Abstract
Stress elicits a variety of psychophysiological responses that show large inter-individual variability. Determining the neural mechanisms that mediate individual differences in the emotional response to stress would provide new insight that would have important implications for understanding stress-related disorders. Therefore, the present study examined individual differences in the relationship between brain activity and the emotional response to stress. In the largest stress study to date, 239 participants completed the Montreal Imaging Stress Task (MIST) while heart rate, skin conductance response (SCR), cortisol, self-reported stress, and blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) signal responses were measured. The relationship between differential responses (heart rate, SCR, cortisol, and self-reported stress) and differential BOLD fMRI data was analyzed. Dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), ventromedial PFC (vmPFC), and amygdala activity varied with the behavioral response (i.e. SCR and self-reported stress). These results suggest the PFC and amygdala support processes that are important for the expression and regulation of the emotional response to stress, and that stress-related PFC and amygdala activity underlie inter-individual variability in peripheral physiologic measures of the stress response.
Keywords: stress, fMRI, prefrontal cortex, amygdala
Stress is a part of everyday life and, although unpleasant, is generally considered an adaptive, allostatic response to perceived threats in our environment (McEwen and Gianaros, 2011). Unfortunately, high levels of prolonged stress can have adverse effects on mental health (Chrousos & Gold, 1992). For example, chronic stress is linked to the development of disorders such as depression and anxiety (Kendler et al., 1999; Havranek et al., 2016). Stress also elicits psychophysiological responses through the activation of the sympathetic branch of the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis. Peripheral measures of ANS activation include changes in heart rate and skin conductance. Cortisol is the primary product of the activation of the HPA axis. These psychophysiological parameters show large inter-individual variability in response to stress. The relationship between stress and each of these peripheral physiological measures (heart rate, skin conductance, and cortisol) at the group level has been extensively studied (Wang et al, 2005; Fechir et al., 2010; Pruessner et al., 2008), as has the basic neural circuitry that mediates the stress response (Masaoka et al., 2003; Kapp et al., 1979; Sullivan et al., 2004; Hilz et al., 2006; Nagai et al., 2004). Group level analyses have demonstrated notable changes in measures such as heart rate, skin conductance, and cortisol in response to stress (Wang et al, 2005; Fechir et al., 2010; Pruessner et al., 2008) and the importance of structures such as the amygdala and prefrontal cortex (PFC) in the expression of the emotional response (LeDoux et al., 1988; Gray et al., 1989; Ochsner et al., 2004; Phan et al., 2005). However, the neural processes that mediate individual differences in these responses have received limited empirical attention. Advancing our understanding of the neural circuitry that mediates individual variability in behavioral, ANS, and HPA axis stress-reactivity may have important implications for our understanding of the nature, onset, course, and concomitants of stress-related disorders.
Prior human and animal model research suggests the PFC and amygdala play an important role in the expression and regulation of the peripheral emotional response (LeDoux et al., 1988; Gray et al., 1989; Ochsner et al., 2004; Phan et al., 2005). The amygdala appears to control the peripheral expression of emotion via projections to the hypothalamus and medulla, which control both the ANS and HPA axis (LeDoux et al., 1988; Gray et al., 1989; Takeuchi et al., 1991; Schwaber et al, 1982). Studies examining the impact of brain lesions demonstrate the amygdala has an important role in the expression of heart rate, skin conductance, and cortisol responses (Masaoka et al., 2003; Kapp et al., 1979; Sullivan et al., 2004). Further, prior functional magnetic resonance imaging (fMRI) research has demonstrated that fMRI signal within the amygdala varies with the peripheral emotional response (e.g., indexed by skin conductance) (Cheng et al., 2006; Critchley, 2002; Knight et al., 2005; Wood et al., 2014). The vmPFC also influences ANS and HPA axis activity through projections that regulate amygdala function (Ochsner et al., 2004; Phan et al., 2005, Phelps et al., 2004). Decreased activation of the vmPFC is associated with increased amygdala activation to emotional stimuli, implicating the vmPFC in the regulation of the emotional response via projections to the amygdala (Hartley and Phelps, 2012). In sum, both lesion and neuroimaging studies indicate the vmPFC is associated with heart rate, skin conductance, and cortisol levels (Hilz et al., 2006).
In the present study, we investigated whether PFC and amygdala activity supports processes that underlie the inter-individual variability of the stress response. Participants completed the Montreal Imaging Stress Task (MIST) while heart rate, skin conductance response (SCR), cortisol, self-reported stress, and blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) signal were measured. Given prior work suggests the amygdala and vmPFC mediate the peripheral expression of emotion (Gray et al., 1989; Hilz et al., 2006 LeDoux et al., 1988; Wheelock et al., In Press), we hypothesized individual variability in the stress response would localize to these regions. More specifically, we hypothesized the stress response would show a positive relationship with amygdala activity and a negative relationship with vmPFC activity.
Methods
Participants
Two-hundred thirty-nine right-handed [126 male, 113 female; age = 19.44 ± 0.07 years (mean ± SEM); range = 17–22 years] volunteers participated in the present study. All participants were screened for exclusion criteria prior to the study. Exclusion criteria included: history of blood or circulation disorders (e.g., anemia or sickle-cell), diabetes, brain or spinal abnormalities, pregnancy, previous or current head injury (e.g., traumatic brain injury, concussion, or loss of consciousness). All volunteers provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Procedure
Montreal Imaging Stress Task (MIST)
Participants completed a modified version of the Montreal Imaging Stress Task (MIST) as described in previous work (Dedovic et al., 2005; Goodman et al., 2016; Wheelock et al., 2016). The MIST consisted of two scans: a Control scan and a Stress scan. Each scan was approximately 7 minutes and 54 seconds in duration and consisted of 54 math trials. Prior to these scans, participants completed practice problems outside of the scanner. These practice problems allowed participants to familiarize themselves with the task and to determine the difficulty of math problems to be presented during scanning. During the fMRI scans, each trial lasted six seconds and contained a unique math problem. At the start of the trial, a math problem and response options (i.e. answers from 0–9) were presented (0.5–5 seconds). Once a participant selected an answer, a fixation cross appeared (0.5–5 seconds). The fixation cross was followed by 0.5 seconds of visual feedback (“Right”, “Wrong”, or “Time out”). Each trial was separated by a fixation cross during a variable inter-trial interval (1–3 seconds). The difficulty of problems presented remained the same during both the Control and Stress scans.
Prior to the Control scan, investigators attempted to lower participant stress levels by telling them “It is OK if you do not answer all of the math problems correctly.” During the Control scan, participants were given five seconds in which to respond to each math problem and received previously recorded positive auditory feedback. In contrast, the investigators attempted to elevate stress levels prior to the Stress scan by telling participants they must answer the questions correctly, and warning that if they did not perform as well as others in the study their data would not be used. Further, during the Stress scan, the participants were given previously recorded negative auditory feedback. Failure was ensured by modulating the time in which the participant could respond in a stair-step manner such that on average participants answered approximately 50% of the problems correctly.
Magnetic Resonance Imaging (MRI)
Blood oxygen level dependent (BOLD) fMRI was acquired on a 3T Siemens Allegra Scanner using a brain-specific RF head coil. Functional MRI data were acquired using a gradient-echo echo planar pulse sequence (TR=2000ms, TE=30ms, FOV=24cm, matrix=64×64, slice thickness=4mm) during two scans of stimulus presentations. High resolution anatomical images (MPRAGE) were also obtained to serve as an anatomical reference (T1 weighted, TR=2300ms, TE=3.9ms, FOV=25.6cm, matrix=256×256, slice thickness=1mm, 0.5mm gap). MRI data were preprocessed using the AFNI software package (AFNI_16.2.06) (Cox, 1996). Functional MRI data were corrected for motion by censoring high-motion TRs, and including the six motion parameters in the first level model. High motion TRs were defined as volumes in which three percent (or greater) of voxels deviated by more than five times the median absolute signal. The fMRI time course for control and stress scans were each separately adjusted to a mean signal of 100 before first level analyses were completed. First level models included a regressor of interest for duration modulated math response (i.e. time from trial onset to button press used to answer math problems) as well as regressors of no interest for linear drift, duration modulated auditory feedback, visual feedback events, joystick movement, and button press responses. A multiple-linear regression analysis was completed and the percent signal change in the fMRI signal was calculated. Functional MRI data were normalized to the MNI 152 template before second level analyses.
Second level analyses (AFNI’s 3dttest++) included a within subject factor for stress (i.e. Stress vs Control conditions) and continuous factors for SCR, heart rate, cortisol, and self-reported stress. These analyses were used to determine the relationship between differential (Stress vs. Control) peak time-course brain activity (fMRI) and observed measures of stress (i.e. skin conductance, heart rate, cortisol, and self-reported stress rating). Analyses were restricted using a gray matter mask of the prefrontal cortex, insula, amygdala, hippocampus, and hypothalamus based on the Automated Anatomical Labeling (AAL) atlas, which were then used to calculate the corrected (i.e. for multiple comparisons) significance threshold. Cluster corrections were performed using AFNI’s 3dclustsim at a family-wise error rate of p < 0.05. The corrected significance threshold was calculated by Monte Carlo simulations (3dclustsim) using an uncorrected threshold of p < 0.01 and a volume correction threshold of 760mm3 (based on AFNI’s spherical autocorrelation function [ACF] with parameters based upon the results from 3dFWHMx). In addition, small volume corrections were performed for the amygdala and hippocampus. Separate masks were created for the amygdala and the hippocampus using the Automated Anatomical Labeling (AAL) atlas, which were then used to calculate corrected significance thresholds as described above. Multiple comparisons corrections were performed using 3dclustim at a family-wise error rate of p < 0.05, based on an uncorrected threshold of p < 0.01. Critical volumes of 152mm3 (amygdala) and 315mm3 (hippocampus) were calculated for these regions.
Autonomic Nervous System (ANS)
Skin Conductance Response.
An MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect skin conductance response (SCR) data. SCR were sampled (10 KHz) using a pair of radio-translucent electrodes (1 cm diameter, Biopac Systems; Goleta, CA) from the thenar and hypothenar eminence of the left hand. SCR data were processed using Biopac Acqknowledge 4.1 software. A 1 Hz low pass digital filter was applied and SCR data were resampled at 250 Hz. The resampled SCR was exported to SCRalyze toolbox for further analysis (version b2.1.8) (Bach et al., 2009). The data were then bandpass filtered with a first order Butterworth filter (highpass cutoff of 0.0159 Hz, lowpass filter of 1.0 Hz) and further downsampled to a 10 Hz sampling rate. The time-series was then normalized (z-transformed and mean centered). The presentation of the math problem was included as a regressor predicting SCR using the general linear model with an assumed SCR function without a time or dispersion derivative (Bach et al., 2009, Bach et al., 2013). Resultant beta coefficients were entered into a two-tailed paired samples t-test in SPSS software to assess SCR to math presentation trials during Control and Stress MIST scans. Beta coefficients of the Control scan were subtracted from beta coefficients of the Stress scan to calculate differential SCR. Differential SCR was then used as a variable in fMRI analyses. Fifty-three participants were excluded from SCR analyses due to equipment failure, SCR non-responsiveness (SCR< 0.05 μS), or as outliers (± 3SD). Therefore, a total of one-hundred eighty-six participants were included in the SCR analyses.
Heart Rate.
An MRI compatible pulse oximeter (Siemens; Munich, Germany) was used to collect cardiac data during Stress and Control scans. Cardiac data were sampled (50 Hz) from the distal phalanx of the left index finger and heart rate was calculated using QRSTool software. Average heart rate during Stress and Control scans was assessed by a two-tailed paired samples t-test comparison using SPSS software. Average heart rate during the Control scan was subtracted from average heart rate during the Stress scan to calculate differential heart rate. Differential heart rate was then used as a variable in fMRI analyses. One-hundred and four participants were excluded from the heart rate analysis due to equipment failure, poor data quality, or as outliers. Thus, a total of one-hundred thirty-five participants were included in heart rate analyses.
Hypothalamic Pituitary Adrenal (HPA) Axis
Participants donated two saliva samples. The first sample (pre-task baseline) was collected sixty minutes after participant arrival, prior to the beginning of the first scan. The second sample (post-task) was collected twenty minutes after the conclusion of the Stress scan. Saliva samples were frozen at −80°C then transported frozen to the Institute for Interdisciplinary Salivary Bioscience Research where they were assayed for cortisol using a commercially available immunoassay (Salimetrics, LLC, Carlsbad, CA). The sample test volume was 25 μl, assay range of sensitivity from .007 to 3 μg/dL, and intra- and inter-assay coefficients of variation were, on average, less than 5% and 15%. All samples were tested in duplicate and the average of the duplicates was used in the statistical analyses. Pre-task baseline and post-task cortisol levels were assessed by a two-tailed paired samples t-test comparison using SPSS software. Pre-task baseline levels were subtracted from post-task levels and this difference score was then used in fMRI analyses. Two participants were excluded from the cortisol analysis due to the high viscosity of the sample or as outliers. Therefore, a total of two-hundred thirty-seven participants were included in cortisol analyses.
Behavioral Assessments
A measure of self-reported stress was also used to assess participant’s emotional response to Control and Stress MIST scans. Following the completion of the MIST, participants completed, outside of the scanner, a self-report questionnaire consisting of eight statements (Wheelock et al., 2016). Participants rated each statement’s applicability on a five point scale where 1 corresponded to “not at all” and 5 corresponded to “Extremely”. Four of the statements were worded positively (e.g., I felt calm) and four were worded negatively (e.g., I felt stressed) for a total possible self-reported stress score of 40. Stress ratings of Control and Stress scans were assessed by two-tailed paired samples t-test comparison using SPSS software. The stress ratings of the Control scan were subtracted from the stress ratings of the Stress scan to calculate differential stress ratings. Differential stress ratings were then used as a variable in fMRI analyses. Self-reported stress ratings were not collected from the first thirteen participants. Therefore, two-hundred twenty-six participants were included in stress rating analyses.
Analytical Strategy
AFNI software was used to complete analyses in the present study. Specifically, 3dttest++ was used with a within subject factor for stress (i.e. Stress vs. Control conditions) and continuous factors for SCR, heart rate, cortisol, and self-reported stress. Because we did not have SCR, heart rate, cortisol, and stress ratings for all participants, analyses were run separately for each index of the emotional response to stress. Prior to assessing the relationship between the fMRI data and behavioral measures, outliers with residuals greater than 3 standard deviations from the overall sample mean were excluded from the analysis. There was considerable variability in SCR, heart rate, cortisol, and self-reported stress across participants and stress measures, including participants that did not show an increase in these measures in response to the stress condition. This type of variability is common in psychophysiological data (Allendorfer et al., 2014; Arasaratnam et al., 2018; Cheng et al., 2006; Dedovic et al., 2009). Therefore, all participants were included regardless of whether or not they displayed an increased response to the Stress condition for any or all stress measures.
Results
Preliminary Analyses: Manipulation Check
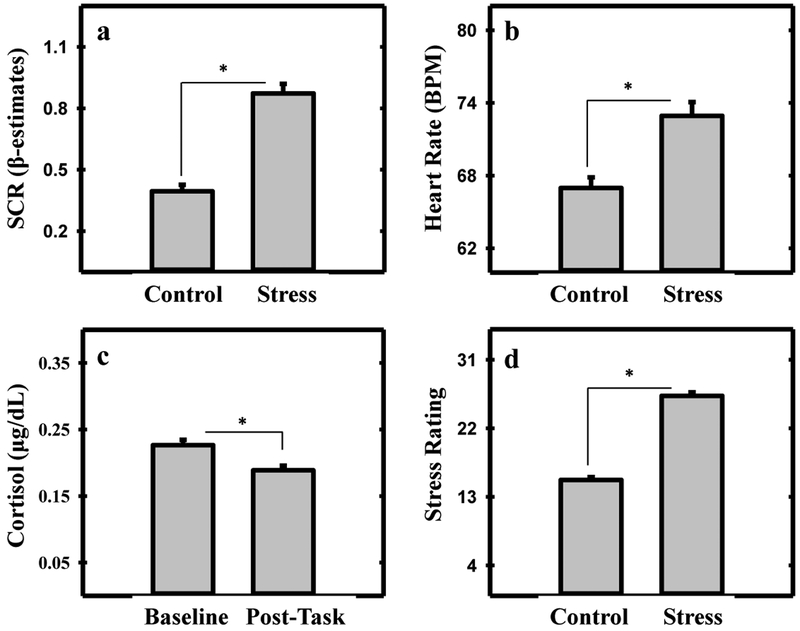
SCR was greater during the Stress (0.85 ± 0.05 beta estimates) than Control (0.40 ± 0.03 beta estimates) condition of the MIST (t[185] = 11.064, p < 0.001; Figure 1a). Heart rate was significantly greater during the Stress (72.25 ± 1.11 BPM) than Control (66.9 ± 0.91 BPM) condition of the MIST (t[134] = 10.66, p < 0.001; Figure 1b). Cortisol levels were higher at baseline (0.23 ± 0.01 μg/dL) than at the conclusion of the Stress scan (0.20 ± 0.01 μg/dL; t[236] = 4.50, p < 0.001; Figure 1c). Participants’ stress ratings were higher for the Stress (26.07 ± 0.45) than Control (15.24 ± 0.39) condition of the MIST (t[225] = 20.27, p < 0.001; Figure 1d). Distributions of participants’ behavioral data for Control and Stress conditions are shown in the supplementary materials.
Fig. (1).
Skin conductance response (SCR), heart rate, cortisol, and stress rating to psychosocial stress. Greater SCR (a) heart rate (b) and stress rating (d) were observed during the Stress than Control scan. Higher cortisol levels (c) were observed at baseline than after the Stress scan. Asterisk denotes a significant difference (p < 0.05).
Main Analyses: Brain and Behavior
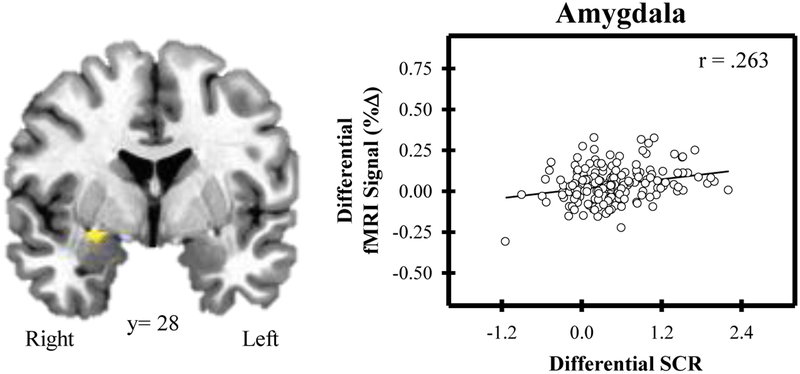
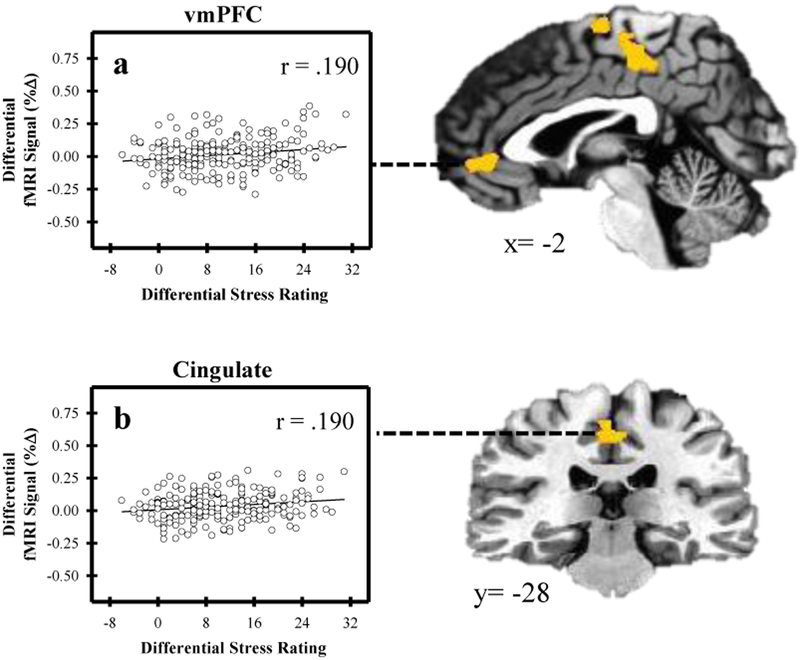
Several brain regions showed a differential fMRI signal response (Stress versus Control) that varied with SCR (Figure 2; Table 1). Specifically, the fMRI signal within the dlPFC, dmPFC, posterior cingulate, hippocampus, and amygdala was positively related to SCR (p < 0.05, corrected). In addition, activity in several brain regions varied with self-reported stress ratings (Figure 3; Table 2). Differential fMRI signal within the dlPFC, vmPFC, and cingulate cortex was positively related to differential stress ratings (p < 0.05, corrected). No significant relationship was observed between either heart rate or cortisol and the fMRI signal response. Distributions of participants’ fMRI data for both Control and Stress conditions are shown in the supplementary materials.
Fig. (2).
Amygdala activity and skin conductance response (SCR) to psychosocial stress. Differential activity (Stress-Control) within the right amygdala varied with differential SCR. As amygdala activity increased, SCR increased.
Table 1.
Regional Brain Activity that Varied with SCR
| MNI Coordinates | SCR | |||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Volume (mm3) | x | y | z | r |
| dmPFC | Bilateral | 2003 | 9 | 3 | 71 | .273 |
| Cingulate | Left | 2000 | −15 | −46 | 38 | .299 |
| dlPFC | Left | 878 | −45 | 34 | 42 | .209 |
| Hippocampus | Right | 593 | 20 | −26 | −13 | .269 |
| Amygdala | Right | 190 | 28 | −1 | −12 | .276 |
Note. dmPFC, dorsomedial prefrontal cortex. dlPFC, dorsolateral prefrontal cortex.
Peak voxel coordinates shown. All p < 0.05 (corrected).
Fig. (3).
Relationship between brain activity and self-reported stress. Differential activity (Stress-Control) within the ventromedial prefrontal cortex (vmPFC) and cingulate cortex varied with differential stress rating. As activity within the vmPFC (a) and the cingulate cortex (b) increased, differential stress rating increased.
Table 2.
Regional Brain Activity that Varied with Stress Rating
| MNI Coordinates | Stress Rating | |||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Volume (mm3) | x | y | z | r |
| dlPFC | Left | 4099 | −36 | 54 | 3 | .298 |
| Cingulate | Bilateral | 2922 | −2 | −28 | 47 | .190 |
| vmPFC | Bilateral | 1117 | 2 | 43 | −4 | .190 |
Note. dlPFC, dorsolateral prefrontal cortex. vmPFC, ventromedial prefrontal cortex.
Peak voxel coordinates shown. All p < 0.05 (corrected).
Discussion
In the largest human stress study to date, we investigated individual differences in the emotional response to stress and found that amygdala and PFC activity varied with stress-induced changes in psychophysiological and self-reported measures of the emotional response. These findings suggest that the amygdala and PFC underlie individual differences in the emotional response to stress.
Amygdala
The amygdala is a critical component of the neural circuit that mediates emotion. More specifically, the amygdala has a well-established role in the peripheral expression of emotion (Knight et al., 2005; LeDoux et al., 1988; Gray et al., 1989; Ochsner et al., 2004; Phan et al., 2005), and prior work indicates the amygdala is an important component of the neural circuit that controls both ANS and HPA axis activity (Masaoka et al., 2003; Kapp et al., 1979; Sullivan et al., 2004). In the present study, we found that amygdala activity varied with the emotional response (i.e. SCR) to psychosocial stress. Specifically, as differential SCR to stress increased, differential activity within the amygdala increased. Although prior emotion research has linked amygdala activity to SCR production, this is the first human stress study to assess the neural correlates of SCR reactivity. Despite differences in task design between prior research and the current study, this relationship between SCR and amygdala activity is consistent with prior fear conditioning and emotion-modulated startle research that has linked the fMRI signal within the amygdala to the generation of SCRs (Cheng et al., 2003; Cheng et al., 2006; Critchley, 2002; Knight et al., 2005; Wood et al., 2014). Taken together with prior work, the present findings indicate the amygdala is an important component of the neural circuit that mediates expression of the peripheral emotional response. Further, the present findings suggest amygdala function underlies individual differences in the emotional response to stress.
vmPFC
The vmPFC is another important component of the neural circuit that mediates emotion (Milad et al., 2007; Phelps et al., 2004). In general, the vmPFC appears to support emotion regulation processes via projections to the amygdala (Ochsner et al., 2004; Phan et al., 2005, Phelps et al., 2004). More specifically, prior research suggests the vmPFC functions to regulate amygdala activity as well as the corresponding emotional response (Milad et al., 2007; Phelps et al., 2004). Consistent with prior work, we also observed vmPFC activity that varied with the emotional response in the present study, specifically self-reported stress. The current results demonstrate that vmPFC activity was greater among individuals who reported more stress during the Stress than Control condition. A popular view of the neural mechanisms of emotion regulation suggests the vmPFC inhibits the expression of the stress response, specifically through projections to the amygdala (Rosenkranz et al., 2003). In fact, there are several prior studies with findings consistent with this view of emotion regulation (Ghashghaei et al., 2007; Kim et al., 2009). However, there is also considerable research that challenges this model (Albert et al., 2015; Damasio et al., 1990; Simpson et al., 2001; Wang et al., 2005). For example, studies have shown vmPFC activity is positively related to self-reported stress and heart rate (Albert et al., 2015; Simpson et al., 2001; Wang et al., 2005). In addition, damage to the vmPFC diminishes the SCR to stress (Damasio et al., 1990). The relationship between the vmPFC and amygdala is complex and utilizes both inhibitory and excitatory connections, potentially explaining the conflicting findings regarding the vmPFC’s function (Myers-Schulz & Koenigs, 2012). In the present study, vmPFC activity showed a positive relationship to self-reported stress. Taken together with prior work, our findings suggest that the vmPFC’s role in the expression of the stress response is not exclusively inhibitory. The present findings suggest the vmPFC plays an important role in regulating perceptions of stress severity. Interestingly, vmPFC activity in the present study only varied with self-reported stress, not heart rate, SCR, or cortisol. Nevertheless, the present findings suggest the vmPFC plays an important role in the individual variability observed in measures of perceived stress (i.e. self-reported stress).
dmPFC
The dmPFC has also been implicated in emotional processing (Dedovic et al, 2009; Maier et al., 2012; Wang et al., 2005). For example, prior work has demonstrated greater dmPFC activity during psychosocial stress (Dedovic et al., 2009). In the present study, dmPFC activity varied with SCR. Specifically, the current study demonstrates that dmPFC activity was greater in individuals with higher SCR to psychosocial stress. This finding suggests the dmPFC plays an important role in the expression of the emotional response to stress and is consistent with prior work that has suggested the dmPFC mediates the emotional response to stress (Critchley et al., 2001, Dedovic et al., 2009, Wang et al., 2005). For example, prior research suggests the dmPFC plays an important role in the appraisal of stressful events and modulates the subsequent emotional response to stress (Dedovic et al, 2009; Maier et al., 2012; Wang et al., 2005). However, the current findings extend this prior work by demonstrating dmPFC activity varies with individual differences in the stress-induced emotional response. The dmPFC has widely distributed connections throughout the brain, particularly to the limbic system (e.g., amygdala), which controls ANS activity (Banks et al., 2007; Gabbott et al., 2012; Gabbott et al., 2005). Coupled with prior work, the present study suggests the dmPFC plays an important role in the expression of ANS activity. The present findings suggest the dmPFC supports the peripheral expression of emotion and underlies individual differences in the emotional response to stress.
dlPFC
The dlPFC is another region that supports a number of executive and cognitive control processes that are important for emotion regulation (Basten et al., 2012). Prior work has demonstrated psychosocial stress elicits dlPFC activity (Dedovic et al., 2009). In the present study, dlPFC activity varied with SCR and self-reported stress. Specifically, dlPFC activity was greater in individuals with higher SCR and self-reported stress ratings to the Stress versus Control conditions. These findings suggest that dlPFC activity has a significant impact on the regulation of the ANS and perceived stress. The present findings are consistent with prior studies that have demonstrated the dlPFC supports emotional processes important to the stress response (Critchley et al., 2001; Chou et al., 2016; Dedovic et al., 2009). Specifically, the present study suggests that the dlPFC modulates important components of the emotional response (ANS and perceived stress) to stress. The dlPFC has extensive connections throughout the brain, including connections to the dmPFC that appear to regulate emotional function (Tanji and Hoshi, 2008; Taren et al., 2011). Therefore, the dlPFC may regulate the emotional response to stress via projections to other regions of the PFC, and thus may play an important role in the individual variability of the stress response.
Activity within the dlPFC and cingulate varied with multiple behavioral and psychophysiological measures of stress. Specifically, SCR and self-reported stress each varied with dlPFC and cingulate activity. However, differences were also observed between the BOLD fMRI signal and each behavioral/psychophysiological measure. For example, regions such as the dmPFC, vmPFC, and amygdala only varied with one index of emotion (SCR or self-reported stress). Amygdala and vmPFC activity would typically be expected to vary with multiple indices of the stress response. However, SCR and self-reported stress index distinct components of the emotional response. SCR is a measure of ANS activity while self-reported stress measures perceived stress. Given that each measure (SCR and self-reported stress) indexes a different component of the stress response, it is possible that the neural substrates associated with the individual variability of each measure may differ. In addition, a large amount of heart rate data was excluded from the present analyses due to equipment failure and poor quality data as described above. This is an important limitation of the present report and may have impacted our ability to identify relationships between heart rate data and brain activity. More specifically, the relatively limited heart rate data included in the present study may have interfered with our ability to detect significant relationships between heart rate and brain activity in regions such as the amygdala.
Limitations
Findings from the current study should be considered in light of a few limitations. Typically, an increase in cortisol, similar to what we observed with SCR, heart rate, and self-reported stress, is expected following stress exposure. Therefore, higher cortisol levels were expected after the stress task (post-task) compared to baseline (pre-stress). However, group cortisol levels did not increase following the stress task in the present study. In fact, cortisol levels were lower after the stress task than at baseline. This decrease in cortisol levels was somewhat unexpected. However, prior psychosocial stress studies completed during fMRI have often found that cortisol levels often fail to increase post-stress (Allendorfer et al. 2014; Dedovic et al., 2009; Gossett et al., 2018). Prior work suggests that the failure to find an increase in cortisol post-stress may be due to elevated baseline cortisol levels in anticipation of the fMRI scan (Muehlhan et al., 2011; Gossett et al., 2018). As a result, cortisol levels may be elevated at baseline, making it appear as though the fMRI task did not elicit a stress response. In addition, cortisol levels fluctuate diurnally with lower basal cortisol levels in the afternoon. In the present study, stress tasks were performed in both the morning and the afternoon due to participant and scanner availability, which potentially impacted cortisol data. However, including the time of day of sample collection did not alter the pattern of results. In addition, self-reported stress levels were determined from a survey that participants completed outside of the scanner after the conclusion of the MIST. Collecting self-report ratings outside the scanner is common (Luettgau et al., 2018 Schechter et al., 2012) and the pattern of self-reported stress results were consistent with other measures of stress (i.e. SCR and heart rate) collected for this study.
Conclusion
Prolonged stress has profound negative effects on the brain which can result in the development of anxiety and depression. A better understanding of the mechanisms that underlie the stress response is crucial to learning how stress-related disorders may develop. In the present study, we investigated individual differences in the emotional and neural response to stress. We observed increased activity within the amygdala, vmPFC, dmPFC, and dlPFC that was linked to changes in SCR and self-reported stress. These findings suggest the PFC and amygdala play important roles in the peripheral emotional response and may underlie individual variability in the emotional response to stress.
Supplementary Material
Acknowledgements:
This research was supported by the National Institutes of Health (grant number MH098348 to SM & DCK).
Footnotes
Disclosure Statement: In the interest of full disclosure we note that DAG is founder and chief Scientific and Strategy Advisor at Salimetrics LLC and Salivabio LLC. The nature of these relationships in managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine.
References
- Albert Kimberly, Pruessner Jens, & Newhouse Paul. (2015). Estradiol Levels Modulate Brain Activity and Negative Responses to Psychosocial Stress across the Menstrual Cycle. Psychoneuroendocrinology, 59, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer JB, Heyse H, Mendoza L, Nelson EB, Eliassen JC, Storrs JM, & Szaflarski JP (2014). Physiologic and cortical response to acute psychosocial stress in left temporal lobe epilepsy — A pilot cross-sectional fMRI study. Epilepsy & Behavior, 36, 115–123. [DOI] [PubMed] [Google Scholar]
- Arasaratnam Punitha, Sadreddini Masoud, Yam Yeung, Kansal Vinay, Dorbala Sharmila, Di Carli Marcelo F., Beanlands Rob S., Merhige Michael E., Williams Brent A., Veledar Emir, Min James K., Chen Li, Ruddy Terrence D., Germano Guido, Berman Daniel S., Shaw Leslee J., & Chow Benjamin J. W. (2018). Prognostic value of vasodilator response using rubidium-82 positron emission tomography myocardial perfusion imaging in patients with coronary artery disease. European Journal of Nuclear Medicine and Molecular Imaging, 45(4), 538–548. [DOI] [PubMed] [Google Scholar]
- Bach DR, Flandin G, Friston KJ, & Dolan RJ (2009). Time-series analysis for rapid event-related skin conductance responses. Journal of Neuroscience Methods, 184(2), 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Friston KJ, & Dolan RJ (2013). An improved algorithm for model-based analysis of evoked skin conductance responses(). Biological Psychology, 94(3), 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U, Stelzel C, & Fiebach CJ (2012). Trait anxiety and the neural efficiency of manipulation in working memory. Cognitive, Affective & Behavioral Neuroscience, 12(3), 571–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, & Helmstetter FJ (2006). Human amygdala activity during the expression of fear responses. Behavioral Neuroscience, 120(6), 1187–1195. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, & Helmstetter FJ (2003). Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience, 117(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Chou P-H, Lin W-H, Hung C-A, Chang C-C, Li W-R, Lan T-H, & Huang M-W (2016). Perceived Occupational Stress is associated with Decreased Cortical Activity of the Prefrontal Cortex: A Multichannel Near-infrared Spectroscopy Study. Scientific Reports, 6, 39089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP and Gold PW (1992) The Concepts of Stress and Stress System Disorders: Overview of Physical and Behavioral Homeostasis. Journal of the American Medical Association, 267, 1244–1252. [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2002). Book Review: Electrodermal Responses: What Happens in the Brain. The Neuroscientist, 8(2), 132–142. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, & Dolan RJ (2001). Neural Activity in the Human Brain Relating to Uncertainty and Arousal during Anticipation. Neuron, 29(2), 537–545. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. (1990) Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 14;41(2), 81–94 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, & Pruessner JC (2005). The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience, 30(5), 319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, & Pruessner JC (2009). Neural correlates of processing stressful information: An event-related fMRI study. Brain Research, 1293, 49–60. [DOI] [PubMed] [Google Scholar]
- Fechir M, Gamer M, Blasius I, Bauermann T, Breimhorst M, Schlindwein P, Schlereth T, & Birklein F (2010). Functional imaging of sympathetic activation during mental stress. NeuroImage, 50(2), 847–854. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Warner T-A, Brown J, Salway P, Gabbott T, & Busby S (2012). Amygdala afferents monosynaptically innervate corticospinal neurons in rat medial prefrontal cortex. The Journal of Comparative Neurology, 520(11), 2440–2458. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, & Busby SJ (2005). Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. The Journal of Comparative Neurology, 492(2), 145–177. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage, 34(3), 905–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Wheelock MD, Harnett NG, Mrug S, Granger DA, & Knight DC (2016). The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience, 339, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett EW, Wheelock MD, Goodman AM, Orem TR, Harnett NG, Wood KH, Mrug S, Knight DC (2018). Anticipatory stress associated with functional magnetic resonance imaging: Implications for psychosocial stress research. International Journal of Psychophysiology, 125, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS (1989). Autonomic Neuropeptide Connections of the Amygdala In Taché Y, Morley JE & Brown MR (Eds.), Neuropeptides and Stress (pp. 92–106). New York, NY: Springer New York. [Google Scholar]
- Hartley CA, & Phelps EA (2012). Anxiety and Decision-Making. Biological Psychiatry, 72(2), 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek MM, Bolliger B, Roos S, Pryce CR, Quednow BB, & Seifritz E (2016). Uncontrollable and unpredictable stress interacts with subclinical depression and anxiety scores in determining anxiety response. Stress, 19(1), 53–62. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Devinsky O, Szczepanska H, Borod JC, Marthol H, & Tutaj M (2006). Right ventromedial prefrontal lesions result in paradoxical cardiovascular activation with emotional stimuli. Brain, 129(12), 3343–3355. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, & Haselton JR (1979). Amygdala central nucleus lesions: Effect on heart rate conditioning in the rabbit. Physiology & Behavior, 23(6), 1109–1117. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, & Prescott CA (1999). Causal Relationship Between Stressful Life Events and the Onset of Major Depression. American Journal of Psychiatry, 156(6), 837–841. [DOI] [PubMed] [Google Scholar]
- Kim MJ, & Whalen PJ (2009). The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(37), 11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, & Bandettini PA (2005). The role of the human amygdala in the production of conditioned fear responses. NeuroImage, 26(4), 1193–1200. [DOI] [PubMed] [Google Scholar]
- LeDoux J, Iwata J, Cicchetti P, & Reis D (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience, 8(7), 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luettgau L, Schlagenhauf F, & Sjoerds Z (2018). Acute and past subjective stress influence working memory and related neural substrates. Psychoneuroendocrinology, 96, 25–34. [DOI] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, et al. (2012) Clarifying the Role of the Rostral dmPFC/dACC in Fear/Anxiety: Learning, Appraisal or Expression?. PLOS ONE 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka Y, Hirasawa K, Yamane F, Hori T, & Homma I (2003). Effects of Left Amygdala Lesions on Respiration, Skin Conductance, Heart Rate, Anxiety, and Activity of the Right Amygdala During Anticipation of Negative Stimulus. Behavior Modification, 27(5), 607–619. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and Allostasis-Induced Brain Plasticity. Annual Review of Medicine, 62, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, & Rauch SL (2007). Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry, 62(5), 446–454. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen H-U, & Kirschbaum C (2011). The scanner as a stressor: Evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. International Journal of Psychophysiology, 79(2), 118–126. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, & Koenigs M (2012). Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Molecular Psychiatry, 17(2), 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, & Dolan RJ (2004). Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. NeuroImage, 22(1), 243–251. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, & Gross JJ (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23(2), 483–499. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, & Tancer ME (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57(3), 210–219. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, & LeDoux JE (2004). Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron, 43(6), 897–905. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, & Lupien S (2008). Deactivation of the Limbic System During Acute Psychosocial Stress: Evidence from Positron Emission Tomography and Functional Magnetic Resonance Imaging Studies. Biological Psychiatry, 63(2), 234–240. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J. Amiel, Moore Holly, & Grace Anthony A. (2003). The Prefrontal Cortex Regulates Lateral Amygdala Neuronal Plasticity and Responses to Previously Conditioned Stimuli. The Journal of Neuroscience, 23(35), 11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, & Peterson BS (2012). An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Social Cognitive and Affective Neuroscience, 7(8), 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber J, Kapp B, Higgins G, & Rapp P (1982). Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. The Journal of Neuroscience, 2(10), 1424–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Drevets WC, Snyder AZ, Gusnard DA, & Raichle ME (2001). Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, & Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Fukui Y, Ichiyama M, Miyoshi S, & Nishimura Y (1991). Direct amygdaloid projections to the superior salivatory nucleus: a light and electron microscopic study in the cat. Brain Research Bulletin, 27(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Tanji J, & Hoshi E (2008). Role of the Lateral Prefrontal Cortex in Executive Behavioral Control. Physiological Reviews, 88(1), 37. [DOI] [PubMed] [Google Scholar]
- Taren AA, Venkatraman V, & Huettel SA (2011). A parallel functional topography between medial and lateral prefrontal cortex: Evidence and implications for cognitive control. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(13), 5026–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, & Davidson RJ (2006). Amygdala and Ventromedial Prefrontal Cortex Are Inversely Coupled during Regulation of Negative Affect and Predict the Diurnal Pattern of Cortisol Secretion among Older Adults. The Journal of Neuroscience, 26(16), 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, & Detre JA (2005). Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America, 102(49), 17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Rangaprakash D, Harnett NG, Wood KH, Orem TR, Mrug S, Granger DA, Deshpande G, & Knight DC (In Press). Psychosocial stress reactivity is associated with decreased whole brain network efficiency and increased amygdala centrality. Behavioral Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Harnett NG, Wood KH, Orem TR, Granger DA, Mrug S, & Knight DC (2016). Prefrontal Cortex Activity Is Associated with Biobehavioral Components of the Stress Response. Frontiers in Human Neuroscience, 10(583). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, & Knight DC (2014). The Amygdala Mediates the Emotional Modulation of Threat-Elicited Skin Conductance Response. Emotion (Washington, D.C.), 14(4), 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.