Abstract
Chemotherapy is currently the major therapeutic method against cancer. However, chemo drugs are usually lacking in the specificity towards cancer cells over normal cells. In this study, we prepared a novel multifunctional trimeric prodrug by linking the chemo drug camptothecin (CPT) and the exceptional photostable near infrared (NIR) croconaine dye (CR) via a glutathione (GSH)-sensitive disulfide linker. Compared with CPT, our novel trimeric CR-(SS-CPT)2 is more hydrophobic and bulky, making it highly effcient to be encapsulated into biocompatible polymeric nanocarrier. The prodrug underwent rapid drug release specifically in cancer cells with high GSH concentration. The hyperthermia produced by CR upon laser irradiation could further accelerate the break of disulfide bond, which makes the release of CPT even more controllable. We further loaded the CR-(SS-CPT)2 into folate modified lipid-polymer nanoparticles, which demonstrated high in vivo tumor accumulation and retention. The biodistribution of these nanoparticles can be directly monitored in real time via NIR fluorescence and photoacoustic imaging. Under the imaging guided chemo- and photothermal- synergistic therapy, the tumors were completely ablated with no recurrence. The design not only highly enhanced the therapeutic specificity and effeciency of CPT, but also provide a “all in one” nanomedicine for imaging-guided dual modality therapy.
Graphical Abstract
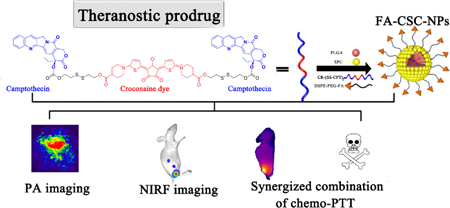
Glutathione-responsive trimeric prodrug with 99% encapsulation efficiency into targeted polymeric nanoparticles is designed for imaging-guided dual chemophotothermal therapy
Introduction
Cancer is currently one of the most lethal disease in global healthcare.1 Over the past several decades, chemotherapy remains a major therapeutic method against cancer.2 However, a single chemotherapy treatment faces the challenge of tremendous side effects due to the nonspecific toxicity of chemotherapeutic drugs to both cancer cells and normal cells, as well as the drug resistance at the high dosage.3 Nanosized drug delivery systems (DDS) are of great interest since they can alter the biodistribution of drugs to achieve better tumor targeting efficiency.4–6 The prodrug strategy,7–10which is referred to chemically modifying drugs in order to keep them inactive until be stimulated by some metabolic process, is another significant way to optimize the distribution and pharmacokinetics of chemotherapeutic agents for better therapeutic effect.
Recently, near-infrared (NIR) photothermal therapy (PTT) has emerged as a new therapeutic method for cancer.11 Upon the laser irradiation, the heat generated by photothermal conversion agents (PTCAs) can selectively ablate the tumor.12 Various nanoagents have been explored as PTCAs for antitumor therapy.13–16 Since there are two “selective” steps: the tumor targeting of PTCAs and the localized exposure to the light, PTT demonstrated high cancer-therapeutic specificity and reduced side effects. Combining NIR PTT and chemotherapy in a synergistic way can improve the therapeutic efficacy, where PTT could selectively remove the majority of tumor cell with minimal damage to normal tissues, while the chemotherapeutic agents could further eradicate hard-to-reach residual and migrated tumor cells. Although many nanoplatforms have been designed for combinational therapy,14, 17, 18 most of them are based on inorganic nanoparticles, facing the difficulty of entering into clinical trials. Several heterodimeric multifunctional prodrugs have also been studied,19–21 however, they usually suffer from insufficient tumor accumulation and instability under laser irradiation.
In this study, we designed a glutathione (GSH)-responsive multifunctional theranostic prodrug CR-(SS-CPT)2, where two molecules of camptothecin were linked together by a croconaine dye (CR) via disulfide bonds (Scheme 1). The camptothecin (CPT) is a traditional class of chemotherapeutic agents for cancer therapy by topoisomerase inhibiting, but failed in clinical trials due to its limited solubility and serious side effect.22 CR, namely 2,5-bis[(4-carboxylicpiperidylamino)thiophenyl]-croconine, is a NIR fluorescence dyeand have recently been proved as an excellent NIR PTCAs owing to high photothermal conversion efficiency and excellent photostability.23 Compared with CPT, our symmetric CR-(SS-CPT)2 is of higher molecular weight and more hydrophobic, resulting in significantly improved drug loading into nanocarriers and fewer premature drug release. These disulfide-linked prodrugs are stable in blood, but undergo rapid drug release in presence of high concentration of GSH inside cancer cells.24 Upon laser irradiation, photo-hyperthermia produced by CR could further accelerate the break of disulfide bonds, which makes the release of CPT highly controllable. To the best of our knowledge, this is the first reported theranostic prodrug, in which an ultrastable NIR PTCA was innovatively used to link two chemotherapeutic CPT together to afford a dual heat- and GSH- responsive trimetric prodrug for improved drug release controllability. The obtained prodrugs have the multi-functionality of diagnosis (photoacoustic imaging and NIR fluorescence imaging), as well as therapy (chemotherapy and photothermal therapy). We further loaded them into folate modified polylactic-co-glycolic acid hybrid nanoparticles (FA-PLGA NPs) for better solubility and tumor targeting. The as-prepared 100 nm FA-CSC-NPs demonstrated great in vivo tumor accumulation and retention ability based on both passive targeting (EPR effects) and active targeting of folate. The intrinsic optical properties of CR provide a non-invasive way to directly monitor the pharmacokinetics and the biodistribution of the trimeric prodrug in real time and further guide the following photothermal therapy. The chemophotothermal synergistic therapy of FA-CSC-NPs completely abated the tumor and prevented potential tumor recurrence in vivo (Scheme 1). We believe that this FA-CSC-NPs design highly enhanced the specificity of CPT chemotherapy, and is a promising “all in one” nanomedicine for imaging-guided dual-modality therapy.
Scheme 1.
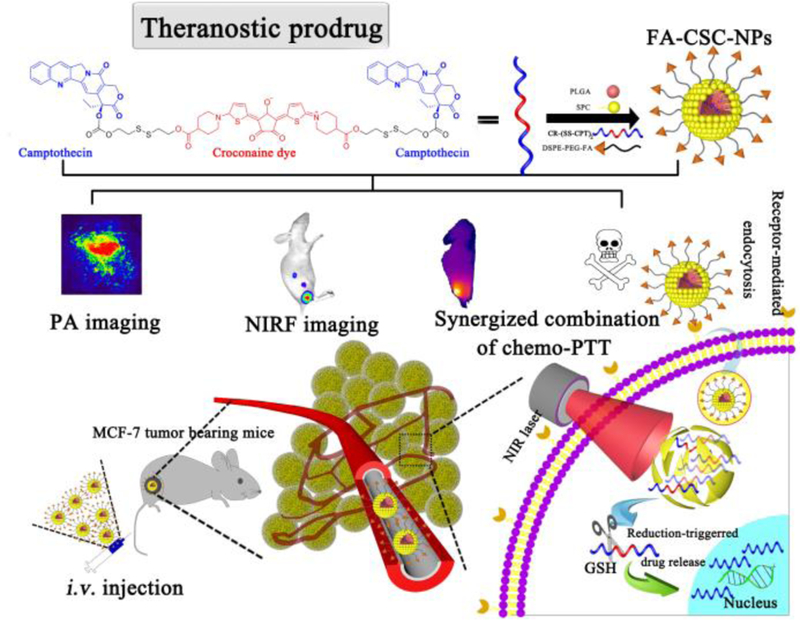
Schematic illustration of FA-CSC-NPs and their theranostic application (CSC: CR-(SS-CPT)2; PLGA: poly lactide-co-glycolide; SPC: soybean phosphatidylcholine; DSPE-PEG-FA: 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate (polyethylene glycol)-2000].).
Results and discussion
The synthetic route of prodrug CR-(SS-CPT)2 is depicted in Fig. S1 CR was synthesized based on a reported method,23 and then conjugated with CPT-SS-OH25 to afford CR-(SS-CPT)2 via esterification reactions. The 1H NMR (Fig.S3–4) and LC/MS (Fig. S5) confirmed that two molecules of camptothecin were linked to one CR in a symmetric way. The UV−Vis spectra (Fig. 1C) have shown that CR-(SS-CPT)2 maintained the major absorption peaks of both CPT and CR qualitatively and quantitatively.
Fig. 1.
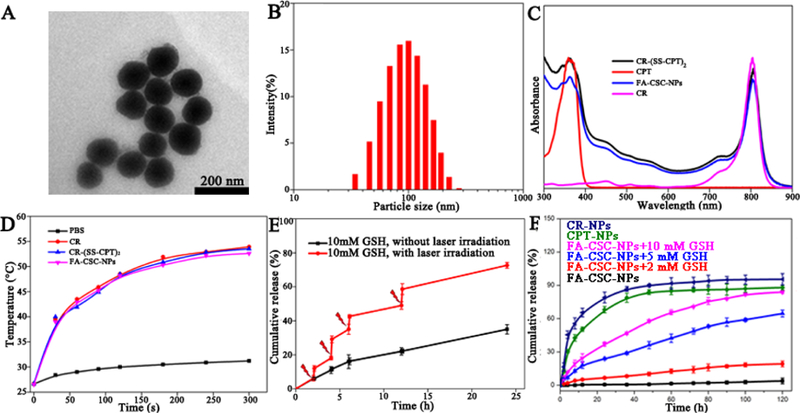
(A) TEM images and (B) DLS characterization of FA-CSC-NPs. (C) UV–Vis absorption spectra of CPT,CR-(SS-CPT)2, CR, and FA-CSC-NPs. (D) The temperature profiles of FA-CSC-NPs, CR-(SS-CPT)2, CR and PBS upon the continuous laser irradiation. (E) NIR-triggered release of CPT from FA-CSC-NPs. The samples were irradiated with an NIR laser (1 W/cm2) for 5 min at different time points as indicated by the arrows. Error bars were based on at least triplicated measurements. (F) In vitro drug release from FA-CSC-NPs under different GSH concentration versus drug or dye release from CPT-NPs and CR-NPs.
The CR-(SS-CPT)2 were then loaded into lipid-polymer hybrid nanovehicles following a modified solvent evaporation method.26 As shown in scheme 1, the final product FA-CSC-NPs had PLGA as the hydrophobic core to load CR-(SS-CPT)2, lipid monolayer composed of soybean phosphatidylcholine(SPC)and 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine(DSPE) as the biomembrane mimetic to increase the cellular internalization, DSPE-PEG to increase blood circulation time, and FA to serve as targeting ligands. All carrier reagents had been approved by the Food and Drug Administration (FDA) for clinical use. It’s worth to mention that CPT is well-known as a hard-to-formulate anticancer drug27 due to their low solubility in both hydrophilic and hydrophobic solvents, compared with which, our CR-(SS-CPT)2 prodrug is more hydrophobic and soluble in most organic solvents, such as tetrahydrofuran and methanol. As a result, the simple solvent evaporation method led to an impressive drug encapsulation efficiency (EE) of 99%, with a drug loading capacity of 16%. In contrast, both CPT and CR had very low EEs (CPT: 15%, CR: 21%) and low loading capacities (CPT: 2.9%; CR: 4%). The increased EE and drug loading capacity demonstrated the significant advantages in nanoformulation of our rationally-designed trimeric prodrug CR-(SS-CPT)2.
The as-prepared NPs were spherical ones with size around 121±55 nm based on the calculation of at least 100 nanoparticles. The representative transmission electron microscopy (TEM) image and scanning electron microscopy(SEM) image are shown in Figure 1A and Figure S8, respectively. Dynamic light scattering (DLS) further demonstrated that these NPs have an overall hydrodynamic size around 102 ± 9 nm (Fig. 1B), which is suitable forthe EPR effect. With a zeta potential around −40 ± 4 mV (Fig. S7), these FA-CSC-NPs maintained excellent stability without any precipitation or phase separation when incubating in PBS or 10% (V/V) plasma/heparin in PBS at 37 °C for 5 d (Fig. S9). We further investigated the photothermal effect of FA-CSC-NPs. CR is reported to have superior photostability over most commercial NIR organic dyes such as ICG (Fig. S11) and IR806.28Both CR-(SS-CPT)2 and FA-CSC-NPs maintained the optical properties and photothermal properties of CR with a photothermal conversion efficiency (η) around 54.3%23(Fig. 1D),comparable with most photothermal agents reported29–31. Upon irradiation with NIR laser (808 nm) at a power density of 1 W/cm2, CR-(SS-CPT)2andFA-CSC-NPs displayed a quick increase of temperature from 28 °C to 53 °C within 300 s (Fig. 1D, S10) and no obvious change in Vis-NIR spectra even under irradiation for 10 min (Fig. S11), similar to the behaviour of CR at the same concentration.
The release experiment was then performed. We first tested the GSH sensitive drug release from FA-CSC-NPs. We incubated the NPs with GSH of different concentrations ranged from 0–10 mM. As expected, both CPT and CR release from the lipid-polymer hybrid nanovehicles were quite fast, with release half life of 10.9 h and 6.3 h, respectively(Fig. 1F). The premature release of CPT during bloodcirculation could potentially cause serious side effects. On the other hand, our CR-(SS-CPT)2 could stay inside the nanovehicles with negligiable drug release in PBS, likely due to its increased molecularweight and lower water solubility. Furthermore, in the presence of GSH,the disulfide could be cleaved,28 resulting in the release of CPT from nanovehicles after a cascaded reaction (Fig. S2). At 2 mM of GSH, the release of CPT is slow with a 19 % release within 120 h. As the concentration of GSH increased, the release rate of CPT from FA-CSC-NPs increased, with ca. 65.5% and 84.7% of CPT released at 5 and 10 mM GSH, respectively. Since GSH tends to be elevated in tumors compared with disease-free tissue,32 it is assumed that the disulfide-linked prodrugs could be relatively stable with little premature drug release in plasma during blood circulation, but undergo rapid drug release in cancer cells. Notably, a triggered burst release of CPT from FA-CSC-NPs was observed upon NIR laser irradiation (808 nm, 1 W/cm2, 5 min)(Fig. 1E). It is believed that the CR component could convert the light to heat which accelerate the drug difusion out of nanovehicles and increased subsequent cleavage rate of disulfide by GSH, leading to the enhanced CPT release. Due to the ultrahigh photostability of CR, the laser triggered release was repeatable. We applied the irradiation several times during the release process (at 2 h, 4 h, 6 h, 12 h respectively), and each time around 10% CPT was released within 15 min. Upon removal of laser irradiation, the CPT release rate restored the same as before. Thus, it is possible to precisely tune the release of CPT by laser irradiation, based on the treatment needs. Overall, we proved that the CR-(SS-CPT)2 had a negligible release from the FA-CSC-NPs in absence of GSH. The cleavage of disufide linkage was triggered by GSH and accelerated by laser irradiation, resulting in the controlled release of CPT from nanovehicle. This dual responisve drug release system will greatly limit the premature drug release in normal tissues leading to reduced the side effect, but promote the release in the tumor tisstues to specifically kill cancer cells.
The cellular internalization behavior of CR-(SS-CPT)2, CSC-NPs and FA-CSC-NPs were evaluated through confocal microscopy using folate receptor expressing MCF-7 cells. Since both CPT (excited at 372 nm, emit at 434 nm) and CR (excited at 395 nm, emit at 780 nm) were fluorescent, no additional fluorescent labeling is required for tracking. As shown in Fig. 2A, lipid-polymer nanovehicle help enhance the cellular internalization of CR(SS-CPT)2 prodrugs. Targeted FA-CSC-NPs entered into cancer cells much more efficiently than non-targeted CSC-NPs. Blocking experiment furthere proved the specificity of FA-CSC-NPs to MCF-7 cells via folate receptor. Similar trends were demonstrated by the flow cytometry analysis (Fig. 2B–C). Interestingly, CPT could be detected both in the cytosol and the nucleus while the signal of CR could only be found in cytosol (Fig. 2A), which confirmed the seperation of CR and CPT and indicated the successfully break of disulfide bond inside MCF-7 cells.
Fig.2.
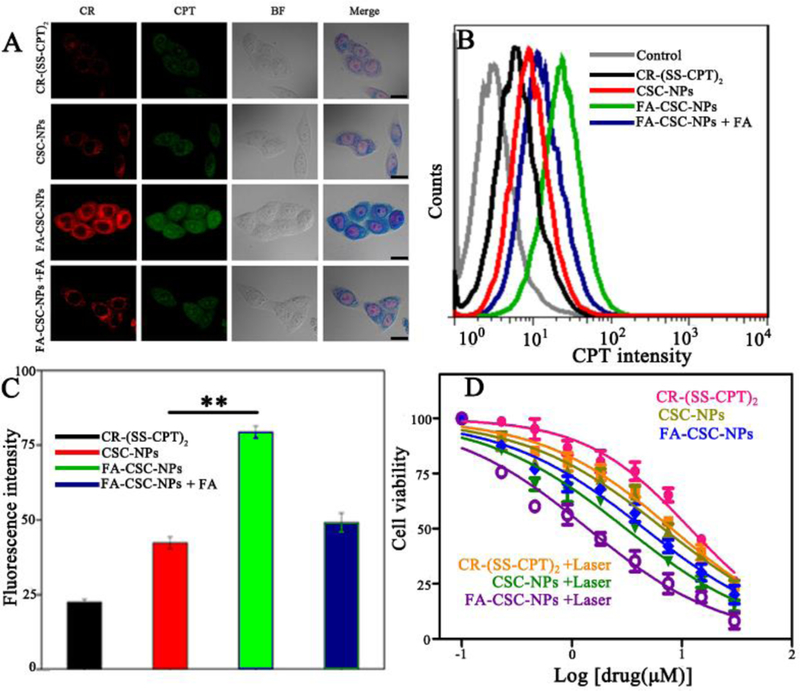
(A) Confocal images of MCF-7 cells incubated with free CR-(SS-CPT)2, CSC-NPs, FA-CSC-NPs and FA-CSC-NPs with FA blocking for 2 h (scale bar = 20 μm). (B) Flow cytometry profiles and (C) Fluorescence measurements of MCF-7 cells incubated with free CR-(SS-CPT)2, CSC-NPs, FA-CSC-NPs and FA-CSC-NPs +FA blocking for 2 h. (D) Cell viability of MCF-7 cells treated with free CR-(SS-CPT)2, CSC-NPs, and FA-CSC-NPs with/without 808-nm laser irradiatin (1 W/cm2, 5 min). *P< 0.05, **P< 0.01.
We then examined the cytotoxic effects of FA-CSC-NPs against MCF-7 cells (Fig. 2D). The CR-(SS-CPT)2 had a IC50 around 11.82 μM, much less toxic than CPT due to the chemical modification.25 Upon laser irradiation at 1.0 W/cm2for 5 min time, the IC50 values of CR-(SS-CPT)2 was decreased to 8.03 μM as a result of photothermal effect(Fig. S13). Due to the enhanced cellular internalization of prodrugs, CSC-NPs and targeted FA-CSC-NPs had much smaller IC50 both without laser irradiation (6.74 μM and 4.47 μM), and under laser irradiation (2.98μM and 1.25 μM). The cytotoxic effect was further confirmed by calcein AM/propidium iodide (PI) staining assay (Fig.S12). Nearly all the cells were dead when treated with FA-CSC-NPs at a concentration of 10 μM and then exposed to laser. Overall, the superior cytotoxic effects of FA-CSC-NPs was contributed to the enhanced cellular internalization and intracellular translocation of CPT, GSH and laser irradiation dual responsive drug release as well as the synergism of hemophotothermal therapy. It’s worth noting that, our FA-CSC-NPs exhibited few cellular internalization (Fig. S14)and dramastically decreased cytotoxicity (Fig. S15) on MCF-10A cells (a normal breast cells), indicating its biosafety against the healthy cells.
We then investigated the biodistribution of FA-CSC-NPs on MCF-7 breast tumor-bearing BALB/c nude mice by NIR fluorescence (NIRF) and photoacoustic (PA) imaging, making use of the intrinsic optical properties of CR. The FA-CSC-NPs showed a blood half-life around 4.17 ± 0.15h (Fig. S16, Table 11). As shown in the real-time fluorescence imaging (Fig. 3A–B), the NPs were gradually accumulated in the tumor and reticuloendothelial system (RES) within 12 h, and decreased at 24 h due to the metabolism. Because of the FA receptor-mediated specific targeting, nearly twice FA-CSC-NPs accumulated in the tumor region than CSC-NPs at equivalent doses 12 h post-injection. PA imaging result was consistent with PL result, but provided much detailed information on tumor microstructure.33 From Fig. 3C–D, we observed that FA-CSC-NPs spread all over the tumor microvessels, distributed more widely and relatively static in the tumor area than CSC-NPs. In addition, ex vivo imaging further confirmed the tissue distribution (Fig. 3E-F). Compared with CSC-NPs, FA-CSC-NPs had significantly higher tumor but lower liver signals.
Fig.3.

In vivo NIRF/PA dual-modal imaging and biodistribution of FA-CSC-NPs in MCF-7 tumor-bearing nude mice. (A) In vivo NIRF imaging and (B) quantitative analysis of the tumor areas at 1.5, 3, 6, 12, and 24 h post-injection of CSC-NPs or FA-CSC-NPs. (C) PA imaging and (D) quantitative analysis of tumor areas in MCF-7 tumor-bearing nude mice at 1.5, 3, 6, 12, and 24 h post- injection of CSC-NPs or FA-CSC-NPs (E) Ex vivo fluorescence images and (F) quantitative analysis of tissues at 12 h post-injection of CSC-NPs or FA-CSC-NPs.. *P< 0.05, **P< 0.01.
Finally, we studied the in vivo chemo- and photothermal therapeutic effect of FA-CSC-NPs. Based on the NIRF and PA imaging results, 12 h post-injection was chosen as the time point for photothermal therapy when the tumor accumulation of nanovehicles was relatively high. The tumor areas were irradiated with NIR laser (808 nm, 1 W/cm2, 5 min), and the real-time temperature variations were recorded by the IR thermal camera (Fig. 4A–B). The group injected with PBS had a negligible increase less than 3 °C. For mice injected with CR-(SS-CPT)2, CSC-NPs and FA-CSC-NPs, the tumor temperature had obviously increased to different extents under the same laser irradiation condition. The group treated with FA-CSC-NPs demonstrated the highest temperature increase up to 54.6 °C while the group treated with CR-(SS-CPT)2 and CSC-NPs increase to 43.6 °C and 50.7 °C, respectively. Since all the three CR-containing agents showed the same photothermal efficiency in phantom, the difference in the in vivo temperature raise should be ascribed to their different tumor accumulation. We then monitored the tumor growth over 2 weeks. As shown in (Fig. 4C–D), the rationally designed trimeric prodrug, capable of dual chemo- and photothermal therapy, significantly inhibited tumor growth after laser irradiation, when compared with the group treated with PBS. Due to high tumor accumulation, the FA-CSC-NPs displayed best therapeutic efficacy, where tumor were completely eliminated with no morbidity or tumor reoccurrence within 14 days (Fig.S19, S20). On the other hand, non-targeted CSC-NPs with laser treatment also showed excellent tumor inhibition, yet significantly inferior to targeted FA-CSC-NPs, demonstrating the advantages of FA targeting. Worth noting, CR-(SS-CPT)2 showed moderate antitumor activities after laser irradiation as well. In addition, neither significant body weight change (Fig. S17) nor obvious damage as determined by the H&E-staining test (Fig. S18) were observed in all treatment groups. We further tested the safety of FA-CSC-NPs by injecting mice with a 5-fold therapeutic dose (25 mg/kg). The mice were found to behave normally, and no significant body weight change was found, indicating a promising wide therapeutic window. All above results indicates that the FA-CSC-NPs are promising theranostic agents capable of multi modalities imaging and dual chemophotothermal therapy.
Fig. 4.
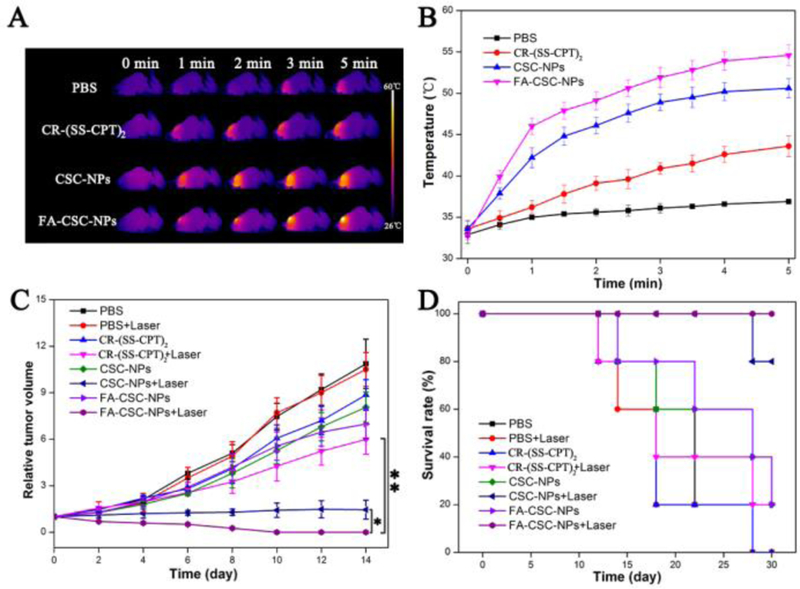
(A) Infrared thermal images and (B) time-dependent tumor temperature increase of MCF-7 tumor-bearing nude mice irradiated by 808-nm laser (1 W/cm2) at 12 h after i.v. injection of PBS, free CR-(SS-CPT)2, CSC-NPs, and FA-CSC-NPs. (C) Tumor growth inhibition profiles. (D) Survival curve of the mice injected with PBS, free CR-(SS-CPT)2, CSC-NPs, and FA-CSC-NPs with/without 808-nm laser irradiation (1 W/cm2, 5 min). *P < 0.05, **P < 0.01.
Conclusions
In summary, we have successfully constructed a novel NIR theranostic trimeric prodrug CR-(SS-CPT)2 by linking two camptothecin molecules and one croconaine dye molecule via GSH-responsive disulfide bonds. Compared with CPT, our symmetric prodrugs is much easier for nanoformulation with an encapsulation efficiency of 99% and a drug loading capacity of16%. The obtained nanoparticles were stable with negligible size changes over several days. Few premature release of CPT was obsereved in PBS from FA-CSC-NPs, which is highly desirable for increased tumor accumulation and reduced side effect. The CPT release was specifically triggered by GSH inside tumor cells, and precisely tuned by the laser irradiation. These FA-CSC-NPs demonstrated high tumor targeting capability. Under the NIR fluorescence and photoacoustic imaging guided chemo-photothermal synergistic therapy, the tumor were effectively ablated with no recurrence. Our design highly improves the bioavailability and tumor-specificity of CPT, and enables new diagnositc functionalities to assist chemotherapy. We believe these “all in one” prodrugs will open more opportunities for clinically cancer theranostics.
Supplementary Material
Acknowledgements
This work was supported by National Key Research and Development Program of China (2016YFA0203600), National Natural Science Foundation of China (51502251, 81571743), the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZRC05). This work is also supported by the Intramural Research Program of NIBIB, NIH.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Notes and references
- 1.Zhang Y, Leonard M, Shu Y, Yang Y, Shu D, Guo P and Zhang X, ACS Nano, 2017, 11, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR and Investigators K-., N. Engl. J. Med, 2016, 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- 3.Denzi A, Della Valle E, Apollonio F, Breton M, Mir LM and Liberti M, J. Membr. Biol, 2017, 250, 31–40. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, He N, Chen M, Zhao L and Li X, Acta. Biomater, 2016, 43, 195–207. [DOI] [PubMed] [Google Scholar]
- 5.Su H, Zhang P, Cheetham AG, Koo JM, Lin R, Masood A, Schiapparelli P, Quinones-Hinojosa A and Cui H, Theranostics, 2016, 6, 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheetham AG, Zhang P, Lin YA, Lock LL and Cui H, J Am Chem Soc, 2013, 135, 2907–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Sun X, Guo Z, Tang J, Shen Y, James TD, Tian H and Zhu W, J. Am. Chem. Soc, 2014, 136, 3579–3588. [DOI] [PubMed] [Google Scholar]
- 8.Su L, Li R, Khan S, Clanton R, Zhang F, Lin YN, Song Y, Wang H, Fan J, Hernandez S, Butters AS, Akabani G, MacLoughlin R, Smolen J and Wooley KL, J. Am. Chem. Soc, 2018, 140, 1438–1446. [DOI] [PubMed] [Google Scholar]
- 9.Huang P, Wang D, Su Y, Huang W, Zhou Y, Cui D, Zhu X and Yan D, J. Am. Chem. Soc, 2014, 136, 11748–11756. [DOI] [PubMed] [Google Scholar]
- 10.Cai K, He X, Song Z, Yin Q, Zhang Y, Uckun FM, Jiang C and Cheng J, J. Am. Chem. Soc, 2015, 137, 3458–3461. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Xu J, Yan D, Zhang P, Zeng F, Li B and Wu S, Chem. Commun. (Camb), 2015, 51, 9567–9570. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Han J, Lim HJ, Ren WX, Lim JY, Kim JH and Kim JS, J. Am. Chem. Soc, 2014, 136, 17836–17843. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Chen Y, Guo W, Gao Y, Song C, Zhang Q, Zheng N, Han X and Guo C, Adv. Funct. Mater, 2018, 28, 1706827 [Google Scholar]
- 14.Guo W, Wang F, Ding D, Song C, Guo C and Liu S, Chem. Mater, 2017, 29, 9262–9274 [Google Scholar]
- 15.Guo W, Qiu Z, Guo C, Ding D, Li T, Wang F, Sun J, Zheng N and Liu S, ACS Appl. Mater. Interfaces, 2017, 9, 9348–9358. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Guo C, Guo W, Zhao X, Liu S and Han X, ACS Appl. Nano Mater, 2018, 1, 820–830. [Google Scholar]
- 17.Chen X, Zhang Q, Li J, Yang M, Zhao N and Xu FJ, ACS Nano, 2018, 12, 5646–5656. [DOI] [PubMed] [Google Scholar]
- 18.Bao T, Yin W, Zheng X, Zhang X, Yu J, Dong X, Yong Y, Gao F, Yan L and Gu Z, Biomaterials, 2016, 76, 11–24. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Ni Q, Jacobson O, Cheng S, Liao A, Wang Z, He Z, Yu G, Song J and Ma Y, Angew. Chem. Int. Ed, 2018. 57, 7066–7070. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Sun Y, Hung R, Cang H, Cai Z and Sun B, Eur. J. Pharm. Biopharm, 2018, 128, 260–271. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Zhai S, Qin H, Yan H, Xing D and Hu X, Biomaterials, 2018, 176, 1–12. [DOI] [PubMed] [Google Scholar]
- 22.Lee DE, Koo H, Sun IC, Ryu JH, Kim K and Kwon IC, Chem. Soc. Rev, 2012, 41, 2656–2672. [DOI] [PubMed] [Google Scholar]
- 23.Tang L, Zhang F, Yu F, Sun W, Song M, Chen X, Zhang X and Sun X, Biomaterials, 2017, 129, 28–36. [DOI] [PubMed] [Google Scholar]
- 24.Zheng M, Zhao P, Luo Z, Gong P, Zheng C, Zhang P, Yue C, Gao D, Ma Y and Cai L, ACS Appl. Mater. Interfaces, 2014, 6, 6709–6716. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Zhu G, Jacobson O, Liu Y, Chen K, Yu G, Ni Q, Fan J, Yang Z, Xu F, Fu X, Wang Z, Ma Y, Niu G, Zhao X and Chen X, ACS Nano, 2017, 11, 8838–8848. [DOI] [PubMed] [Google Scholar]
- 26.Hadinoto K, Sundaresan A and Cheow WS, Eur. J. Pharm. Biopharm, 2013, 85, 427–443. [DOI] [PubMed] [Google Scholar]
- 27.Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG and Ithakissios DS, J. Control. Release, 2002, 79, 123–135. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Piao Y and Hyeon T, Chem. Soc. Rev, 2009, 38, 372–390. [DOI] [PubMed] [Google Scholar]
- 29.Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu M, Yang S, Wang J, Wang J and Hu J, ACS Nano, 2011, 5, 9761. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Li C, Xing B, Yang P and Lin J, J. Mater. Chem. B, 2016, 4, 4884–4894. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Guo W, Wei J, Li C, Liang XJ and Yin M, ACS Nano, 2017, 11, 3797–3805. [DOI] [PubMed] [Google Scholar]
- 32.Gupta N, Kancharla J, Kaushik S, Ansari A, Hossain S, Goyal R, Pandey M, Sivaccumar J, Hussain S, Sarkar A, Sengupta A, Mandal SK, Roy M and Sengupta S, Chem. Sci, 2017, 8, 2387–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhi X, Yang M, Zhang J, Lin L, Zhao X, Hou W, Zhang C, Zhang Q, Pan F, Alfranca G, Yang Y, de la Fuente JM, Ni J and Cui D, Theranostics, 2017, 7, 1650–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


