Abstract
Facioscapulohumeral dystrophy is one of the most common forms of muscular dystrophies worldwide. It is a complex and heterogeneous disease secondary to insufficient epigenetic repression of D4Z4 repeats and aberrant expression of DUX4 in skeletal muscles. Type 1 facioscapulohumeral muscular dystrophy (FSHD) is caused by contraction of D4Z4 repeats on 4q35, whereas type 2 FSHD is associated with mutations of the SMCHD1 or DNMT3B gene in the presence of a disease-permissive 4qA haplotype. Classical FSHD is a slowly progressive disorder with gradual-onset of muscle atrophy and a descending pattern of muscle weakness. In contrast, early-onset FSHD is associated with a large deletion of D4Z4 repeats and a more severe disease phenotype, including early loss of independent ambulation as well as extramuscular manifestations, such as retinal vasculopathy, hearing loss, and central nervous system (CNS) involvement. However, the correlation between D4Z4 repeats and disease severity remains imprecise. The current standard of care guidelines offers comprehensive assessment and symptomatic management of secondary complications. Several clinical trials are currently underway for FSHD. New and emerging treatments focus on correcting the transcriptional misregulation of D4Z4 and reversing the cytotoxic effects of DUX4. Other potential therapeutic targets include reduction of inflammation, improving muscle mass, and activating compensatory molecular pathways. The utility of disease-modifying treatments will depend on selection of sensitive clinical endpoints as well as validation of muscle magnetic resonance imaging (MRI) and other biomarkers to detect meaningful changes in disease progression. Correction of the epigenetic defects using new gene editing as well as other DUX4 silencing technologies offers potential treatment options for many individuals with FSHD.
Keywords: facioscapulohumeral muscular dystrophy, review, new emerging treatments
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is a progressive genetic myopathy with variable onset of symptoms, distribution of weakness, and disease severity, as recently reviewed by several authors, including Tawil et al,1 Sacconi et al,2 Daxinger et al,3 and Knopp et al.4 It is one of the most common types of muscular dystrophies with an estimated incidence of 1 in 15,0005 and a prevalence of 1 in 8,000 to 20,000 in the general population.6,7 Up to 30% of genetically confirmed cases of FSHD can be asymptomatic,8 especially among those with smaller deletions (8 to 10 copies of D4Z4 repeats).9,10 Mosaic mutations may also be associated with a milder phenotype.11 In contrast, infantile FSHD is an uncommon but more severe spectrum of FSHD. The purpose of this article is to provide a pediatric review of FSHD, including the diagnosis, standard of care, and new emerging treatment for children and adults with this disease.
Clinical Features
Symptoms of classical FSHD typically present during the second or third decade of life with facial weakness, scapular winging, as well as limitation in shoulder abduction and elbow flexion (see ►Fig. 1). Asymmetric muscle involvement is common.12 Progression of weakness is usually slow and follows a descending pattern with gradual involvement of the trunk, hip girdle, and distal lower extremities muscles. Over time, most affected individuals have a constellation of clinical signs related to FSHD, including eye closure weakness, reduced facial expression, transverse smile, inability towhistle or suck from a straw, periscapular muscle atrophy, high riding scapula, horizontal clavicles, Beevor’s sign, increased lumbar lordosis as well as hip girdle and foot extensor weakness.13–15 Atypical features, with relative facial sparing, calf hypertrophy, and predominantly lower extremities weakness, have been described.16–18 In general, dysphagia,19 cardiac arrthymias,20 cardiomyopathy,21 and severe respiratory insufficiency22 are uncommon complications in classical FSHD; additional investigations may be required to exclude non-FSHD-related or double-trouble etiologies.17,23 Individuals with FSHD have an increased risk of developing chronic musculoskeletal pain and fatigue.16,24 Life expectancy is unaffected; however, between 10 to 30% of individuals may become nonambulatory due to progressive muscle weakness.25
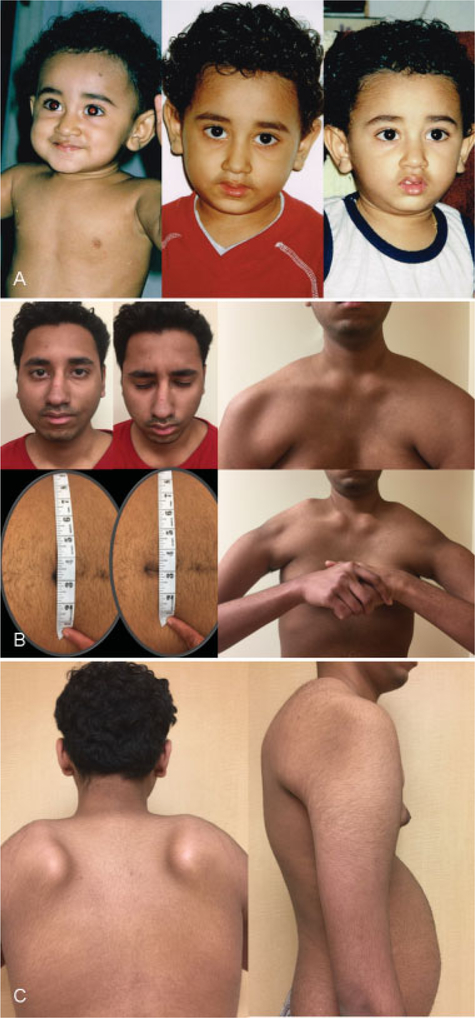
Fig. 1.
Clinical features of early-onset FSHD. (A) Evidence of mild asymmetrical facial weakness with onset after 1 year of age (right panel). (B) Clinical features of FSHD at 16 years of age, including eye closure weakness (left upper panel), Beevor’s sign (left lower panel; the umbilicus moved upward with neck flexion), pectus excavatum (right upper panel), high-riding scapula, horizontal clavicles, and poly-hill sign due to prominent periscapular muscle atrophy (right lower panel). (C) Scapular winging (left panel) and lumbar lordosis (right panel). FSHD, facioscapulohumeral muscular dystrophy.
Early-Onset FSHD
Early-onset FSHD makes up approximately 4 to 21% of the total FSHD population26,27 and nearly half of the pediatric FSHD population;27 the rest have the classical phenotype of late adolescent or adult onset disease. The term “infantile FSHD” was initially coined by Brooke in 1977 to denote an early-onset form of the disease.28 The diagnostic criteria for infantile FSHD was further defined by Brouwer et al29 to include affected individuals with onset (signs and symptoms) of facial weakness before 5 years of age plus sign and symptoms of shoulder girdle weakness before 10 years of age. In addition to facial diplegia and shoulder weakness, affected children may present with generalized hypotonia, global developmental delay, dysarthria, and dysphagia shortly after birth.30 Rarely, features resembling Mobius syndrome with ophthalmoplegia have been described.31,32 Additional manifestations, such as retinal vasculopathy,33 intellectual disability, epilepsy,34 and cochlear dysfunction,35 are more common among individuals with early-onset FSHD and large D4Z4 deletions. On occasion, these extramuscular features may be the initial presenting complaints, and thus, are particularly noteworthy for pediatric neurologists as potentially early signs of FSHD.27,29,30,36 Prompt recognition is important for optimal treatment and family counseling.
Early-onset FSHD is generally associated with fewer number of D4Z4 repeats, younger age at loss of independent ambulation, and a greater risk of disease severity.27,30,37,38 However, the correlation between disease severity and D4Z4 repeat number is inconsistent; infantile FSHD may be influenced by other genetic and environmental modifiers.39,40
Pathogenesis
FSHD results from a highly unusual and complex disease mechanism, in which the mutation does not reside within the gene or genes responsible for the disease. Type 1 FSHD (FSHD1, Online Mendelian Inheritance in Man (OMIM) #158900; https://www.omim.org/entry/158900) is an auto-somal dominant disorder associated with deletions of integral copies of a 3.3 kb tandem repeat unit termed D4Z4 in the subtelomeric region of chromosome 4q35.41 In FSHD1 patients, there are only 1 to 10 copies of D4Z4 repeats at the 4q35 region, while 11 to 100 copies are found in the normal population. Studies showed that the deletion causes DNA hypomethylation of D4Z4 repeats and transcriptional derepression of the region.42 Less than 5% of individuals with FSHD have normal-sized repeat array on chromosome 4 but still exhibit DNA hypomethylation of D4Z4 repeats and carry a permissive 4qA allele; they are considered to have Type 2 FSHD (FSHD2, OMIM #158901; https://www.omim.org/entry/ 158901) due to additional mutations involving the structural maintenance of chromosomes flexible hinge domain-containing protein 1 (SMCHD1) gene on chromosome 18p1143 or the DNA methyltransferase 3B (DNMT3B) gene on chromosome 20q11.44 Both types of mutations are associated with insufficient epigenetic repression of D4Z4 repeats leading to aberrant expression of double homeobox protein 4 (DUX4) in skeletal muscles and subsequently disease progression.3 While initially considered as “junk” DNA, each D4Z4 repeat contains an open reading frame for the DUX4 gene. DUX4 belongs to a family of genes encoding double homeodomain proteins.45 A homologue of DUX4, known as DUX4c, is located distally nearby DUX4. The two genes share the same DNA binding homeodomain. However, DUX4c lacks the C-terminus transactivation domain and is not cytotoxic when expressed in muscle cells. Previous studies suggested that DUX4c may play important roles in muscle regeneration, while DUX4 expression adversely affects muscle cell survival and differentiation.46–50 DUX4 is a nuclear transcription factor that is normally expressed in germ cells.51,52 Abnormal DUX4 expression leads to dysregulation of molecular pathways that are involved in muscle differentiation, oxidative stress responses, immune responses, and protein turnover.51–53 A recent study showed cytoplasmic localization of DUX4 during muscle differentiation in culture; however, its function in the cytoplasm is not clear.54 The precise mechanism of how changes in these pathways cause FSHD remains to be elucidated.
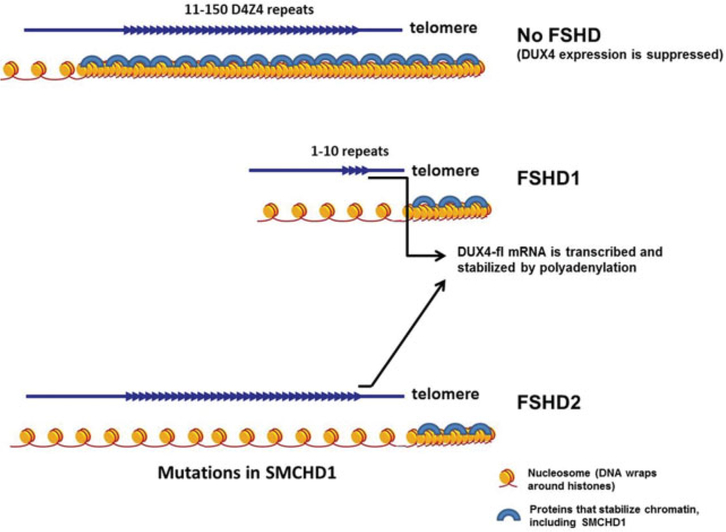
Based on recent findings, it is believed that a combination of two genomic features is required to result in FSHD. The first feature is a mutation that causes derepression of DUX4 gene, which allows for the DUX4 transcripts to be transcribed. The second genomic feature is a functional polyadenylation signal in the pLAM region distal to the D4Z4 repeat array, which is associated with the FSHD-permissive 4qA haplotypes.43,55 Without the polyadenylation signal, the DUX4 transcripts are quickly degraded. However, when the transcripts from the last D4Z4 repeat are polyadenylated, it stabilizes the DUX4 mRNA and allows for the translation of the DUX4 protein (see ►Fig. 2).
Fig. 2.
Genetic mechanisms of FSHD. Two genomic features are required to cause FSHD. The first is a contraction of the D4Z4 array from 11 to 150 repeats to 1 to 10 repeats in patients with FSHD1 and mutations in SMCHD1 in patients with FSHD2. Both loosen chromatin structure of the D4Z4 region and allow transcription of DUX4. The second feature is a functional polyadenylation signal (PAS) downstream of the last D4Z4 repeat, which allows the DUX4 transcript to be stabilized for protein translation. FSHD, facioscapulohumeral muscular dystrophy.
In FSHD1, shortening of the D4Z4 repeat array destabilizes the chromatin structure in the 4q35 region, resulting in the transcriptional derepression of the DUX4 gene. In many patients with FSHD2, mutations in the SMCHD1 gene lead to haploinsufficiency of SMCHD1, which derepresses the D4Z4 region and allows for DUX4 expression in the presence of a permissive 4qA allele.43 Recent reports showed that SMCHD1, a member of the condensin/cohesin family of chromatin compaction complexes, is also a genetic modifier of FSHD1. Patients with FSHD1 developed more severe disease phenotypes when they carry mutations in SMCHD1 in addition to contraction of the D4Z4 repeat array.56,57 In addition to SMCHD1, mutations in the DNMT3B gene have also been found to modify disease severity and may be responsible for some cases of FSHD2.44
Genetic Diagnosis
According to a recent European Neuromuscular Centre (ENMC) workshop, conventional Southern blot-based methods remain the key diagnostic tools for FSHD; the authors further emphasized the importance of having detailed clinical description at the time of referral for gene testing.58 Currently, commercial genetic testing for FSHD is limited to FSHD1. Exclusion criteria for FSHD include evidence of an alternative diagnosis based on atypical clinical features, muscle biopsy, or other available investigations.
For individuals with clinical features of FSHD, the diagnosis can be confirmed by determining the size of the D4Z4 repeat on chromosome 4q using Southern blot or molecular combing methods.58 Tawil et al confirmed that finding a D4Z4 contraction on chromosome 4q35 is highly sensitive (93%) and specific (98%) for the diagnosis of clinically defined FSHD; the degree of specificity is unlikely to be further enhanced in typical cases by testing for the 4qA variant.38
Additional genetic analysis or referral to a more specialized laboratory for further DNA tests (such as methylation analysis or FSHD gene panel) may be required if the standard FSHD test is negative. Determining the occurrence of a D4Z4 contraction on a 4qA haplotype is warranted for atypical FSHD, especially in the absence of a first-degree relative with a genetically confirmed diagnosis.38 Methylation analysis using methylation-sensitive restriction enzymes56 or bisul-fite sequencing of one or more D4Z4/pLAM sequences59 can be diagnostic for the presence of pathologic variants in D4Z4-chromatin modifiers. Additional genetic testing for SMCHD1 or DNMT3B may be indicated based on correlation with D4Z4 methylation status.44
Standard of Care for FSHD
Beyond confirming the diagnosis, a recent publication on standard of care offered guidelines on comprehensive assessment and treatment of complications related to FSHD.38,60 Important lifestyle strategies include regular physical activities, well-balanced diet, and management of musculoskeletal pain that can be offered to children and adults with FSHD after confirmation of the diagnosis. Scapular fixation in centers experienced with FSHD is potentially safe and effective for carefully selected patients, once they have achieved skeletal maturity. Other anticipatory care includes screening for extramuscular manifestations, particularly among children with early-onset disease.
Extramuscular Manifestations in Early-Onset FSHD
Retinal Vasculopathy
Small (1968) first described the association of bilateral retinal exudative telangiectasia, neurosensory deafness, and mental retardation among four siblings with FSHD.61 A Dutch population survey subsequently found that 3 out of 256 (1.7%) patients with FSHD had retinal vasculopathy with features resembling Coats’ disease.7 More recently, Statland et al estimated the prevalence of retinal vasculopathy in FSHD1 to be at 0.8% (95% CI: 0.2–2.2%); there was a negative association between D4Z4 repeat number and the risk of Coats’ disease.33 Based on a systematic review, Tawil et al concluded that although up to 25% (95% CI: 20.9–30.8%) of patients with FSHD had abnormalities on retinal examination, only 0.6% (95% CI: 0.2–1.5%) had symptomatic retinal disease.38
To ensure optimal vision, children with FSHD, especially those with large deletions (10–20 kb or less than five D4Z4 repeats) should be referred to an experienced ophthalmologist for dilated indirect ophthalmoscopy. Subsequent monitoring will depend on the degree of retinal vasculopathy at initial screening. Also, children with unspecified retinal vasculopathy should have careful neurological evaluation to exclude early-onset FSHD.
Neurosensory Hearing Loss
Lutz et al found that hearing loss preferentially affects individuals with large deletions (<20 kb); one-third (32%; 95% CI:16.7–51.4%) of their patients in this group had hearing loss and some were progressive.35 Tawil et al concluded in their systematic review that although 15.5% (95% CI: 12.1–19.4%) of patients with FSHD had evidence of audiometric abnormalities, the estimate was hampered by the significant variability in the frequencies reported in the literature.38
The current guideline therefore recommends that young children with FSHD should have hearing assessment at diagnosis and yearly thereafter until entry into the school. Older children do not need formal evaluation if their language development is normal and hearing is routinely monitored in school. Similarly, adults with FSHD generally do not require audiograms unless they are symptomatic.
Central Nervous System Involvement
Rarely, FSHD can be associated with cognitive disability and epilepsy in childhood, particularly among those with very few D4Z4 repeats.62–66 In line with previous studies, Chen et al found that epilepsy frequently occurred with mental retardation among patients with very short EcoRI fragments (10–12 kb or less than three D4Z4 repeats).30 Given the risk of epilepsy in these individuals, education regarding seizure recognition and epilepsy safety should be offered at diagnosis; a sleep-deprived electroencephalogram (EEG) may be indicated for those with suspected seizures. Also, facial weakness and auditory or visual disturbance could be obscured by the presence of profound intellectual disability in early-onset FSHD.67 Formal visual and auditory assessments on a regular basis plus timely intervention are required to ensure optimal developmental outcomes for these children.
Cardiac and Respiratory Involvement
Respiratory insufficiency and cardiomyopathy are relatively uncommon in FSHD. Routine cardiac screening is not required in the absence of any cardiac symptoms. However, given that children may not be able to express their symptoms, an initial echocardiogram and electrocardiogram (ECG) assessment is reasonable for those diagnosed with early-onset disease. Similarly, a baseline pulmonary function test is recommended for all patients with FSHD, especially those with severe involvement. In a recent pediatric study, respiratory volumes were found to correlate with disease severity, and the expiratory phase appeared to be specifically affected in early-onset FSHD compared with other muscular dystrophies.68 Those with abnormal baseline pulmonary function, significant weakness, or other comorbid conditions that may affect ventilation should be monitored regularly or referred to a specialist in pulmonary and sleep medicine for consideration of overnight sleep studies or noninvasive positive-pressure ventilation.
Clinical Evaluations of FSHD
Accurate assessments are essential for clinical care and research; these can provide information regarding the extent of functional impairment and detect potential response to disease-modifying treatments. Common clinical evaluations for FSHD include quantitative muscle strength testing (QMT), timed function test, and clinical severity scores.38 The use of age-appropriate motivating audiovisual feedback such as the Cooperative International Neuromuscular Research Group quantitative muscle testing (QMT) system can enable maximal isometric contraction and reliable muscle testing in young children.69 Recently, Eichinger et al described the use of timed function tests, including the 6 minute walk test (6MWT), the Timed Up and Go (TUG), and the 30 foot Go/Timed 10 m test, as measures of mobility among participants with FSHD.70 They found the 6MWT to be reliable and consistent when compared across two study sites using standardized protocols. The 6MWT also showed moderate to strong correlations with other assessments of FSHD disease severity, including measures of strength and function. These timed function tests are likely be included as outcome measures for future natural history studies and clinical trials for FSHD.
Lamperti et al developed a clinical evaluation scale (CES)71 to enable functional quantification of muscle weakness for patients with FSHD. The sum of six regional (facial, scapular, upper limb, distal leg, pelvic girdle, and abdominal) muscle groups generates a FSHD clinical severity score with total scores ranging from 0 to 15. The score can be further grouped into grades ofdiseaseinvolvement, for example, 0 (unaffected), 1–2 (mild), 3–6 (moderate), or 7–15 (severe). More recently, Ricci et al revised the FSHD clinical form to expand the phenotypic spectrum as observed in FSHD.72 The new comprehensive clinical evaluation form (CCEF) allows for precise categorization of patients; furthermore, it may potentially be useful for genetic counseling, selection of subjects in treatment trials, as well as research into other genetic factors contributing to the clinical variability in FSHD.72 Both the FSHD CES and the CCEF can be adapted for assessment of pediatric-onset FSHD.
Additional Evaluations
Muscle Magnetic Resonance Imaging
Muscle MRI is increasingly used to identify specific pattern of involvement in different types of muscular dystrophies. It is particularly useful in detecting selective muscle involvement of nonclinically testable muscles; furthermore, MRI can potentially be used as outcome measures in clinical trials as well as longitudinal studies of FSHD.73,74 However, young children or those with cognitive disability may require sedation or general anesthesia to undergo a formal muscle MRI study.
Recently, Gerevini et al detected a specific pattern of muscle fatty replacement and atrophy, particularly in upper girdle muscles using MRI. According to Gerevini et al, the most frequently affected muscles, including paucisymptomatic and severely affected FSHD patients, were the trapezius, teres major, and serratus anterior; moreover, asymmetric muscle involvement was significantly higher in FSHD as compared with non-FSHD patients.74 In another study, Tasca et al reported that the combined involvement of abdominal and hamstring muscles with iliopsoas sparing was relatively common in FSHD (67% of the patients). They also found that the extent of lesions on lower limb MRI showed a high correlation with overall clinical severity, with one-fourth of their nonpenetrant gene carriers showed abnormalities on MRI.73 Furthermore, potential markers of active disease, as indicated by hyperintensities on short-tau inversion recovery sequences, were found in muscles without signs of fatty replacement in one-third of their patients. The authors suggested that the hyperintensities were indicative of early stages of muscle involvement in FSHD.73
Biomarker Exploration for FSHD
Biomarkers that can be used to evaluate the effects of treatment in much more acute time frames are highly desirable, especially those that are quantitative in nature and not subjected to effort or bias. Surrogate biomarkers available from a less invasive procedure such as blood collection (instead of muscle biopsies) will be a valuable outcome measure for evaluating therapeutic efficacy in clinical studies for FSHD, especially in children with significant muscle weakness. Previous studies identified both protein and miRNA biomarker candidates in patients’ sera.75,76 Recently, Petek et al utilized the sensitive, specific, high-throughput SomaLogic proteomics platform of 1,129 proteins to identify proteins with levels that correlated with FSHD severity. They reported that the levels of creatine kinase (both MM and MB isoforms), carbonic anhydrase III, and troponin I type 2 were found to reliably predict the disease state and correlated with disease severity.77 Moreover, the novel biomarkers may help reveal other mechanisms of disease in FSHD. Further confirmatory studies are pending.
New Emerging Treatments for FSHD
Although there is presently no effective pharmacological treatment, recent discoveries have improved our understanding on the pathogenesis of FSHD and allowed researchers to focus their efforts on epigenetic and transcriptional regulation of the aberrantly expressed DUX4 in FSHD. DUX4 is a retro-transposed gene with several alternative splice variants.78 Snider et al reported that the full-length splice variants of DUX4 (DUX4-fl) are aberrantly expressed in FSHD muscle cells but not in the muscles of healthy individuals. In addition, expression of the DUX4-fl transcript is restricted to testis and possibly germline cells in healthy individuals. Several studies showed that DUX4 upregulated germline genes in FSHD muscle cells when ectopically expressed in muscle cells.53,79,80 DUX4 is toxic independent of tissue types when it is expressed ectopically in frogs, zebra fish, and mice.81,82 In addition, it is proapoptotic when aberrantly expressed in mouse and human muscle cells.51,81,83 Additional molecular pathways misregu lated by DUX4 include oxidative stress responses,84,85 immune responses,52 transcription regulation,54,86,87 protein turnover,88,89 and myogenesis program.53 Currently, therapeutic strategies targeting different steps in the cascade of DUX4 regulation are under investigation and include (a) restoring epigenetic state at the D4Z4 region, (b) knocking down the aberrantly expressed DUX4 (c) reducing the cytotoxic effects downstream of DUX4, and (d) counteracting the pathological changes by activating compensatory pathways. The following are examples of treatment strategies that have been advanced into preclinical or clinical studies for FSHD.
Oligonucleotide-Based Gene Therapy
In the past three decades, different oligonucleotide-based gene silencing strategies have been developed to target specific genes for treating infectious and genetic diseases. Some of these gene-silencing strategies are being evaluated in clinical trials and two of these are United States Food and Drug Administration (FDA) approved drugs (eteplirsen and nusinersen) for neuromuscular diseases. Different modifications of existing strategies were developed and investigated to overcome common problems associated with these gene-silencing strategies, including (a) poor stability, (b) inefficient delivery into target cells, (c) off-target/toxicity effects, and (d) immunogenicity.90,91 Several backbone modifications of these oligo-nucleotides improve their stability as RNA-silencing agents. Protein peptides or other molecules are conjugated to the oligonucleotides to increase delivery efficiency. However, such modifications often increased toxicity and/or immunogenicity. To date, oligonucleotide strategies are still evolving quickly, and they will require further preclinical studies.
For FSHD, several approaches have been developed to sequester or degrade the target mRNAs, including shRNA, miRNA, siRNA, and antisense oligonucleotides (AON).92–95 Before DUX4 was considered as the causative gene of FSHD, candidate genes in the 4q35 region were investigated for their potential involvement in FSHD. These were the facioscapulohumeral muscular dystrophy region gene 1 (FRG1), facioscapulohumeral muscular dystrophy region gene 2 (FRG2), and adenine nucleotide trans-locator 1 (ANT1).96 The shRNA and miRNA against FRG1 were delivered using adeno-associated viral (AAV) vector, which successfully reduced the FRG1 expression in vivo.93,94 Later, miRNA against DUX4 was delivered by AAV vector into mouse muscles ectopically expressing DUX4 and was able to reduce DUX4 and improve pathologies induced by it.95 In addition, morpholinos were shown to either successfully reduce DUX4 expression or inhibit translation of a DUX4-regulated gene, termed paired-like homeodomain transcription factor 1 (Pitx1).89,90,98 One major challenge of AON therapy is the limited uptake by muscle in vivo. Currently, several new compounds with gymnosis property are under investigation.
Reduce Muscle Inflammation by Immune Modulatory Agents
Previous studies reported that severe inflammatory responses were found in approximately 30% of patients with FSHD. Atypical inflammatory responses, including activation of both innate and acquired immune responses, were shown in FSHD muscles, although autoantibodies that are commonly detected in myositis were not detected in FSHD.79,98,99 In addition, molecular studies reported activation of immune-relatedgenes when DUX4 is activated in muscle cells.51,52 These findings suggested active roles of immune system in disease mechanisms of FSHD. Pharmaceutical agents that modulate immune responses may potentially benefit the disease. A pilot trial of prednisone was conducted and showed no significant effect, although this may have been due to the short trial period to detect significant improvement or delay in disease progression.100 Currently, clinical trials testing an immune modulatory compound, ATYR1940, arebeing conductedby ATyr Pharma Inc. The compound is a physiocrine-based protein (histidyl tRNA synthetase)withmultiplefunctionsand is believed to modulate immune responses in skeletal muscles.101,102
Improve Muscle Mass and Pathology by Activating Compensatory Pathways
Previous studies showed that aberrant expression of DUX4 led to activation of molecular pathways involved in skeletal muscle atrophy.89 One potential therapeutic strategy will be to activate compensatory pathways that will counteract the muscle atrophy induced by DUX4. Myostatin is a member of the transforming growth factor-β superfamily. It negatively regulates muscle mass during fetal development and in postnatal muscles.103 Several species with mutated myostatin have hypertrophic muscles, supporting the role of myostatin as a negative regulator of muscle mass in the whole body. Myostatin is downregulated during normal muscleregenerationand in muscular dystrophies.104–108 Several animal studies have demonstrated beneficial effects of myostatin inhibitors109–113 although this has not been tested in a FSHD model. A clinical studyexamining safetyof a neutralizing antibody to myostatin, MYO-029, was conducted and concluded that the agent was relatively safe.114 Clinical studies on locally-acting myostatin inhibitor, ACE-083, are currently conducted by Acceleron Pharma.
Other Treatments
Other potential treatments include exercise therapy, cell therapy, as well as studies of compounds that target molecular pathways and are misregulated by DUX4. The latter includes a clinically approved tyrosine kinase inhibitor (sunitinib),115 poly ADP ribose polymerase (PARP1) inhibitors,116 and antioxidants.117 FSHD cells have been reported to be more susceptible to oxidative stress. A clinical study with vitamin C, vitamin E, zinc, and selenium supplements indicated potential benefits to the disease.118 Exercise and behavioral intervention have also shown beneficial effects for individuals with FSHD.119–121 Several clinical trials testing different regiments of exercises are currently ongoing.
A recent study investigated the fusion of FSHD myoblasts with primary myoblasts from healthy individuals to correct differentiation defects in culture.122 The authors concluded that more than 50% of normal myoblasts were required to correct the phenotypical and functional defects observed in FSHD; therefore, cell therapy in the FSHD is potentially feasible but with limitations. In addition to genome editing, a CRISPR/dCas9 transcriptional inhibitor was targeted to the D4Z4 array to silence the aberrantly expressed DUX4.123 The study demonstrated the potential of using CRISPR/dCas9 transcriptional inhibitor to correct the epigenetic defects in FSHD.
Conclusion
FSHD is a heterogeneous disease; children with early-onset disease are at a risk of more rapid decline in motor function, early loss of independent ambulation, as well as extramuscular manifestations. The elucidation of a unifying model for FSHD has provided several potential therapeutic approaches to treat FSHD. Early diagnosis, ongoing support as per standard of care guidelines, and collaborationwith global disease registries will be essential for optimizing the health outcome of affected individuals, as well as clinical trial readiness for FSHD.
Acknowledgment
The authors would like to thank the FSH Society, Muscular Dystrophy Canada, the FSHD Global Research Foundation, as well as patients and families for their support of the early onset FSHD study.
References
- 1.Tawil R, van der Maarel SM, Tapscott SJ. Facioscapulohumeral dystrophy: the path to consensus on pathophysiology. Skelet Muscle 2014;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacconi S, Salviati L, Desnuelle C. Facioscapulohumeral muscular dystrophy. Biochim Biophys Acta 2015;1852(04):607–614 [DOI] [PubMed] [Google Scholar]
- 3.Daxinger L, Tapscott SJ, van der Maarel SM. Genetic and epigenetic contributors to FSHD. Curr Opin Genet Dev 2015; 33:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopp P, Krom YD, Banerji CR, et al. DUX4 induces a transcriptome more characteristic of a less-differentiated cell state and inhibits myogenesis. J Cell Sci 2016;129(20):3816–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanigan KM, Coffeen CM, Sexton L, Stauffer D, Brunner S, Leppert MF. Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord 2001;11(6–7):525–529 [DOI] [PubMed] [Google Scholar]
- 6.Deenen JC, Arnts H, van der Maarel SM, et al. Population-based incidence and prevalence of facioscapulohumeral dystrophy. Neurology 2014;83(12):1056–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padberg GW, Frants RR, Brouwer OF, Wijmenga C, Bakker E, Sandkuijl LA. Facioscapulohumeral muscular dystrophy in the Dutch population. Muscle Nerve Suppl 1995;2:S81–S84 [PubMed] [Google Scholar]
- 8.Padberg GW, Lunt PW, Koch M, Fardeau M. Diagnostic criteria for facioscapulohumeral muscular dystrophy. Neuromuscul Disord 1991;1(04):231–234 [DOI] [PubMed] [Google Scholar]
- 9.Lemmers RJ, Wohlgemuth M, van der Gaag KJ, et al. Specific sequence variations within the 4q35 region are associated with facioscapulohumeral muscular dystrophy. Am J Hum Genet 2007;81(05):884–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scionti I, Fabbri G, Fiorillo C, et al. Facioscapulohumeral muscular dystrophy: new insights from compound heterozygotes and implication for prenatal genetic counselling. J Med Genet 2012;49(03):171–178 [DOI] [PubMed] [Google Scholar]
- 11.van der Maarel SM, Deidda G, Lemmers RJ, et al. De novo facioscapulohumeral muscular dystrophy: frequent somatic mosaicism, sex-dependent phenotype, and the role of mitotic transchromosomal repeat interaction between chromosomes 4 and 10. Am J Hum Genet 2000;66(01):26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tawil R, McDermott MP, Mendell JR, Kissel J, Griggs RC; FSH-DY Group. Facioscapulohumeral muscular dystrophy (FSHD): design of natural history study and results of baseline testing. Neurology 1994;44(3, Pt 1):442–446 [DOI] [PubMed] [Google Scholar]
- 13.Pradhan S Poly-Hill sign in facioscapulohumeral dystrophy. Muscle Nerve 2002;25(05):754–755 [DOI] [PubMed] [Google Scholar]
- 14.Shahrizaila N, Wills AJ. Significance of Beevor’s sign in facioscapulohumeral dystrophy and other neuromuscular diseases. J Neurol Neurosurg Psychiatry 2005;76(06):869–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mul K, Lassche S, Voermans NC, Padberg GW, Horlings CG, van Engelen BG. What’s in a name? The clinical features of facioscapulohumeral muscular dystrophy. Pract Neurol 2016;16(03): 201–207 [DOI] [PubMed] [Google Scholar]
- 16.van der Kooi EL, Kalkman JS, Lindeman E, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol 2007;254(07):931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastorello E, Cao M, Trevisan CP. Atypical onset in a series of 122 cases with Facioscapulohumeral muscular dystrophy. Clin Neurol Neurosurg 2012;114(03):230–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Kooi AJ, Visser MC, Rosenberg N, et al. Extension of the clinical range of facioscapulohumeral dystrophy: report of six cases. J Neurol Neurosurg Psychiatry 2000;69(01):114–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wohlgemuth M, de Swart BJ, Kalf JG, Joosten FB, Van der Vliet AM, Padberg GW. Dysphagia in facioscapulohumeral muscular dystrophy. Neurology 2006;66(12):1926–1928 [DOI] [PubMed] [Google Scholar]
- 20.van Dijk GP, van der Kooi E, Behin A, et al. High prevalence of incomplete right bundle branch block in facioscapulohumeral muscular dystrophy without cardiac symptoms. Funct Neurol 2014;29(03):159–165 [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji M, Kinoshita M, Imai Y, Kawamoto M, Kohara N. Facioscapulohumeral muscular dystrophy presenting with hypertrophic cardiomyopathy: a case study. Neuromuscul Disord 2009; 19(02):140–142 [DOI] [PubMed] [Google Scholar]
- 22.Scully MA, Eichinger KJ, Donlin-Smith CM, Tawil R, Statland JM. Restrictive lung involvement in facioscapulohumeral muscular dystrophy. Muscle Nerve 2014;50(05):739–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacconi S, Salviati L, Bourget I, et al. Diagnostic challenges in facioscapulohumeral muscular dystrophy. Neurology 2006; 67(08):1464–1466 [DOI] [PubMed] [Google Scholar]
- 24.Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil 2008;89(02):320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Statland JM, Tawil R. Risk of functional impairment in facioscapulohumeral muscular dystrophy. Muscle Nerve 2014;49(04): 520–527 [DOI] [PubMed] [Google Scholar]
- 26.Dorobek M, van der Maarel SM, Lemmers RJ, et al. Early-onset facioscapulohumeral muscular dystrophy type 1 with some atypical features. J Child Neurol 2015;30(05):580–587 [DOI] [PubMed] [Google Scholar]
- 27.Klinge L, Eagle M, Haggerty ID, Roberts CE, Straub V, Bushby KM. Severe phenotype in infantile facioscapulohumeral muscular dystrophy. Neuromuscul Disord 2006;16(09–10):553–558 [DOI] [PubMed] [Google Scholar]
- 28.Brooke MH. A Clinician’s View of Neuromuscular Diseases Baltimore, MD: Williams & Wilkins; 1977 [Google Scholar]
- 29.Brouwer OF, Padberg GW, Wijmenga C, Frants RR. Facioscapulohumeral muscular dystrophy in early childhood. Arch Neurol 1994;51(04):387–394 [DOI] [PubMed] [Google Scholar]
- 30.Chen TH, Lai YH, Lee PL, et al. Infantile facioscapulohumeral muscular dystrophy revisited: Expansion of clinical phenotypes in patients with a very short EcoRI fragment. Neuromuscul Disord 2013;23(04):298–305 [DOI] [PubMed] [Google Scholar]
- 31.Hanson PA, Rowland LP. Möbius syndrome and facioscapulohumeral muscular dystrophy. Arch Neurol 1971;24(01):31–39 [DOI] [PubMed] [Google Scholar]
- 32.Kolski HK, Leonard NJ, Lemmers RJ, Bamforth JS. Atypical facet of Möbius syndrome: association with facioscapulohumeral muscular dystrophy. Muscle Nerve 2008;37(04):526–529 [DOI] [PubMed] [Google Scholar]
- 33.Statland JM, Sacconi S, Farmakidis C, Donlin-Smith CM, Chung M, Tawil R. Coats syndrome in facioscapulohumeral dystrophy type 1: frequency and D4Z4 contraction size. Neurology 2013;80(13): 1247–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grosso S, Mostardini R, Di Bartolo RM, Balestri P, Verrotti A. Epilepsy, speech delay, and mental retardation in facioscapulohumeral muscular dystrophy. Eur J Paediatr Neurol 2011;15(05): 456–460 [DOI] [PubMed] [Google Scholar]
- 35.Lutz KL, Holte L, Kliethermes SA, Stephan C, Mathews KD. Clinical and genetic features of hearing loss in facioscapulohumeral muscular dystrophy. Neurology 2013;81(16):1374–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bindoff LA, Mjellem N, Sommerfelt K, et al. Severe fascioscapulohumeral muscular dystrophy presenting with Coats’ disease and mental retardation. Neuromuscul Disord 2006;16 (9–10):559–563 [DOI] [PubMed] [Google Scholar]
- 37.Lunt PW, Jardine PE, Koch MC, et al. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35-facioscapulohumeral muscular dystrophy (FSHD). Hum Mol Genet 1995;4(05):951–958 [DOI] [PubMed] [Google Scholar]
- 38.Tawil R, Kissel JT, Heatwole C, Pandya S, Gronseth G, Benatar M; Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Evidence-based guideline summary: evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2015;85(04):357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goselink RJ, Schreuder TH, Mul K, et al. Facioscapulohumeral dystrophy in children: design of a prospective, observational study on natural history, predictors and clinical impact (iFocus FSHD). BMC Neurol 2016;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolic A, Ricci G, Sera F, et al. Clinical expression of facioscapulohumeral muscular dystrophy in carriers of 1–3 D4Z4 reduced alleles: experience of the FSHD Italian National Registry. BMJ Open 2016;6(01):e007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Deutekom JC, Wijmenga C, van Tienhoven EA, et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol Genet 1993; 2(12):2037–2042 [DOI] [PubMed] [Google Scholar]
- 42.van Overveld PG, Lemmers RJ, Sandkuijl LA, et al. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat Genet 2003;35(04):315–317 [DOI] [PubMed] [Google Scholar]
- 43.Lemmers RJ, Tawil R, Petek LM, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet 2012;44(12):1370–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Boogaard ML, Lemmers RJ, Balog J, et al. Mutations in DNMT3B Modify epigenetic repression of the D4Z4 repeat and the penetrance of facioscapulohumeral dystrophy. Am J Hum Genet 2016;98(05):1020–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabriëls J, Beckers MC, Ding H, et al. Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene 1999;236(01): 25–32 [DOI] [PubMed] [Google Scholar]
- 46.Ansseau E, Laoudj-Chenivesse D, Marcowycz A, et al. DUX4c is up-regulated in FSHD. It induces the MYF5 protein and human myoblast proliferation. PLoS One 2009;4(10):e7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostlund C, Garcia-Carrasquillo RM, Belayew A, Worman HJ. Intracellular trafficking and dynamics of double homeodomain proteins. Biochemistry 2005;44(07):2378–2384 [DOI] [PubMed] [Google Scholar]
- 48.Coppée F, Mattéotti C, Ansseau E, et al. The DUX gene family and FSHD In: Upadhyaya M, Cooper DN, eds. Facioscapulohumeral Muscular Dystrophy: Clinical Medicine and Molecular Cell Biology. Oxford, UK: BIOS Scientific Publishers; 2004 [Google Scholar]
- 49.Yip DJ, Picketts DJ. Increasing D4Z4 repeat copy number compromises C2C12 myoblast differentiation. FEBS Lett 2003;537 (1–3):133–138 [DOI] [PubMed] [Google Scholar]
- 50.Beckers M, Gabriëls J, van der Maarel S, et al. Active genes in junk DNA? Characterization of DUX genes embedded within 3.3 kb repeated elements. Gene 2001;264(01):51–57 [DOI] [PubMed] [Google Scholar]
- 51.Sharma V, Harafuji N, Belayew A, Chen YW. DUX4 differentially regulates transcriptomes of human rhabdomyosarcoma and mouse C2C12 cells. PLoS One 2013;8(05):e64691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng LN, Yao Z, Snider L, et al. DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev Cell 2012;22(01):38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winokur ST, Chen YW, Masny PS, et al. Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Hum Mol Genet 2003;12(22):2895–2907 [DOI] [PubMed] [Google Scholar]
- 54.Ansseau E, Eidahl JO, Lancelot C, et al. Homologous transcription factors DUX4 and DUX4c associate with cytoplasmic proteins during muscle differentiation. PLoS One 2016;11(01):e0146893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemmers RJ, van der Vliet PJ, Klooster R, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 2010;329(5999):1650–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen M, Rost S, El Hajj N, et al. Diagnostic approach for FSHD revisited: SMCHD1 mutations cause FSHD2 and act as modifiers of disease severity in FSHD1. Eur J Hum Genet 2015;23(06): 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacconi S, Lemmers RJ, Balog J, et al. The FSHD2 gene SMCHD1 is a modifier of disease severity in families affected by FSHD1. Am J Hum Genet 2013;93(04):744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemmers RJ, O’Shea S, Padberg GW, Lunt PW, van der Maarel SM. Best practice guidelines on genetic diagnostics of facioscapulohumeral muscular dystrophy: workshop 9th June 2010, LUMC, Leiden, The Netherlands. Neuromuscul Disord 2012;22(05): 463–470 [DOI] [PubMed] [Google Scholar]
- 59.Calandra P, Cascino I, Lemmers RJ, et al. Allele-specific DNA hypomethylation characterises FSHD1 and FSHD2. J Med Genet 2016;53(05):348–355 [DOI] [PubMed] [Google Scholar]
- 60.Tawil R, van der Maarel S, Padberg GW, van Engelen BG. 171st ENMC international workshop: Standards of care and management of facioscapulohumeral muscular dystrophy. Neuromuscul Disord 2010;20(07):471–475 [DOI] [PubMed] [Google Scholar]
- 61.Small RG. Coats’ disease and muscular dystrophy. Trans Am Acad Ophthalmol Otolaryngol 1968;72(02):225–231 [PubMed] [Google Scholar]
- 62.Akiyama C, Suzuki H, Nonaka I. A case of facioscapulohumeral muscular dystrophy with infantile spasms, sensorineural deafness and retinal vessel abnormality [Article in Japanese]. No To Hattatsu 1991;23(04):395–399 [PubMed] [Google Scholar]
- 63.Miura K, Kumagai T, Matsumoto A, et al. Two cases of chromo-some 4q35-linked early onset facioscapulohumeral muscular dystrophy with mental retardation and epilepsy. Neuropediatrics 1998;29(05):239–241 [DOI] [PubMed] [Google Scholar]
- 64.Funakoshi M, Goto K, Arahata K. Epilepsy and mental retardation in a subset of early onset 4q35-facioscapulohumeral muscular dystrophy. Neurology 1998;50(06):1791–1794 [DOI] [PubMed] [Google Scholar]
- 65.Hobson-Webb LD, Caress JB. Facioscapulohumeral muscular dystrophy can be a cause of isolated childhood cognitive dys-function. J Child Neurol 2006;21(03):252–253 [DOI] [PubMed] [Google Scholar]
- 66.Trevisan CP, Pastorello E, Tomelleri G, et al. Facioscapulohumeral muscular dystrophy: hearing loss and other atypical features of patients with large 4q35 deletions. Eur J Neurol 2008;15(12): 1353–1358 [DOI] [PubMed] [Google Scholar]
- 67.Saito Y, Miyashita S, Yokoyama A, et al. Facioscapulohumeral muscular dystrophy with severe mental retardation and epilepsy. Brain Dev 2007;29(04):231–233 [DOI] [PubMed] [Google Scholar]
- 68.Trucco F, Pedemonte M, Fiorillo C, et al. Respiratory pattern in a FSHD pediatric population. Respir Med 2016;119:78–80 [DOI] [PubMed] [Google Scholar]
- 69.Escolar DM, Henricson EK, Mayhew J, et al. Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. Muscle Nerve 2001;24(06):787–793 [DOI] [PubMed] [Google Scholar]
- 70.Eichinger K, Heatwole C, Heininger S, et al. ; FSHD Clinical Trials Research Network. Validity of the 6 minute walk test in facioscapulohumeral muscular dystrophy. Muscle Nerve 2017;55(03): 333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamperti C, Fabbri G, Vercelli L, et al. A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: the FSHD clinical score. Muscle Nerve 2010;42(02): 213–217 [DOI] [PubMed] [Google Scholar]
- 72.Ricci G, Ruggiero L, Vercelli L, et al. A novel clinical tool to classify facioscapulohumeral muscular dystrophy phenotypes. J Neurol 2016;263(06):1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tasca G, Monforte M, Ottaviani P, et al. Magnetic resonance imaging in a large cohort of facioscapulohumeral muscular dystrophy patients: pattern refinement and implications for clinical trials. Ann Neurol 2016;79(05):854–864 [DOI] [PubMed] [Google Scholar]
- 74.Gerevini S, Scarlato M, Maggi L, et al. Muscle MRI findings in facioscapulohumeral muscular dystrophy. Eur Radiol 2016; 26(03):693–705 [DOI] [PubMed] [Google Scholar]
- 75.Matsuzaka Y, Kishi S, Aoki Y, et al. Three novel serum biomarkers, miR-1, miR-133a, and miR-206 for limb-girdle muscular dystrophy, facioscapulohumeral muscular dystrophy, and Becker muscular dystrophy. Environ Health Prev Med 2014;19(06):452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Statland J, Donlin-Smith CM, Tapscott SJ, van der Maarel S, Tawil R. Multiplex screen of serum biomarkers in facioscapulohumeral muscular dystrophy. J Neuromuscul Dis 2014;1(02):181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petek LM, Rickard AM, Budech C, et al. A cross sectional study of two independent cohorts identifies serum biomarkers for facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul Disord 2016;26(07):405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snider L, Geng LN, Lemmers RJ, et al. Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene. PLoS Genet 2010;6(10):e1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tasca G, Pescatori M, Monforte M, et al. Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS One 2012;7(06): e38779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahimov F, King OD, Leung DG, et al. Transcriptional profiling in facioscapulohumeral muscular dystrophy to identify candidate biomarkers. Proc Natl Acad Sci U S A 2012;109(40): 16234–16239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallace LM, Garwick SE, Mei W, et al. DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann Neurol 2011;69(03):540–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wuebbles RD, Long SW, Hanel ML, Jones PL. Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol 2010;3(04):386–400 [PMC free article] [PubMed] [Google Scholar]
- 83.Kowaljow V, Marcowycz A, Ansseau E, et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul Disord 2007;17(08):611–623 [DOI] [PubMed] [Google Scholar]
- 84.Winokur ST, Barrett K, Martin JH, et al. Facioscapulohumeral muscular dystrophy (FSHD) myoblasts demonstrate increased susceptibility to oxidative stress. Neuromuscul Disord 2003; 13(04):322–333 [DOI] [PubMed] [Google Scholar]
- 85.Turki A, Hayot M, Carnac G, et al. Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction. Free Radic Biol Med 2012;53(05):1068–1079 [DOI] [PubMed] [Google Scholar]
- 86.Feng Q, Snider L, Jagannathan S, et al. A feedback loop between nonsense-mediated decay and the retrogene DUX4 in facioscapulohumeral muscular dystrophy. eLife 2015. doi: 10.7554/eLife.04996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rickard AM, Petek LM, Miller DG. Endogenous DUX4 expression in FSHD myotubes is sufficient to cause cell death and disrupts RNA splicing and cell migration pathways. Hum Mol Genet 2015; 24(20):5901–5914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Homma S, Beermann ML, Boyce FM, Miller JB. Expression of FSHD-related DUX4-FL alters proteostasis and induces TDP-43 aggregation. Ann Clin Transl Neurol 2015;2(02):151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanderplanck C, Ansseau E, Charron S, et al. The FSHD atrophic myotube phenotype is caused by DUX4 expression. PLoS One 2011;6(10):e26820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pandey SN, Lee YC, Yokota T, Chen YW. Morpholino treatment improves muscle function and pathology of Pitx1 transgenic mice. Mol Ther 2014;22(02):390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moreno PM, Pêgo AP. Therapeutic antisense oligonucleotides against cancer: hurdling to the clinic. Front Chem 2014;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thakker DR, Natt F, Hüsken D, et al. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci U S A 2004;101(49):17270–17275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bortolanza S, Nonis A, Sanvito F, et al. AAV6-mediated systemic shRNA delivery reverses disease in a mouse model of facioscapulohumeral muscular dystrophy. Mol Ther 2011;19(11): 2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallace LM, Garwick-Coppens SE, Tupler R, Harper SQ. RNA interference improves myopathic phenotypes in mice over-expressing FSHD region gene 1 (FRG1). Mol Ther 2011;19(11):2048–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallace LM, Liu J, Domire JS, et al. RNA interference inhibits DUX4-induced muscle toxicity in vivo: implications for a targeted FSHD therapy. Mol Ther 2012;20(07):1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gabellini D, Green MR, Tupler R. Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell 2002;110(03):339–348 [DOI] [PubMed] [Google Scholar]
- 97.Chen JC, King OD, Zhang Y, et al. Morpholino-mediated knockdown of DUX4 toward facioscapulohumeral muscular dystrophy therapeutics. Mol Ther 2016;24(08):1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frisullo G, Frusciante R, Nociti V, et al. CD8(þ) T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J Clin Immunol 2011;31(02): 155–166 [DOI] [PubMed] [Google Scholar]
- 99.Hengstman GJ, van Brenk L, Vree Egberts WT, et al. High specificity of myositis specific autoantibodies for myositis compared with other neuromuscular disorders. J Neurol 2005; 252(05):534–537 [DOI] [PubMed] [Google Scholar]
- 100.Tawil R, McDermott MP, Pandya S, et al. ; FSH-DY Group. A pilot trial of prednisone in facioscapulohumeral muscular dystrophy. Neurology 1997;48(01):46–49 [DOI] [PubMed] [Google Scholar]
- 101.Lo WS, Gardiner E, Xu Z, et al. Human tRNA synthetase catalytic nulls with diverse functions. Science 2014;345(6194):328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou JJ, Wang F, Xu Z, et al. Secreted histidyl-tRNA synthetase splice variants elaborate major epitopes for autoantibodies in inflammatory myositis. J Biol Chem 2014;289(28):19269–19275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodriguez J, Vernus B, Chelh I, et al. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci 2014;71(22):4361–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Babcock LW, Knoblauch M, Clarke MS. The role of myostatin and activin receptor IIB in the regulation of unloading-induced myofiber type-specific skeletal muscle atrophy. J Appl Physiol (1985) 2015;119(06):633–642 [DOI] [PubMed] [Google Scholar]
- 105.Jespersen JG, Nedergaard A, Andersen LL, Schjerling P, Andersen JL. Myostatin expression during human muscle hypertrophy and subsequent atrophy: increased myostatin with detraining. Scand J Med Sci Sports 2011;21(02):215–223 [DOI] [PubMed] [Google Scholar]
- 106.Durieux AC, Amirouche A, Banzet S, et al. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology 2007;148(07):3140–3147 [DOI] [PubMed] [Google Scholar]
- 107.Kirk S, Oldham J, Kambadur R, Sharma M, Dobbie P, Bass J. Myostatin regulation during skeletal muscle regeneration. J Cell Physiol 2000;184(03):356–363 [DOI] [PubMed] [Google Scholar]
- 108.Chen YW, Nagaraju K, Bakay M, et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 2005;65(06):826–834 [DOI] [PubMed] [Google Scholar]
- 109.Latres E, Pangilinan J, Miloscio L, et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skelet Muscle 2015;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang L, Rajan V,Lin E,et al. Pharmacologicalinhibition ofmyostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 2011;25(05):1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gilson H, Schakman O, Combaret L, et al. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007;148(01):452–460 [DOI] [PubMed] [Google Scholar]
- 112.Holzbaur EL, Howland DS, Weber N, et al. Myostatin inhibition slows muscle atrophy in rodent models of amyotrophic lateral sclerosis. Neurobiol Dis 2006;23(03):697–707 [DOI] [PubMed] [Google Scholar]
- 113.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 2002;52(06):832–836 [DOI] [PubMed] [Google Scholar]
- 114.Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 2008;63(05):561–571 [DOI] [PubMed] [Google Scholar]
- 115.Moyle LA, Blanc E, Jaka O, et al. Ret function in muscle stem cells points to tyrosine kinase inhibitor therapy for facioscapulohumeral muscular dystrophy. eLife 2016;5:1–35. Doi: 10.7554/eLife.11405.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma V, Pandey SN, Khawaja H, Brown KJ, Hathout Y, Chen YW. PARP1 differentially interacts with promoter region of DUX4 gene in FSHD myoblasts. J Genet Syndr Gene Ther 2016;7(04):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Emilie P, Maurice H, Gilles C, Joel P, Jacques M, Dalila C. Oxidative stress and dystrophy facioscapulohumeral: effects of vitamin C, vitamin E, zinc gluconate and selenomethionine supplementation. Free Radic Biol Med 2014;75(Suppl 1):S14. [DOI] [PubMed] [Google Scholar]
- 118.Passerieux E, Hayot M, Jaussent A, et al. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: a double-blind randomized controlled clinical trial. Free Radic Biol Med 2015; 81:158–169 [DOI] [PubMed] [Google Scholar]
- 119.Voet N, Bleijenberg G, Hendriks J, et al. Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology 2014;83(21):1914–1922 [DOI] [PubMed] [Google Scholar]
- 120.Bankolé LC, Millet GY, Temesi J, et al. Safety and efficacy of a 6-month home-based exercise program in patients with facioscapulohumeral muscular dystrophy: a randomized controlled trial. Medicine (Baltimore) 2016;95(31):e4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pasotti S, Magnani B, Longa E, et al. An integrated approach in a case of facioscapulohumeral dystrophy. BMC Musculoskelet Disord 2014;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dib C, Bou Saada Y, Dmitriev P, et al. Correction of the FSHD myoblast differentiation defect by fusion with healthy myo-blasts. J Cell Physiol 2016;231(01):62–71 [DOI] [PubMed] [Google Scholar]
- 123.Himeda CL, Jones TI, Jones PL. CRISPR/dCas9-mediated transcriptional inhibition ameliorates the epigenetic dysregulation at D4Z4 and represses DUX4-fl in FSH muscular dystrophy. Mol Ther 2016;24(03):527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]