Abstract
A number of mechanisms ensure that the intestine is protected from pathogens and also against our own intestinal microbiota. The outermost of these is the secreted mucus, which entraps bacteria and prevents their translocation into the tissue. Mucus contains many immunomodulatory molecules and is largely produced by the goblet cells. These cells are highly responsive to signals they receive from the immune system, and are also able to deliver antigens from the lumen to dendritic cells in the lamina propria. In this Review, we will give a basic overview of mucus, mucins, and goblet cells and explain how each of these contributes to immune regulation in the intestine.
Online summary:
• Mucins are highly O-glycosylated molecules that have gel-like properties. The mucin family is made up of transmembrane and gel-forming mucins. The transmembrane mucins cover the apical surfaces of the enterocytes and form the glycocalyx. The gel-forming mucins are secreted from goblet cells as large multimers that form the mucus skeleton and cover all epithelial surfaces.
• Mucus in the small intestine forms a diffusion barrier where antimicrobial substances keep the epithelium free from microorganism. Mucus in colon forms a dense inner mucus layer that bacteria are unable to penetrate creating a bacteria free zone at the epithelial surface.
• Some, but not all, bacteria stimulate the formation of a functional mucus system with removable mucus in the small intestine and a stratified impenetrable inner mucus layer in colon.
• Mucus in the intestine creates a niche for bacteria with digestible glycans providing a stable energy source, but mucus also traps and removes bacteria. Bacteria in loose mucus are planktonic and less virulent.
• The small intestinal goblet cells can sample luminal material during mucus secretion and transfer the antigens to lamina propria dendritic cells something that also happens in colon if bacterial numbers are decreased. This communication with the immune system has tolerogenic effects.
• Intestinal pathogens have mechanisms that can circumvent the mucus protection to reach the epithelium. These include good motility and secretion of enzymes that can degrade the otherwise protease resistant mucins.
Introduction
Our bodies are continuously exposed to hazardous and infectious agents and we require efficient protective mechanisms to cope with these assaults. The skin surface is made up of keratinized dead cells. However, this surface is small in comparison to the lining of the gastrointestinal and respiratory tracts, which are wet barrier surfaces composed of a single layer of epithelial cells. These surfaces are protected by mucus, which traps and transports debris and bacteria, lubricates, and lowers the mechanical stress on the epithelium. The mucus serves as a first line of innate defense and is produced by surface goblet cells, other epithelial cells, and glands that are all intimately coupled to other parts of the innate and adaptive immune systems. The mucus is composed of many different molecules with mucins forming the basic skeleton. Once the mucus is secreted, it is largely out of cellular control and reach. To fulfill their function mucus and mucins have many inherent properties that can be controlled from the host1. The mucus system has evolved ever since the first metazoans and no doubt contains many undiscovered innate immunity secrets2. In this Review, we explain our view of the protective intestinal mucus system and put this in the context of immune regulation.
The intestinal mucus
The intestine is protected by mucus, which in the small intestine forms a single easily removable mucus layer and in the colon forms a double layer, with the inner mucus layer firmly attached to the epithelium (Fig 1). In the small intestine the mucus layer is penetrable, but the bacteria are kept away from the epithelium by antibacterial mediators. In the large intestine, the inner mucus layer is impenetrable to bacteria whereas the outer mucus layer is expanded and by this become the habitat for the bacteria (Fig 1). The major building blocks giving mucus its properties are large glycoproteins called mucins.
Figure 1 |. A general overview of the mucus protection in small intestine and colon.
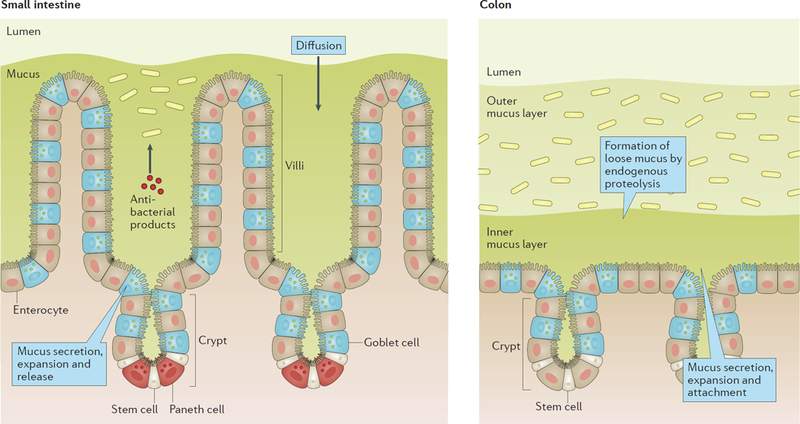
In the small intestine the mucus is not attached and forms a diffusion barrier with antibacterial products that limit penetration by bacteria. In colon bacteria are compartmentalized to the outer loose mucus layer while the inner attached layer is almost free of bacteria and protect the epithelium.
Mucins.
Mucins are defined as glycoproteins with more than 50% of their mass as O-glycans. The names of the specific mucins are confusing and unrelated to their structure and function. However, two major types of mucins can be functionally distinguished; transmembrane mucins and gel-forming mucins (Fig 2)3,4.
Figure 2 |. A schematic presentation of the domain structure of gel-forming and transmembrane mucins normally expressed in the intestine.
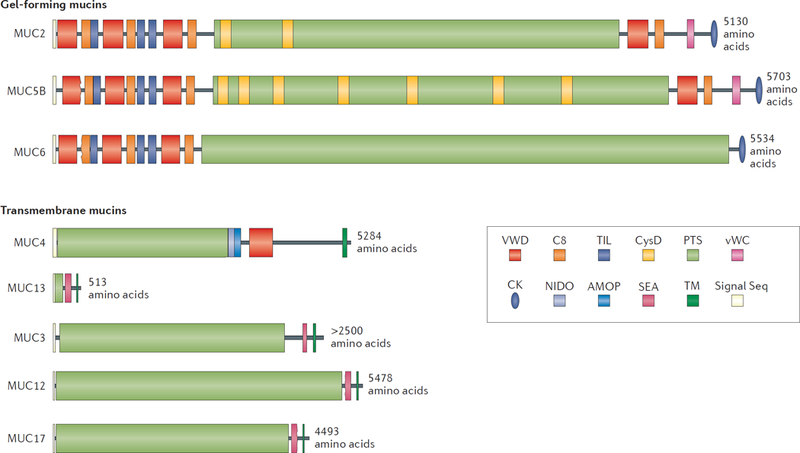
The gel-forming mucins and the transmembrane mucins are presented group vise to scale. The PTS domains become heavily O-glycosylated to form the mucin domains. These are rod-like and extended, looking like a bottle brush. The non-PTS parts of the gel-forming mucins are rich in Cys amino acids and form compact structures.
The key elements in mucins are the mucin domains that are extensively decorated by O-linked glycans which bind water and give mucus gel-like properties (Fig 2). The protein sequences of these domains are rich in the amino acids proline, threonine and serine (called the PTS domain) that after addition of glycans create mucin domains4. The mucin domains form an extended, stiff, and voluminous rod with a central protein core. Their terminal glycans make up a three-dimensional surface that can interact with cells or microorganisms.
Transmembrane mucins.
The transmembrane mucins are of type 1 class and have a single membrane spanning domain close to the C-terminus (Fig 2). The N-terminus is found on the apical cell surface. The C-terminal can be involved in intracellular signaling3. In the intestinal tract, the transmembrane mucins MUC3, MUC4, MUC12, MUC13 and MUC17 are normally expressed, and MUC1 and MUC16 are upregulated in response to infection and cancer (Table 1)5,6. The transmembrane mucins vary in length, with MUC13 being the shortest and MUC16 being the longest. With the exception of MUC13, these transmembrane mucins are cleaved in the stalk region between the membrane-spanning domain and the PTS domain. This occurs within the SEA domain for all mucins, except for MUC4 that instead has a von Willebrand D-domain (vWD) combined with NIDO, AMOP domains3. In all cleaved transmembrane mucins the two subunits remain associated and may be separated by mechanical forces or released from the membrane by proteases as for MUC17. The intracellular C-terminal domains have interaction and phosphorylation sites involved in signal transduction where MUC1 is the most complex with respect to potential interactions3.
Table1.
Expression of mucins and mucus-associated proteins in the intestine
| Protein | Expressed by cell type | Expression location | References |
|---|---|---|---|
| MUC2 | Goblet cell/Paneth cell | Throughout intestine | 117,118 |
| MUC4 | Goblet cell/Enterocyte | Colon | 117 |
| MUC13 | Enterocyte | Throughout intestine | 119 |
| MUC3 | Enterocyte | Small intestine | 11,120 |
| MUC12 | Enterocyte | Colon | 12 |
| MUC17 | Enterocyte | Small intestine | 11 |
| MUC5B | Goblet cell | Colon | 8 |
| MUC6 | Gland cell | Brunners glands | 121 |
| CLCA1 | Goblet cell | Throughout intestine | 122 |
| FCGBP | Goblet cell | Throughout intestine | 123 |
| TFF3 | Goblet cell | Throughout intestine | 124 |
| ZG16 | Goblet cell | Throughout intestine | 125 |
| DEFENSINS | Paneth cell | Small intestine | 33 |
| Lysozyme | Paneth cell | Small intestine | 33 |
| DMBT1 | Paneth cell/Enterocyte | Throughout intestine | 126 |
| IgA | B cell | Throughout intestine | 41,52 |
Gel-forming mucins.
Gel-forming mucins are secreted from goblet cells and make up the skeleton of the mucus layer. These mucins have cysteine-rich N- and C-terminal parts that mediate oligomerization. In the intestine, the main gel-forming mucin is MUC2 (Table 1). MUC6 is expressed by glands in the stomach and duodenum and low levels of MUC5B can be found in human colon8. MUC5AC is normally only found on the stomach surface, but can be induced in the intestine during infection9. The domain structure of the gel-forming mucins is a combination of von Willebrand assemblies (vWD-C8-TIL domains), mucin domains interspersed with CysD domains, C-terminally located vWD, vWC and a cysteine knot domain (Fig. 2). Mucin multimerization is initiated via disulfide bonds between the cysteine knot domains forming dimers in the far C-terminus. The O-glycosylated dimers then form multimers with intermolecular disulfide bonds between the N-terminal vWD3 assemblies either in a trimeric way as for MUC2 or in a linear way as shown for MUC5B1,10. The oligomeric nature of MUC5AC and MUC6 is still not elucidated.
Mucin-producing cells
Enterocytes.
Enterocytes, the major cell type in the intestinal epithelium, have transmembrane mucins covering their apical cell membrane. These mucins reach further out into the intestinal lumen than any other membrane protein and generate a local, attached glycan-rich diffusion barrier, called glycocalyx, protecting the enterocyte cell membrane. The MUC3, MUC12 and MUC17 mucin group have mucin domains of >4,000 amino acids that extend for up to 1 µm into the intestinal lumen. MUC3 is expressed in the whole intestine, most prominently in the duodenum11, whereas MUC12 is mainly found in the colon12. By contrast, MUC17 is mainly expressed in the small intestine, with high expression in the duodenum, but is also found in the transverse colon 11. The function of these mucins is not well understood, but they are probably involved in both sensing and regulating the local milieu at the enterocyte surfaces. This is suggested from studies showing that the localization of MUC17 (wrongly annotated as Muc3 in mouse) is oppositely synchronized with the surface recruitment of the CFTR ion channel from intracellular vesicular pools upon carbachol stimulation; this localization is partly controlled by the C-terminal PDZ-binding motif interacting with scaffold PDZ proteins13. MUC13 is also expressed throughout the intestine and has a shorter mucin domain compared to the other transmembrane mucins and, as such, does not extend as far from the enterocyte surface. It has been suggested that MUC13 can protect from dextran sulfate sodium (DSS)-induced colitis by inhibiting enterocyte apoptosis14.
Goblet cells.
The intestinal mucus is produced and secreted by the goblet cells that are specialized for this task. In the small intestine they are found at higher relative numbers in the crypt than on the villi where they are more interspersed by enterocytes. In the colon they are also abundant, especially in the upper crypts where they have large mucus filled granulae. Goblet cells with smaller mucus granulae are also present on the flat colon surface. The goblet cells are derived from the secretory epithelial cells lineage and their maturation is controlled, at least in part, by the transcription factor SAM pointed domain-containing Ets transcription factor (SPDEF)15. As the predominant intestinal gel-forming mucin MUC2 forms large, multimeric, net-like, and insoluble molecules their biosynthesis is highly demanding for the cell. There are two goblet cell-specific endoplasmic reticulum (ER) proteins that are suggested to be required for normal MUC2 biosynthesis; anterior gradient protein 2 homolog (AGR2) and ER-to-nucleus signalling 2 ERN2 (also known as IRE1β)16,17. The control of mucin biosynthesis in goblet cells is still poorly understood, but there seems to be a large element of translational control as a discrepancy between mucin mRNA and protein levels is common.
In the late secretory pathway the mucus vesicles store compactly packed glycosylated MUC2 multimers. Packaging is enabled by interactions of the N-terminal vWD domains forming concatenated ring structures in the low pH and high Ca2+ environment (Fig 3)1. Mucus is likely released from the goblet cells at a basal rate by vesicle fusion at the apical membrane as observed in the airways18–20. However, when goblet cells are stimulated they can respond by a dramatic release process known as compound exocytosis where most of the mucus granulae in the theca are fused and released emptying the whole cell interior and exposing the cell cytoplasm21. This dramatic secretion leaves small thin goblet cells that are not easily identified, something that has contributed to the misunderstanding that inflammation causes ‘goblet cell depletion’. Mucus granulae release studies with modern techniques combined with enteroid systems will reveal details of these processes22,23. The importance of concomitant endocytosis and autophagy with mucus release has recently been illustrated as loss of components involved in these processes generated goblet cells that accumulated mucus24. Interestingly, inflammasome components, such as NOD-, LRR- and pyrin domain-containing 6 (NLRP6), have also been suggested to be involved in controlling mucus release from goblet cells25. In fact, we recently discovered that the colon crypt opening is guarded by one sentinel goblet cell (senGC) that senses TLR2, TLR4 and TLR5 ligands - and activates NLRP626. This causes a Ca 2+-dependent compound exocytosis of mucus from the senGC as well as an intercellular gap junction Ca 2+-signal that induces MUC2 secretion from adjacent goblet cells around the upper crypt. Finally, the senGC is expelled into the lumen. The coordinated mucus release expels bacterial intruders and protects the colonic crypt.
Figure 3 |. The release mechanism of mucins in the small intestine.
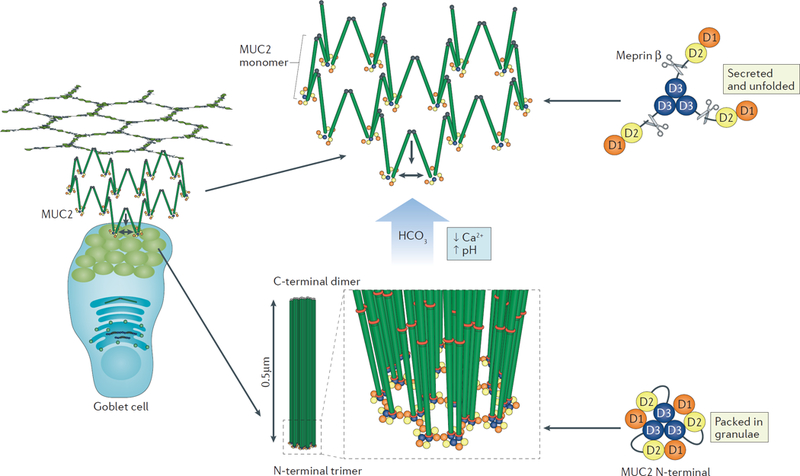
MUC2 is packed in goblet cell granulae and secreted into the lumen. Mucus is secreted into a bicarbonate-rich environment as generated by the CFTR channel in the small intestine, and by raising pH and lowering Ca2+ concentration it allows the packed molecules to expand into net-like sheets. The expanded conformation allows the protease Meprinβ to digest the N-terminal part of MUC2 releasing the attached mucus from the epithelium. This process is important for the clearance of mucus that has trapped bacteria.
Secreted mucus
For proper mucus expansion upon secretion from the goblet cell acidic granulae, the densely and well-packed mucins must be exposed to increased pH and decreased calcium levels1. In the small intestine, this is mediated by bicarbonate secreted by enterocyte-expressed CFTR, a chloride and bicarbonate channel, while the ion channels involved in mucus secretion in colon are not well understood27. The calcium removal weakens the N-terminal interactions allowing a dramatic expansion of the MUC2 mucin into large flat sheets as water rushes in and binds to the mucin domain glycans (Fig 3)1. These newly secreted MUC2 sheets interact with the previously formed ones. The mucins are not released free into the lumen, but are attached to the epithelium28.
Small intestine.
The mucus in the small intestine is released from its epithelial attachment by the activated protease meprin β (MEP1B), which cleaves and releases the MUC2 mucin28. In bacteria-colonized small intestine, the MUC2 mucin is almost instantly cleaved and detached allowing the mucus to be transported distally. The mucus in the small intestine is relatively porous, which is important for efficient nutritional uptake, but this also means that particles as large as bacteria can penetrate the mucus29. However, bacteria are still kept away from the epithelial cells by antibacterial agents contained within the mucus, such as defensins and IgA, which form a gradient due to their slow diffusion through the mucus29–32 (Fig 1). The highest concentration of antibacterial peptides will be in the crypts and at the crypt openings as these components largely are produced by Paneth cells found at the bottom of the crypts33.
Large instestine.
Mouse caecum, with its special fermentation, has bacteria in contact with the epithelium, but mouse proximal colon shows some separation by the mucus29,34. Humans, on the other hand, show a mucus protective system that keeps bacteria away from the epithelium throughout the large intestine32. The inner mucus layer is most prominent in the distal colon where it has a stratified structure of layered sheets made up by MUC2 multimers1,35. This dense and well-organized structure is anchored to the epithelium and limits bacterial penetration into the mucus, creating a zone that is almost completely free from bacteria (Fig 1). The colonic epithelium absorbs water and sodium chloride creating a compact fecal material of microorganisms and undigested fibers. The NaCl uptake is mediated by exchange of sodium/hydrogen and chloride/bicarbonate coupled transport mediated by channels from the SLC26A and SLC9A families with some contribution of electrogenic sodium uptake via ENaC36. The slow distal propulsion of this compact fecal material generates mechanical stress and leads to long exposure times of the epithelium by the numerous microorganisms in the lumen. To maintain homeostasis it is important that the mucus is constantly and quickly renewed from the epithelial side, something that is accomplished by secretion from the surface goblet cells18. In the distal colon of mice, the 50 µm inner mucus layer is replaced every hour18. The attached inner colonic mucus layer is converted by endogenous proteases to a detached, less dense outer mucus layer35. This is important as the mucus can follow the fecal stream, provide lubrication, and cover the fecal material37. The mucin glycans of the expanded outer mucus layer allows the microbiota to create a specialized niche for mucus-associated microorganisms such as Akkermansia muciniphila and Mucispirillum spp.38–40.
Immunological role of mucus
Immune mediators found in mucus.
Mucus contains, in addition to the mucins, a number of molecules with impact on the gut microbiota for which the mucin network with its bound water provides a diffusion barrier. IgA and its joining (J) chain are secreted into the lumen and found at high concentrations in mucus of duodenum and ileum with lower levels in colon41,42. Binding of IgA to bacteria or viruses cause aggregation leading to slower diffusion and reduced bacterial mobility in the mucus43. However, in colon IgA is not necessary for the compartmentalization of the microbiota in the mucus, as mice lacking the polymeric Ig receptor (PigR), which are deficient in IgA translocation, still have mucus separating bacteria from the epithelium44,45. Antibacterial products such as defensins, lysozyme, and deleted in malignant brain tumors 1 (DMBT1) are produced by Paneth cells located in the bottom of small intestinal crypts33. Paneth cells also produce MUC2, but most mucus is secreted at the crypt opening where the antibacterial peptides mix with and follow the mucus out and cover the space between the villi. Additional bactericidal products, like Reg3γ in mouse or the human counterpart REG3A that is bactericidal to gram-positive bacteria, are produced by the epithelial cells46–48. Reg3β has also been observed in the small intestinal epithelium at the villus/crypt opening, but its bactericidal effect is still unclear49. Increased ROS production from dual oxidase 2 (DUOX2) at the upper villi produces hydrogen peroxide that affect the microbiota50. In the intestinal lumen, the microbiota composition is also balanced by bacterial competition and the presence of bacteriophages that seem to adhere to the mucus51. The bactericidal activity is very prominent in the small intestinal mucus42,52.
In addition to the mucins and other immune mediators, mucus contains a number of other molecules52. Specific mucus components such as FCGBP, CLCA1, ZG16, AGR2, TFF3 and KLK1 are together with MUC2 produced and secreted by goblet cells and are integrated into the mucus15,35. However, so far little is known about the immunological roles or other functions of these molecules.
Immunological role of goblet cells
As the goblet cell is the major producer of the mucus and thus provide the first line of defense against bacteria it could be considered to be more central to the immune system than previously appreciated. In fact, unknown goblet cell functions confirming this have recently started to be revealed. The goblet cells of the small intestine sense luminal content upon mucus secretion53 (Fig 4). Actively secreting goblet cells can take up intraluminal antigenic material and, in a manner not yet not fully understood, deliver these antigens to dendritic cells (DCs) in the lamina propria. These routes for antigen transport are called goblet cell-associated antigen passages (GAPs), and when fluorescent dextran was followed by imaging these passages actually look like gaps in the epithelium53. The luminal material taken up is from the neighborhood of the secreting cell and is subjected to mucus filtering where molecules larger than 70 kDa are limited and of 2,000 kDa are excluded53. It is not fully understood whether the mechanism of goblet cell uptake is vesicle mediated or if the whole cytoplasm is filled up (Fig 4). However, the material seems to be transported directly to lamina propria DCs without leaking out into lamina propria. These DCs are CD103+, CD11c+-type cells that interact with Treg-cells and are suggested to induce tolerance to food antigens54.
Figure 4 |. Goblet cell uptake of luminal material with transfer to dendritic cells.
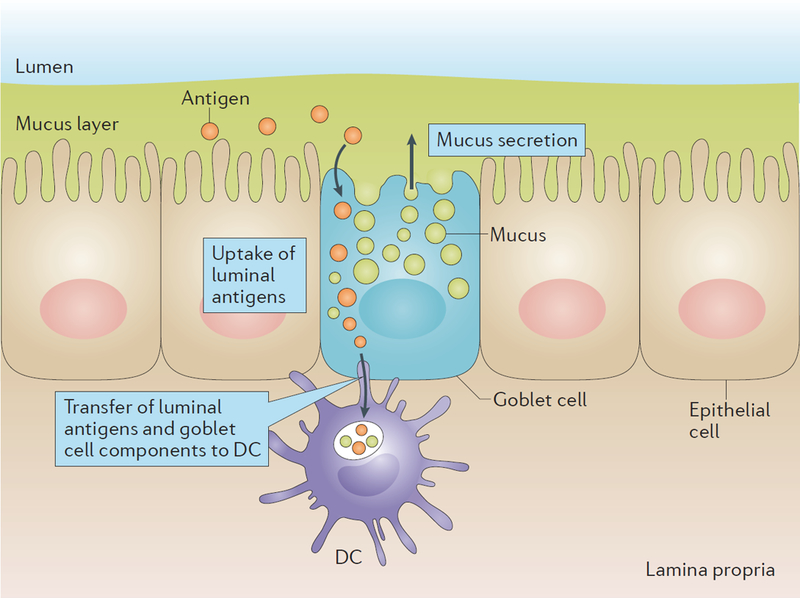
During mucus secretion small intestinal goblet cells sample the luminal content. How this uptake occurs is not known, but it could be proposed to occur through endocytosis and vesicle transport or by free diffusion in the cytoplasm. The luminal material is transferred together with goblet cell components to CD103+ DCs in the lamina propria that docks up against the secreting goblet cell.
A more well-known system is the sampling of the luminal antigens by M-cells on lymphoid follicles, in particular in Peyers patches55. Whole bacteria can be taken up by these cells, but there might be some limitations in the numbers that reach these follicle-associated epithelial surfaces as they are covered by non-attached mucus29.
Under normal conditions, the colon goblet cells ability to form GAPs is shut down and they do not sample luminal material. This process seems to be turned off by the microbiota and is dependent on goblet cell expression of MYD88, as mice with a goblet cell-specific deficiency in MYD88 have more GAPs56. Germ-free mice maintain the ability to sample and transport colonic luminal material to dendritic cells, also to antigen presenting cells. Bacteria reduction by antibiotics treatment also induce GAP formation with increased size of molecules that can be taken up as also bacteria can be transferred to DCs and mesenteric lymph nodes, a dramatic effect observed also after a single antibiotic dose57. A number of details of GAP formation and DC delivery are still unclear and require further studies.
Have the mucins a direct immunological effect?
Over the years mucins and their glycans have been suggested to have direct immunological effects by binding to the numerous lectin-like proteins found on immune cells. The surface of the mucin domains formed by the glycan arrangement is important for receptor binding as for example when selectin proteins are used to recruit immune cells to inflammatory sites58. Single glycan-protein interactions are usually of low affinity, but binding to glycan surfaces could quickly reach high affinities. More recently the MUC2 mucin has been suggested to imprint DC tolerance after direct DC uptake54. This was suggested to occur when MUC2 or other mucins were given orally. The way these experiments were performed reflect effects of the highly glycosylated mucin domains. In this case, the glycan repertoire should be important, but this did for unknown reasons not seem to matter. A potential role of glycans and mucins in tolerogenic mechanisms needs to be further explored.
Immunomediators and goblet cells.
The immune system is acting on the goblet cell as observed by hyperplasia and mucus hypersecretion in Th2 cell responses via interleukin IL-4, IL-5, IL-9 and IL-1359,60. IL-13 is highly increased at parasitic helminth infections and triggers STAT6 signaling in goblet cells causing hyperplasia61. Mice overexpressing IL-9 also induce IL-13 mediated goblet cell hyperplasia62. The Th2 cell responses can be initiated by another secretory cell type in the epithelium, the tuft cell, which expresses IL-2563,64. Allergic responses, including asthma, are Th2 cell-mediated and the goblet cell transcription factor SPDEF in the respiratory tract is required for Th2-type inflammatory responses and increased IL-13 and IL-3365. SPDEF is also expressed in intestinal goblet cells and might have a similar role also here15. The Th17 cell-associated cytokine IL-22 might also be involved in goblet cell regulation as IL-22-deficient mice failed to show increased goblet cell number and mucus filling after N. brasiliensis and T. muris infection66. The mucus barrier and goblet cells are also influenced by IL-10 and IL-18, as IL-10−/− animals have a defective colonic mucus layer and IL-18−/− mice show goblet cells that are less67,68. IL-18 expression is induced by bacteria and coupled to NLRP6 that can orchestrate colonic epithelial cell responses in host–microbial interactions25,69.
Mucus and the intestinal microbiota
Gut bacteria in mucus.
Although increased absolute numbers of bacteria are seen in the distal small intestine, the small intestinal microbiota is less diverse and stable than its colonic counterpart70,71. In the distal small intestine bacteria are found intermixed with mucus in the lumen and upper intervilli region as this mucus is penetrable. In the colon, the expanded outer colon mucus layer allows the microbiota to enter and utilize the exposed mucin glycans as attachment sites and as a nutritional source. This outer mucus layer is thus the natural habitat for the commensal bacteria. The mucin domain surface exposes terminal glycans that interact with bacterial adhesins72. As mucin glycosylation is typical for each species, it is probably important for the specific selection of bacteria colonizing each host species73,74. This interaction is likely most important at the outer side of the inner mucus layer where the glycans still are relatively intact and reflect the host glycan repertoire. The mucin glycans provide a stable energy source for the microbiota as bacteria have enzymes that release and utilize different glycans; indeed, for some bacteria mucins can be more or less their only energy source38,75,76. Most bacteria prefer the different non-digested food polysaccharides and thus the food composition will affect the intestinal bacterial composition77,73,78. Food restriction will on the other hand promote expansion of bacteria that can utilize mucin glycans. In a fiber limited diet, as in the western world, bacteria have to rely more on the mucus glycans as energy source, something that might have an impact on mucus homeostasis75. Mucus also has effects on the function of bacteria. Motile bacteria can stay in a planktonic and motile state in mucus, something that prevents them from forming biofilms and adhere to the underlying surface79.
Effects of bacteria on mucus development in the small intestine.
Under physiological conditions, the small intestinal mucus is non-attached, but in germ-free conditions the mucus cannot be removed from the epithelium. Following bacterial colonization of germ-free animals, the amounts of defensins and IgA found in the mucus peaks within the first few weeks70. Four weeks after colonization, there is a dramatic alteration in the bacterial composition primarily in the small intestine with reduced relative abundance of Clostridium spp. and an increase in Bacteroidia and Bacilli. At the same time the segmented filamentous bacteria (SFB) become undetectable71. This attached mucus is poorly cleared and the intervilli mucus is heavily colonized. This is also observed in mice lacking a functional CFTR channel that also show small intestinal bacterial overgrowth27,80. Five weeks after colonization, the mucus quality is shifted into its normal detached form and the microbiota composition is quickly returning to the one found in wild-type animals. This suggests that having movable mucus is a vital step in maintaining small intestinal homeostasis46,47,71. The importance of mucus in the small intestine is also illustrated in Muc2-deficient mice as these animals have increased bacterial burden that drives increased epithelial cell proliferation, inflammation, tumor development, and increased production of antibacterial immune components35,81.
Effects of bacteria on mucus development in colon.
Colonic mucus is normally impenetrable to bacteria and beads the size of bacteria. By contrast, such beads can penetrate the mucus all the way down to the epithelial cell surface in germ-free conditions71. Colonization of germ-free mice is quickly associated with reduced numbers of filled colonic goblet cells due to increased mucus secretion82. Recovery of the colonic mucus to its normally impenetrable phenotype takes five weeks post bacterial colonization71. It has been commonly assumed that germ-free animals are normalized two weeks after colonization, but more time (up to 8 weeks) is required to develop a normal mucus system and a stable complex colon microbiota71,83. The shift in bacterial composition and the mucus alterations appear first in the small intestine and soon after in the colon, suggesting an important role for the small intestine in bacterial selection. The stimulatory effect of bacteria on the mucus could be general to all bacteria, but recent observations suggest that this is more specific. Only certain, so far, undefined bacteria have such mucus stimulating capacity as suggested from studies of two mouse colonies42. The bacterial effect on the host mucus properties can be speculated to be mediated by small compounds that diffuse over the inner mucus layer.
Effects of antibiotic treatment on intestinal mucus.
Studies exploring the effects of antibiotics on the intestinal bacteria and mucus are often used as an alternative to studies in germ-free animals. However, these studies must be interpreted with caution due to the specific effects of antibiotics on different bacteria and other more or less direct effects of antibiotics on the epithelium84. Treatment of mice with metronidazole caused thinning of the normally impenetrable colonic mucus layer, but this was not observed with streptomycin treatment85. On the other hand, a combination of four antibiotics promoted a slight increase in the thickness of the colonic mucus71. One important conclusion is that antibiotic treatment does not revert the intestinal mucus system into that of the germ-free animals and the 6–8 weeks it takes to normalize the mucus after colonization suggest that the microbiota has non-reversible effects on epithelial cells.
Bacterial products affect mucus production.
Toll-like receptor (TLR) family members are key mediators of microbial-host communication promoting homeostasis with implications for mucus formation86,87. For example, mice with an intestinal epithelial cell-specific deletion of Myd88 show defective mucosal barrier functions that are characterized by decreased mRNA expression of Muc2 and increased translocation of bacteria into the lamina propria45,88. Bacterial metabolites, such as short-chain fatty acids (SCFAs), influence the epithelial protective function. Butyrate is especially important as it is a preferred energy source for colonic epithelial cells and decreased butyrate absorption is associated with both intestinal inflammation and carcinogenesis89. Goblet cells use butyrate as an important energy source for MUC2 production when other energy sources are limited as in distal colon90.
Intestinal pathogens and mucus.
The colonic inner mucus layer is an efficient physical barrier to both commensal bacteria and pathogens. Just as important as the pore size excluding bacteria is the continuous mucus renewal from the epithelium, something that is even more evident for the small intestine. The importance of mucus for expulsion of pathogens is clearly shown by the Th2-mediated goblet cell hyperplasia and coordinated expulsion of the helminths91. In fact, all pathogens that aim to invade the epithelium have developed strategies to overcome the mucus protection (Fig 5). The small intestinal mucus is loose and penetrable, but the direction of the mucus transport requires bacterial mobility to swim against the flow, sometimes promoted by bacterial mucin degrading proteases (Fig 5b). Mobility in mucus is important for Salmonella typhimurium that depends on flagella and chemotaxis to penetrate the mucus layer and reach the epithelium92, as also observed for Shigella flexneri and Vibrio cholerae93,94. Listeria takes advantage of secreting goblet cells for transcytosis, but if GAP formation is involved is not known95. As discussed, attached small intestinal mucus causes problems as it cannot clear the bacteria efficiently which cause bacterial overgrowth (Fig 5a).
Figure 5 |. Effects of mucus defects and how pathogens can circumvent the mucus protection in the intestine.
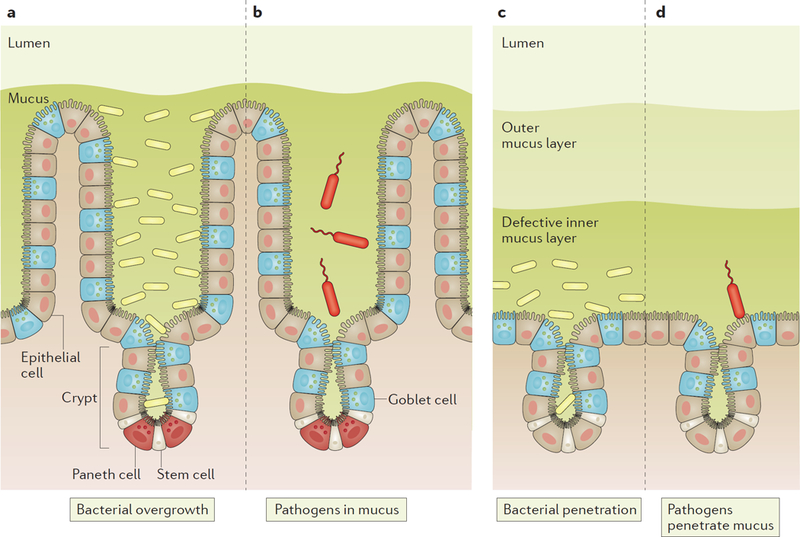
a, Defective release of the mucus from the epithelium in the small intestine results in stagnant mucus that allows bacteria to grow in the mucus (overgrowth). b, Pathogens that infect the small intestine must be motile and swim against the mucus flow and have an advantage if able to degrade the mucus. c, Defects in the inner mucus layer properties in colon allow bacteria to penetrate and reach the epithelium and penetrate into the crypts, something that triggers inflammation. d, Pathogens that infect colon must produce proteases able to degrade the inner mucus layer to be able to penetrate and reach the epithelial cells.
The colon with its strong physical mucus barrier requires microbally derived proteases to dissolve the mucus (Fig 5d). The colonic infection by Citrobacter rodentium require mucus penetration, but once the epithelial surface has been reached the bacteria can attach and remain96,97. Also large parasites as Entamoeba histolytica use proteolytic activity to dissolve the colon mucus by a specific cleavage in the MUC2 mucin that allow them to penetrate the inner mucus and invade the underlying epithelium98.
Mucus and intestinal inflammation
Mice lacking the Muc2 mucin have no mucus, which causes the animals to develop inflammation with diarrhea, bleeding, rectal prolapse and increased epithelial cell proliferation often leading to tumor development at older age35,81,99. Without mucus, the bacteria can come in direct contact with the epithelium, translocate, and trigger the submucosal immune system to elicit an overt immune response.
Bacterial role in inflammation.
The inflammation in colitic mouse models is dependent on the microbiota and the severity is dependent on the type of bacteria and the response of the host. Mice with an increased inflammatory tone typically have elevated levels of Proteobacteria42,100. Several mouse models that show mucus defects have an inner mucus layer that is more penetrable to bacteria and develop inflammation (Fig 5c). These models include mice with defects in ion transport proteins resulting in poorly organized mucus67,101,102 and shorter mucin glycans reducing the time it takes for bacteria to degrade and dissolve the mucus polymeric structure67,103. In susceptible hosts, the commensal B. thetaiotaomicron cause colitis utilizing a sulfatase activity required for bacterial outer membrane vesicles to penetrate the mucus and cause inflammation104. The commonly used DSS-induced colitis model in mice instantly renders the inner mucus layer penetrable to bacteria105. Due to mice diurnal drinking cycle, the animals can partly recover during the first days until prolonged bacterial contact with the epithelium causes DSS induced colitis after 3–5 days. This is the best model to study initiation of colon inflammation and it shows the importance of the inner mucus layer in protecting the intestine.
Immune mechanisms and inflammation.
Many mouse models with immune deficiencies spontaneously develop colitis or show increased susceptibility to DSS-mediated colitis. The most well-studied colitis model is the IL-10−/− deficient mouse model, which was developed already by 1993106. Interestingly, it was recently shown that these mice have comparably thick inner and outer mucus layers to wild-type mice, but their inner mucus layer is penetrable to bacteria67. The reason for this is not understood, but again emphasizes the importance of an intact inner mucus layer. Mouse models with genetic defects resulting in decreased Muc2 expression or a thinner mucus layer are also more susceptible to DSS16,45,107. DSS has also been used to illustrate alterations in colitis severity in interleukin deficient mice108,109,110. More bacteria in contact with the colon epithelial cells trigger attempts to clear the bacteria. The first system to be activated is the senGC guarding cell that triggers mucus secretion to wash bacteria away at the crypt opening26. There are more systems yet not fully understood illustrated in experiments where the colon blood flow was blocked for one hour during which the surface mucus was lost and bacteria penetrated into the crypts111. When the blood flow was turned on again, most crypt goblet cells emptied their accumulated mucus to wash away all bacteria and restore the inner mucus layer. The goblet cells must continuously be renewed and refilled, something that is challenging as the MUC2 is large and complex and its biosynthesis requires specific aiding proteins (Ern2 and Agr2)16,17. Prolonged mucus hypersecretion will thus cause the system to become overloaded and accumulate unfolded proteins in ER, leading to lower production and secretion of mucus. The high demand on the ER folding systems will result in ER stress as observed in mice with Muc2 mutations causing a Th17 dominated inflammation112. This inflammation could be alleviated by IL-23 inhibition and glucocorticoids 100,113,114. Protection from DSS colitis was also achieved by epithelial cell specific deletion of IL-18, its epithelial receptor IL-18r1, while deletion of the IL-18 negative regulator IL18bp worsens the disease68.
Ulcerative colitis.
The human inflammatory bowel disease ulcerative colitis (UC) is likely sharing features with mouse models having colon mucus defects. UC patients with active disease have mucus that is penetrable to bacteria, something that this is also observed in a subgroup of patients in remission67. This could indicate a role for mucus defects in disease development in some patient of this heterogeneous patient group. The mucus in patients with active ulcerative colitis is thinner and has an altered glycosylation profile not seen in patients in remission8,67,115. Once an overt inflammation has developed, the immune system drives the goblet cells to extensive mucus release in attempts to remove the intruders. This is overwhelming the system and is reflected in the emptied goblet cell phenotype seen during colitis. Dampening the immune system will give the goblet cells more time to recover. One way of upregulating the mucus protection is by inducing a Th2 cell response that will lead to increased number of goblet cells. This approach has been therapeutically tested in inflammatory bowel diseases using helminth infections116
Conclusion
The mucus covering the intestinal surface provides the outer protection of the intestine, but is also the interface with our microbiota. It has numerous intrinsic functions developed to meet the requirements at different locations of the intestine. The mucus system is dynamic and highly responsive to the immune system. Mucus secretion by goblet cells mediates delivery of intestinal material to dendritic cells. Goblet cells and their produced mucus is part of our innate immunity and intimately linked to the adaptive immune system. We are just in the beginning of answering fundamental questions in relation to the diverse repertoire of goblet cells and different mucus components. Even more important is to understand the signaling systems connecting the intestinal microbiota, epithelium including goblet cells, and the immune systems in relation to intestinal inflammation.
Box 1. Immunological aspects of respiratory mucus and mucins.
The respiratory mucus/mucin system is more complex than the intestinal one as it includes two mucins; MUC5B from glands and MUC5AC for surface goblet cells4,10. The system also varies between rodents, that essentially lack glands, and humans, where the glands are important for the daily housekeeping of the lungs. The respiratory mucus is moved upwards by the cilia and in this respect it is similar to the small intestinal mucus. On the other hand, the stagnant mucus observed at COPD and CF probably resembles the normal colonic mucus system. Airway goblet cells are also responding to signaling from the immune system and also act as immune effector cells by secreting cytokines65. Comparisons of the intestinal and respiratory tract mucus/mucins and goblet cells will be important for understanding the two systems.
Glossary terms
- CFTR
Cystic fibrosis transmembrane regulator is an ion channel defect in the disease cystic fibrosis that transports chloride and bicarbonate ions.
- Dextran sulfate sodium (DSS)-induced colitis
A model of colitis induced in rodents by the addition of DSS to drinking water; this causes the inner colon mucus layer to become penetrable to bacteria, disrupts the epithelial layer and leads to intestinal inflammation.
- SEA
Domain found in Sea urchin sperm protein, Enterokinase, and Agrin that are found on the outside of the membrane in several transmembrane mucins and autocatalytically cleaved during folding in the endoplasmic reticulum.
- SAM pointed domain-containing Ets transcription factor (SPDEF)
Transcription factor that is a master regulator of goblet cell lineage differentiation and maturation
- Theca
The cluster of large mucin filled granulae typical for goblet cells.
- SFB
Segmented filamentous bacteria infect the small intestine of mouse as they attach to the enterocyte membranes, but not humans, and support Th17 cell responses in the mouse intestine.
Biography
Gunnar C. Hansson
Gunnar C. Hansson is an M.D., Ph.D. and full professor at the Department of Medical Biochemistry, University of Gothenburg, Sweden. After his post-doc at NIH he has devoted 30 years to the studies of mucins and mucus of especially the gastrointestinal tract. Lately he has further expanded his mucin research to the respiratory tract. He is a member of the Swedish Royal Academy of Sciences. http://www.medkem.gu.se/mucinbiology/
Malin E.V. Johansson
Malin E.V. Johansson received her PhD in 2009 and is now an Associate professor and group leader at the Department of Medical Biochemistry, University of Gothenburg, Sweden. Her work is focused on mucus protection, with functional studies on its different components, and goblet cell function in the intestine. http://biomedicine.gu.se/ominst/avd/medkem/forskare/malin-johansson
References
- 1.Ambort D et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. U. S. A 109, 5645–5650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang T, Hansson GC & Samuelsson T Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. U. S. A 104, 16209–16214 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattrup CL & Gendler SJ Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol 70, 431–457 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Corfield AP Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 1850, 236–252 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Linden SK, Florin TH & McGuckin MA Mucin dynamics in intestinal bacterial infection. PLoS. ONE 3, e3952 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibahara H et al. Pathobiological implications of mucin (MUC) expression in the outcome of small bowel cancer. PLoS. ONE 9, e86111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thathiah A & Carson DD MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem. J 382, 363–373 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson JM et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel. Dis 17, 2299–2307 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Hasnain SZ, Gallagher AL, Grencis RK & Thornton DJ A new role for mucins in immunity: insights from gastrointestinal nematode infection. Int. J. Biochem. Cell Biol 45, 364–374 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Ridley C et al. Assembly of the respiratory mucin MUC5B: a new model for a gel-forming mucin. J. Biol. Chem 289, 16409–16420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gum JR, Crawley SC, Hicks JW, Szymkowski DE & Kim YS MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun 291, 466–475 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Williams SJ et al. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res 59, 4083–4089 (1999). [PubMed] [Google Scholar]
- 13.Pelaseyed T, Gustafsson JK, Gustafsson IJ, Ermund A & Hansson GC Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. Am. J. Physiol Cell Physiol 305, C457–C467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng YH et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 60, 1661–1670 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Gregorieff A et al. The Ets-Domain Transcription Factor Spdef Promotes Maturation of Goblet and Paneth Cells in the Intestinal Epithelium. Gastroenterology 137, 1333–1345 (2009). The transcription factor Spdef is a major regulator of goblet and paneth cell maturation and control goblet cell specific gene expression. [DOI] [PubMed] [Google Scholar]
- 16.Park SW et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. U. S. A 106, 6950–6955 (2009). A sentinel goblet cell (senGC) is guarding the colonic crypt opening and defends this by stimulating mucus secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuru A et al. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc. Natl. Acad. Sci. U. S. A 110, 2864–2869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson ME Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS. ONE 7, e41009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neutra M & Leblond CP Synthesis of the carbohydrate of mucus in the golgi complex as shown by electron microscope radioautography of goblet cells from rats injected with glucose-H3. J. Cell Biol 30, 119–136 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis CW & Dickey BF Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol 70, 487–512 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Specian RD & Neutra MR Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol 85, 626–640 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato T et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Tran DT, Masedunskas A, Weigert R & Ten Hagen KG Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat. Commun 6, 10098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel KK et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J 32, 3130–3144 (2013). Goblet cell secretion can be initiated via endocytosis and require activation of autophagy proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wlodarska M et al. NLRP6 Inflammasome Orchestrates the Colonic Host-Microbial Interface by Regulating Goblet Cell Mucus Secretion. Cell 156, 1045–1059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birchenough GMH, Nystrom ELN, Johansson MEV & Hansson GC A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science in press, (2016). [DOI] [PMC free article] [PubMed]
- 27.Gustafsson JK et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med 209, 1263–1272 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schutte A et al. Microbial-induced meprin beta cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc. Natl. Acad. Sci. U. S. A 111, 12396–12401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ermund A, Schutte A, Johansson ME, Gustafsson JK & Hansson GC Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol Gastrointest. Liver Physiol 305, G341–G347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooper LV & Macpherson AJ Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol 10, 159–169 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Meyer-Hoffert U et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57, 764–771 (2008). [DOI] [PubMed] [Google Scholar]
- 32.van der Waaij LA et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm. Bowel. Dis 11, 865–871 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Bevins CL & Salzman NH Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol 9, 356–368 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Swidsinski A, Loening-Baucke V, Lochs H & Hale LP Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J. Gastroenterol 11, 1131–1140 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson ME et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U. S. A 105, 15064–15069 (2008). The well.structured inner mucus layer of a two-layered mucus system separates the colon bacteria from the epithelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato A & Romero MF Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol 73, 261–281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S & Doerffel Y Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 135, 568–579 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Li H et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun 6, 8292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derrien M, Collado MC, Ben-Amor K, Salminen S & de Vos WM The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol 74, 1646–1648 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson BR et al. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int. J. Syst. Evol. Microbiol 55, 1199–1204 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Macpherson AJ, McCoy KD, Johansen FE & Brandtzaeg P The immune geography of IgA induction and function. Mucosal. Immunol 1, 11–22 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Jakobsson HE et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16, 164–177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slack E, Balmer ML & Macpherson AJB cells as a critical node in the microbiota-host immune system network. Immunol. Rev 260, 50–66 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Rogier EW, Frantz AL, Bruno ME & Kaetzel CS Secretory IgA is Concentrated in the Outer Layer of Colonic Mucus along with Gut Bacteria. Pathogens 3, 390–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frantz AL et al. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal. Immunol 5, 501–512 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaishnava S et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loonen LM et al. REG3gamma-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal. Immunol 7, 939–947 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Cash HL, Whitham CV, Behrendt CL & Hooper LV Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Earle KA et al. Quantitative Imaging of Gut Microbiota Spatial Organization. Cell Host. Microbe 18, 478–488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sommer F & Backhed F The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal. Immunol 8, 372–379 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Barr JJ et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. U. S. A 110, 10771–10776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Pineiro AM et al. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am. J. Physiol Gastrointest. Liver Physiol 305, G348–G356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDole JR et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012). Small intestinal goblet cells take up lumenal material at secretion and deliver this to lamina propria dendritic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan M et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraehenbuhl JP & Neutra MR Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol 16, 301–332 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Knoop KA, McDonald KG, McCrate S, McDole JR & Newberry RD Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal. Immunol 8, 198–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knoop KA, McDonald KG, Kulkarni DH & Newberry RD Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut (2015). [DOI] [PMC free article] [PubMed]
- 58.Rosen SD Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol 22, 129–156 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Khan WI & Collins SM Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol 26, 319–326 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Oeser K, Schwartz C & Voehringer D Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal. Immunol 8, 672–682 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Finkelman FD et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev 201, 139–155 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Steenwinckel V et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol 182, 4737–4743 (2009). [DOI] [PubMed] [Google Scholar]
- 63.von Moltke J, Ji M, Liang HE & Locksley RM Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerbe F et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajavelu P et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Invest 125, 2021–2031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turner JE, Stockinger B & Helmby H IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS. Pathog 9, e1003698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansson ME et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014). Colitic mice and ulcerative colitis patients have a defect inner mucus layer allowing bacteria to penetrate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowarski R et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 163, 1444–1456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy M et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 163, 1428–1443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El AS et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal. Immunol 5, 567–579 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Johansson ME et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host. Microbe 18, 582–592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Juge N Microbial adhesins to gastrointestinal mucus. Trends Microbiol 20, 30–39 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Ley RE et al. Evolution of mammals and their gut microbes. Science 320, 1647–1651 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rawls JF, Mahowald MA, Ley RE & Gordon JI Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433 (2006). Host mechanisms are important for the selection of host-specific microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marcobal A, Southwick AM, Earle KA & Sonnenburg JL A refined palate: bacterial consumption of host glycans in the gut. Glycobiology 23, 1038–1046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pudlo NA et al. Symbiotic Human Gut Bacteria with Variable Metabolic Priorities for Host Mucosal Glycans. MBio 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonnenburg JL et al. Glycan Foraging in Vivo by an Intestine-Adapted Bacterial Symbiont. Science 307, 1955–1959 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Sonnenburg ED et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caldara M et al. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol 22, 2325–2330 (2012). Bacteria can swim in freshly prepared mucus and remain in a planktonic state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norkina O, Burnett TG & De Lisle RC Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect. Immun 72, 6040–6049 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velcich A et al. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science 295, 1726–1729 (2002). Mice lacking the Muc2 mucin has no protective mucus and develop cancer. [DOI] [PubMed] [Google Scholar]
- 82.El AS et al. Transient inflammatory-like state and microbial dysbiosis are pivotal in establishment of mucosal homeostasis during colonisation of germ-free mice. Benef. Microbes 5, 67–77 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Wrzosek L et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC. Biol 11, 61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willing BP, Russell SL & Finlay BB Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol 9, 233–243 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Wlodarska M et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun 79, 1536–1545 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S & Medzhitov R Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 118, 229–241 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Vijay-Kumar M et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest 117, 3909–3921 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhinder G et al. Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses. Infect. Immun 82, 3753–3763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamer HM et al. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther 27, 104–119 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Gaudier E et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol Gastrointest. Liver Physiol 287, G1168–G1174 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Artis D & Grencis RK The intestinal epithelium: sensors to effectors in nematode infection. Mucosal. Immunol 1, 252–264 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Stecher B et al. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun 72, 4138–4150 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navarro-Garcia F et al. Pic, an autotransporter protein secreted by different pathogens in the Enterobacteriaceae family, is a potent mucus secretagogue. Infect. Immun 78, 4101–4109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silva AJ, Pham K & Benitez JA Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149, 1883–1891 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Nikitas G et al. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med 208, 2263–2277 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bergstrom KS et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS. Pathog 6, e1000902 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhullar K et al. The Serine Protease Autotransporter Pic Modulates Citrobacter rodentium Pathogenesis and Its Innate Recognition by the Host. Infect. Immun 83, 2636–2650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lidell ME, Moncada DM, Chadee K & Hansson GC Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc. Nat. Acad. Sci. Usa 103, 9298–9393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Sluis M et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Huang EY et al. Using corticosteroids to reshape the gut microbiome: implications for inflammatory bowel diseases. Inflamm. Bowel. Dis 21, 963–972 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C et al. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am. J. Physiol Cell Physiol 305, C121–C128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao F et al. Slc26a3 deficiency is associated with loss of colonic HCO secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol (Oxf)(2013). [DOI] [PubMed]
- 103.Fu J et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest 121, 1657–1666 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hickey CA et al. Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host. Microbe 17, 672–680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johansson ME et al. Bacteria Penetrate the Inner Mucus Layer before Inflammation in the Dextran Sulfate Colitis Model. PLoS. ONE 5, e12238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuhn R, Lohler J, Rennick D, Rajewsky K & Muller W Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993). [DOI] [PubMed] [Google Scholar]
- 107.Bertolotti A et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J. Clin. Invest 107, 585–593 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bersudsky M et al. Non-redundant properties of IL-1alpha and IL-1beta during acute colon inflammation in mice. Gut 63, 598–609 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Imaeda H et al. Interleukin-33 suppresses Notch ligand expression and prevents goblet cell depletion in dextran sulfate sodium-induced colitis. Int. J. Mol. Med 28, 573–578 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Ito R et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun 377, 12–16 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Grootjans J et al. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut 62, 250–258 (2013). A concept of how mucus secretion from the crypt can clear bacteria and reconstitute an inner mucus layer. [DOI] [PubMed] [Google Scholar]
- 112.Heazlewood CK et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS. Med 5, e54 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Das I et al. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J. Exp. Med 210, 1201–1216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang R et al. Neutralizing IL-23 is superior to blocking IL-17 in suppressing intestinal inflammation in a spontaneous murine colitis model. Inflamm. Bowel. Dis 21, 973–984 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Pullan RD et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35, 353–359 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heylen M et al. Of worms, mice and man: an overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol. Ther 143, 153–167 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Audie JP et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem 41, 1479–1485 (1993). [DOI] [PubMed] [Google Scholar]
- 118.Weiss AA, Babyatsky MW, Ogata S, Chen A & Itzkowitz SH Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J. Histochem. Cytochem 44, 1161–1166 (1996). [DOI] [PubMed] [Google Scholar]
- 119.Williams SJ et al. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem 276, 18327–18336 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Chang SK et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology 107, 28–36 (1994). [DOI] [PubMed] [Google Scholar]
- 121.HO SB et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res 55, 2681–2690 (1995). [PubMed] [Google Scholar]
- 122.Gruber AD et al. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics 54, 200–214 (1998). [DOI] [PubMed] [Google Scholar]
- 123.Johansson ME, Thomsson KA & Hansson GC Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome. Res 8, 3549–3557 (2009). [DOI] [PubMed] [Google Scholar]
- 124.Madsen J, Nielsen O, Tornoe I, Thim L & Holmskov U Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem 55, 505–513 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Tateno H et al. Human ZG16p recognizes pathogenic fungi through non-self polyvalent mannose in the digestive system. Glycobiology 22, 210–220 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Kang W & Reid KB DMBT1, a regulator of mucosal homeostasis through the linking of mucosal defense and regeneration? FEBS Lett 540, 21–25 (2003). [DOI] [PubMed] [Google Scholar]


