Abstract
Background and Purpose
We used a decision analysis approach to analyze triage strategies for patients with acute stroke symptoms while accounting for pre-hospital large vessel occlusion (LVO) screening methods and key time metrics.
Methods
Our decision analysis compared anticipated functional outcomes for patients within the tissue plasminogen activator (IV-tPA) treatment window in the “mothership” and “drip-and-ship” frameworks. Key branches of the model included IV-tPA eligibility, presence of an LVO, and endovascular therapy eligibility. Our decision analysis evaluated two pre-hospital LVO screening approaches: 1) no formal screening and 2) the use of clinical LVO screening scales. An excellent outcome was defined as modified Rankin Scale scores 0-1. Probabilities and workflow times were guideline-based or imputed from published studies. In sensitivity analyses, we individually and jointly varied transport time to the nearest primary stroke center (PSC), additional time required to transport directly to a comprehensive stroke center (CSC), and LVO screening scale predictive probabilities. We evaluated two separate scenarios: one in which ideal time metrics were achieved and one under current real-world metrics.
Results
In the ideal metrics scenario, the drip-and-ship strategy was almost always favored in the absence of formal LVO screening. For patients screened positive for an LVO, mothership was favored if the additional transport time to the CSC was less than 3-23 minutes. Under real-world conditions, in which PSC workflow is slower than ideal, the mothership strategy was favored in more scenarios, regardless of formal LVO screening. For example, mothership was favored with an additional transport time to the CSC of 32-99 minutes for patients screened positive for an LVO and 28-39 minutes in the absence of screening.
Conclusions
Joint consideration of LVO probability, screening, workflow times, and transport times may improve pre-hospital stroke triage. Drip-and-ship was more favorable when more ideal PSC workflow times were modeled.
Keywords: Stroke, Large Vessel Occlusion, Triage, Decision Analysis
Subject Terms: Health services, Cerebrovascular disease/stroke, Ischemic stroke
Introduction
Stroke is the fifth leading cause of death in the United States. Ischemic stroke accounts for 87% of strokes,1 and up to 12% of ischemic stroke is caused by a large vessel occlusion (LVO).2 In addition to intravenous tissue plasminogen activator (IV-tPA), endovascular therapy is a highly effective treatment option for patients with stroke caused by LVO.3–5 Recanalization therapy, whether with IV-tPA or endovascular therapy, is time-sensitive.4–6
In the United States, the Joint Commission provides eligible stroke centers with two types of certification: Primary Stroke Center (PSC) and Comprehensive Stroke Center (CSC). While both PSCs and CSCs offer IV-tPA, CSCs also provide endovascular therapy around the clock. This key difference has given rise to two triage strategies for patients with potential LVO. In the “drip-and-ship” paradigm, patients are first transported to the closest PSC for evaluation and IV-tPA treatment before transferring to a CSC for endovascular therapy when indicated. In the “mothership” paradigm, Emergency Medical Services (EMS) personnel bypass the PSC and transport patients directly to the nearest CSC for IV-tPA and endovascular therapy, if indicated. Based on expert consensus, the American Heart Association/American Stroke Association (AHA/ASA)’s Mission: Lifeline Stroke algorithm recommends the mothership approach if bypassing a PSC to go to a CSC leads to an additional travel time of less than 15 minutes.7
While prior observational studies did not find differences in functional outcomes between triage strategies,8, 9 a recent, multi-center registry found the drip-and-ship strategy to be associated with worse functional outcomes.10 Due to the paucity of conclusive clinical data, modeling has also been used to inform triage practices. For example, an analysis using probabilistic modeling of stroke care in Alberta, Canada suggested that the mothership approach may be preferable when the additional travel time between non-endovascular capable hospitals and endovascular capable hospitals is less than 60 minutes.11–13 Additionally, a recent decision analysis found that mothership was superior until this additional transport time exceeded 44 minutes.14 Prior analyses demonstrated that such models are sensitive to and must account for a multitude of variables.15 We designed a comprehensive decision analysis model to assess the effect of hospital and patient level time metrics and the variable predictive abilities of LVO screening scales on pre-hospital triage, while specifically evaluating model output in two distinct scenarios: one simulating an idealized stroke system of care and another simulating current, real-world time metrics.
Methods
Model Design
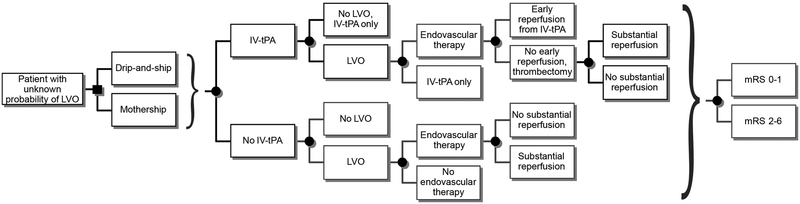
We constructed a decision tree model (Figure 1) to identify the optimal triage strategy in terms of the probability of an excellent functional outcome for patients with acute stroke symptoms. Our model took the view of first responders seeing a patient with acute stroke symptoms and faced with the decision of whether to take the patient to a PSC (drip-and-ship) or directly to a CSC (mothership). After the main decision point, subsequent decisions pertained to delivering IV-tPA, the presence of an LVO, and referral for endovascular therapy. The outcomes following all treatments (IV-tPA and endovascular therapy with or without reperfusion) were the 3-month modified Rankin Scale (mRS) score 0–1 versus 2–6.16 Input parameters for the decision analysis were derived from guidelines and published studies and are described below in detail. All analyses were conducted using TreeAge Pro 2017 (Williamstown, MA). No patient data were used for this model, so institutional review board approval was not sought. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Figure 1. Schematic of pre-hospital stroke triage decision tree model.
The key nodes in our analysis were triage strategy (drip-and-ship vs. mothership), treatment with IV-tPA, presence of an LVO, treatment with endovascular therapy, and anticipated outcomes for all treatment types. The decision analysis model used time-dependent and time-independent probabilities derived from published estimates and guidelines. Squares are decision nodes representing a decision-making point; circles represent chance nodes followed by subsequent event probabilities. mRS, modified Rankin Scale; LVO, large vessel occlusion; IV-tPA, intravenous tissue plasminogen activator; drip-and-ship, transporting to the closest primary stroke center first; mothership, transporting directly to a comprehensive stroke center.
Model Assumptions
We made several assumptions for our model. First, EMS was activated for an individual with stroke symptoms with clear time of last known well within the 4.5 hour IV-tPA onset-to-treatment window. This assumption was made because individuals presenting with symptoms in excess of 4.5 hours from last known well would not be eligible for IV-tPA and therefore would not necessarily benefit from rapid triage to a PSC. Second, the nearest PSC is closer to the patient’s location at the time of EMS contact than the nearest CSC. We made this assumption because the mothership approach would always be favored if a CSC is closer. Third, PSCs and CSCs had similar door-to-needle times to ensure that the analyses were performed without favoring either triage strategy. However, this assumption was interrogated in a sensitivity analysis and by comparing ideal and real-world scenarios.
Model Parameters
A variety of treatment eligibility and outcome probabilities were derived from guidelines and published studies and imputed into the decision analysis model (Table I in the online-only Data Supplement). The key treatment eligibility probabilities pertained to IV-tPA, the presence of an LVO, and endovascular therapy. The probability of receiving IV-tPA accounted for the individual probability of having no contraindications (e.g. hemorrhage) to IV-tPA use and that of actually receiving IV-tPA.17 The probability of receiving endovascular therapy accounted for the probability of LVO and the probability of being eligible for endovascular therapy among patients with LVO.2 For the probability of having an LVO, we evaluated a range of probabilities based on the likelihood of an LVO in the context of no formal LVO screening and in the setting of clinical LVO screening scales of varying predictive ability. When we considered the use of LVO screening scales, we varied the probability of an LVO based on the full range of positive predictive values for patients screened positive and false negative rates (1-negative predictive value) for those screened negative. These probabilities were calculated from published sensitivities and specificities using Bayes’ theorem under two LVO prevalence estimates (4.9% and 11.8%) (Table II in the online-only Data Supplement).2, 18–22
Both time-independent2, 5, 17, 18, 23 (Table 1) and time-dependent probabilities5, 6, 23 (Table I in the online-only Data Supplement) were imputed for possible outcomes following stroke treatment. The probability of an excellent functional outcome for patients who did not receive IV-tPA was time-independent.23 The probability of an excellent functional outcome in patients who received IV-tPA treatment only was time-dependent.6, 23 The probability of early reperfusion from IV-tPA was considered in patients receiving both IV-tPA and endovascular therapy.5 The probability of an excellent functional outcome after endovascular therapy accounted for the probability of substantial reperfusion, defined as achieving modified Thrombolysis in Cerebral Infarction scale scores of 2b or 3.5 The time-varying probabilities of excellent functional outcomes for endovascular therapy, by reperfusion status, were derived from a meta-analysis of endovascular therapy trial data.5 Formulae used to calculate these time-varying probabilities are shown in Table I in the online-only Data Supplement.
Table 1.
Time-independent probabilities and key time variables in base case.
| Variable name |
Description | Value* |
|---|---|---|
| Time-independent probabilities | ||
| Pel | Probability of not having IV-tPA contraindication | 0.3517 |
| Ptpa1 | Probability of receiving IV-tPA treatment among those without contraindication for IV-tPA | 0.8217 |
| Ptpa | Probability of receiving IV-tPA (=Pel*Ptpa1) | 0.287 |
| Plvo | Probability of LVO | 0.048718 |
| Pendo | Probability of endovascular therapy eligibility among LVO patients | 0.8652 |
| Pearly | Probability of early reperfusion from IV-tPA among LVO patients | 0.0545 |
| Pre | Probability of substantial reperfusion in endovascular therapy | 0.715 |
| Pgnp1 | Probability of mRS 0-1 in no LVO patients who did not receive IV-tPA | 0.423 |
| Pgnp2 | Probability of mRS 0-1 in LVO patients who did not receive IV-tPA or endovascular therapy | 0.2523 |
| Key time variables | ||
| Time spent outside of hospital | ||
| ttrans | Scene to closest PSC | 20 |
| t1 | Onset to PSC arrival | 45 |
| t2 | Transfer time from PSC to CSC | 20 |
| t3 | Additional time if transported directly to CSC compared to directly to PSC | 20 |
| t4 | Onset to CSC arrival in mothership strategy | 65 |
| Drip-and-ship | ||
| to_evt1 | Onset-to-puncture time for endovascular therapy | 160 |
| to_evtr1 | Onset-to-reperfusion time for endovascular therapy | 190 |
| Mothership | ||
| to_evt2 | Onset-to-puncture time for endovascular therapy | 130 |
| to_evtr2 | Onset-to-reperfusion time for endovascular therapy | 160 |
LVO, Large vessel occlusion; PSC, primary stroke center; CSC, comprehensive stroke center.
Except where reference specified, the value is either an assumption or result of a calculation; Detailed explanations to these assumptions can be found in the supplemental tables.
Key time variables included in the decision analysis were pre-hospital times, transport times, and in-hospital treatment workflow times (Table III in the online-only Data Supplement). Importantly, two sets of time metrics were considered: one to simulate an ideal stroke system of care and a second to simulate current real-world times. For the ideal time metrics, the time variables were derived from clinical guidelines and data reported from high-performing stroke centers participating in clinical trials.5, 24, 25 Time variables in the real-world scenario were derived from a large, multi-center United States registry of patients treated with endovascular therapy for acute ischemic stroke.10 The main differences between ideal and real-world assumptions included differences in door-in-door-out time at the PSC, which constituted door-to-needle and IV-tPA to PSC departure.
Statistical Analyses
To simulate the experience of an EMS crew evaluating and triaging a patient, the base case was a hypothetical adult patient presenting with symptoms of stroke of known duration but of unknown etiology (e.g. ischemic versus hemorrhagic). No formal LVO screening was assumed in the base case, so the probability of having an LVO in the base case was 4.9%.18 The total transport time was up to 3.5 hours, which made IV-tPA treatment within 4.5 hours possible. The base case was examined using ideal time metrics, with a door-to-needle time of 35 minutes at both the PSC and CSC.5 The total time from symptom onset to arrival at the closest PSC was 45 minutes, including time from onset to EMS arrival at scene and scene to hospital. Twenty minutes of additional transport time was required for the patient to be taken directly to the closest CSC.
The favorability of drip-and-ship versus mothership was compared in one-way sensitivity analyses in which we varied key variables while holding all other variables constant using base case values. The following variables were subjected to one-way sensitivity analyses: the likelihood of an LVO (to reflect various LVO prevalence estimates and predictive abilities of LVO screening scales), the probability of substantial reperfusion after endovascular therapy,26 the possible additional door-to-needle time at PSC (to account for potential differences in IV-tPA workflow at PSCs versus CSCs), the time from symptom onset to EMS arrival, the transport time from scene to the nearest PSC, the additional time required to reach a CSC, and the additional detour time in the drip-and-ship model that results from going to a PSC that is not exactly on the route to the CSC .
We performed several two-way sensitivity analyses of the base case. First, we jointly varied the probability of LVO and the possible additional door-to-needle time at PSCs as compared to CSCs. Second, we jointly varied the probability of LVO and the door-in-door-out time at PSCs. Third, to understand the interaction between pre-hospital times to the PSC and CSC, we performed a two-way sensitivity analysis jointly varying the transport time from scene to arrival at the closest PSC and the additional transport time necessary to bypass a PSC and go to a CSC. Then, we created a three-way sensitivity analysis by adding LVO probability, as a function of a range of LVO screening methods, to pre-hospital times to the PSC and CSC. We performed two-way and three-way sensitivity analyses under the ideal and real-world assumptions. The three-way sensitivity analysis also accounted for two estimates of LVO prevalence (4.9% and 11.8%).2, 18 Additionally, the three-way sensitivity analysis was repeated to simulate an urban environment under real-world assumptions by reducing the detour time to 0 to reflect the presence of PSCs directly on the route to CSCs, changing the range of transport time to PSC to 0–30 minutes, and changing the range of additional transport time to CSC to 0–15 minutes.27, 28 Last, to account for scenarios with longer stroke onset to alarm time and longer alarm to EMS arrival time, we conducted an analysis with an onset to EMS arrival time of 60 minutes.
Results
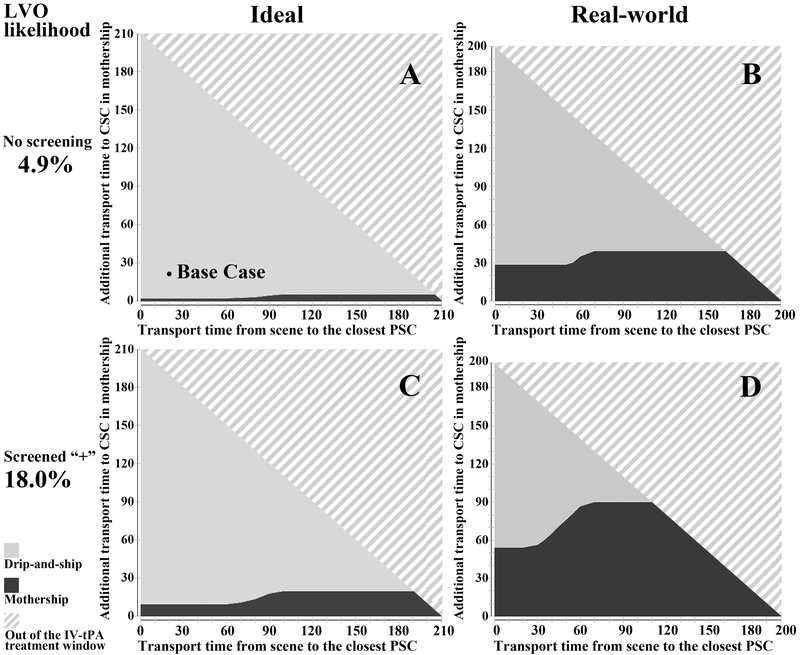
In the idealized base case, with a total time from symptom onset to arrival at PSC of 45 minutes, additional transport time to CSC of 20 minutes, and no formal LVO screening, we found that the drip-and-ship strategy was preferred (Figure 2). The probability of an excellent outcome under the drip-and-ship paradigm was 44.7% while it was 44.3% under the mothership paradigm. In contrast, when the real-world time metrics were applied, the mothership paradigm was preferred with a 44.3% probability of an excellent outcome as compared to 43.8% under the drip-and-ship paradigm.
Figure 2. Selected results of three-way sensitivity analyses under ideal and real-world time metrics.
Three-way sensitivity analysis was conducted by jointly varying transport time from scene to primary stroke center, additional transport time if taken directly to a comprehensive stroke center, and the probability of a large vessel occlusion (LVO). (A) and (B) represent ideal and real-world time metrics, respectively, in the absence of formal LVO screening; (C) and (D) represent ideal and real-world scenarios, respectively, for patients screening positive with an LVO screening scale of moderate sensitivity and specificity (post-test probability of 18%). The solid dot in (A) represents the base case result; dark gray shading represents favorability of the direct-to-mothership approach, light gray shading represents favorability of the drip-and-ship approach, and the angled line pattern represents patients outside of the IV-tPA treatment window who were not included in this model. LVO, large vessel occlusion; PSC, primary stroke center; CSC, comprehensive stroke center.
One-way sensitivity analysis
We then varied a range of values for key input parameters individually under ideal assumptions (Figure I in the online-only Data Supplement). First, when the probability of LVO was below 0.39 (after LVO screening), drip-and-ship was preferred. Second, probabilities of substantial reperfusion (from 70% to 95%) after endovascular therapy did not affect triage preference to the PSC in the base case scenario. Third, when the additional door-to-needle time at the PSC compared to the CSC was longer than 16.5 minutes, then mothership became the favored triage option. Fourth, when analyzing the time variables individually, we found that varying the time from onset to EMS arrival (0–120 minutes), the transport time from scene to arrival at the PSC, or the additional detour time (0–40 minutes) alone did not change triage strategy selection. While varying additional transport time to CSC alone, mothership was favored only when the additional transport time to CSC in mothership was less than 2 minutes. When these analyses were repeated using the real-world assumptions, individually varying these variables did not affect triage strategy selection, except for when examining the variable of additional transport time to the CSC. If the additional transport time to a CSC was less than 28 minutes, then mothership was preferred.
Two-way sensitivity analysis
In the first two-way sensitivity analysis, we examined the interplay of the probability of LVO and the possible additional door-to-needle time at the PSC compared to the CSC. When the additional door-to-needle time at the PSC was more than 20 minutes longer than at the CSC, mothership was the preferred strategy across the full range of probabilities of LVO (Figure II in the online-only Data Supplement). When this was repeated in the real-world scenario, if the door-to-needle time at the PSC was more than 5 minutes longer than at a CSC, then mothership was always preferred. In the second two-way sensitivity analysis, we jointly varied the probability of LVO and the door-in-door-out time at the PSC and found that drip-and-ship was preferred regardless of door-in-door-out time until the probability of LVO reached 15%. Beyond that, for drip-and-ship to be preferred, the permissible door-in-door-out time at the PSC decreased steeply as the probability of an LVO increased (Figure II in the online-only Data Supplement). Under real-world assumptions, mothership was preferred regardless of the door-in-door-out time. Third, we jointly varied the time from onset to arrival at the closest PSC in drip-and-ship and the additional transport time required for direct transport to the nearest CSC. In the absence of formal LVO screening, drip-and-ship was favored regardless of transport time to the PSC, unless going directly to a CSC took less than 2–5 minutes in the ideal scenario and 28–39 minutes in the real-world scenario.
The results of our models are most informative when jointly considering PSC and CSC transport times, LVO prevalence, LVO screening methods, and whether workflow times reflect ideal or real-world metrics. To do this, we performed three-way sensitivity analyses accounting for PSC and CSC transport times and LVO probabilities under several scenarios. In the three-way sensitivity analysis, variables were varied continuously (Video in online-only Data Supplement); however, we report results for three discrete sets of LVO screening scale test characteristics to facilitate comparison (Table 2) and graphically present results for select scenarios (Figure 2). Drip-and-ship was almost always favored for non-screened patients and those screening negative on an LVO scale under ideal metrics. However, under real-world assumptions, additional transport times to CSC as long as 23–39 minutes were permitted when bypassing PSCs for these same patients. For patients screening positive on an LVO scale under ideal conditions, the duration of bypass time permitted to favor bypassing the PSC varied from 3 to 23 minutes, depending on the LVO screening tool used. In contrast, under real-world conditions, the permissible additional transport time was 32–99 minutes for these patients. In general, under real-world conditions, mothership was preferred for considerably longer bypass times. When assuming a higher baseline LVO prevalence, the thresholds were generally longer in all scenarios. When testing a hypothetical LVO screening tool with a positive predictive value of 0.9, mothership was the superior strategy when the additional transport time was up to 1 hour. In the urban scenario, where all transport times were shorter to reflect greater hospital density, the mothership strategy was always preferred regardless of transport times, LVO prevalence and LVO screening status. Last, when the stroke symptom onset to EMS arrival time was changed to 60 minutes, longer bypass times to the CSC were permitted to favor the mothership strategy (Table IV in the online-only Data Supplement).
Table 2.
Time thresholds for mothership to be the preferred strategy.
| LVO Screening methods |
Probability of LVO |
Ideal time metrics | Real-world time metrics | ||
|---|---|---|---|---|---|
| Scene to PSC arrival time (min)* |
Additional transport time to CSC (min) |
Scene to PSC arrival time (min)* |
Additional transport time to CSC (min) |
||
| LVO prevalence = 0.0487 | |||||
| Screened Negative29† | 0.02 | <60 | <1 | <40 | <23 |
| >100 | <2 | >70 | <28 | ||
| No Screening18 | 0.0487 | <60 | <2 | <40 | <28 |
| >100 | <5 | >70 | <39 | ||
| Screened Positive‡ | |||||
| High sensitivity/ Low specificity30 |
0.07 | <60 | <3 | <40 | <32 |
| >100 | <8 | >70 | <46 | ||
| Moderate sensitivity/ Moderate specificity29 |
0.18 | <60 | <9 | <20 | <50 |
| >100 | <18 | >70 | <83 | ||
| Low sensitivity/ High specificity31 |
0.23 | <60 | <11 | <20 | <59 |
| >100 | <23 | >70 | <99 | ||
| LVO prevalence = 0.118 | |||||
| Screened Negative29† | 0.06 | <60 | <3 | <40 | <30 |
| >100 | <7 | >70 | <43 | ||
| No Screening2 | 0.118 | <60 | <6 | <40 | <40 |
| >100 | <12 | >70 | <63 | ||
| Screened Positive‡ | |||||
| High sensitivity/ Low specificity30 |
0.16 | <60 | <8 | <40 | <47 |
| >100 | <16 | >70 | <77 | ||
| Moderate sensitivity/ Moderate specificity29 |
0.36 | <60 | <18 | <20 | <103 |
| >100 | <33 | >70 | <135 | ||
| Low sensitivity/ High specificity31 |
0.45 | <60 | <23 | <20 | <130 |
| >100 | <39 | >70 | <158 | ||
LVO, Large vessel occlusion; PSC, primary stroke center; CSC, comprehensive stroke center.
The dichotomized time ranges presented for scene to PSC arrival time were generated from both individual sensitivity analyses and visual inspection of the two-way sensitivity analysis figures.
Probability of LVO calculated from using a single scale with moderate sensitivity (0.63), moderate specificity (0.85).
We calculated positive predictive values across the full range of reported sensitivity/specificity, and chose to present 3 representative combinations: 1. high sensitivity (0.83), low specificity (0.40), and positive likelihood ratio (1.8); 2. moderate sensitivity (0.63), moderate specificity (0.85), and positive likelihood ratio (4.3); and 3. low sensitivity (0.30), high specificity (0.95), and positive likelihood ratio (6.0).
Discussion
The favorability of triage strategies depended on transport times, the likelihood of an LVO as a function of LVO screening scale accuracy and LVO prevalence, and whether the stroke system of care met ideal metrics, particularly with regards to PSC door-to-needle and door-in-door-out times. For patients screening positive for an LVO under ideal assumptions, direct transport to the CSC was preferable if the additional transport time was no longer than 3–23 minutes, with the precise duration of permissible additional transport time varying with the positive predictive value of the LVO screening scale. However, under current real-world conditions, the permissible additional transport time to the CSC was much longer.
Our analyses build upon prior approaches with respect to methodology and findings. To the best of our knowledge, apart from an ongoing randomized clinical trial (RACECAT),32 there are limited conclusive data regarding pre-hospital stroke triage.8–10 Additionally, small absolute differences in clinical outcomes for individual patients, as seen in our base case results, suggests that statistical power of randomized trials may be limited. Several groups have used modeling approaches to address this gap in the literature, including a recently published decision analysis,14 advanced mathematical,15 and conditional probability modeling studies.11–13 While earlier modeling studies only included patients with known LVO11, 12, recent analyses have attempted to more accurately reflect the clinical uncertainty faced by EMS.13–15 Our model complements these analyses in two key aspects. First, we examined our model output in two distinct scenarios: under ideal and real-world assumptions. Because of this, our model reflects the importance of the door-in-door-out time at PSCs. Second, our model was designed to simultaneously assess the impact of numerous variables in combination with a large range of LVO screening scale predictive abilities and two reasonable estimates of LVO prevalence. This allows us to provide estimates of thresholds for direct transport to CSC under a variety of scenarios, with an emphasis on how these thresholds differ between ideal and real-world conditions.
Under ideal conditions, for patients screened positive by LVO screening scales, the mothership approach was favored when the additional transport time was up to 3–23 minutes, depending on the predictive ability of LVO screening scales. This range includes the 15 minute threshold recommended by the current AHA/ASA Mission: Lifeline Stroke triage algorithm.7 The thresholds, under ideal conditions, identified in our analysis were generally shorter than the 44 and 60 minute thresholds identified by other modeling approaches.13, 14 However, under real-world conditions, our results were more aligned with prior models, with thresholds of additional permissible transport time to the CSC ranging from 32 to 99 minutes. Overall our approach confirms findings of other modeling approaches: bypass thresholds are highly sensitive to joint variations in model input parameters.13–15 Specifically, because we evaluated model output under ideal and real-world conditions, our results revealed that while bypass times could be longer than recommended by the AHA/ASA algorithm under current real-world conditions, the AHA/ASA guidelines would be appropriate if stroke systems of care were optimized to achieve ideal workflow times.
Total door-in-door-out time at the PSC, including door-to-needle time and IV-tPA to PSC departure time, was considerably longer in the real-world than under ideal assumptions. This was likely a major contributor to the favorability of the mothership approach in the real-world scenario. Prior modeling studies have emphasized the importance of the door-to-needle time in decision model outcomes.13, 15 By comparing the deconstructed workflow times in the ideal and real-world scenarios, our model also demonstrates the important contribution of the overall door-in-door-out time and the IV-tPA to departure time. The PSC door-in-door-out time is increasingly an area of interest for quality improvement because it contributes substantially to delays in recanalization in the drip-and-ship paradigm.33 Our model results underscore the importance of innovative strategies to increase the speed of post-tPA care at PSCs in order for drip-and-ship to retain its favorability. The delay from IV-tPA to PSC departure was reported to be related to waiting for ambulance arrival in one analysis,10 which could be reduced by having the ambulance that initially transported the patient to the PSC remain on scene until a decision regarding transfer is made (“hold, drip and go”).34 Taking an integrated network approach in establishing PSC LVO protocols may improve triaging and outcomes for patients with suspected LVO.35
Our three-way sensitivity analysis video allows us to visually represent the joint effect of LVO probability, as a function of LVO screening and baseline LVO prevalence, with other important variables. In general, higher LVO prevalence increased the additional transport time permitted for mothership favorability. The additional transport time thresholds varied with the test characteristics of LVO screening scales; use of an LVO screening scale with a higher positive likelihood ratio, as opposed to sensitivity or specificity alone, allowed longer permissible additional transport time to a CSC for mothership to be favored. Advanced LVO detection strategies such as a portable transcranial sonography36 and clinical screening scales that incorporate demographics and vital signs37 may reach sufficient accuracy to facilitate direct referral to endovascular centers with greater certainty despite longer additional transport times. Dynamic triage tools, such as the FAST-ED mobile application38 and the tool presented by Ali et al15 could incorporate additional variables such as the door-in-door-out time at the PSC and baseline LVO prevalence to parlay the complex interaction of important factors into simple triage decision support.
We compared pre-hospital triage strategies using decision analysis, a commonly used method in healthcare decision-making, to comprehensively and jointly assess multiple important variables and their combined influence on stroke outcomes. Our models used real-world data, reasonable assumptions, and tested a wide range of possibilities for key inputs. However, our results should be interpreted with caution given several limitations inherent to modeling studies. First, although the data imputed into our models were taken from high quality studies and clinical guidelines, our model cannot be applied directly to patient care as many individual patient-level and healthcare system-level factors were not accounted for in our model. Second, our model does not account for different occlusion sites among patients with LVO. Third, we did not model cost-effectiveness and quality of life; mRS scores were directly reported in clinical trials and were directly imputed into our models without further assumptions. Last, we did not account for mobile stroke units as this technology is not in widespread use.
Conclusions
Using a decision analysis model, we found that the favorability of choosing transport to a PSC versus a CSC for patients with acute stroke symptoms was strongly influenced by the use and predictive abilities of pre-hospital LVO screening tools and PSC door-in-door-out time in addition to transport distances.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Parikh was supported by NIH/NINDS (T32-NS07153; PI: Elkind).
Footnotes
Social media handle: @NealSParikhMD
Disclosures
Dr. Elkind serves as the Chairman of the Advisory Committee to the American Stroke Association and on the National, Founders Affiliate, and New York City boards of the American Heart Association. He receives royalties for chapters on stroke from UpToDate. No other authors had disclosures.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360 [DOI] [PubMed] [Google Scholar]
- 2.Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. Journal of Neurointerventional Surgery. 2017;9:722–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 4.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279–1288 [DOI] [PubMed] [Google Scholar]
- 6.Kim JT, Fonarow GC, Smith EE, Reeves MJ, Navalkele DD, Grotta JC, et al. Treatment With Tissue Plasminogen Activator in the Golden Hour and the Shape of the 4.5-Hour Time-Benefit Curve in the National United States Get With The Guidelines-Stroke Population. Circulation. 2017;135:128–139 [DOI] [PubMed] [Google Scholar]
- 7.American Heart Association Mission:Lifeline. Severity-Based Stroke Triage Algorithm for EMS. https://mlnetwork.heart.org/resources/364. Accessed August 6, 2018
- 8.Gerschenfeld G, Muresan IP, Blanc R, Obadia M, Abrivard M, Piotin M, et al. Two Paradigms for Endovascular Thrombectomy After Intravenous Thrombolysis for Acute Ischemic Stroke. JAMA Neurology. 2017;74:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishihara H, Oka F, Oku T, Shinoyama M, Suehiro E, Sugimoto K, et al. Safety and Time Course of Drip-and-Ship in Treatment of Acute Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases. 2017;26:2477–2481 [DOI] [PubMed] [Google Scholar]
- 10.Froehler MT, Saver JL, Zaidat OO, Jahan R, Aziz-Sultan MA, Klucznik RP, et al. Interhospital Transfer Before Thrombectomy Is Associated With Delayed Treatment and Worse Outcome in the STRATIS Registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136:2311–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holodinsky JK, Williamson TS, Kamal N, Mayank D, Hill MD, Goyal M. Drip and Ship Versus Direct to Comprehensive Stroke Center: Conditional Probability Modeling. Stroke. 2017;48:233–238 [DOI] [PubMed] [Google Scholar]
- 12.Milne MS, Holodinsky JK, Hill MD, Nygren A, Qiu C, Goyal M, et al. Drip ‘n Ship Versus Mothership for Endovascular Treatment: Modeling the Best Transportation Options for Optimal Outcomes. Stroke. 2017;48:791–794 [DOI] [PubMed] [Google Scholar]
- 13.Holodinsky JK, Williamson TS, Demchuk AM, Zhao H, Zhu L, Francis MJ, et al. Modeling Stroke Patient Transport for All Patients With Suspected Large-Vessel Occlusion. JAMA Neurology. 2018;75;1477–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit JL, Khatri P, Adeoye OM, Broderick JP, McMullan JT, Scheitz JF, et al. Prehospital Triage of Acute Ischemic Stroke Patients to an Intravenous tPA-Ready versus Endovascular-Ready Hospital: A Decision Analysis. Prehospital Emergency Care. 2018;22:722–733 [DOI] [PubMed] [Google Scholar]
- 15.Ali A, Zachrison KS, Eschenfeldt PC, Schwamm LH, Hur C. Optimization of Prehospital Triage of Patients With Suspected Ischemic Stroke. Stroke. 2018;49:2532–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasner SE. Clinical interpretation and use of stroke scales. The Lancet Neurology. 2006;5:603–612 [DOI] [PubMed] [Google Scholar]
- 17.Messe SR, Khatri P, Reeves MJ, Smith EE, Saver JL, Bhatt DL, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87:1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dozois A, Hampton L, Kingston CW, Lambert G, Porcelli TJ, Sorenson D, et al. PLUMBER Study (Prevalence of Large Vessel Occlusion Strokes in Mecklenburg County Emergency Response). Stroke. 2017;48:3397–3399 [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Kent DM, Bulsara KR, Leung LY, Lichtman JH, Reeves MJ, et al. Accuracy of Prediction Instruments for Diagnosing Large Vessel Occlusion in Individuals With Suspected Stroke: A Systematic Review for the 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke. 2018;49:e111–e122 [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Coote S, Pesavento L, Churilov L, Dewey HM, Davis SM, et al. Large Vessel Occlusion Scales Increase Delivery to Endovascular Centers Without Excessive Harm From Misclassifications. Stroke. 2017;48:568–573 [DOI] [PubMed] [Google Scholar]
- 21.Scheitz JF, Abdul-Rahim AH, MacIsaac RL, Cooray C, Sucharew H, Kleindorfer D, et al. Clinical Selection Strategies to Identify Ischemic Stroke Patients With Large Anterior Vessel Occlusion: Results From SITS-ISTR (Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Registry). Stroke. 2017;48:290–297 [DOI] [PubMed] [Google Scholar]
- 22.El-Ghanem M, Al-Mufti F, Thulasi V, Singh IP, Gandhi C. Expanding the treatment window for ischemic stroke through the application of novel system-based technology. Neurosurgical Focus. 2017;42:E7. [DOI] [PubMed] [Google Scholar]
- 23.NINDS. Tissue plasminogen activator for acute ischemic stroke. The New England Journal of Medicine. 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 24.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 25.English JD, Yavagal DR, Gupta R, Janardhan V, Zaidat OO, Xavier AR, et al. Mechanical Thrombectomy-Ready Comprehensive Stroke Center Requirements and Endovascular Stroke Systems of Care: Recommendations from the Endovascular Stroke Standards Committee of the Society of Vascular and Interventional Neurology (SVIN). Interventional Neurology. 2016;4:138–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vagal AS, Khatri P, Broderick JP, Tomsick TA, Yeatts SD, Eckman MH. Time to angiographic reperfusion in acute ischemic stroke: decision analysis. Stroke. 2014;45:3625–3630 [DOI] [PubMed] [Google Scholar]
- 27.Parikh NS, Chatterjee A, Diaz I, Pandya A, Merkler AE, Gialdini G, et al. Modeling the Impact of Interhospital Transfer Network Design on Stroke Outcomes in a Large City. Stroke. 2018;49:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz BS, Adeoye O, Sucharew H, Broderick JP, McMullan J, Khatri P, et al. Estimated Impact of Emergency Medical Service Triage of Stroke Patients on Comprehensive Stroke Centers: An Urban Population-Based Study. Stroke. 2017;48:2164–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qureshi S JA, Raza SA, Brasher C, Anderson A, Belagaje S, Ranagaraju S, et al. Stroke Screening Tools Have High Specificity for Detecting Large Vessel Occlusion in a Southeastern Us Prospective Cohort Study [Abstract]. Stroke. 2016;47(suppl 1):WMP67 [Google Scholar]
- 30.Katz BS, McMullan JT, Sucharew H, Adeoye O, Broderick JP. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;46:1508–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turc G, Maier B, Naggara O, Seners P, Isabel C, Tisserand M, et al. Clinical Scales Do Not Reliably Identify Acute Ischemic Stroke Patients With Large-Artery Occlusion. Stroke. 2016;47:1466–1472 [DOI] [PubMed] [Google Scholar]
- 32.Direct Transfer To An Endovascular Center Compared to Transfer To The Closest Stroke Center in Acute Stroke Patients with Suspected Large Vessel Occlusion (RACECAT). NCT02795962. https://clinicaltrials.gov/ct2/show/nct02795962. Accessed Nov 1, 2018.
- 33.McTaggart RA MK, Oliver LA, Dibiasio EL, Baird GL, Hemendinger ML, Haas RA, et al. Door-in-Door-Out Time at Primary Stroke Centers May Predict Outcome for Emergent Large Vessel Occlusion Patients. Stroke. 2018;49:2969–2974 [DOI] [PubMed] [Google Scholar]
- 34.McTaggart RA MK, Oliver LA, Hemendinger ML, Yaghi S, Baird G, Haas RA, et al. Door in Door Out Time at Primary Stroke Centers Predicts Outcome for Patients With Large Vessel Occlusion Stroke [Abstract]. Stroke. 2018;49:A135. [DOI] [PubMed] [Google Scholar]
- 35.McTaggart RA, Yaghi S, Cutting SM, Hemendinger M, Baird GL, Haas RA, et al. Association of a Primary Stroke Center Protocol for Suspected Stroke by Large-Vessel Occlusion With Efficiency of Care and Patient Outcomes. JAMA Neurology. 2017;74:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorpe SG, Thibeault CM, Canac N, Wilk SJ, Devlin T, Hamilton RB. Decision Criteria for Large Vessel Occlusion Using Transcranial Doppler Waveform Morphology. Frontiers in Neurology. 2018;9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Pardo J, Fuentes B, Alonso de Lecinana M, Ximenez-Carrillo A, Zapata-Wainberg G, Alvarez-Fraga J, et al. The Direct Referral to Endovascular Center criteria: A proposal for pre-hospital evaluation of acute stroke in the Madrid Stroke Network. European Journal of Neurology. 2017;24:509–515 [DOI] [PubMed] [Google Scholar]
- 38.Nogueira RG, Silva GS, Lima FO, Yeh YC, Fleming C, Branco D, et al. The FAST-ED App: A Smartphone Platform for the Field Triage of Patients With Stroke. Stroke. 2017;48:1278–1284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.