Abstract
Purpose: To compare the recovery of erections and potency following the transection of accessory pudendal arteries (APAs) in men undergoing robot-assisted radical prostatectomy (RARP) compared with men with normal vascular anatomy.
Materials and Methods: A total of 880 consecutive patients who underwent RARP from January 1, 2007 to December 31, 2014 were included with prospectively collected data in cross-sectional analysis. Erectile function (EF) was assessed preoperatively and postoperatively at 3, 6, 12, and 24 months using the International Index of Erectile Function (IIEF)-5, a percent erection fullness compared to preoperative status, and two Expanded Prostate Cancer Index (EPIC) questions: (1) are erections firm enough for penetration and (2) are they satisfactory?
Results: Two hundred thirty-one (33.1%) men had APAs transected. There were no significant differences in baseline demographics or clinical characteristics in men with or without APAs transected. Multivariate analyses demonstrated that age (confidence interval [95% CI]: 0.94, 0.99) and baseline IIEF-5 (95% CI: 1.15, 1.26) strongly correlated with recovery of erections and potency. Transection of APAs was not a significant predictor of erectile dysfunction (ED).
Conclusion: Good surgical technique dictates the preservation of APAs. However, when preservation is questioned, we found that APA transection had no measurable effect on recovery of erections or potency regardless of age, preoperative ED, or number of APAs transected.
Keywords: : redundant blood supply, robotic prostatectomy, accessory pudendal artery, postoperative sexual function, prostate cancer
Introduction
Radical prostatectomy (RP) represents a heterogeneous procedure with functional outcomes highly dependent on patient and provider determinants. Preservation of erectile function (EF) depends on patient preoperative characteristics, including baseline sexual function, age, as well as comorbidity.1,2 Moreover, technical factors such as surgeon skill and technique in nerve-sparing dealing primarily with avoiding inadvertent neurovascular resection, transection, and thermal or traction injury have been previously shown to improve functional outcomes.3 As an isolated issue, direct vascular injury is rarely discussed or cited.4
Polascik and Walsh were the first to publish a surgical series of vascular injuries and EF, reporting a 4% incidence of accessory pudendal arteries (APAs) among patients with efforts set forth at preserving APAs.5 They were unable to demonstrate any benefit from preservation of APAs and EF in that publication. In 2004, the same group reported a larger but similar series which noted a possible benefit in EF with preservation of APAs.6 Beginning in 2005, Guilloneau and associates reported a series of articles defining a laparoscopic surgical experience with APAs. They found an APA incidence of ∼30% and demonstrated two distinct variants of APAs: lateral APAs, which branch off the terminal branches of the hypogastric artery and course along the lateral aspect of the prostate superficial to the endopelvic fascia, and apical APAs, which originate from the external iliac or femoral artery and penetrate through the levator ani behind the endopelvic fascia in close proximity of the apex of the prostate.7–9 Since these initial publications, the analysis of APAs has been stagnant, with no reports addressing the controversy since 2012.
As an early adopter of robot-assisted RP (RARP), in June of 2002, our initial concern in the first 50 patients was perioperative patient safety and oncologic results, specifically regarding positive surgical margins.10 Beginning in 2003, we started and then subsequently published an approach that included defatting the anterior prostate and transecting the puboprostatic ligaments and stapling the dorsal vein complex (DVC).11 The defatting process exposed all lateral APAs, while incising the endopelvic fascia at the apex exposed all apical APAs. While we were encouraged with improvement of positive surgical margin rate, we were unsure what to make of the more than occasional APAs seen coursing along the lateral edge of the prostate and at the apex. We did, however, note that our incidence and anatomic findings remarkably paralleled those of Guilloneau.8 While these APAs were clearly seen, they were not well understood, especially with regard to sexual function. In 2009, using validated instruments, we reported our 2-year potency outcomes in men operated on between 2004 and 2006 who were <66 years of age with normal preoperative sexual function after adopting an athermal approach.12 With newly-found concern for APAs, we evaluated the impact of APA transection on potency outcomes in the same group of ideal men (i.e., <66 years with normal sexual function) and found no reduction in potency recovery.13
The present cross-sectional cohort analysis14 augments our previous report on APA transection by examining a more recent and robust group of men of all ages and preoperative sexual function. While our previous report was limited to young potent men, the present study applies to all men undergoing RARP regardless of their baseline sexual function. By comparing the natural control group of men with normal vascular anatomy to those with APAs transected, we seek to compare the impact of APA transection on the postoperative recovery of “erections” and “potency” post-RARP.
Materials and Methods
Patient selection and data collection
Between January 1, 2007 and December 31, 2014, 880 consecutive patients underwent RARP. Standard clinical characteristics were prospectively entered into an electronic database under an approved institutional review board protocol. All patients had the presence and type of APA (i.e., apical or lateral) recorded intraoperatively by the surgeon (T.E.A.) using a template diagram. Pre- and postoperative outcomes were obtained using self-administered validated questionnaires. Data management was blinded to the presence of APAs. All patients were instructed to use daily low-dose phosphodiesterase-5 inhibitors for the first year following surgery and as needed thereafter, without knowledge of the presence of APAs.
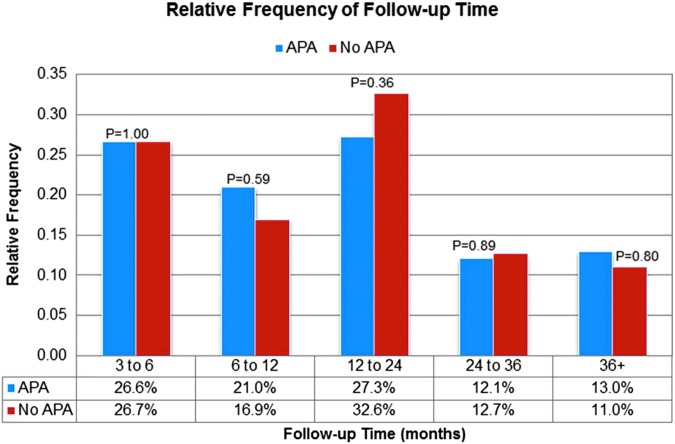
A cross-sectional cohort analysis was conducted in 2015. Men were excluded for adjuvant administration of hormonal and/or radiation therapy (n = 32), or less than 90-day follow-up and/or incomplete responses to validated questionnaires (n = 152) (Supplemental Fig. S1). After exclusions, 696 (79%) men had assessable outcomes. As Figure 1 demonstrates, cross-sectional analysis captured an even distribution of questionnaires at various follow-up time points in APA vs no APA groups (3–6, 6–12, 12–24 months, etc.). Median follow-up for the entire cohort was 15 months (range: 3–102 months). For subtle analysis, preoperative characteristics were matched between APA vs no APA groups (p > 0.05).
FIG. 1.
Cross-sectional study design—a comparison of the relative frequency of follow-up between the APA and no APA groups. APA = accessory pudendal artery.
Operative technique
Since 2003, we have completely defatted the anterior prostate to expose the anterior prostatic surface, dorsal vein complex (DVC), and the puboprostatic ligaments. Lateral APAs are seen at this point emanating from the terminal branches of the internal iliac artery and then coursing superficial or above the endopelvic fascia. The endopelvic fascia is incised, and apical APAs are seen at this point at the apex emanating through the levator ani. The prostate is completely freed from the urogenital diaphragm as much as the notch of the urethra. The puboprostatic ligaments are sharply divided, and the DVC is skeletonized and stapled to better define the apex and reduce anterior apical positive surgical margins. As a consequence of stapling, all APAs coursing along the DVC to the penis are sacrificed. We used a descending nerve-sparing approach.
Main outcome measures
Sexual function was evaluated in a stepwise approach, aptly differentiated between “erections” and “potency.” “Erections” were assessed using the International Index of Erectile Function-5 (IIEF-5) as a continuous variable and a fullness of erection scale (i.e., 0%, 25%, 50%, 75%, or 100%) compared with preoperative baseline at 3, 6, 12, and 24 months postoperatively. “Potency” was assessed two ways: a “yes/yes” response to “are your erections firm enough for penetration?” and “are they satisfactory?” taken from the Expanded Prostate Cancer Index (EPIC)-24 questionnaire and with categorical IIEF-5 scores ≥15, 17, or 21.
Statistical analyses
We used the two-sided Student's t-test to compare continuous variables while Fisher's exact and Pearson's chi-square tests were used to compare categorical variables. Univariate associations between patient characteristics and postoperative erectile function (IIEF-5 or percent fullness score) were examined using Pearson correlations. Multivariate associations between patient characteristics and postoperative IIEF-5 (continuous) and percent fullness (continuous) were analyzed using general linear models. Independent variables which had significant univariate associations were included in the initial multivariate model, with stepwise elimination of variables which did not reach significance with p < 0.15. To examine the impact of APAs on postoperative EF after adjusting for covariates, APA status was added to the linear model after including all significant independent predictors.
Logistic regression was used to examine associations between patient characteristics and potency (“yes and yes”; dichotomous). The impact of APAs on potency was investigated after adjusting for independent predictors identified through stepwise analysis. Similar analyses for potency recovery were conducted using postoperative IIEF-5 (<15 vs ≥15) and fullness (<75% vs ≥75%) as dichotomous outcome variables. A two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.2 (SAS, Cary, NC).
Results
Characteristics of the study population according to APA(s) transected (yes or no) are described in Table 1. Aberrant APA(s) were identified and transected in 231 (49.68%) men. In the APA group, the total number of APAs transected was 1 in 154 (67%) and 2 or more in 77 (33%). Both groups had nerve-sparing as defined as none, unilateral or bilateral preservation performed in 97%, 674/696 (3%, 18/696 had bilateral wide excision). As noted in Table 1, there were no statistically significant differences in baseline sexual function based on the presence of APAs. Further analysis confirmed no difference in baseline IIEF-5 scores between men with one APA vs two APAs (mean 18.9 vs 19.7, p = 0.432). Furthermore, there was no difference in positive surgical margin rates between men with and without APAs (9%, 41/465 vs 6%, 14/217 p = 0.204) nor between men with a single APA vs multiple APAs (6%, 10/161 vs 6%, 4/70 p = 0.204).
Table 1.
Patient Characteristics of Entire Cohort According to Accessory Pudendal Artery(s) Transected
| No APA, n = 465 (mean, SD) | APA group, n = 231 (mean, SD) | p | |
|---|---|---|---|
| Age | 61.8 (7.7) | 61.8 (7.4) | 0.997 |
| BMI (kg/m2) | 27.1 (3.4) | 27.3 (3.7) | 0.481 |
| Prostate weight (g) | 55.0 (19.8) | 53.2 (17.9) | 0.250 |
| EBL | 105.2 (33.6) | 101.0 (33.2) | 0.121 |
| Preoperative IIEF-5 | 19.3 (7.1) | 19.1 (7.2) | 0.783 |
| Preoperative PSA | 6.63 (5.3) | 6.26 (5.0) | 0.367 |
| Clinical Gleason, n (%) | |||
| ≤6 | 111 (25) | 61 (26) | 0.285 |
| 7 | 304 (65) | 153 (66) | |
| ≥8 | 47 (10) | 17 (7) | |
| Unknown | 3 (<1) | 0 | |
| Clinical stage, n (%) | |||
| T1a/b/c | 326 (70) | 158 (68) | 0.720 |
| T2a/b/c | 128 (28) | 69 (30) | |
| T3 | 11 (2) | 4 (2) | |
| Nerve sparing | |||
| None | 14 (3) | 4 (2) | 0.549 |
| Unilateral | 62 (13) | 30 (13) | |
| Bilateral | 389 (84) | 197 (85) | |
| No. of APA transected, n (%) | |||
| 0 | 465 (100) | N/A | N/A |
| 1 | N/A | 154 (67) | |
| 2 | N/A | 76 (33) | |
| Potency recovery, n (%) | |||
| Entire cohorta | 208/444 (46.8) | 101/222 (45.5) | 0.751 |
| ≤65, IIEF-5 22–25b | 103/137 (75.1) | 58/79 (73.42) | 0.725 |
21 and 9 patients were not assessable, due to adjuvant therapy.
Age less than or equal to 65, with baseline IIEF-5 22–25 at an average follow-up of 15 months.
APA = accessory pudendal artery; BMI = body mass index; IIEF-5 = International Index of Erectile Function-5; SD = standard deviation.
Cross-sectional analysis of erections and potency was evenly distributed between men with and without APAs (Fig. 1) throughout the follow-up period. In multivariate analysis (Table 2), age (p < 0.001), prostate weight (p = 0.030), and preoperative IIEF-5 (p < 0.001) were significant independent predictors of postoperative IIEF-5 score. After adjusting for these covariates, the presence of APAs was not associated with postoperative erections (IIEF-5 score). Adjusted mean postoperative IIEF-5 scores for 0, 1, or 2 APAs were 12.1, 12.0, and 12.9, respectively (p = 0.800). Coefficients for significant covariates were not affected by the addition of APA to the model. Similar correlations were observed between independent variables and percent fullness score. In multivariate analysis, age (odds ratio [OR]: 0.97, confidence interval [95% CI]: 0.95, 1.00, p = 0.033), prostate weight (OR: 0.99, 95% CI: 0.98, 1.00, p = 0.072), and preoperative IIEF-5 (OR: 1.23, 95% CI: 1.15, 1.31, p < 0.001) also significantly predicted percent fullness score as the outcome variable. After adjusting for significant co-variables, the presence of APAs was not associated with percent fullness (p = 0.545). In addition, no impact on postoperative erections (IIEF-5 scores) was found in subgroup analysis which was conducted with the following groups: IIEF-5 22–25, <66 and ≥66 years and IIEF-5 15–21, <66 and ≥66 years.
Table 2.
Multivariate Prediction of Postoperative Outcome IIEF-5 Score (Continuous) from Preoperative Characteristics
| Estimate of effects | F-ratio | ||
|---|---|---|---|
| Constant | 14.2 | 18.0 | <0.001 |
| Age | −0.19 | 16.1 | <0.001 |
| Prostate weight | −0.04 | 4.9 | 0.030 |
| Preoperative IIEF-5 | 0.58 | 130.8 | <0.001 |
| APA (level = 0) | −0.26 | 0.3 | 0.800 |
| APA (level = 1) | −0.27 |
| Factor = APAa | N | LS mean | SE |
|---|---|---|---|
| Level = 0 | 444 | 12.1 | 0.39 |
| Level = 1 | 154 | 12.0 | 0.66 |
| Level = 2 | 67 | 12.9 | 0.99 |
Level = 0 is Control, no APA, Level = 1 is one APA, Level = 2 two or more APAs.
In multivariate analysis, only age and baseline IIEF-5 score significantly impacted postoperative recovery of potency (Table 3). Higher body mass index (BMI) and prostate weight were associated with lower odds for potency; however, results did not reach statistical significance (p = 0.060 and 0.070, respectively). After adjusting for independent preoperative factors associated with potency, the presence of one or more transected APAs did not impact recovery of potency (p = 0.770 and 0.760, respectively). Two additional analyses defining recovery of potency as an IIEF ≥15 or percent fullness ≥75% again found no interaction with transection of one or multiple APAs; only age and preoperative sexual function status were statistically significant. Furthermore, the same analysis using potency defined as an IIEF ≥17 and ≥21 was conducted, but failed to show significant change in any of the outcomes. There was no difference in recovery of potency in subgroup analysis (as defined above) between men with or without APA(s). In our experience, <5% of men with baseline IIEF-5 of less than 15 recover potency; hence, all three analyses were repeated while excluding these men and again saw no changes in any of the above findings.
Table 3.
Multivariate Prediction of Postoperative Potency (Dichotomous) from Baseline Characteristics
| 95% confidence interval | ||||||
|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | p | Odds ratio | Lower | Upper |
| Constant | −0.03 | 1.3 | 0.980 | |||
| Age (continuous) | −0.03 | 0.01 | 0.008 | 0.97 | 0.94 | 0.99 |
| BMI (continuous) | −0.05 | 0.03 | 0.071 | 0.95 | 0.90 | 1.00 |
| Prostate weight (continuous) | −0.01 | 0.01 | 0.091 | 0.99 | 0.98 | 1.00 |
| Preop IIEF-5 (continuous) | 0.18 | 0.02 | <0.001 | 1.20 | 1.15 | 1.26 |
| APA [1 vs 0 (ref)] | −0.06 | 0.21 | 0.770 | 0.94 | 0.62 | 1.43 |
| APA [2 vs 0 (ref)] | 0.09 | 0.30 | 0.760 | 1.09 | 0.61 | 1.96 |
Discussion
During the course of open, laparoscopic, or robotic RP, surgeons will likely not encounter APAs if the approach does not defat the prostate and incise/dissect the endopelvic fascia (i.e., the veil of Aphrodite approach).16 However, most open and robotic surgeons do defat and incise the endopelvic fascia during lateral mobilization of the prostate. In 1995, Walsh and associates were the first to specifically note a 4% APA incidence when performing an open RP.17 It was another 10 years until they expanded on this initial report to suggest a benefit to preserving APAs. However, from 2005 to 2010, three contemporary laparoscopic or robotic “surgical” series4,13,18 reported a similar incidence of 25% to 33%. Angiographic and cadaveric studies have reported much higher incidences (70%–85%), but, from the technical view of preserving APAs, the question of incidence defaults to the ∼30% visually encountered if a surgeon defats and incises the endopelvic fascia.19
In this study, we performed a cross-sectional cohort analysis on a consecutive group of patients who underwent RARP. This study design is distinct because it reduces bias of outcome follow-up by taking a snapshot assessment of prospectively collected data at a single time point.14 As presented in Table 1, it is important to note that men with APAs had no differences in baseline characteristics (age, IIEF-5, prostate weight, BMI). In addition, of note, in the 17% (152/880) of men without follow-up there were no statistically significant differences in baseline characteristics compared with men with follow-up. All men were assessed identically without prior knowledge of APA status. To identify any effect on recovery of erections, we analyzed postoperative IIEF-5 scores and percent erection fullness (compared with preoperative) as continuous variables to examine factors suspected to impact the recovery of EF, including (transected) APAs in univariate and multivariate analysis (Table 2).
Recovery of potency as a dichotomous variable was also examined using three common definitions. The primary confirmation of potency was two affirmative answers to questions taken from EPIC as described above. A postoperative IIEF-5 score >15, 17, 21 and percent erection fullness >75% were also defined as “potent.” The baseline factor with the greatest impact on potency recovery was IIEF-5 score, followed by age. For men 65 and under with preoperative IIEF-5 22–25, with an average follow-up of just 15 months, 74.5% recovered potency (75.1% in the APA group and 73.42% in the no APA group, p = 0.725). Regardless of age and baseline sexual function, transection of APA(s) had no impact on the recovery of potency. Moreover, when men with baseline IIEF-5 scores <15 were removed, no impact was seen with transection of APAs. Finally, we found no change in potency findings when defined as a postoperative IIEF-5 score of ≥15, 17, or 22.
Why use men without APAs as our control rather than men with preserved APAs? Selecting to compare men who have preservation of an APA actually addresses a separate question. We stress “presumably” as dissecting and preserving are not equivalent as it is not known how well any arteries (especially small ones) are preserved from a flow viewpoint. Comparing preservation of APAs reflects the skill of the surgeon. At the core, transecting APA(s) questions whether there is subsequent adequate redundant flow.
In 2017, a meta-analysis by Henry and colleagues introduced two potential issues.20 First, they recommend preservation of the APA in 5.4% of men with penile blood supplied solely by an APA—an observation based on a cadaveric study by Droupy and colleagues in 1996.21 It is important to recognize that the presence or absence of internal pudendal arteries (IPA) cannot be determined because these arteries course outside of the levator ani. The second issue is atherosclerosis in patients with Type II penile blood supply from both the APA and IPA. In our 2011 analysis, we identified no compromise in potency recovery with APA transection for men less than 65 years old with normal preoperative sexual function (IIEF 22–25).13 The present study expands these findings to include men of all ages with mild-to-moderate erectile dysfunction (ED) (IIEF-5 > 15). In this analysis and in subset analysis for men over the age of 65 (data not shown), there is no statistically significant difference in erections or potency recovery with APA transection. This finding was further confirmed regardless of the number and location of APA(s) transected (Table 2).
Few articles have been published about the consequences of either preserving or sacrificing APAs. Mulhall and colleagues summarized the beneficial roles of APAs on postoperative recovery of sexual function. Based on available evidence of seemingly improved sexual function recovery, they advocated for preservation of APAs during RP.4 Remarkably, a systematic review in 2006 on the topic indicated equivocal evidence that sacrifice appreciably impaired recovery of sexual function.22 The present study is the first publication on surgical implications of APAs since 2010 and reveals that adequate redundant arterial supply to the penis is strongly suggested. The human body is replete with examples of redundant blood supply that ensures our most important organs receive proper vasculature. For example, the anterior cerebral circle (Circle of Willis) ensures that our most vital organ, the brain, has several layers of redundant blood supply.23 Other examples include limbs, heart, and hepatic alternatives of blood supply, but, most pertinently the pelvis has long been recognized for redundant redundancy. Dr. Kelly in 1893 described bilateral internal iliac and ovarian artery ligation for bleeding cervical cancer at the time of hysterectomy.24 In 1961, Siegel and Mengert stressed the anatomic basis of redundant circulation for the safety of bilateral iliac artery ligation.25 Other angiographic studies have long demonstrated numerous routes of inflow to the pelvis in addition to the internal iliac arteries accounting for the safety of their ligation.26,27
Our results need to be interpreted in the context of the study design. First, we are not suggesting that transection of an APA is preferred over attempted preservation. However, if dissection is complicated by bleeding or other issues transection appears to be safe because of redundant flow. Second, this is a cross-sectional analysis of prospectively collected data on patients with and without APAs. The classification of APA status is a limitation. While both cohorts were similar, we cannot infer a direct cause and effect relationship from the present data. Third, we limited our analysis to a single high-volume surgeon and these results may not be generally applicable, particularly during the learning curve. However, prior research has shown heterogeneous results regarding EF even among well-experienced surgeons.28
Conclusions
While surgical preservation of APAs is optimal, we present data from a robust patient cohort that sacrifice of APA(s) during RARP did not lessen recovery of EF. We found no evidence that transecting one or more APA(s) reduced recovery of erections or potency in normal baseline function of men. This was also true in patients with mild-to-severe preoperative ED and/or advanced age.
Supplementary Material
Abbreviations Used
- APA
accessory pudendal artery
- BMI
body mass index
- DVC
dorsal venous complex
- EF
erectile function
- IIEF-5
International Index of Erectile Function-5
- PSA
prostate specific antigen
- RARP
robot-assisted radical prostatectomy
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stanford JL, Feng J, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: The Prostate Cancer Outcomes study. JAMA 2000;283:354–360 [DOI] [PubMed] [Google Scholar]
- 2.Kandu SD, Rhoel KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol 2004;172:2227–2231 [DOI] [PubMed] [Google Scholar]
- 3.Alemozaffar M, Duclos A, Hevelone ND, Lipsitz RS, Borza T, Hua-Yin Y, et al. Technical refinement and learning curve for attenuating neurapraxia during robotic-assisted radical prostatectomy to improve sexual function. Eur Urol 2012;6:1222–1228 [DOI] [PubMed] [Google Scholar]
- 4.Mulhall JP, Secin FP, Guillonneau B. Artery sparing radical prostatectomy—Myth or reality? J Urol 2008;179:827–831 [DOI] [PubMed] [Google Scholar]
- 5.Polascik TJ, Walsh PC. Radical retropubic prostatectomy: The influence of accessory pudendal arteries on the recovery of sexual function. J Urol 1995;154:150–152 [DOI] [PubMed] [Google Scholar]
- 6.Rogers CG, Trock BP, Walsh PC. Preservation of accessory pudendal arteries during radical retropubic prostatectomy: Surgical technique and results. Urology 2004;64:148–151 [DOI] [PubMed] [Google Scholar]
- 7.Secin F, Karanikolas N, Kuroiwa K, Vickers A, Touijer K, Guillonneau B. Positive surgical margins and accessory pudendal artery preservation during laparoscopic radical prostatectomy. Eur Urol 2005;48:786–792 [DOI] [PubMed] [Google Scholar]
- 8.Secin F, Touijer K, Mulhall J, Guillonneau B. Anatomy and preservation of accessory pudendal arteries in laparoscopic radical prostatectomy. Endo Urol 2007;51:1229–1235 [DOI] [PubMed] [Google Scholar]
- 9.Secin FP, Karanikolas N, Touijer AK, Salamanca JI, Vickers AJ, Guillonneau B. Anatomy of accessory pudendal arteries in laparoscopic radical prostatectomy. J Urol 2005;174:523–526 [DOI] [PubMed] [Google Scholar]
- 10.Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: A comparison of one surgeon's outcomes. Urology 2004;63:819–822 [DOI] [PubMed] [Google Scholar]
- 11.Ahlering TE, Eichel L, Edwards RA, Lee DI, Skarecky DW. Robotic radical prostatectomy: A technique to reduce pt2 positive margins. Urology 2004;64:1224–1228 [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez E, Finley DS, Skarecky D, Ahlering TE. Single institution 2-year patient reported validated sexual function outcomes after nerve sparing robot assisted radical prostatectomy. J Urol 2009;181:259–263 [DOI] [PubMed] [Google Scholar]
- 13.Box GN, Kaplan AG, Rodriguez E, Skarecky DW, Osann KE, Finley DS, et al. Sacrifice of accessory pudendal arteries in normally potent men during robot-assisted radical prostatectomy does not impact potency. J Sex Med 2010;7:298–303 [DOI] [PubMed] [Google Scholar]
- 14.Hudson JI, Pope HG, Jr, Glynn RJ. The cross-sectional cohort study: An underutilized design. Epidemiology 2005;16:355–359 [DOI] [PubMed] [Google Scholar]
- 15.Skarecky D, Gordon A, Ahlering TE. Evaluation of a potency fullness scale at 3 months is predictive of overall 2 year outcomes after RARP. J Urol 2016;195:e632–e633 [Google Scholar]
- 16.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol 2010;57:179–192 [DOI] [PubMed] [Google Scholar]
- 17.Polascik TJ, Walsh PC. Radical retropubic prostatectomy: The influence of accessory pudendal arteries on the recovery of sexual function. J Urol 1995;154:150–152 [DOI] [PubMed] [Google Scholar]
- 18.Matin SF. Recognition and preservation of accessory pudendal arteries during laparoscopic radical prostatectomy. Urology 2006;67:1012–1015 [DOI] [PubMed] [Google Scholar]
- 19.Juhan CM, Padula G, Huguet JH. Angiography in male impotence. In: Bennett AH, ed. Management of Male Impotence. Baltimore: Williams & Wilkins, 1982, pp. 73–107 [Google Scholar]
- 20.Henry BM, Pekala PA, Vikse J, Sanna B, Skinningsrud B, Sganiak K, et al. Variations in the arterial blood supply to the penis and the accessory pudendal artery: A meta-analysis and review of implications in radical prostatectomy. J Urol 2017;198:345–353 [DOI] [PubMed] [Google Scholar]
- 21.Droupy S, Benoit G, Giuliano G, Jardin A. Penile arteries in humans, origin—Distribution—Variations. Surg Radiol Anat 1996;19:161–167 [DOI] [PubMed] [Google Scholar]
- 22.Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: A systematic review of prognostic indicators for a successful outcome. Eur Urol 2006;50:711–720 [DOI] [PubMed] [Google Scholar]
- 23.Niederberger E, Gauvrit JY, Morandi X, Carsin-Nicol B, Gauthier T, Ferré J. Anatomic variants of the anterior part of the cerebral arterial circle at multidetector computed tomography angiography. J Neuroradiol 2010;37:139–147 [DOI] [PubMed] [Google Scholar]
- 24.Kelly HA. Ligation of both internal iliac arteries for hemorrhage in hysterectomy for carcinoma uteri. Bull Johns Hopkins Hosp 1894;5:53–54 [Google Scholar]
- 25.Siegel P, Mengert WF. Internal iliac artery ligation in obstetrics and gynecology. JAMA 1961;178:1059–1062 [DOI] [PubMed] [Google Scholar]
- 26.Shafiroff BG, Grillo EB, Baron H. Bilateral ligation of hypogastric arteries. Am J Surg 1959;98:34–40 [DOI] [PubMed] [Google Scholar]
- 27.Chait A, Moltz A, Nelson JH. The collateral arterial circulation in the pelvis an angiographic study. Am J Roentgenol Radium Ther Nucl Med 1968;102:392–400 [DOI] [PubMed] [Google Scholar]
- 28.Vickers A, Savage C, Bianco F, Mulhall J, Sandhu J, Guillonneau B, et al. Cancer control and functional outcomes after radical prostatectomy as markers of surgical quality: Analysis of heterogeneity between surgeons at a single cancer center. Eur Urol 2011;59:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.