants who are not exclusively breast-fed [24–26]. However, systematic reviews and meta-analyses on clinical studies have shown contradictory findings on the efficacy of hydrolyzed formulas in preventing allergic dieseases in infants [24–28]. Molecular struture of proteins and peptides is important for the digestibility and allergenicity, but it was not well characterized in infant formulas and its changes were not annotated along infants’ digestive tract, which could be a confounding variable in epidemiological analyses.
2.2. Food composition
Baby foods typically consist of multiple components besides proteins, and non-protein components may have chemical interactions with the proteins. For example, cereal grains like sorghum contain polyphenols, polysaccharides and phytic acid that can crosslink with or bind to proteins, thus decreasing their digestibility [16]. Reducing sugars react with proteins in food during thermal processing through non-enzymatic browning, the Maillard reaction, modifying the extent of proteolysis and the bioavailability of proteins [29, 30]. In addition to interacting with proteins of the food, naturally occurring inhibitors from legumes and bovine colostrum can inhibit human trypsin without adequate processing [31].
Baby foods often go through processing procedures such as spray-drying or extensive cooking that are necessary for safety. However, processing operations can cause unintended consequences on proteins in baby foods, changing their structures and interactions. Thermal processing can reduce antinutrients, like tannins and phytic acid in legumes, and improve protein digestibility [32], whereas denaturation, crosslinking between amino acid residues, and the Maillard reaction decrease protein digestibility. Infant formula is particularly susceptible to the Maillard reaction due to the high content of the reducing sugar lactose, which reacts with lysine and reduces its availability [30, 33]. Lysine is an essential amino acid critical for infant growth; therefore, the lysine blockage has been raised as a nutritional concern [33]. This concern has led to advancement in recommendations for formula composition (6.7 g of available lysine per 100 g protein), analytical methods to measure lysine bioavailability, and processing conditions to optimize the quality of infant formulas [30, 33, 34]. Steam-cooking of meat-based baby foods seems to hinder in vitro proteolysis, although it reduces allergenic responses in children with atopic dermatitis compared to raw meat [35].
2.3. Food structure
Baby foods are diverse in their physical structure: the spatial arrangement of carbohydrates, lipids, and proteins varies considerably from sweet potato mash to meat puree [36–39]. The structure of baby food affects the rate and extent of its protein digestion, for digestive processes are to convert a structured food into a bioavailable form. A milk emulsion of the native milk fat globule structure has a lower rate of proteolysis during in vitro gastric digestion compared to homogenized or homogenized/pasteurized infant formulas [40]. The adsorption of proteins to lipid interfaces in an emulsified food or the protein-lipid interactions can change the accessibility of cleavage sites to digestive enzymes and modify the local flexibility of substrates [41]. For grains, such as rice, maize and sorghum, the structure of protein bodies and endosperm cell walls affects the stability of their storage proteins during cooking and digestion [42, 43]. Since food properties are variables affecting proteolysis, characterization of baby foods is valuable to obtain insights into infant protein digestion.
3. Infant physiology that relates to protein digestion
Digestion is a complex process of mechanical and chemical breakdown of food that involves multiple organs and tissues [44]. To reduce its complexity for studies, this process is generally dissected into three physiological phases: oral phase, gastric phase, and intestinal phase.
3.1. Oral phase
During the oral phase, food materials are broken down and lubricated with saliva before being swallowed [45]. These processes change the particle size and viscosity of the ingested food, increasing protein accessibility. The oral phase is often neglected when modeling infant digestion because of the relatively short transit time of liquid foods in newborn infants’ oral cavity [46]. However, significant oral processing of food occurs with teething and neural development of oral movement control. Infants are introduced to semi-solid and solid foods during 6 months to 2 years, and they respond with different chewing and swallowing behaviors to help food breakdown [47, 48].
As infants’ oral cavity and nervous system develop, the main pattern of their oral movement shifts from suckling to chewing (Figure 1). At the age of 4 to 6 months, infants move the tongue gently back and forth as food enters mouth [49]. At 6 months, most infants are able to remove pureed foods by full lip occlusion around the spoon, yet they have difficulty retaining viscous and solid foods [47]. From 6 to 10 months, infants can eat foods with tiny lumps without gagging [49]. Around 8 months, teeth erupt [50]; from 12 to 24 months, initiation of chewing becomes predominant [47, 48]. In parallel, their eating behavior becomes more efficient, including better lip control, increased tongue mobility, and decreased involvement of lips and cheeks in swallowing [47]. Also, both the time of chewing and the number of chewing cycles to process food before swallowing decrease as infants grow [51]. One hypothesis for these decreases is that older children, with increased eating efficiency, require less efforts to achieve the same particle size or viscosity of the food after chewing, which may hold constant across ages [47]. Unfortunately, the particle size and texture of bolus before swallowing by infants are unknown, since obtaining such information requires subjects to spit out the chewed bolus before swallowing, which is challenging to implement with infants. Though bite force of infants is unknown, data from children indicates a positive correlation between bite force and age. In particular, bite force increases from 196.0±96.1 to 480.2±162.4 N in children from 3–5 to 12–14 years old, respectively [52]. No significant difference exists between pre-pubescent boys and girls [52, 53].
Figure 1.

Development of oral behaviors in response to foods.
Ben-Aryeh et al. [54] analyzed whole unstimulated saliva of healthy infants aged 3 days to 12 months, and compared to data from adults. Significant differences of salivary compositions were not found in terms of diet, tooth eruption or gender, but were observed between age groups.
3.2. Gastric phase
Gastric juice contains water, hydrochloric acid, intrinsic factor, enzymes, and mucus. Infants’ gastric secretory capacity were studied by Agunod et al. [55] and Rodbro et al. [56]. Their findings show that gastric secretion in infants was below adult level (Table 1a and 1b).
Table 1a.
Gastric outputs after stimulation with Histalog (1.0 mg/kg body weight) in full-term infants (1–110 days), children (4–9 years), and adults. Adapted from Agunod et al. [55]
| Subjects | Body weight (kg) | Volume (mL/hr) | Acid (mEq/hr) | Pepsin (mg/hr) | |
|---|---|---|---|---|---|
| 1 day | Mean | 3.4 | 3.3 | 0.03 | 0.18 |
| (n=10) | Range | 0.8–9.3 | 0.01–0.9 | 0–0.53 | |
| 3–8 days | Mean | 3.3 | 3.7 | 0.06 | 0.21 |
| (n=7) | Range | 1.3–4.7 | 0.01–0.12 | 0.11–0.44 | |
| 10–11 days | Mean | 3.0 | 4.0 | 0.12 | 0.46 |
| (n=5) | Range | 2.0–5.4 | 0.11–0.15 | 0.29–0.67 | |
| 14–17 days | Mean | 3.4 | 6.4 | 0.19 | 0.88 |
| (n=4) | Range | 1.5–12.0 | 0.04–0.37 | 0.20–1.46 | |
| 25–32 days | Mean | 3.9 | 3.1 | 0.08 | 0.32 |
| (n=3) | Range | 3.0–3.2 | 0.03–0.14 | 0.21–0.38 | |
| 67–110 days | Mean | 4.9 | 13.4 | 0.47 | 1.34 |
| (n=4) | Range | 7.1–19.9 | 0.14–0.65 | 1.25–1.44 | |
| 4–9 years | Mean | - | 42.5 | 4.88 | 18.5 |
| (n=2) | Range | 35.0–50.0 | 3.89–5.88 | 14.2–29.8 | |
| Adults | Mean | 70.0 | 143.2 | 13.06 | 41.9 |
| - | Range | - | 7.17–18.94 | 32.7–51.0 | |
Table 1b.
Gastric outputs after stimulation with histamine acid phosphate (40 μg/kg body weight) in healthy infants (9–30 months) and adults. Adapted from Rodbro et al. [64]
| Subjects | Body weight (kg) | Volume (mL/hr) | Acid (mEq/hr) | Pepsin (mg/hr) | |
|---|---|---|---|---|---|
| 9–30 months | Mean | 11.3 | 49 | 1.9 | 12.5 |
| (n=18) | Range | 8.0–13.9 | 25–105 | 0.3–3.7 | 2.2–26.0 |
| Adults | Mean | - | 238 | 20.0 | 80.9 |
| (n=12) | Range | 148–339 | 6.8–34.9 | 32.4–153.2 | |
A direct measurement to elucidate effects of gastric acid on protein digestion is gastric pH in response to a meal. Fluctuation in pH leads to conformational changes of enzymes and proteins, affecting enzyme-substrate interactions. Figure 2 shows the change of gastric pH after meal in healthy infants and adults [57–63]. While postprandial gastric pH of infants is relatively high compared to adults, it depends on gastric acid secretion as well as meal content. When food is ingested, parietal cells in the gastric glands are stimulated to secrete acid into the stomach lumen [44]. This process, regulated by the nervous and endocrine system, is limited by the secretory capacity of infants [55, 56, 64]. Meanwhile, foods like breast milk, with a strong buffering capacity and a slow gastric emptying, can slow down the decrease of gastric pH [57, 65]. Compared to formula, clear liquid (glucose-electrolyte solution) resulted in a lower gastric pH in preterm infants (Figure 3) [65].
Figure 2.

Postprandial gastric pH of healthy infants and adults.
Figure 3.
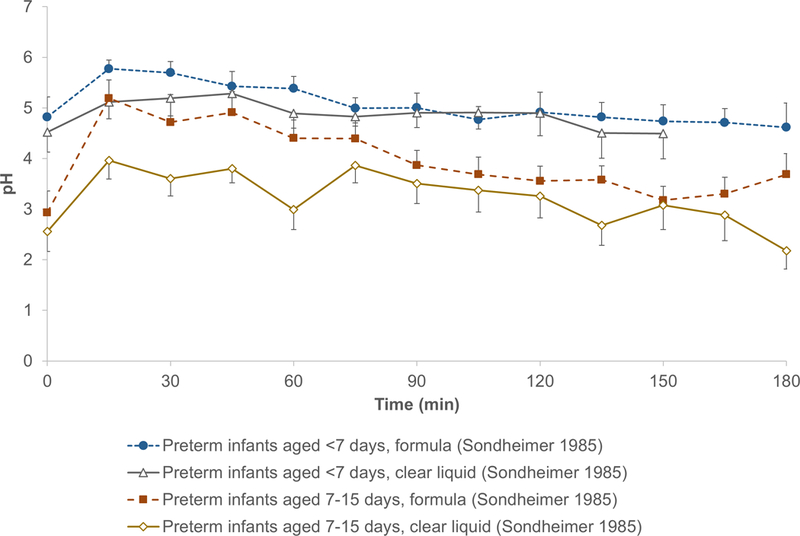
Postprandial gastric pH of preterm infants after formula and clear liquid feeding.
The major digestive protease in human stomach is pepsin. Pepsinogen is secreted by gastric chief cells and activated into pepsin via autocatalytic cleavage of an N-terminal peptide when it comes in contact with acid. As an aspartic protease, pepsin is most active in acidic environments around pH 2, and inactivated at pH above 4.5 [44]. Questions remain whether pepsin acts in the stomach of newborns [46], since the postprandial pH in infant stomach stays above 4.5 for about two hours (Figure 2). The capacity of pepsin output after stimulation is shown in Table 1a and 1b. From birth to 3 months, pepsin secretion rises progressively, but remains well below adult levels [55]. The lower pepsin secretion coupled with higher gastric pH indicates less protein is hydrolyzed by pepsin in an infant’s stomach.
Gastric emptying rate determines the length of time for interactions between hydrochloric acid, pepsin and dietary proteins. Human milk generally empties faster than infant formula, although large variations in gastric half-emptying have been reported [12]. Van Den Driessche et al. [66] reported that the mean half-emptying time was 65 (range 27–98) minutes for formula and 47 (range 16–86) minutes for breast milk. While gastric emptying is often considered slower in infants than in older children and adults, many covariates exist and this speculation may be more relevant to properties of the tested food rather than age [67, 68]. Although regulations of gastric emptying are not fully understood, the type of protein is a factor that influences gastric emptying. Specifically, whey protein empties faster than casein as whey protein is soluble while casein clots in the stomach, affecting the amino acid absorption and whole body protein anabolism [69–71]. These findings have implications for infants with delayed gastric emptying and infants with low body weight, suggesting that protein composition and structure could be formulated specifically for particular infants to achieve optimal growth and metabolism.
3.3. Intestinal phase
Hydrolysis of dietary proteins begins in the stomach, while major proteolysis occurs in the small intestine [44, 72]. Specificity of pancreatic proteases and their activity in infants are summarized in Table 2 [72–75]. In the intestine of healthy newborns aged 3–15 days, concentrations of trypsin during fasting and after bottle-fed with breast milk were 119.5±78.3 and 93.8±49.0 μg/mL, and concentrations of chymotrypsin during fasting and after feeding were 100.3±66.5 and 86.5±45.0 μg/mL, respectively [76]. Lower concentrations and activities of these enzymes indicate less proteolysis occurs in infant intestine.
Table 2.
Pancreatic proteases in infants
| Enzyme | Specificity [72] | Activity in infants |
|---|---|---|
| Trypsin | Endopeptidase, cleaves bonds at basic amino acids - lysine and arginine | In newborns (at birth and 30 days) 90% to 100% of activity in children (>2 years) after pancreozymin-secretin stimulation [74]. At birth 8% and at 1 month 25 % of activity in children (9 months to 13 years) [73]. |
| Chymotrypsin | Endopeptidase, cleaves bonds at aromatic amino acids - phenylalanine, tyrosine, tryptophan | In newborns (at birth and 30 days) 50% to 60% of activity in children (>2 years) [74]. |
| Elastase | Endopeptidase, cleaves bonds at small amino acids - alanine, glycine, serine | Low activity in infancy, develops during the first 2 years of life [75]. |
| Carboxypeptidases | Exopeptidase, carboxypeptidase A cleaves C-terminal residues with aromatic or aliphatic side chains, carboxypeptidase B cleaves C-terminal residues with basic side chains. | In newborns (at birth and 30 days) 10% to 25% of carboxypeptidase B activity in children (>2 years) [74]. |
In contrast to different levels of enzymes in infants and adults, intestinal pH is generally similar across age groups. Bicarbonate is released from the pancreas into the duodenum to neutralize the acidic chyme from the stomach. In healthy young and elderly adults, duodenal pH is typically between 6 and 7, but it can be temporarily reduced to 5.4 after a meal [60, 62, 77]. Similarly, duodenal pH in normal infants and children aged from 1 month to 12 years is 6 to 7, and the average pH of duodenal content collected within 1-hour following a 40% cream meal is between 5 to 6 [78].
Motility in the small intestine is to propel intestinal contents, mix the contents with digestive juice, and retain them long enough for digestion and absorption [44]. Differences in the amplitude of non-migrating contractions and the number of pressure waves have been observed among preterm infants, term infants and adults [79]. However, information about intestinal transit time of ingested food in infants is limited. Preterm infants (gestational age 26–33 weeks, postnatal age 6–37 days) fed with expressed human milk had gastric half-emptying time of 1.0 (range 0.5–3.0) hours and orocecal transit time of 3.1 (range 1.3–6.1) hours [80], suggesting that small intestinal transit time is around 2 hours. The orocecal transit time has been measured in children, but results varied greatly: 90.2±20 minutes for children aged 1–5 years fed with 15 mL lactulose in 100 mL water [81], and 255 (range 165–390) minutes for children aged 3–17 years fed with 250 mg lactose-[13C]ureide in 200 mL chocolate milk [82].
Assimilation of dietary proteins in the small intestine is incomplete. 5.73% of cooked egg protein and 35.10% of raw egg protein have been found to escape digestion and absorption in the small intestine in healthy adults (mean age 27 years) [83]. Dietary proteins, peptides, or amino acids that escape assimilation can be fermented by gut bacteria, yielding branched chain fatty acids, amines, phenols, and indoles [84, 85]. Interestingly, Piper et al. [86] measured major bacterial metabolites along the gastrointestinal tract of weaned piglets, and results suggest that considerable protein fermentation has already occurred in the stomach. However, most information is generated from animal models or fecal samples; little is known about the gut environment for protein fermentation in human infants due to limited accessibility. Some clinical studies on human infants indicate that cow’s milk proteins and cow’s milk formulas are associated with infantile colic, or excessive crying [87]. However, many hypotheses exist about causes of infantile colic, ranging from protein intolerance to microbiota shifts [88, 89], and yet none have been resolved since the molecular mechanism is unclear.
4. Approaches to studying infant protein digestion
4.1. In vitro approaches
Advantages of using in vitro models to study mechanisms of protein digestion include no ethical concerns, better control, higher repeatability, lower cost, and high-throughput potential compared to in vivo models (Figure 4). To study infant protein digestion, in vitro models should mimic conditions of the infant gastrointestinal tract that are relevant to protein digestion. Physiological conditions of the infant digestive system have been neglected for in vitro studies until the last decade, as researchers have started to establish dedicated infant in vitro gastrointestinal models. Major adjustments include higher gastric pH, reduced enzyme concentration, and longer transit time compared to adult models [29, 40, 90–96]. Recent studies on this subject are summarized in Table 3.
Figure 4.

Comparison of in vitro and in vivo approaches of studies on infant protein digestion.
Table 3.
Recent studies on infant protein digestion using in vitro models
| Model | Food | Analytical techniques | Adaptations for infants | Reference |
|---|---|---|---|---|
| Static infant gastric digestion model (with porcine pepsin or human neonatal gastric juice) | Whey protein concentrate, infant formula | SDS-PAGE, HPLC, N-terminal sequencing, western blot | (1) Human neonatal gastric juice for in vitro digestion; (2) pH 6.5,5.0, 4.0, 3.5, 3.0 and 2.0; (3) 1hr incubation | [90] |
| Static infant gastrointestinal model | Human milk with and without pasteurization (62.5°C for 30min) | LC-MS/MS | (1) Gastric pH 4.0; (2) 15min for gastric digestion; (3) 5min for intestinal digestion. | [96] |
| Static infant gastrointestinal model | Human milk, infant formula | LC-MS/MS | (1) Gastric pH 3.5; (2) 30min for gastric digestion; (3) 60min for intestinal digestion. | [91] |
| Static infant gastrointestinal model | Human milk, infant formula | LC-MS/MS, ELISA | (1) Gastric pH 4.0; (2) 100U pepsin/mg protein; (3) 5mg pancreatin and 30mg bile salt extract/mL sample for duodenal digestion. | [110] |
| Static infant gastroduodenal model (with enzymes and surfactants) | Milk protein solutions, Maillard reaction products, food matrices (milk and yogurt) with different processing | SDS-PAGE, ELISA, immunoblotting, RP-HPLC, LC-MS/MS | (1) Gastric pH 3.0 instead of 2.5; (2) 22.75U pepsin/mg substrate (8-fold reduction compared to adult model); (3) 1mM duodenal bile salt (4-fold reduction), 3.45U trypsin/mg substrate (10-fold reduction), 0.04U chymotrypsin/mg substrate (10-fold reduction). | [29, 92, 93] |
| Semi-dynamic infant gastric model | Model formulas with different processing | Chemical characterization for lipids and proteins, structural characterization for emulsions | Three subsequent stages: 0–60min at pH 6, 60–120min at pH 5, 120–180min at pH 4. | [40] |
| pH static and dynamic gastric models for infants and adults | Milk protein solutions, protein emulsions | SDS-PAGE, light scattering and fluorescence microscopy | (1) gastric digestion time 240min (120min for adults); (2) pH gradients from 6.5 to 3.5 (4.5 to 1.5 for adults) in dynamic models, and pH 2.5 in static models; (3) 210U pepsin/mg protein (240U for adults). | [94] |
| Dynamic gastric model mimicking infants aged 9–12 months | Human milk | SDS-PAGE, LC-MS/MS, immunoblotting | (1) pH gradients from 7 to 2 over 60min; (2) enzymatic content; (3) contraction; (4) half gastric emptying at 48min. | [95] |
Although parameters have been adjusted to adapt digestion models to infant conditions, the extent of adjustments vary among research groups because of limited understanding of infant physiology. Additionally, the composition and the amount of food material as well as the techniques to analyze proteolysis are diverse across studies. All these differences lead to difficulties in comparing results of protein digestion among research groups. Nevertheless, several studies offer invaluable comparisons between digestion models, revealing how varying conditions affect proteolysis.
Chatterton et al. [90] treated human milk with gastric juice from a human neonate at different pH in vitro, and meanwhile collected gastric aspirates from two infants following administration of human milk (in vivo). Analysis by electrophoresis indicated that a large amount of human milk proteins remained intact for at least 1 hour after ingestion both in vitro and in vivo, but patterns of proteolysis as shown on gels were different between these two approaches. Such discrepancy may result from the variation of samples or subjects. It may also indicate that the in vitro digestion model, using neonate gastric juice that was collected, centrifuged and frozen, missed a crucial factor that plays an important role in vivo. Dupont et al. [92] compared effects of an in vitro infant model and an adult model on the hydrolysis of purified food proteins. Their infant and adult models differed only in pH and enzyme concentrations, leading to different rates of proteolysis: ovalbumin and β-casein were digested slowly, while β-lactoglobulin was degraded more extensively in the infant model. This research group also compared kinetics of formula proteolysis in an in vitro dynamic infant gastrointestinal model to in vivo data collected from piglets. A good correlation was shown between the two models based on extent of proteolysis and residual gastric volume [97], but whether these results correlate with data from human infants is unclear. The Lesmes group [94] tested milk proteins and their emulsions with a pH-static and pH-dynamic model for both infants and adults. They showed that proteolysis and emulsion stability differed between constant pH and dynamic pH as well as between infants and adults. Overall, varied modeling conditions alter the specificity, rate, and extent of proteolysis.
4.2. In vivo approaches
Animal and human studies offer valuable in vivo data on food digestion. In vivo studies provide a biological environment that is hard to replicate in vitro, because the gastrointestinal tract is within a complex biological system containing delicate feedback controls. For example, secretion of digestive juice and intestinal motility in response to a meal are automatically controlled in vivo, but are difficult to reproduce through in vitro experiments. Nevertheless, disadvantages of in vivo studies include ethical concerns, sampling limitations, subject variations, and high costs. Before conducting an in vivo trial, issues such as subject selection, sample collection, and analytical techniques need to be considered. Studies for infant protein digestion on animal and human infants are summarized in Table 4
Table 4.
Recent studies related to infant protein digestion using in vivo approaches
| Subjects | Description | Diet | Analysis | Reference |
|---|---|---|---|---|
| Mice | Male, weaning, n=6 per group | Beans and rice. | Chemical analysis of diet and feces. Protein digestibility-corrected amino acid scores. | [111] |
| 0 and 8-day-old | Colostrum and mature milk from lactating mice. | SDS-PAGE, western blot, and amino acid sequencing of stomach content. | [112] | |
| Piglets | Fed from day 7 to 28, n = 28 per group | Adequate- or high-protein formulas. | Growth measurements, hormone assays, western blot, adipocyte diameter, real-time PCR of blood and adipose tissue. | [101] |
| Fed from day 2 to 28, n = 18 | Infant formula adapted to piglets. | Chemical analysis, SDS-PAGE, immunoblotting, ELISA, and LC-MS/MS of content from stomach, proximal jejunum, median jejunum, and ileum. | [102] | |
| Piglets weaned at 25-day-old and fed respective diets for 3 weeks, n = 6 per group | Diets high (26%) and low (18%) in protein and with or without two different sources of carbohydrates. | Chemical analysis for major bacterial metabolites from stomach, jejunum, ileum, caecum, proximal and distal colon. | [86] | |
| Piglets weaned at 17-day-old, and fed experimental diets from day 27 to 34, n = 5 per group | Casein diet, casein sesame expeller diet, or casein soya bean meal diet. | Chemical analysis of diet and ileum content for ileal digestibility calculation. | [100] | |
| Human infants | Term, 4 to 12-day-old, n = 3 | Expressed breast milk. | LC-MS/MS analysis of gastric content collected via a nasogastric tube. | [106, 107] |
| Premature, 1 to 28-day-old, n = 56 in total | Human milk with an ultraconcentrated liquid human milk fortifier or a powder human milk fortifier. | Nutritional intake analysis, growth measurements, blood analysis. | [113] | |
| Birth to 12-month-old, n = 305 in total | Low-protein formula with probiotics, standard formula, or breast milk, and complementary foods. | Growth measurements, blood analysis for biomarkers of protein metabolism (blood urea nitrogen, insulin growth factor-1, insulinogenic amino acids). | [114] | |
| Premature, gestation <32 weeks, n = 60 in total | Breast milk with high-protein fortifier or low-protein fortifier. | Growth measurements, blood analysis for clinical chemical parameters (albumin, cystatin C, and urea). | [115] | |
Among common animal models, piglets are similar to human infants in terms of anatomy, histology, and digestive physiology [98, 99]. Particularly, a 3-week-old piglet is comparable with a 3-month-old human infant in the activity of major proteases and fecal measures of protein digestibility; however, large differences exist between the species at birth. Moreover, the gut capacity of a 3-week-old piglet is double that of a 3-month-old infant [98, 99]. Thus, appropriate physiological stage and meal portion are needed to acquire physiologically comparable results.
With animals, several methods are available for sample collection and subsequent analysis. (1) Fecal collection: this non-invasive method of sampling aims at end products coming out of the body, revealing effects on food molecules after the whole process of digestion, absorption and excretion, and is applicable to human as well [98], offering opportunities for model validation. (2) Dynamic collection of digestive contents: digestive contents from the gastrointestinal tract can be collected through a cannula [100]; it allows information to be obtained at various time points after feeding, and reduces certain inter-individual variability, as treatments are repeatedly tested on the same animal. However, surgery and special management for animals are required, such as antibiotic treatments, which may modify their digestion process. (3) Multi-point sample collection after slaughter: collecting digested contents and tissue samples enables versatile analysis [86, 101, 102], revealing the fate of food molecules along the gastrointestinal tract and the effects of diet on blood or adipose tissues. The drawback of this method is that sample collection is only at one specific time, so studying digestion over time requires extensive resources. If resources are available, these methods of sample collection can be combined to obtain multi-aspect information that is impossible to be obtained from human infants and provide insights into the mechanism of protein digestion.
Compared to animal models, studies on human infants are constrained by ethical considerations and difficulties in subject recruitment. Most studies on infant digestion have been performed in hospitals. Hospitalization may affect infants’ digestion, even though the reason of hospitalization is not related to digestive system. Anthropometric measurements can be taken on infants, such as whole body protein synthesis and breakdown, metabolic and energy balance, and sometimes blood tests [103, 104]. Besides input and output measures, the process of protein digestion has been explored by analyzing contents from infant gastrointestinal tract, which were collected through nasogastric and transintestinal tubes [59, 105–107]. In addition to improved methods for sample collection, advancements in analytical techniques provide more detailed information of proteolysis in human infants. In the past, total nitrogen balance was calculated from ingested food and secreted feces [105]; current analyses like liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) have disclosed peptides that are released in infants’ stomach [106, 107]. Advances in in vivo studies have improved our understanding of protein digestion under physiological conditions, revealing the fate of dietary proteins and their impact on infants.
5. Challenges
Protein digestion of baby foods is a highly complex process. Gaining a thorough understanding of this process and revealing its implications for feeding practices are challenging. Although new technology has facilitated steady progress in the last few decades, there remain challenges limiting breakthroughs in this field:
-
(1)
Comprehensive characterization of baby food properties. Composition of baby food is often analyzed in studies, but chemical modifications of dietary proteins and physical structure of the food are less commonly considered, which are also important for protein digestion.
-
(2)
Collection of detailed data from human infants. Data collection from human infants is technically and ethically difficult. Current knowledge of infant digestion is mainly confined to the upper gastrointestinal tract, while detailed information on the fate of dietary proteins in infants’ lower digestive tract is missing, which veils the impact of diet on infant health.
-
(3)
Evaluation of infant digestion models. At present, how appropriate the measured proteolysis in an animal or in vitro model representing a human infant is undefined. Even if many in vitro parameters are set based on available infant data, critical factors are invariably missing from models. The output of protein digestion in different models has not been systematically evaluated. Without proper evaluations of research models, the translation of laboratory findings to infant feeding is restricted.
6. Future directions
The emphasis of protein science is moving from amino acid provision to a dynamic nourishment and a complex bioactive signaling system. As scientists recognize the biological activities from proteins to specific peptides and their actions along the entire biogeography of the gastrointestinal tract, the role of specific proteolysis is becoming central to the success in protein nourishment – delivering beneficial functions and managing health risks such as intolerance and allergies. Improving the efficacy of protein nourishment for infants requires quantitative estimates of the performance of infant models, for example, dependence of the outcome (characteristics of proteolysis) on each independent factor (pH, enzyme concentration, bacterial contribution, etc.). The relationship of in vitro dissolution and in vivo bioavailability has been studied extensively in pharmaceutics, which has advanced drug discoveries [108, 109]. While foods are more complicated than drugs, the concept and principle of in vitro-in vivo correlation can be applied to studying food digestion, such as identifying fundamental variables which define protein digestion in infants. This information will guide the development of models that grasp a greater dimensionality of protein nourishment in time and space. New knowledge of infant protein digestion and its consequences will provide opportunities for baby food development, precise treatment, and effective diet and health management for infants.
7. Conclusions
Developmental events continuously take place during infancy. Food properties significantly influence protein digestion and individual health, and physiology of the infant digestive system is distinct from adult, so baby food should be developed to reflect these differences. Food and nutrition are getting to the stage where detailed and quantitative scientific knowledge will guide precise health management. Knowledge about infant protein digestion will be obtained by systematic scientific research with various approaches. The quality of research models determines the accuracy of our understanding of how dietary proteins are processed in infants. Study approaches need to be refined to consider food properties and infant specificity. Advances in understanding protein digestion of baby foods at a molecular and cellular level will empower biological discoveries and food innovations to improve infant health effectively.
Acknowledgements
This work was supported by the UC Davis RISE program and the National Institutes of Health Awards R01AT008759 and RO1AT007079.
Footnotes
Conflict of interest
The authors have declared no conflicts of interest.
References
- [1].Michaelsen KF, Greer FR, Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [DOI] [PubMed] [Google Scholar]
- [2].Nelson WE, Kliegman R, Nelson textbook of pediatrics, Saunders, Philadelphia, PA: 2011. [Google Scholar]
- [3].Lonnerdal B, Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [DOI] [PubMed] [Google Scholar]
- [4].Galli SJ, Tsai M, Piliponsky AM, The development of allergic inflammation. Nature 2008, 454, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grimshaw KEC, Maskell J, Oliver EM, Morris RCG, et al. , Introduction of complementary foods and the relationship to food allergy. Pediatrics 2013, 132, e1529–e1538. [DOI] [PubMed] [Google Scholar]
- [6].Complementary feeding: report of the global consultation, and summary of guiding principles for complementary feeding of the breastfed child, World Health Organization; 2003. [Google Scholar]
- [7].Eidelman AI, Schanler RJ, Johnston M, Landers S, et al. , Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [DOI] [PubMed] [Google Scholar]
- [8].Agostoni C, Decsi T, Fewtrell M, Goulet O, et al. , Complementary feeding: a commentary by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [DOI] [PubMed] [Google Scholar]
- [9].Victora CG, Bahl R, Barros AJD, França GVA, et al. , Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. The Lancet 2016, 387, 475–490. [DOI] [PubMed] [Google Scholar]
- [10].Brown K, Dewey K, Allen L, Complementary feeding of young children in developing countries: a review of current scientific knowledge, World Health Organization, Geneva: 1998. [Google Scholar]
- [11].Neville MC, Anderson SM, McManaman JL, Badger TM, et al. , Lactation and neonatal nutrition: defining and refining the critical questions. J. Mammary Gland Biol. Neoplasia 2012, 17, 167–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bourlieu C, Menard O, Bouzerzour K, Mandalari G, et al. , Specificity of infant digestive conditions: some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [DOI] [PubMed] [Google Scholar]
- [13].Poquet L, Wooster TJ, Infant digestion physiology and the relevance of in vitro biochemical models to test infant formula lipid digestion. Mol. Nutr. Food Res. 2016, 60, 1876–1895. [DOI] [PubMed] [Google Scholar]
- [14].Wal JM, Cow’s milk proteins/allergens. Ann. Allergy, Asthma Immunol. 2002, 89, 3–10. [DOI] [PubMed] [Google Scholar]
- [15].Shan L, Molberg O, Parrot I, Hausch F, et al. , Structural basis for gluten intolerance in Celiac Sprue. Science 2002, 297, 2275–2279. [DOI] [PubMed] [Google Scholar]
- [16].Duodu KG, Taylor JRN, Belton PS, Hamaker BR, Factors affecting sorghum protein digestibility. Journal of Cereal Science 2003, 38, 117–131. [Google Scholar]
- [17].Liu DS, Wang YY, Yu Y, Hu JH, et al. , Effects of enzymatic dephosphorylation on infant in vitro gastrointestinal digestibility of milk protein concentrate. Food Chem. 2016, 197, 891–899. [DOI] [PubMed] [Google Scholar]
- [18].de Jongh HHJ, Robles CL, Timmerman E, Nordlee JA, et al. , Digestibility and IgE-binding of glycosylated codfish parvalbumin. Biomed Res. Int. 2013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boonvisut S, Whitaker JR, Effect of heat, amylase, and disulfide bond cleavage on the in vitro digestibility of soybean proteins. J. Agric. Food Chem. 1976, 24, 1130–1135. [DOI] [PubMed] [Google Scholar]
- [20].Hamaker BR, Kirleis AW, Butler LG, Axtell JD, Mertz ET, Improving the in vitro protein digestibility of sorghum with reducing agents. Proc. Natl. Acad. Sci. U. S. A. 1987, 84, 626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Breiteneder H, Mills ENC, Molecular properties of food allergens. J. Allergy Clin. Immunol. 2005, 115, 14–23. [DOI] [PubMed] [Google Scholar]
- [22].Sicherer SH, Sampson HA, Food allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [DOI] [PubMed] [Google Scholar]
- [23].Host A, Frequency of cow’s milk allergy in childhood. Annals of Allergy Asthma & Immunology 2002, 89, 33–37. [DOI] [PubMed] [Google Scholar]
- [24].Hays T, Wood RA, A systematic review of the role of hydrolyzed infant formulas in allergy prevention. Arch. Pediatr. Adolesc. Med. 2005, 159, 810–816. [DOI] [PubMed] [Google Scholar]
- [25].Alexander DD, Cabana MD, Partially hydrolyzed 100% whey protein infant formula and reduced risk of atopic dermatitis: a meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 422–430. [DOI] [PubMed] [Google Scholar]
- [26].Szajewska H, Horvath A, Meta-analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Curr. Med. Res. Opin. 2010, 26, 423–437. [DOI] [PubMed] [Google Scholar]
- [27].Greer FR, Sicherer SH, Burks AW, Comm N, Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [DOI] [PubMed] [Google Scholar]
- [28].Boyle RJ, Ierodiakonou D, Khan T, Chivinge J, et al. , Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta-analysis. BMJ-British Medical Journal 2016, 352, i974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moscovici AM, Joubran Y, Briard-Bion V, Mackie A, et al. , The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants. Food Funct. 2014, 5, 1898–1908. [DOI] [PubMed] [Google Scholar]
- [30].Mehta BM, Deeth HC, Blocked lysine in dairy products: formation, occurrence, analysis, and nutritional implications. Comprehensive Reviews in Food Science and Food Safety 2016, 15, 206–218. [DOI] [PubMed] [Google Scholar]
- [31].Feeney RE, Means GE, Bigler JC, Inhibition of human trypsin, plasmin, and thrombin by naturally occurring inhibitors of proteolytic enzymes. J. Biol. Chem. 1969, 244, 1957–1960. [PubMed] [Google Scholar]
- [32].Rehman ZU, Shah WH, Thermal heat processing effects on antinutrients, protein and starch digestibility of food legumes. Food Chem. 2005, 91, 327–331. [Google Scholar]
- [33].Finot PA, Aeschbacher HU, Hurrell RF, Liardon R, The Maillard reaction in food processing, human nutrition and physiology, Birkhauser Verlag, Basel: 1990. [Google Scholar]
- [34].Finot PA, Historical perspective of the Maillard reaction in food science. Ann. N. Y. Acad. Sci. 2005, 1043, 1–8. [DOI] [PubMed] [Google Scholar]
- [35].Restani P, Fiocchi A, Restelli R, Velona T, et al. , Effect of technological treatments on digestibility and allergenicity of meat-based baby foods. J. Am. Coll. Nutr. 1997, 16, 376–382. [DOI] [PubMed] [Google Scholar]
- [36].Ahmed J, Ramaswamy HS, Viscoelastic properties of sweet potato puree infant food. J. Food Eng. 2006, 74, 376–382. [Google Scholar]
- [37].Ahmed J, Ramaswamy HS, Viscoelastic and thermal characteristics of vegetable puree-based baby foods. J. Food Process Eng. 2006, 29, 219–233. [Google Scholar]
- [38].Ahmed J, Ramaswamy HS, Dynamic rheology and thermal transitions in meat-based strained baby foods. J. Food Eng. 2007, 78, 1274–1284. [Google Scholar]
- [39].Ramamoorthi L, Lee Y, Brewer S, Effect of food matrix and heat treatment on the rheological properties of salmon-based baby food. J. Food Eng. 2009, 95, 432–437. [Google Scholar]
- [40].Bourlieu C, Menard O, De La Chevasnerie A, Sams L, et al. , The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during in vitro gastric digestion. Food Chem. 2015, 182, 224–235. [DOI] [PubMed] [Google Scholar]
- [41].Mackie A, Macierzanka A, Colloidal aspects of protein digestion. Current Opinion in Colloid & Interface Science 2010, 15, 102–108. [Google Scholar]
- [42].Oria MP, Hamaker BR, Axtell JD, Huang CP, A highly digestible sorghum mutant cultivar exhibits a unique folded structure of endosperm protein bodies. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 5065–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duodu KG, Nunes A, Delgadillo I, Parker ML, et al. , Effect of grain structure and cooking on sorghum and maize in vitro protein digestibility. Journal of Cereal Science 2002, 35, 161–174. [Google Scholar]
- [44].Johnson LR, Gastrointestinal physiology, Elsevier Health Sciences; 2013. [Google Scholar]
- [45].Chen JS, Food oral processing - a review. Food Hydrocolloids 2009, 23, 1–25. [Google Scholar]
- [46].Hamosh M, Digestion in the newborn. Clin. Perinatol. 1996, 23, 191–209. [PubMed] [Google Scholar]
- [47].Stolovitz P, Gisel EG, Circumoral movements in response to three different food textures in children 6 months to 2 years of age. Dysphagia 1991, 6, 17–25. [DOI] [PubMed] [Google Scholar]
- [48].Blossfeld I, Collins A, Kiely M, Delahunty C, Texture preferences of 12-month-old infants and the role of early experiences. Food Qual. Prefer. 2007, 18, 396–404. [Google Scholar]
- [49].Carruth BR, Skinner JD, Feeding behaviors and other motor development in healthy children (2–24 months). J. Am. Coll. Nutr. 2002, 21, 88–96. [DOI] [PubMed] [Google Scholar]
- [50].Bailey KV, Dental development in new guinean infants. J. Pediatr. 1964, 64, 97–100. [DOI] [PubMed] [Google Scholar]
- [51].Archambault M, Millen K, Gisel EG, Effect of bite size on eating development in normal children 6 months to 2 years of age. Phys. Occup. Ther. Pediatr. 1991, 10, 29–47. [Google Scholar]
- [52].Kamegai T, Tatsuki T, Nagano H, Mitsuhashi H, et al. , A determination of bite force in northern Japanese children. Eur. J. Orthod. 2005, 27, 53–57. [DOI] [PubMed] [Google Scholar]
- [53].Le Reverend BJD, Edelson LR, Loret C, Anatomical, functional, physiological and behavioural aspects of the development of mastication in early childhood. Br. J. Nutr. 2014, 111, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Benaryeh H, Lapid S, Szargel R, Benderly A, Gutman D, Composition of whole unstimulated saliva of human infants. Arch. Oral Biol. 1984, 29, 357–362. [DOI] [PubMed] [Google Scholar]
- [55].Agunod M, Yamaguch N, Lopez R, Luhby AL, Glass GBJ, Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am. J. Dig. Dis. 1969, 14, 400–414. [DOI] [PubMed] [Google Scholar]
- [56].Rødbro P, Krasilnikoff PA, Christiansen PM, Parietal cell secretory function in early childhood. Scand. J. Gastroenterol. 1967, 2, 209–213. [DOI] [PubMed] [Google Scholar]
- [57].Mason S, Some aspects of gastric function in the newborn. Arch. Dis. Child. 1962, 37, 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Harries JT, Fraser AJ, The acidity of the gastric contents of premature babies during the first fourteen days of life. Biol. Neonat. 1968, 12, 186–193. [DOI] [PubMed] [Google Scholar]
- [59].Cavell B, Postprandial gastric acid secretion in infants. Acta Paediatr. Scand. 1983, 72, 857–860. [DOI] [PubMed] [Google Scholar]
- [60].Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, et al. , Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 1990, 7, 756–761. [DOI] [PubMed] [Google Scholar]
- [61].Roman C, Carriere F, Villeneuve P, Pina M, et al. , Quantitative and qualitative study of gastric lipolysis in premature infants: Do MCT-enriched infant formulas improve fat digestion? Pediatr. Res. 2007, 61, 83–88. [DOI] [PubMed] [Google Scholar]
- [62].Russell TL, Berardi RR, Barnett JL, Dermentzoglou LC, et al. , Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [DOI] [PubMed] [Google Scholar]
- [63].Calbet JAL, Holst JJ, Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [DOI] [PubMed] [Google Scholar]
- [64].Rødbro P, Krasilnikoff P, Bitsch V, Gastric secretion of pepsin in early childhood. Scand. J. Gastroenterol. 1967, 2, 257–260. [DOI] [PubMed] [Google Scholar]
- [65].Sondheimer JM, Clark DA, Gervaise EP, Continuous gastric pH measurement in young and older healthy preterm infants receiving formula and clear liquid feedings. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 352–355. [DOI] [PubMed] [Google Scholar]
- [66].Van Den Driessche M, Peeters K, Marien P, Ghoos Y, et al. , Gastric emptying in formula-fed and breast-fed infants measured with the C-13-octanoic acid breath test. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 46–51. [DOI] [PubMed] [Google Scholar]
- [67].Maes BD, Ghoos YF, Geypens BJ, Hiele MI, Rutgeerts PJ, Relation between gastric emptying rate and energy intake in children compared with adults. Gut 1995, 36, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bonner JJ, Vajjah P, Abduljalil K, Jamei M, et al. , Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm. Drug Disposition 2015, 36, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Boirie Y, Dangin M, Gachon P, Vasson MP, et al. , Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 14930–14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jarvenpaa AL, Rassin DK, Raiha NCR, Gaull GE, Milk protein quantity and quality in the term infant II. Effects on acidic and neutral amino acids. Pediatrics 1982, 70, 221–230. [PubMed] [Google Scholar]
- [71].Tolia V, Lin CH, Kuhns LR, Gastric emptying using three different formulas in infants with gastroesophageal reflux. J. Pediatr. Gastroenterol. Nutr. 1992, 15, 297–301. [DOI] [PubMed] [Google Scholar]
- [72].Whitcomb DC, Lowe ME, Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007, 52, 1–17. [DOI] [PubMed] [Google Scholar]
- [73].Zoppi G, Andreotti G, Pajnofer F, Njai DM, Gaburro D, Exocrine pancreas function in premature and full term neonates. Pediatr. Res. 1972, 6, 880–886. [DOI] [PubMed] [Google Scholar]
- [74].Lebenthal E, Lee PC, Development of functional response in human exocrine pancreas. Pediatrics 1980, 66, 556–560. [PubMed] [Google Scholar]
- [75].Borulf S, Lindberg T, Cathodal elastase in duodenal Juice from children with gastrointestinal disorders. Pediatr. Res. 1981, 15, 1051–1054. [DOI] [PubMed] [Google Scholar]
- [76].Norman A, Ojamae O, Strandvi. B, Bile acids and pancreatic enzymes during absorption in the newborn. Acta Paediatr. Scand. 1972, 61, 571–576. [DOI] [PubMed] [Google Scholar]
- [77].McCloy R, Greenberg G, Baron J, Duodenal pH in health and duodenal ulcer disease: effect of a meal, Coca-Cola, smoking, and cimetidine. Gut 1984, 25, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Klumpp TG, Neale AV, The gastric and duodenal contents of normal infants and children: the duodenal enzyme activity and the gastric and duodenal reactions (H-ion). Am. J. Dis. Child. 1930, 40, 1215–1229. [Google Scholar]
- [79].Dumont RC, Rudolph CD, Development of gastrointestinal motility in the infant and child. Gastroenterol. Clin. North Am. 1994, 23, 655–671. [PubMed] [Google Scholar]
- [80].Bode S, Dreyer T, Greisen G, Gastric emptying and small intestinal transit time in preterm infants: a scintigraphic method. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 378–382. [DOI] [PubMed] [Google Scholar]
- [81].Myo K, Bolin TD, Oo T, Nyunt TW, et al. , Investigation of small-intestinal transit time in normal and malnourished children. J. Gastroenterol. 1999, 34, 675–679. [DOI] [PubMed] [Google Scholar]
- [82].Van den Driessche M, Van Malderen N, Geypens B, Ghoos Y, Veereman-Wauters G, Lactose- C-13 ureide breath test: a new, noninvasive technique to determine orocecal transit time in children. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 433–438. [DOI] [PubMed] [Google Scholar]
- [83].Evenepoel P, Claus D, Geypens B, Hiele M, et al. , Amount and fate of egg protein escaping assimilation in the small intestine of humans. American Journal of Physiology-Gastrointestinal and Liver Physiology 1999, 277, G935–G943. [DOI] [PubMed] [Google Scholar]
- [84].Windey K, De Preter V, Verbeke K, Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [DOI] [PubMed] [Google Scholar]
- [85].Fan PX, Li LS, Rezaei A, Eslamfam S, et al. , Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Peptide Sci. 2015, 16, 646–654. [DOI] [PubMed] [Google Scholar]
- [86].Pieper R, Boudry C, Bindelle J, Vahjen W, Zentek J, Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Arch. Anim. Nutr. 2014, 68, 263–280. [DOI] [PubMed] [Google Scholar]
- [87].Lucassen P, Assendelft WJJ, Gubbels JW, van Eijk JTM, et al. , Effectiveness of treatments for infantile colic: systematic review. Br. Med. J. 1998, 316, 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Camilleri M, Park SY, Scarpato E, Staiano A, Exploring hypotheses and rationale for causes of infantile colic. Neurogastroenterol. Motil. 2017, 29, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].de Weerth C, Fuentes S, Puylaert P, de Vos WM, Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 2013, 131, E550–E558. [DOI] [PubMed] [Google Scholar]
- [90].Chatterton DEW, Rasmussen JT, Heegaard CW, Sorensen ES, Petersen TE, In vitro digestion of novel milk protein ingredients for use in infant formulas: research on biological functions. Trends Food Sci. Technol. 2004, 15, 373–383. [Google Scholar]
- [91].Hernandez-Ledesma B, Quiros A, Amigo L, Recio I, Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int. Dairy J. 2007, 17, 42–49. [Google Scholar]
- [92].Dupont D, Mandalari G, Molle D, Jardin J, et al. , Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol. Nutr. Food Res. 2010, 54, 767–780. [DOI] [PubMed] [Google Scholar]
- [93].Dupont D, Mandalari G, Molle D, Jardin J, et al. , Food processing increases casein resistance to simulated infant digestion. Mol. Nutr. Food Res. 2010, 54, 1677–1689. [DOI] [PubMed] [Google Scholar]
- [94].Shani-Levi C, Levi-Tal S, Lesmes U, Comparative performance of milk proteins and their emulsions under dynamic in vitro adult and infant gastric digestion. Food Hydrocolloids 2013, 32, 349–357. [Google Scholar]
- [95].Zhang Q, Cundiff JK, Maria SD, McMahon RJ, et al. , Differential digestion of human milk proteins in a simulated stomach model. J. Proteome Res. 2014, 13, 1055–1064. [DOI] [PubMed] [Google Scholar]
- [96].Wada Y, Lonnerdal B, Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Pediatr. Res. 2015, 77, 546–553. [DOI] [PubMed] [Google Scholar]
- [97].Menard O, Cattenoz T, Guillemin H, Souchon I, et al. , Validation of a new in vitro dynamic system to simulate infant digestion. Food Chem. 2014, 145, 1039–1045. [DOI] [PubMed] [Google Scholar]
- [98].Darragh AJ, Moughan PJ, The three-week-old piglet as a model animal for studying protein digestion in human infants. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 387–393. [DOI] [PubMed] [Google Scholar]
- [99].Moughan P, Birtles M, Cranwell P, Smith W, Pedraza M, The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. Nutritional triggers for health and in disease 1992, 67, 40–113. [DOI] [PubMed] [Google Scholar]
- [100].Aguilera A, de Souza TCR, Mariscal-Landin G, Escobar K, et al. , Standardized ileal digestibility of proteins and amino acids in sesame expeller and soya bean meal in weaning piglets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 728–736. [DOI] [PubMed] [Google Scholar]
- [101].Morise A, Seve B, Mace K, Magliola C, et al. , Impact of intrauterine growth retardation and early protein intake on growth, adipose tissue, and the insulin-like growth factor system in piglets. Pediatr. Res. 2009, 65, 45–50. [DOI] [PubMed] [Google Scholar]
- [102].Bouzerzour K, Morgan F, Cuinet I, Bonhomme C, et al. , In vivo digestion of infant formula in piglets: protein digestion kinetics and release of bioactive peptides. Br. J. Nutr. 2012, 108, 2105–2114. [DOI] [PubMed] [Google Scholar]
- [103].Catzeflis C, Schutz Y, Micheli JL, Welsch C, et al. , Whole body protein synthesis and energy expenditure in very low birth weight infants. Pediatr. Res. 1985, 19, 679–687. [DOI] [PubMed] [Google Scholar]
- [104].Decsi T, Veitl V, Szasz M, Pinter Z, Mehes K, Plasma amino acid concentrations in healthy, full-term infants fed hydrolysate infant formula. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 62–67. [DOI] [PubMed] [Google Scholar]
- [105].Hirata Y, Matsuo T, Kokubu H, Digestion and absorption of milk protein in infants’ intestine. Kobe J. Med. Sci. 1965, 11, 103–109. [Google Scholar]
- [106].Dallas DC, Guerrero A, Khaldi N, Borghese R, et al. , A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J. Nutr. 2014, 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Holton TA, Vijayakumar V, Dallas DC, Guerrero A, et al. , Following the digestion of milk proteins from mother to baby. J. Proteome Res. 2014, 13, 5777–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Amidon GL, Lennernas H, Shah VP, Crison JR, A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [DOI] [PubMed] [Google Scholar]
- [109].Emami J, In vitro-in vivo correlation: from theory to applications. Journal of Pharmacy and Pharmaceutical Sciences 2006, 9, 169–189. [PubMed] [Google Scholar]
- [110].Su MY, Broadhurst M, Liu CP, Gathercole J, et al. , Comparative analysis of human milk and infant formula derived peptides following in vitro digestion. Food Chem. 2017, 221, 1895–1903. [DOI] [PubMed] [Google Scholar]
- [111].Kannan S, Nielsen SS, Mason AC, Protein digestibility-corrected amino acid scores for bean and bean-rice infant weaning food products. J. Agric. Food Chem. 2001, 49, 5070–5074. [DOI] [PubMed] [Google Scholar]
- [112].Yoneda M, Shiraishi J, Kuraishi T, Aoki F, et al. , Gastric proteinase digestion of caseins in newborn pups of the mouse. J. Dairy Sci. 2001, 84, 1851–1855. [DOI] [PubMed] [Google Scholar]
- [113].Olsen IE, Harris CL, Lawson ML, Berseth CL, Higher protein intake improves length, not weight, z scores in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 409–416. [DOI] [PubMed] [Google Scholar]
- [114].Inostroza J, Haschke F, Steenhout P, Grathwohl D, et al. , Low-protein formula slows weight gain in infants of overweight mothers. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Maas C, Mathes M, Bleeker C, Vek J, et al. , Effect of increased enteral protein intake on growth in human milk-fed preterm infants: a randomized clinical trial. JAMA Pediatrics 2017, 171, 16–22. [DOI] [PubMed] [Google Scholar]


