Abstract
Introduction
The phases of fracture healing have been well characterized. However, the exact source and genetic profile of the skeletal progenitors that participate in bone repair is somewhat unclear. Sox9 expression in skeletal elements precedes bone and cartilage formation and a Sox9+ cell type is retained in the adult periosteum. We hypothesized that Sox9+ periosteal cells are multipotent skeletal progenitors normally participating in fracture repair.
Methods
To test this hypothesis we used tamoxifen (TM)-mediated lineage tracing of Sox9+ cells in Sox9CreErt2:Td-Tomato mice. Intact femora were analyzed with immunostaining and RNA sequencing to evaluate the skeletal distribution and gene expression profile of Td-Tomato positive, Sox9-descendent cells in the adult femur. To assess the role of Td-tomato+ cells in the fracture healing process, mice underwent a closed mid-diaphyseal femoral fracture. Fractured hind limbs were analyzed by X-ray, histology and immuno-staining at 3, 9 or 56 days post-fracture.
Results
In the intact adult mouse femur, Td-Tomato-labeled cells were observed in the primary spongiosa, periosteum and endosteum. RNA sequencing showed that Td-Tomato positive periosteal cells were co-enriched for Sox9 transcripts, and mRNAs for osteoblast and chondrocyte specific genes. In a femoral fracture model, we showed that pre-labeled Td-Tomato positive descendent cells were mobilized during the early stages of bone repair (day 3 post-op) contributing to the fracture repair process by differentiating into chondrocytes, osteoblasts and osteocytes.
Conclusion
A Sox9+ skeletal progenitor population resides in the adult periosteum. Fate tracing studies show that descendants of the Sox9+ periosteal progenitors give rise to chondrocytes, osteoblasts and mature cortical osteocytes in repair of the fractured femur. To our knowledge this is the first report of a reparative Sox9+ progenitor population in the periosteum of the adult long bone. Taken together with developmental studies, our data suggest a broad role for Sox9+ osteochondroprogenitors in development and repair of the mammalian skeleton.
Keywords: fracture healing, skeletal progenitors, periosteal cells, Sox9
1. Introduction
Fracture healing is a complex process that involves the well-orchestrated participation of growth factors, cytokines and several cell types [1]. Most fractures are treated with a form of fixation that provides stability, but allows for some degree of motion (sling or cast immobilization, external fixation, intramedullary fixation). Thus the majority of fractures heal by secondary or indirect bone healing, a process that involves both intramembranous and endochondral ossification [2]. Secondary bone healing involves three major phases: the reactive phase (hematoma and inflammatory response), the reparative phase (soft and hard callus formation) and the remodeling phase [3]. Briefly, a fracture leads to surrounding soft tissue trauma, damage to local blood vessels and disruption of the bone marrow structure. Wound healing pathways are activated and a hematoma is formed at the area of the injury. Inflammatory cells and activated platelets soon infiltrate the hematoma and start secreting cytokines that can stimulate angiogenesis and initiate cellular events associated with the later stages of fracture healing [4]. Before the inflammation stage subsides, the repair process is initiated. An early indication of skeletal repair is the appearance of a chondrocyte-derived cartilage template that bridges and temporarily stabilizes the fractured bone fragments (soft callus). The cartilaginous callus serves as a template for formation of the hard bony callus by osteoblasts. Eventually the cartilage is eliminated from the callus that is composed of woven bone. Finally a remodeling process, dominated by osteoblasts, osteocytes and osteoclasts, returns the newly formed bone to its original bone configuration.
Despite the fact that the phases of bone healing have been well characterized, the cellular origins and molecular pathways underlying bone healing are somewhat unclear. Several possible sources of skeletal progenitor cells for bone healing have been reported [5] including endosteum [6,7], periosteum [7–10], bone marrow [8,11,12], vascular walls [13] and adjacent soft tissue [14]. Of these, a pivotal role for periosteum-derived progenitor cells in bone healing has been confirmed in several in vitro and in vivo studies, though it is not clear if this is a general property of periosteal cells, or a property restricted to distinct osteochondroprogenitors within this tissue [15].
In contrast to bone repair, the cellular mechanisms underlying bone development during embryogenesis have been well documented. Here, the SRY (Sex Determining Region Y)-Box9 (Sox9) transcription factor plays an essential role in determining skeletal progenitor cells’ fate prior to overt chondrocyte and osteoblast development [16]. Thereafter, this osteochondroprogenitor cell population segregates into Sox9+ chondrocyte progenitors and Sox9−, Runx2/Sp7+ osteoblast progenitors that deposit cartilage and bone, respectively [16,17]. Sox9 is necessary for establishing skeletal elements in the cranial, axial and appendicular systems [18–20]. In addition, Sox9 is sufficient to initiate chondrogenic programs when activated in mesenchymal stem cells, embryonic stem cells and even fibroblasts [21–24].
Fracture healing has been characterized by many as the postnatal analogue of embryonic skeletal development, since many of the molecular mechanisms that control differentiation and growth during embryogenesis recur during fracture repair [25]. Since Sox9 defines osteochondroprogenitor cells during skeletogenesis and a similar differentiation program is likely shared between skeletal development and adult long bone repair, we hypothesized that Sox9 might play a major role in adult long bone repair. In this study, we demonstrate that an osteochondroprogenitor cell population positive for Sox9 resides in the periosteum of adult long bones and that upon activation by fracture stimulation, these osteochondroprogenitor cells direct fracture repair, differentiating into chondrocytes, osteoblasts and osteocytes.
2. Material and Methods
2.1 Mouse lines and lineage tracing
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Southern California (IACUC # 11892) and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. A double heterozygous Sox9CreErt2:Td-Tomato mouse line was used for the lineage tracing experiments. These double heterozygous mice, carrying one allele of Sox9CreErt2 driver and one allele of Td-Tomato reporter, were generated by crossing heterozygous Sox9CreErt2 (Sox9tm1(cre/ERT2)Haak) [26] with homozygous Td-Tomato (Gt(ROSA)26Sortm14(CAG-tdTomato)Hze) [27] mice purchased from the Jackson Laboratory (https://www.jax.org. Bar Harbor, ME). The mice received 3 intraperitoneal injections of 100μL tamoxifen (TM) solution (20 mg/mL diluted in corn oil, Cat# T5648-1g, Sigma-Aldrich, St. Louis, MO), at 48-hour intervals, 2 weeks pre-euthanasia or pre-operatively depending upon the group, to induce Td-Tomato reporter expression in Sox9-expressing cells and their descendants. Thirty-six, 12–16-week old, Sox9CreErt2:Td-Tomato mice, were used in the study; 24 mice for analysis of intact adult femora and 12 for the femoral fracture assay. Intact mouse femora were harvested 2 weeks after the last tamoxifen injection and analyzed with a) frozen histology and immunostaining, to evaluate the distribution of Sox9CreErt2+ descendent cells in adult long bones and b) RNA sequencing, to determine the gene expression profile of periosteal cells of the femur. The remaining mice were used to assess the contribution of Sox9CreErt2+ cells at different stages of the fracture healing process.
2.2 Femoral fracture
Twelve animals received 3 consecutive doses of TM, 2 weeks before a closed mid-diaphyseal femoral fracture was created unilaterally using an established fracture model [28–30]. Briefly, the mice were anesthetized with inhalational anesthesia (2% isoflurane) and their left hind limbs were shaved and prepared with three alternating scrubs of betadine and 70 % isopropyl alcohol. Using aseptic surgical technique, a 3 mm incision was made medial to the patellar tendon. The patella was dislocated laterally to expose the femoral condyles. A small hole was then drilled into the trochlear groove and a 26-gauge needle was inserted in retrograde fashion into the femoral intramedullary canal, not exiting through the greater trochanter. The dislocated patella was reduced and a careful closure with absorbable sutures was performed. A closed, mid-diaphyseal femoral fracture was then created using a modified Bonnarens & Einhorn’s fracture apparatus described by Marturano et al [28,29]. Radiographic images of the fractured femora were obtained right after intramedullary fixation/fracture creation to verify production of a transverse, mid-diaphyseal fracture. Post-operatively, mice received buprenorphine subcutaneously for 2 days and antibiotics through the drinking water for 5 days. The animals were allowed to bear weight immediately and to eat and drink ad libitum.
The mice were euthanized at different time points post-operatively (1, 3, 9, and 56 days) and fractured and contralateral normal limbs harvested for further analysis (radiographic evaluation, H&E and safranin O/Fast green histology and frozen histology and subsequent immunostaining).
2.3 Radiographic evaluation
Radiographs of the fractured femora were obtained using a Faxitron X-ray device (Faxitron Bioptics, Tucson, AZ) immediately post-fracture, to verify the type of fracture and initial pin fixation, and at the time of euthanasia, to monitor callus formation at different time points (Postoperative days 1, 3, 9, 28 and 56).
2.4 Histologic Analysis
After limb harvesting, dissection of the adjacent soft tissue, and removal of the intramedullary fixator for the fractured femora, limb specimens were processed for histology. Fractured femora were analyzed with both standard and frozen decalcified histology, whereas intact femora were processed for frozen histology only.
For standard histology, fractured femora were fixed in 10% formalin for 24 hours, decalcified in 10% EDTA for 14 days at room temperature, then embedded in paraffin and cut longitudinally. Sections were stained with H&E or Safranin O/Fast green and imaged using a Zeiss Axio Imager 2 microscope (Carl Zeiss Microscopy, Thornwood, NY).
For decalcified frozen histology specimens were fixed in 4% paraformaldehyde/PBS (PFA) for 4 hours and decalcified in 14% EDTA/PBS for 14 days. Next, samples were soaked in 30% sucrose/PBS overnight at 4°C. After embedding in Tissue Tek OCT compound (Cat#25608-930; VWR, Radnor, PA), samples were cut longitudinally with a cryostat (Leica, Nussloch, Germany) to generate 8–10 μm sections which were mounted onto glass slides. Sections were stored at −20°C until use.
2.5 Immunostaining
Immunostaining was carried out following a previously established protocol [22]. Briefly, frozen sections were washed with PBS for 3 × 5 minutes, then fixed again in 4% PFA for 15 minutes. Fixed sections were treated with 0.1 M Glycine/PBS for 25 minutes and washed three times in 0.1% Tween-20 /PBS (PBST). Sections were blocked with 3% bovine serum albumin (Cat#A7960; Sigma-Aldrich, St. Louis, MO) in 1% heat inactivated sheep serum (Cat#S2263; Sigma-Aldrich, St. Louis, MO) in PBST for 30 minutes before applying antibodies. The following antibodies were used in the study: anti-Sox9 (1:500; Cat#AB5535; Millipore, Billerica, MA), anti-Sp7 (1:5,000; Cat#AB22552; Abcam, Cambridge, MA), anti-Col1a1 (1:1000, Cat#600-401-103-0.5, Rockland Immunochemicals Inc. Limerick, PA), anti-CD31 (1:1000, Cat#553370, BD Pharmingen, San Jose, CA), Alexa Fluor 488 (1:500; Cat#21206, Life Technologies, Carlsbad, CA), Alexa Fluor 633 (1:500; Cat#A21094, Life Technologies, Carlsbad, CA) and Phalloidin conjungated with Alexa Fluor 635 (1:300; Cat#A34054, Life Technologies, Carlsbad, CA). Primary antibodies were incubated overnight at 4°C in blocking buffer at the dilutions specified above, followed by six, 6 minute washes in PBST prior to an 1 hour incubation with secondary antibodies in blocking buffer at room temperature. Sections were washed five times, then treated with DAPI (1:50,000 in PBS, Cat#H-1200; Vector laboratories, Burlingame, CA) for 5 minutes to label nuclei. Stained sections were mounted with Vectashield Mounting Medium before imaging. Images were taken on a Zeiss 780 confocal microscope or a Zeiss Axioscan scanner (Carl Zeiss Microscopy, Thornwood, NY).
2.6 RNA sequencing
Three month old Sox9CreErt2:Td-Tomato mice received three, 2mg injections of TM at 48 hour intervals and were then euthanized 14 days after the last dose of TM. Both hind limbs were harvested for cell isolation and RNA sequencing. In brief, after carefully removing the surrounding soft tissues, the distal and proximal ends of each femur (including both epiphysis and metaphysis) were cut off and the bone marrow flushed from the intramedullary canal to collect the diaphysis for further analysis. Femoral diaphyses were digested in a liberase solution (100ug/mL in PBS, Cat # 5401119001, Roche) for 2 hours in a fast shaking rocker at 37 °C, 100 rpm to obtain diaphyseal periosteum cells. Digested cells were filtered and single cell suspensions were subjected to FACS sorting to collect Td-Tomato positive and negative cells. About 10,000 Td-Tomato positive cells and 10,000 Td-Tomato negative control cells were isolated after two collections from 18 femora (9 mice). Total RNA was extracted from each cell population separately with Qiagen RNAasy micro kit (Cat# 74004, Qiagen) with DNaseI digestion according to the manufacturer’s instructions. Duplicated experimental RNA sequencing libraries were made with a SMARTer Universal Low Input RNA Kit (Cat# 634938, Clontech) and 75 nucleotide paired-end sequencing performed on an Illumina Nextseq 500 sequencer in USC’s Epigenomics Core facility. Sequence reads were mapped by the Star aligner and quantified within the Partek Genomics Suite software (http://www.partek.com) to identify differentially expressed genes. The resulting gene list was uploaded to DAVID GO analysis tool (https://david.ncifcrf.gov/) to extract the enriched gene ontology terms of TdTomato positive periosteal cells. Note, RNA-seq data has been deposited at GEO (accession number GSE98587).
3. Results
3.1 Expression of Sox9 in intact adult mouse femora
In the intact adult mouse femora, a TdT signal was observed not only in the articular and growth plate cartilage, but also in the primary spongiosa, periosteum, and endosteum, 14 days after the last dose of TM (Figure 1B). In contrast, no such expression was seen in uninjected control mice (Figure 1A). The Td-Tomato positive primary spongiosa cells were located in a vascular (CD31+) and osteoblast (Sp7+) rich environment with an abundant Col1a1 matrix (Figure 1C–E and 1Ea–Ec); Sox9 was not detected in these cell populations (Figure 1F). In contrast, the periosteum contained a subset of Td-Tomato+; Sox9+ cells (Figure 1Ga–Gc and 1Ha–Ib) that was more prevalent in proximal than medial diaphyseal domains (Figure 1Ga–Gc and Ha–Hc). As expected, we also observed occasional periosteal cells that were positive for Sox9 but did not exhibit Td-Tomato activity in the medial diaphysis that most likely reflect cells failing to undergo TM-mediated reporter activation. In the medial, diaphyseal periosteum and endosteum, Td-Tomato positive cells were negative for Sox9 but positive for osteocalcin (Ocn) indicative of a mature bone cell fate (Figure 1, Ia–Ic and Ja–Jc respectively).
Figure 1.

Short term Sox9CreErt2:Td-Tomato lineage tracing in representative frozen sections of intact adult mouse femora. The contribution of Td-Tomato positive cells was examined in the femurs of uninjected control (A) and TM-injected mice (B) 14 days after a final TM injection. In the primary spongiosa/growth plate area, expression and cellular localization of CD31+ endothelial cells (C), Col1a1+ osteoblasts (D), Sp7+ osteoblast progenitors and osteoblasts (E) and endogenous Sox9+ cell types (F). Periosteal and endosteal localization of Sox9 descendant Td-Tomato+ cells (red) was compared with endogenous Sox9+ cells (green in G, H) or Ocn+ cells (green in I, J). Nuclei were labeled by DAPI (blue). The left panel (a) shows a high magnification overlay of Td-Tomato, Sox9 or Ocn and DAPI signals. The middle panel (b) separates the Td-Tomato and DAPI signal, and the right panel (c) the Sox9/Ocn and DAPI signal from panel (a). The white arrow indicates subpopulations of Td-Tomato+/Sox9+ (G, H) and Td-Tomato+/Ocn+ (I) cells. GP: growth plate, PS: primary spongiosa, EO: endosteum, pPO: proximal diaphyseal periosteum, mPO: medial diaphyseal periosteum
3.2 Characterization of pre-labeled Sox9-derived periosteal cells
RNA sequencing and subsequent analysis of periosteal cells isolated from intact mouse femur showed that Td-Tomato positive periosteal cells were enriched in Sox9 expression as expected, as well as in the expression of various osteoblast and chondrocyte specific genes such as Bglap and Col2a1 (Table 1 and Supplementary Table S1). Gene Ontology (GO) analysis further supported the finding that Td-Tomato positive periosteal cells are associated with various osteogenic and chondrogenic functions (Figure 2 and Supplementary Table S2). Together, these results suggest that Sox9 is present in a subset of cells within the periosteum of the long bone and there are Sox9 positive periosteal cells that maintain both osteogenic and chondrogenic properties.
Table 1.
Comparison of RNA-seq profiles of Td-Tomato positive and Td-Tomato negative periosteal cells identifies the top 20 Td-Tomato postitive enriched genes (fold change >2 and p value <0.05).
| Gene name | Gene ID | Fold change (Td-Tomato+/Td-Tomato−) | P-value |
|---|---|---|---|
| Sox9 | ENSMUSG00000000567.5 | 3.083529553 | 0.010370343 |
| Col2a1 | ENSMUSG00000022483.16 | 229.1660366 | 8.98E-10 |
| Mia | ENSMUSG00000089661.9 | 142.251931 | 1.12E-06 |
| Bglap | ENSMUSG00000074483.2 | 14.04341473 | 1.25E-06 |
| Chad | ENSMUSG00000039084.8 | 14.81212568 | 4.74E-06 |
| Bglap2 | ENSMUSG00000074486.2 | 8.928815352 | 6.10E-06 |
| Col9a2 | ENSMUSG00000028626.5 | 58.22796051 | 1.44E-05 |
| 3110079O15Rik | ENSMUSG00000026258.5 | 68.5267276 | 1.89E-05 |
| Comp | ENSMUSG00000031849.8 | 8.923529253 | 2.42E-05 |
| Sparc | ENSMUSG00000018593.12 | 4.204180358 | 7.12E-05 |
| 1500015O10Rik | ENSMUSG00000026051.8 | 15.01158853 | 8.85E-05 |
| Col9a3 | ENSMUSG00000027570.15 | 42.20714482 | 9.61E-05 |
| Mgp | ENSMUSG00000030218.2 | 3.848808039 | 0.000169295 |
| Lect1 | ENSMUSG00000022025.13 | 43.64745613 | 0.000199769 |
| Gpha2 | ENSMUSG00000024784.13 | 18.39908795 | 0.000199813 |
| Ctgf | ENSMUSG00000019997.11 | 2.426398441 | 0.000338354 |
| Col1a1 | ENSMUSG00000001506.10 | 3.961099725 | 0.000348882 |
| Col1a2 | ENSMUSG00000029661.16 | 2.975894342 | 0.000407987 |
| Cytl1 | ENSMUSG00000062329.4 | 23.96015457 | 0.000419167 |
| Clu | ENSMUSG00000022037.14 | 13.15160407 | 0.000457251 |
| Col2a1 | ENSMUSG00000022483.16 | 229.1660366 | 8.98E-10 |
| Mia | ENSMUSG00000089661.9 | 142.251931 | 1.12E-06 |
| Bglap | ENSMUSG00000074483.2 | 14.04341473 | 1.25E-06 |
| Chad | ENSMUSG00000039084.8 | 14.81212568 | 4.74E-06 |
Figure 2.
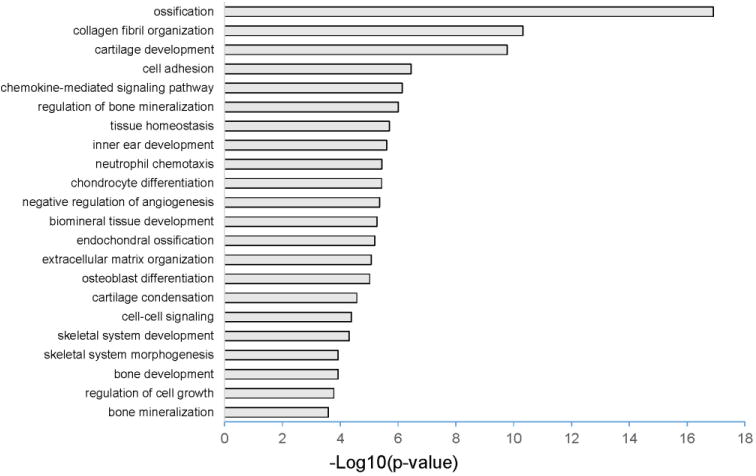
RNA sequencing of Td-Tomato positive periosteal cells.
The top 20 gene functions predicted from DAVID GO analysis of RNA-seq profiling of Td-Tomato + periosteal cells. Td-Tomato positive periosteal cells are associated with various osteogenic and chondrogenic functions. The X axis depicts the -log10 transformation of p-values from Supplementary Table S2.
3.3 Femoral fracture healing in Sox9CreErt2:Td-Tomato mice
Radiographs were taken over a time course of 2 months to document callus formation and union in fractured femora. Analysis at 1, 3 and 9 days post-fracture showed new bone first appearing at day 9 at the edges of the callus while radiolucency persisted in the center of the callus. Fully mineralized bridging was observed 14 days post fracture, union was apparent by day 28, whereas remodeling, with complete disappearance of the fracture site, was accomplished 2 months post-fracture (Supplementary Figure S1A–D).
The radiographic results were confirmed with histologic analysis of H&E and safranin-O/fast green-stained femora at 1, 3, 9 and 56 days post-operatively. At days 1 and 3 a hypercellular area, consistent with acute inflammation and early stages of bone repair, was present at the fracture site. Histological analysis 9-days post fracture revealed a callus bridging the fractured bony ends; the callus was mostly cartilaginous, with a few mineralized boney areas at the proximal and distal ends of the callus. Complete healing of the fracture, with callus remodeling and reconstitution of the normal morphology of the bony cortex and medullary canal was noted 2 months post-fracture. (Supplementary Figure S1E–J)
3.4 Contribution of Sox9 positive periosteal cells in long bone fracture repair
To determine the role of Sox9 cells in fracture healing, Sox9CreErt2:Td-Tomato mice were injected with tamoxifen to activate the Td-Tomato reporter in Sox9+ cells prior to injury, and follow their Td-Tomato+ descendants in the femoral fracture repair process.
By 3 days post-fracture, the diaphyseal periosteum adjacent to the fracture site thickened and Td-Tomato positive cells expanded and migrated towards the fracture site (Figure 3A–B). Immunodetection showed that these Td-Tomato positive, periosteal-derived cells were positive for Sox9 and Sp7 (Figure 3C–D, Figure 3Ea–Ec and 3Fa–Fc). Thus, Sox9 positive cells in normal bone participate in subsequent fracture repair. Not all Sox9+ and Sp7+ cells were Td-Tomato positive. This result may reflect mosaic labeling of the Sox9 pool or de novo activation of Sox9 in another cell population following injury (see Discussion).
Figure 3.

Representative images of femoral fracture site 3 days post-operatively, demonstrating the expansion and activation of Td-Tomato+ periosteal cells on injury, in lower (A) and higher magnification (B) views. Td-Tomato+ cells co-express Sox9 (C) or Sp7 (D) within the fracture site. Panels C and D present higher magnification views of the overlap in Td-Tomato, Sox9 (Ea–Ec) and Sp7 (Fa–Fc) activity at the fracture site. The left panel (a) presents an overlay of Td-Tomato, Sox9 or Sp7, and DAPI (nuclear) signals. The middle panel (b) separates the Sox9 or Sp7 signal and the right panel (c) the nuclear signal (DAPI) from the overlay in (a). The bone cortices are demarcated with dashed lines.
At 9 days post-fracture, a large number of Td-Tomato positive cells were present in both the cartilaginous soft callus and the bony hard callus. In the soft callus, this Td-Tomato positive population was also positive for Sox9 (Figure 4A–A′, Da–Dc). A few cells at the periphery of the soft callus were Sp7 positive consistent with pre-hypertrophic chondrocytes. However, no Sp7 labeling was observed in the immature chondrocytes located at the center of the soft callus (Figure 4G). In the hard callus, the Td-Tomato signal co-localized with Col1a1 and Sp7 (Figure 4B–B′, 4C–C′ and Ea–Ec, respectively). As expected, no Sox9 expression was observed in the hard callus area (Figure 4F). Thus, Sox9 positive cells labeled prior to fracture give rise to both chondrocytes and osteoblasts in the soft and hard callus, respectively. Furthermore, the periosteal labeling pre-injury and periosteal activation post-injury suggests that the periosteum is the major site of origin of this reparative cell population. Some variability was observed between samples (A vs A′) possibly due to the mosaic labeling of the Sox9 pool although a similar dosing regimen was followed in all animals: three doses of tamoxifen administered at 48-hour intervals with the last injection 2 weeks pre-operatively.
Figure 4.

Representative images of two mouse femora 9-days post fracture, showing the contribution of Td-Tomato+ cells to bone repair in the fracture-induced callus. Td-Tomato signal within cells was compared with Sox9 (A and A′), Col1a1 (B and B′) and Sp7 (C and C′) by immunostaining. (Da–Dc) presents a magnified view of the soft callus visualizing Td-Tomato (red), Sox9 (green) and nuclear (DAPI, blue) signals. (Ea-Ec) presents a magnified view of the hard callus visualizing Td-tomato (red), Sp7 (green) and nuclear (DAPI, blue) signals. As expected, Td-Tomato + cells in the hard callus were negative for Sox9 (F). The majority of Td-Tomato+ cells in the soft callus were negative for Sp7, except for a population of pre-hypertrophic chondrocytes located at the proximal and distal ends of the soft callus (G). These findings in (F) and (G) demonstrate the specificity for the overlapping signals observed in (D) and (E), respectively.
After 2 months, Td-Tomato positive cells were still observed in the operated femur (Figure 5B and 5D); Td-Tomato positive cells were significantly more prevalent in the cortex and periosteum of the healed bone compared to the contralateral non-operated intact femur though some cells were also observed in the marrow (Figure 5A and 5C). Sox9 positive cells in the cortical bone showed typical osteocyte morphology though immunostaining of F-actin using conjugated phalloidin demonstrated that these osteocytes were organized irregularly (Figure 5F) when compared to osteocytes in the intact control femur (Figure 5E). These observations suggest that remodeling at the level of osteocyte alignment is incomplete 2 months post-fracture.
Figure 5.

Representative frozen sections of mouse femora 2 months post-fracture. Distribution of Td-Tomato+ cells in the healed femur (B, D) and contralateral intact femur (A, C) in lower and higher magnification views, respectively. Phalloidin labelling of F-actin shows a distinct osteocyte organization comparing normal cortical bone (E) with cortices generated on fracture repair of the femur (F). Double headed arrows indicate the long axis of osteocyte orientation.
4. Discussion
The important role of periosteum in bone healing has been confirmed in several in vitro and in vivo studies [15]. Previous studies have shown that in vitro cultured periosteal cells can differentiate into osteoblasts and chondrocytes [31], supporting the hypothesis that the periosteum harbors osteoprogenitor and chondroprogenitor or osteochondroprogenitor cells that could potentially participate in fracture healing. When implanted heterotopically into nude mice, these cells give rise to bone and/or cartilage. [32–34] Moreover, studies in murine segmental bone graft transplantation models have demonstrated that periosteal progenitor cells play a critical role in the initiation of bone graft healing. [35–37] Consistent with the critical role of periosteal cells in bone healing, periosteal stripping impairs healing in long bone fracture and bone defect models [38–40].
In this study, we identified a rare, Sox9 positive cell population normally residing within the periosteum of the adult mouse long bone. Fate mapping identified Sox9+ cell descendants of Sox9+ cells in articular and growth plate chondrocytes of the adult femur as expected, but also in the primary spongiosa, diaphyseal periosteum and endosteum. Moreover, RNA sequencing analysis of Td-Tomato positive periosteal cells isolated from uninjured adult femora demonstrated that these cells express various osteogenic and chondrogenic genes, and thus have a gene expression profile analogous to that of osteochondroprogenitors. A prior study examining Sox9-LacZ adult mice failed to detect Sox9 in periosteal or endosteal cells of uninjured long bones [9]. The difference here is likely to reflect a great sensitivity of the Td-Tomato fluorescent labeling approach as we observe Sox9 protein directly in a subset of cells [27].
In fracture repair, fate mapping shows that this resident Sox9 positive periosteal cell population participates in bone healing, proliferating and migrating towards the fracture site during the first few days post injury, then differentiating into chondrocytes, osteoblasts and osteocytes. Previous studies have also attempted to perform a similar cell lineage analysis and provide information on the cellular identity of periosteal skeletal progenitors during fracture repair. Transgene-expressing periosteal cells in Rosa26:LacZ; Prx1CreER:GFP adult mice were able to differentiate into chondrocytes and osteoblasts during the healing process of femoral and ulna fractures [41]. In a different study, using a Sox9:LacZ mouse line and X-gal staining in a tibia fracture model, Murao et al showed that Sox9 is activated after a fracture stimulus and is then expressed in chondrocytes in the soft callus [9]. Moreover, these fracture-induced Sox9 positive progenitor cells have been shown to be able to give rise to osteoblasts and osteocytes during fracture repair. In our model, only a subset of the chondrocytes and osteoblasts derived from pre-labelled resident Sox9+ cells consistent with additional mechanisms of injury directed induction of Sox9.
The bone healing sequence described above is similar to the developmental process of skeletogenesis. In early skeletal development Sox9-labeled osteochondroprogenitors have been shown to play a major role in endochondral ossification [18]. Cell fate mapping using a reporter mouse demonstrated Sox9-positive limb bud mesenchymal cells give rise to both chondrocytes and osteoblasts during embryogenesis [16]. Moreover, inactivation of the Sox9 gene in limb buds before mesenchymal condensations resulted in an inhibition in subsequent cartilage and bone formation. The data herein suggest a Sox9 positive, osteochondroprogenitor cell population persists, or forms de novo, in the adult long bone, and this population plays a substantial role in fracture repair.
However, although RNA-seq analysis of the periosteal Sox9+ is consistent with an osteochondroprogenitor phenotype, and the lineage tracing shows a marked periosteal response and expansion of Sox9-descendant cells into the fracture site as an early response, Sox9-directed Cre activity is broader than the periosteal Sox9 population of interest. Definitive evidence will require the development of new genetic tools that uniquely demarcate this cell type; unfortunately, no clear driver genes that could provide a more direct insight have been documented.
In conclusion, a resident Sox9 positive cell population participates in fracture repair of the adult femur. This population has distinct characteristics of osteochondroprogenitors and the most-likely source is the periosteum. In addition, there may be alternative injury invoked mechanisms that recruit additional cells into directed differentiation to skeletal cell types.
Supplementary Material
Highlights.
A Sox9+ osteochondroprogenitor population resides in the periosteum of adult long bones.
RNA-seq analysis of the periosteal Sox9+ cells is consistent with an osteochondroprogenitor phenotype.
Bone fracture stimulates the mobilization of Sox9+ cells to effect fracture repair
Acknowledgments
Work in APM’s laboratory was supported by a grant from the NIH (DK056246). The modified Bonnarens & Einhorn’s fracture apparatus was purchased through USC’s Regenerative Medicine Initiative. We thank Gohar Saribekyan, Lora Barsky, Bernadette Masinsin, Seth Ruffins, Riana Parvez and Charles Meyer Nicolet from the USC Histology Core, the USC Flow Cytometry Core, the USC Imaging Center, and the USC Epigenome Center for technical assistance. The authors would also like to thank Amy Tang for the H&E and Safranin O/Fast green staining of the mouse femora sections. We are also grateful to Drs. Gage Crump and Mariani Francesca for discussion, and Drs. Henry M. Kronenberg, Clifford J. Tabin, and Matthew L. Warman for their helpful inputs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Einhorn T. The cell and molecular biology of fracture healing. Clin Orthop. 1998;355S:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19(10 Suppl):S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 3.Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci. 2015 Jan;28(1):57–71. doi: 10.3967/bes2015.006. [DOI] [PubMed] [Google Scholar]
- 4.Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200(2):165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- 5.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers TJ, Longobardi L, Willcockson H, et al. BMP2 Regulation of CXCL12 Cellular, Temporal, and Spatial Expression is Essential During Fracture Repair. Journal of Bone and Mineral Research. 2015;30:2014–2027. doi: 10.1002/jbmr.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stafford HJ, Roberts MT, Oni OO, Hay J, Gregg P. Localisation of bone-forming cells during fracture healing by osteocalcin immunocytochemistry: an experimental study of the rabbit tibia. J Orthop Res. 1994;12(1):29–39. doi: 10.1002/jor.1100120105. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki A, Tsunoda M, Kinoshita S, Saura R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J Orthop Sci. 2000;5:64–70. doi: 10.1007/s007760050010. [DOI] [PubMed] [Google Scholar]
- 9.Murao H, Yamamoto K, Matsuda S, Akiyama H. Periosteal cells are a major source of soft callus in bone fracture. J Bone Miner Metab. 2013;31:390–398. doi: 10.1007/s00774-013-0429-x. [DOI] [PubMed] [Google Scholar]
- 10.Utvåg SE, Grundnes O, Reikeraos O. Effects of periosteal stripping on healing of segmental fractures in rats. J Orthop Trauma. 1996;10(4):279–284. doi: 10.1097/00005131-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331(1):31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 12.Devine MJ, Mierisch CM, Jang E, Anderson PC, Balian G. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 2002;20(6):1232–1239. doi: 10.1016/S0736-0266(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 13.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 14.Alaee F, Hong SH, Dukas AG, Pensak MJ, Rowe DW, Lieberman JR. Evaluation of osteogenic cell differentiation in response to bone morphogenetic protein or demineralized bone matrix in a critical sized defect model using GFP reporter mice. J Orthop Res. 2014;32(9):1120–1128. doi: 10.1002/jor.22657. [DOI] [PubMed] [Google Scholar]
- 15.Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9(Suppl 1):S45–64. doi: 10.1089/10763270360696978. 2003. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama H, Kim JE, Nakashima K, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farquharson C. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. In: Hall Brian K., editor. Br Poult Sci. Second. 2015. [Google Scholar]
- 18.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi W, Huang W, Whitworth DJ, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Do HJ, Yang HM, et al. Overexpression of SOX9 in mouse embryonic stem cells directs the immediate chondrogenic commitment. Experimental & molecular medicine. 2005;37:261–268. doi: 10.1038/emm.2005.35. [DOI] [PubMed] [Google Scholar]
- 22.Ohba S, He X, Hojo H, McMahon AP. Distinct Transcriptional Programs Underlie Sox9 Regulation of the Mammalian Chondrocyte. Cell Rep. 2015;12:229–243. doi: 10.1016/j.celrep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Outani H, Okada M, Yamashita A, Nakagawa K, Yoshikawa H, Tsumaki N. Direct induction of chondrogenic cells from human dermal fibroblast culture by defined factors. PLoS One. 2013:e77365. doi: 10.1371/journal.pone.0077365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZH, Li XL, He XJ, Wu BJ, Xu M, et al. Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz J Med Biol Res. 2014;47(4):279–286. doi: 10.1590/1414-431X20133539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 26.Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis. 2010;48:635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 29.Marturano JE, Cleveland BC, Byrne MA, O’Connell SL, Wixted JJ, Billiar KL. An improved murine femur fracture device for bone healing studies. J Biomech. 2008:1222–1228. doi: 10.1016/j.jbiomech.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004:687–695. doi: 10.1097/00005131-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 31.De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006:1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 32.Nakahara H, Bruder SP, Goldberg VM, Caplan AI. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop Relat Res. 1990:223–232. [PubMed] [Google Scholar]
- 33.Nakahara H, Dennis JE, Bruder SP, Haynesworth SE, Lennon DP, Caplan AI. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp Cell Res. 1991;195:492–503. doi: 10.1016/0014-4827(91)90401-f. [DOI] [PubMed] [Google Scholar]
- 34.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9(4):465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Xie C, Lin AS, et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20(12):2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karaoglu S, Baktir A, Kabak S, Arasi H. Experimental repair of segmental bone defects in rabbits by demineralized allograft covered by free autogenous periosteum. Injury. 2002;33:679–683. doi: 10.1016/s0020-1383(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 37.Tiyapatanaputi P, Rubery PT, Carmouche J, Schwarz EM, O’Keefe RJ, Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254–1260. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Utvåg SE, Grundnes O, Reikeraos O. Effects of periosteal stripping on healing of segmental fractures in rats. J Orthop Trauma. 1996;10(4):279–284. doi: 10.1097/00005131-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Utvåg SE, Grundnes O, Reikerås O. Early muscle-periosteal lesion inhibits fracture healing in rats. Acta Orthop Scand. 1999;70(1):62–66. doi: 10.3109/17453679909000960. [DOI] [PubMed] [Google Scholar]
- 40.Grundnes O, Reikerås O. The role of hematoma and periosteal sealing for fracture healing in rats. Acta Orthop Scand. 1993;64(1):47–49. doi: 10.3109/17453679308994527. [DOI] [PubMed] [Google Scholar]
- 41.Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386(3):477–82. doi: 10.1016/j.bbrc.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


