Abstract
Human CD4+ T regulatory cells (Tregs) are a population of phenotypically and functionally diverse cells that downregulate inflammatory and autoimmune responses. As Th17 cells play an important role in the pathogenesis of autoimmune diseases, it is critical to elucidate the mechanisms regulating these cells. In this study, we examined the molecular basis underlying the phenotypic and functional diversity of human Tregs expressing the ectonucleotidase CD39. CD4+CD25hiCD39+ Tregs inhibit the proliferative response and the secretion of IL-17 and IFN-γ of autologous CD4+ T effector cells, while CD4+CD25hiCD39− Tregs only suppress IFN-γ production. We demonstrate that activated human CD4+CD25hiCD39+ Tregs express the Th17-associated surface markers CCR6 and IL-23R, and phosphorylate the transcription factor Stat3. Moreover, suppression of IL-17 by CD4+CD25hiCD39+ Tregs occurs via a Stat3-dependent mechanism as inhibition of Stat3 activation in the CD39+ Tregs reverses their ability to suppress IL-17. CD4+CD25hiCD39− Tregs are not endowed with the ability to inhibit IL-17 as they do not upregulate CCR6 or the IL-23R, and furthermore, they secrete IL-17. Our findings provide the first evidence that human Treg functional diversity is matched to the type of immune response being regulated and reveal a new role for Stat3 in controlling Treg function.
Keywords: : CD39+ Treg cells, Stat3, IL-17
Introduction
Human thymus-derived naturally occurring T regulatory cells (Tregs) are an essential subset of CD4+ T cells that maintain immune homeostasis, control immune responses to different pathogens, and prevent autoimmunity. Tregs were initially described as a population of CD4+ T cells expressing high levels of CD25 (CD25hi) that suppressed the proliferation of CD4+CD25neg T cells in vitro (Sakaguchi and others 1995; Baecher-Allan and others 2001). The transcription factor Foxp3 was shown to be expressed predominantly by CD4+CD25hi Tregs (Bennett and others 2001; Brunkow and others 2001; Wildin and others 2001; Fontenot and others 2003; Hori and others 2003; Bacchetta and others 2006). Identifying and isolating human Tregs is challenging as expression of CD25 and Foxp3 increases transiently upon activation on CD4+ T effector cells (Teffs) (Lipkowitz and others 1984; Morgan and others 2005). Foxp3 expression is intracytoplasmic and cannot be used for isolating Tregs. Other potential “signature” markers for human Tregs include CTLA-4 (CD152) and CD27 (Dieckmann and others 2001; Jonuleit and others 2001; Koenen and others 2005; Ruprecht and others 2005) and lack of expression of CD127, the IL-7Rα (Liu and others 2006; Seddiki and others 2006; Hartigan-O'Connor and others 2007).
CD39 and CD73, ectonucleotidases responsible for hydrolysis of ATP to adenosine, are coexpressed on nearly all murine Tregs (Borsellino and others 2007; Deaglio and others 2007). In humans, by contrast, CD39 expression is highly variable and restricted to a subset of CD4+CD25hiFoxp3+ Tregs (Borsellino and others 2007; Fletcher and others 2009; Mandapathil and others 2009). CD39 and CD73 coexpression is observed in a low percentage of human Tregs (Dwyer and others 2010; Mandapathil and others 2010). The difference in expression of these ectonucleotidases between murine and human Treg cells underscores that extrapolation from murine to human Treg cells may not always be applicable. Furthermore, human, but not murine, Th17 cells were described as being resistant to Treg suppression (Annunziato and others 2002; Evans and others 2007). Interestingly, CD4+CD25hiCD39+ Tregs are endowed with the ability to suppress IL-17 production by CD4+ Teffs (Fletcher and others 2009; Ye and others 2011).
The Th17-associated chemokine receptor, CCR6, is expressed on human and mouse Tregs (Kleinewietfeld and others 2005; Acosta-Rodriguez and others 2007; Kluger and others 2014). CCR6+ Tregs express Foxp3, exhibit Treg functional properties, migrate in response to the CCR6 ligand CCL20, express memory cell markers, and produce IL-10. CCR6 was expressed on human Tregs that secrete IL-17 directly ex vivo (Ayyoub and others 2009) or on activation (Voo and others 2009).
The suppressive mechanisms used by Tregs to inhibit immune responses are complex and not completely understood. The suppressive mechanisms are cell–cell contact dependent and include the release of inhibitory cytokines (IL-10, TGF-β, or IL-35) (Nakamura and others 2001; Levings and others 2002; Li and others 2006, 2007; Collison and others 2007, 2009; Niedbala and others 2007; Chaturvedi and others 2011; Chaudhry and others 2011; Huber and others 2011), modulation of antigen-presenting cell function (CTLA-4) (Read and others 2000; Takahashi and others 2000; Oderup and others 2006; Onish and others 2008), cytolysis (granzymes and perforin) (Grossman and others 2004), generation of suppressive metabolites (adenosine) (Huang and others 1997; Fredholm and others 2003; Naganuma and others 2006), and metabolic disruption (consumption of IL-2) (de la Rosa and others 2004; Busse and others 2010). Tregs were also reported to inhibit Th17 cells via a latency-associated peptide (LAP)-dependent mechanism (Ye and others 2011). It is possible that Tregs use other mechanisms to inhibit specific T effector cell responses.
Several groups demonstrated that murine Tregs expressed canonical CD4+ T effector cell-associated transcription factors to control polarized Th1-, Th2-, and Th17-driven immune responses (Chaudhry and others 2009; Koch and others 2009; Zheng and others 2009). Murine Tregs upregulate the expression of the transcription factors T-bet, IRF4, and GATA-3, and Stat3 to efficiently suppress Th1 (Koch and others 2009), Th2 (Zheng and others 2009), and Th17 responses (Chaudhry and others 2009), respectively. Similar lineage-specific Tregs are yet to be identified in humans.
In the present study, we describe a subset of human CD39-expressing CD4+CD25hiFoxp3+ Tregs that upregulate expression of the Th17-associated chemokine receptor CCR6, upregulate the IL-23 cytokine receptor, and phosphorylate Stat3 on activation. These Tregs inhibit the proliferation, as well as IFN-γ and IL-17 production, of autologous CD4+ Teffs. We demonstrate that suppression of IL-17 production by these cells occurs via a Stat3-dependent mechanism. This study suggests that CD4+CD25hiCD39+CCR6+IL-23R+Foxp3+ Tregs represent human Th17-specific regulatory T cells (Treg17).
Materials and Methods
Isolation of peripheral blood mononuclear cells
Human subjects age 23–55 years old with no known autoimmune conditions were recruited from the campus of the University of Medicine and Dentistry of New Jersey-New Jersey Medical School (now Rutgers-NJMS) in Newark, NJ. Research protocols were approved by the Institutional Review Board of NJMS and performed in accordance with the regulatory guidelines. Written informed consent was obtained from each subject. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Histopaque gradient centrifugation of heparinized venous blood as described in Liu and Rohowsky-Kochan (2008).
Antibodies
The following fluorochrome-conjugated mouse monoclonal antibodies specific for human markers were used in this study. APC-Cy7-anti-CD4 (clone RPA-T4), Pacific Blue-anti-Foxp3 (clone 259D), APC-anti-CD39 (clone TU66), and Brilliant Violet 421 anti-CD73 (clone AD2) were obtained from BioLegend (San Diego, CA). PE-anti-CD25 (clone M-A251), Alexa Fluor700-anti-CD25 (clone M-A251), and PE-Cy5-anti-CD152 (CTLA-4, clone BNI3) were obtained from BD Pharmingen (San Jose, CA). APC-anti-CCR6 (clone #53103) and FITC-anti-IL-23R (clone #218213) were obtained from R&D Systems (Minneapolis, MN). LEAF™ purified anti-human CD3 (clone HIT3a) Abs from BioLegend were used for activation.
Surface and intracellular phenotyping of Tregs
Resting, nonactivated, or “ex vivo isolated” PBMC were examined for expression of surface CD4, CD25, CD39, CD73, CCR6, IL-23R, and CTLA-4 using fluorochrome-conjugated Abs. For intracellular staining, cells were fixed, permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience; San Diego, CA), and stained for expression of Foxp3 and/or CTLA-4. For all staining protocols, the buffer was 0.1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS) buffer, and heat-inactivated normal human serum was used for blocking. Immunofluorescence flow cytometry data were acquired using the 4-laser LSRII flow cytometer (BD Biosciences, San Jose, CA) in the NJMS Flow Cytometry Core Facility. Data were analyzed using FlowJo software (Version 7.6.4; TreeStar, Stanford, CA). All gates for individual markers were set based on fluorescence minus 1 (FMO) control technique, in which cells are stained with all of the markers except 1 and gates are drawn based on fluorescence of the missing fluorochrome within its individual FMO tube.
Isolation of T cell subsets by fluorescence-activated cell sorting
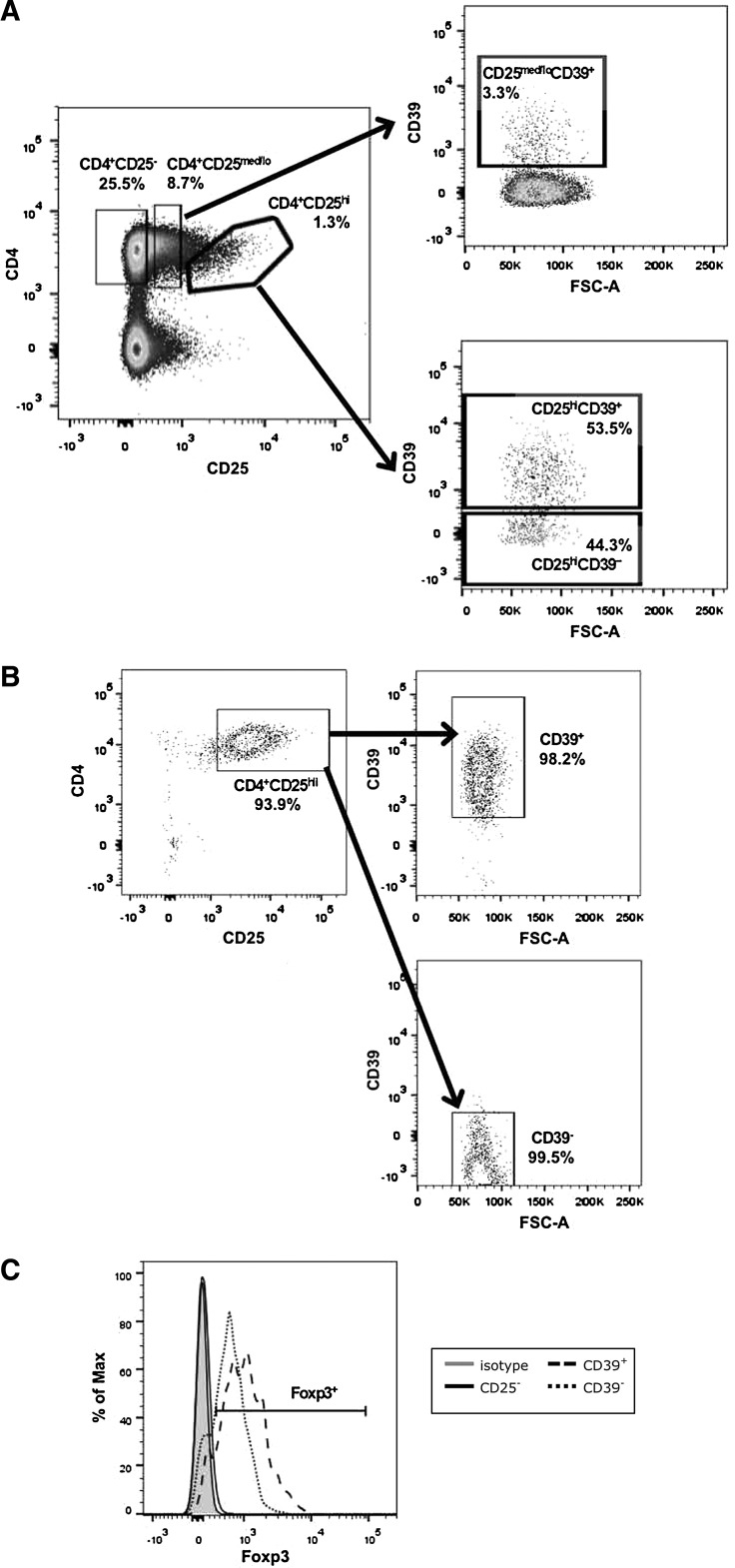
For Treg cell functional experiments, PBMC were stained with fluorochrome-conjugated CD4, CD25, and CD39 antibodies and separated into Treg and Teff populations by fluorescence-activated cell sorting (FACS) using the 4-laser FACSAria II cell sorter (BD Biosciences) in the NJMS Flow Cytometry Core Facility. Stringent gates were set using FMO, as described above, and CD4+ CD25hi Tregs were <1.5% of CD4+ T cells. The gating strategy for CD25 and sorting experiments is shown in Fig. 1. All cells were collected into 5-mL polypropylene tubes containing 40% fetal bovine serum in RPMI-1640 (Life Technologies, Grand Island, NY) complete medium supplemented with 1% penicillin–streptomycin–glutamine. The purity of the sorted cells was greater than 96%.
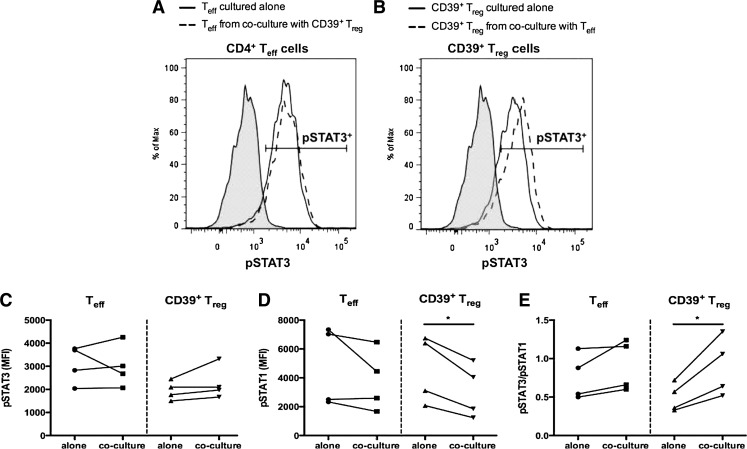
FIG. 1.
Distinct populations of CD4+CD25hi Tregs based on CD39 expression. PBMC were stained for surface expression of CD4, CD25, and CD39. (A) PBMC were first gated on lymphocytes using an FSC-A versus SSC-A dot plot, gated on singlets using an FSC-W versus FSC-H dot plot (not shown), gated on CD4+CD25hi or CD4+CD25med/lo populations using a CD4 versus CD25 dot plot, and then gated on CD39+ and CD39− populations using a CD39 versus FSC-A dot plot. (B) FACS was used to collect CD4+CD25− Teffs, CD4+CD25hiCD39+ Tregs, CD4+CD25hiCD39− Tregs, and CD4+CD25med/loCD39+ T cells. The purity of the sorted CD4+CD25hiCD39+ and CD4+CD25hiCD39− Tregs from 1 representative donor is shown and (C) expression of Foxp3 in the sorted CD4+CD25hiCD39+ and CD4+CD25hiCD39− Tregs is shown. FACS, fluorescence-activated cell sorting; FSC-A, forward scatter area; FSC-H, forward scatter height; FSC-W, forward scatter width; PBMC, peripheral blood mononuclear cells; SSC-A, side scatter area; Tregs, T regulatory cells.
Suppression assay
CD4+CD25− Teffs (4 × 104 cells/well) were stimulated with 0.5 μg plate-bound anti-CD3 plus irradiated allogeneic CD3− antigen-presenting cells (APCs; 1 × 105 cells/well) and cocultured with or without sorted Tregs at a 1:1 ratio in 96-well round-bottomed plates for 6 days at 37°C in a humidified incubator with 5% CO2 (Fletcher and others 2009). To ensure HLA MHC class II mismatch, CD3− cells were collected from the PBMC of 3 donors, by depletion of CD3+ T cells by the Human CD3 MicroBeads kit (Miltenyi Biotec, Auburn, CA). The CD3− APCs from all 3 donors were mixed and irradiated at 20 Gy before culture. Proliferation was assessed after addition of thymidine (3H-TdR; 1 μCi/well) for 18 h and measured using the RackBeta Liquid Scintillation counter (LKB Wallac, Turku, Finland). For experiments in which proliferation was not measured, cells were harvested on day 6 and stained for expression of various markers, including CD4, CD25, CD39, CD73, CTLA4, CCR6, IL-23R, and Foxp3.
Cytokine quantitation by enzyme-linked immunosorbent assay
IL-17 was measured by enzyme-linked immunosorbent assay (ELISA) using an anti-human IL-17 antibody (Clone No. 41809, 2,000 ng/mL; R&D Systems) as the capture antibody and a biotinylated polyclonal anti-human antibody (75 ng/mL; R&D Systems) as the detection antibody with quantification by reference to an rIL-17 standard (R&D Systems). IFN-γ was measured using a commercially available OptEIA kit (BD Pharmingen), according to the manufacturer's instructions. For all the ELISAs, 1% BSA in PBS was used as the blocking and dilution buffer. All samples were measured in duplicate wells, OD values were measured at an absorbance of 450 nm, and means of the duplicates were compared to a standard curve of known cytokine concentrations. The sensitivity of the ELISA was 7.8 pg/mL for IL-17 and 4.7 pg/mL for IFN-γ.
Intracellular staining for Stat phosphorylation
Phosphorylation of Stat3 and Stat1 was analyzed using intracellular staining following 6 days of activation of FACS-sorted T cells as described (Purvis and others 2014). Cells were stained for surface expression of CD4 and CD25, fixed with 2% paraformaldehyde, permeabilized with 100% ice-cold methanol overnight, blocked with human serum, and stained with pStat antibodies (pY705;clone 4/P-Stat3 or pY701;clone4a/P-Stat1) both from BD Pharmingen for 1 h. Data were acquired using the LSRII flow cytometer and analyzed using FlowJo software, as described above.
Stat3 inhibition
CD4+CD25− Teffs and CD4+CD25hi T cells with or without CD39 expression were isolated by FACS, as described above. FACS-sorted CD4+ T cells and Tregs were then incubated at 37°C for 16 h in the absence or presence of Stattic V, a small molecule inhibitor of Stat3 activation and dimerization (50 ng/mL; Sigma-Aldrich). Following pretreatment with Stattic V, treated and untreated CD4+CD25− Teffs, CD4+CD25hiCD39+ Tregs, and CD4+CD25hiCD39− T cells (4 × 104 cells/well) were cultured in anti-CD3-coated wells in the presence of irradiated allogeneic CD3− APCs (1 × 105/well). Untreated CD4+CD25− Teffs were also cultured with treated and untreated CD4+CD25hiCD39+ Treg cells and CD4+CD25hiCD39− T cells at a 1:1 ratio. After 5 days, cells were harvested and stained for analysis of Stat3 and Stat1 phosphorylation by flow cytometry, and supernatants were collected for analysis of IFN-γ and IL-17 production by ELISA.
Statistical analysis
Data were analyzed by looking at mean differences in fluorescence, cytokine levels and suppression of proliferation between the different cell populations or culture conditions using a repeated measures 1-way ANOVA (F-test) with a 1-tailed alpha level of 0.05 or Student's t-test, as appropriate, using Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA). Tukey's posttest was used to compare pairs of data sets following ANOVA. All experiments were analyzed utilizing appropriate statistical tests for parametric and nonparametric analyses on consultation with the biostatistics core facility at NJMS.
Results
CD39+human Tregs suppress Teff proliferation, IFN-γ, and IL-17 production
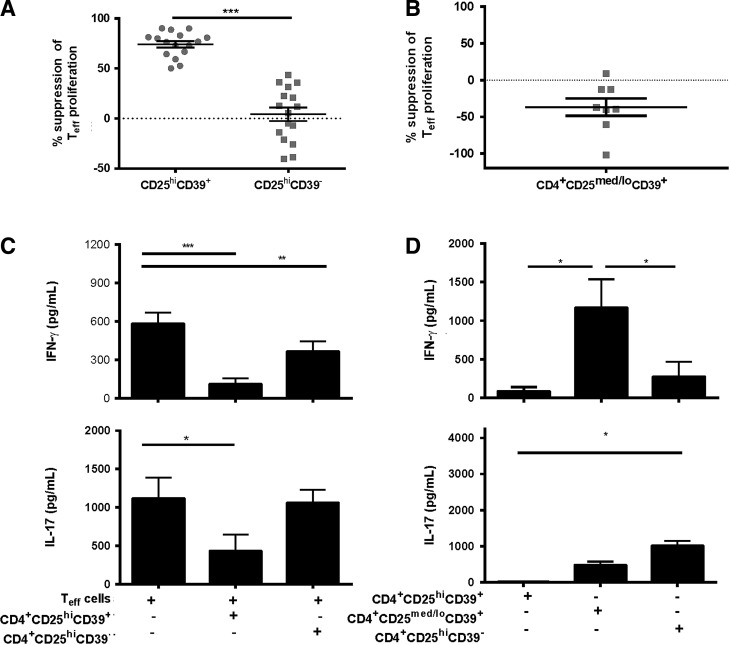
To access the functional role of CD39 on Tregs, we examined the ability of FACS-sorted CD4+CD25hiCD39+ and CD4+CD25hiCD39− Tregs to suppress proliferation and cytokine production of CD4+CD25− Teffs. Human CD4+ T cells were sorted into highly purified CD4+CD25-, CD4+CD25hiCD39+, CD4+CD25hiCD39−, and CD4+CD25med/loCD39+ T cell populations, using the gating strategy shown in Fig. 1A. Purity of the sorted populations was >98% (Fig. 1B). Foxp3 expression was higher in the CD4+CD25hiCD39+ compared to the CD4+CD25hiCD39− T cells (Fig. 1C). CD4+CD25hiCD39+ T cells suppressed the proliferation of CD4+CD25− Teffs (mean ± standard error of the mean, 74% ± 12%), but CD4+CD25hiCD39− T cells were not capable of suppressing this response (Fig. 2A). To determine whether expression of CD39 or high CD25 expression was critical for the suppressive ability of these Tregs, we examined the ability of CD4+CD25med/loCD39+ T cells to inhibit the proliferation of CD4+CD25− Teffs. Similar to the CD4+CD25hiCD39− T cells, CD4+CD25med/loCD39+ T cells were unable to suppress proliferation of CD4+CD25− Teffs (Fig. 2B).
FIG. 2.
CD39+ Tregs suppress proliferation, IFN-γ production, and IL-17 production of CD4+CD25− Teffs. CD4+CD25− Teff cells, CD4+CD25hiCD39+ Treg cells, CD4+CD25hiCD39− Treg cells, and CD4+CD25med/loCD39+ T cells were collected by FACS. (A) CD4+CD25− Teffs were stimulated with plate-bound anti-CD3 plus irradiated allogeneic CD3− APCs and cultured alone or with sorted CD4+CD25hiCD39+ Tregs or CD4+CD25hiCD39− Tregs at a 1:1 ratio for 6 days in 96-well round-bottomed plates. Proliferation was determined by measuring tritiated thymidine (3H-TdR) incorporation during the final 18 h of the assay. Percent suppression was calculated using the formula [1 − (co-culture cpm/Teff alone cpm)] × 100. (B) CD4+CD25− Teffs were stimulated as in (A) and cultured alone or with CD4+CD25med/loCD39+ T cells at a 1:1 ratio for 6 days and proliferation was measured by the amount of radiolabel uptake. (C) Supernatants from cultures in (A) were collected on day 5 and IFN-γ and IL-17 were measured by ELISA. (D) CD4+CD25hiCD39+ Tregs, CD4+CD25hiCD39− Tregs, and CD4+CD25med/loCD39+ T cells were cultured alone or with plate-bound anti-CD3, and irradiated allogeneic APCs and supernatants were collected on day 5 and levels of IFN-γ and IL-17 were measured by ELISA. Data are expressed as the mean ± SEM. For (A) n = 16 different donors, (B) n = 8 different donors, (C, D) n = 9 different donors. Statistical analysis was performed using repeated measures ANOVA with Tukey's post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. APCs, antigen-presenting cells; ELISA, enzyme-linked immunosorbent assay; SEM, standard error of the mean.
We next examined the ability of the CD4+CD25hiCD39+ and CD4+CD25hiCD39− T cell subsets to inhibit cytokine production, as it has been demonstrated that proliferation and cytokine production are separate functions (Sojka and Fowell 2011). IFN-γ production by CD4+CD25− Teffs was inhibited both by the CD4+CD25hiCD39+ and CD4+CD25hiCD39− T cells (P < 0.001 and P < 0.01, respectively), with slightly stronger inhibition by the CD39+ Treg cells (Fig. 2C). Moreover, the CD4+CD25hiCD39+ T cells significantly inhibited IL-17 production by CD4+CD25− Teffs (P < 0.05), whereas the CD4+CD25hiCD39− T cells did not inhibit IL-17 production (Fig. 2C). Thus, CD39 expression on CD4+CD25hi T cells denotes a subset of Tregs that suppress IL-17 production, while inhibiting T cell proliferation and IFN-γ production, confirming a previous report (Fletcher and others 2009). In contrast, the CD4+CD25hiCD39− T cells have regulatory activity that is restricted to inhibition of IFN-γ production.
To compare the ability of the CD25-expressing CD4+ Tregs to secrete cytokines, we measured cytokine levels produced by CD4+CD25hiCD39+, CD4+CD25med/loCD39+, and CD4+CD25hiCD39− T cells on activation. The CD4+CD25hiCD39+ Tregs produced very low levels of IFN-γ and undetectable amounts of IL-17 (Fig. 2D). The CD4+CD25hiCD39− T cell subset produced higher (not significant) amounts of IFN-γ and significantly higher levels of IL-17 on activation (P < 0.05) compared to the CD4+CD25hiCD39+ Tregs. The nonregulatory CD4+CD25med/loCD39+ T cells produced significantly higher levels of IFN-γ (P < 0.05) and slightly but not significantly higher levels of IL-17 compared to the CD4+CD25hiCD39+ Tregs (Fig. 2D).
Phenotypic analysis of CD39+ human Tregs
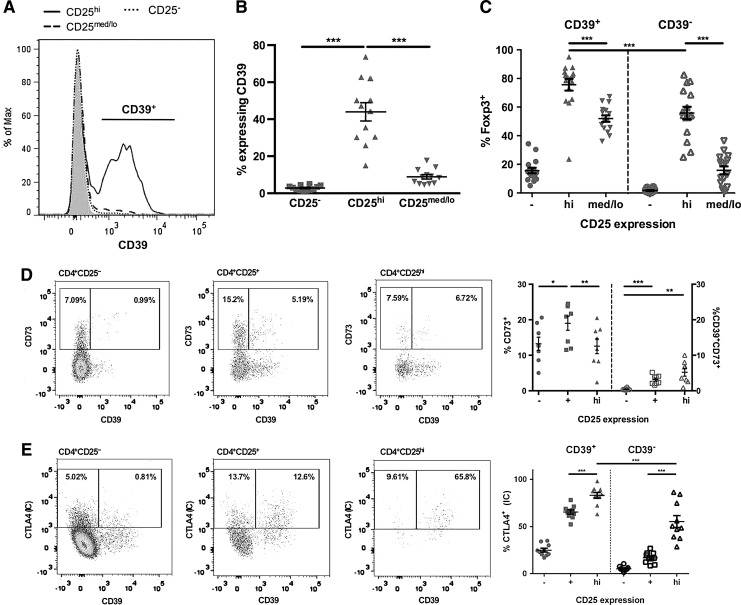
The finding that human CD4+CD25hiCD39+ and CD4+CD25hiCD39− Tregs were functionally different prompted us to perform a phenotypic analysis on these regulatory subsets. CD39 expression was significantly higher on the CD4+CD25hi Tregs (44% ± 17%) in comparison to CD4+CD25− (2.8% ± 1%; P < 0.001) and CD4+CD25med/lo (9% ± 4%; P < 0.001) T cells (Fig. 3A, B). CD39 expression on CD4+CD25hi Tregs was highly variable between donors, with expression ranging from 15% to 76% (Fig. 3B). Analysis of the Treg-specific transcription factor Foxp3 revealed that CD4+CD25hiCD39+ Tregs (76% ± 16%) expressed significantly higher levels (P < 0.001) compared to CD4+CD25hiCD39− Tregs (56% ± 17%) (Fig. 3C). CD73 surface coexpression with CD39 was very low in CD4+CD25hi Tregs ranging from below 1% to 10% (Fig. 3D). CD4+CD25+ T cells include both CD4+CD25hi and CD4+CD25med/lo T cells. CD73 was expressed without CD39 on a minor fraction of CD25hi Tregs. We observed very low levels of intracellular CD73 in the different T cell subsets, with almost no coexpression of CD39 and intracellular CD73 (data not shown). The CD4+CD25hiCD39+ Tregs have a significantly higher (P < 0.05) percentage of CD27+ CD127− cells (82.0% ± 4.3%) than the CD4+CD25hiCD39− Tregs (66.1% ± 8.4%). CD45RO was expressed on almost all of the CD4+CD25hiCD39+ Treg cells and CD4+CD25hiCD39− Treg cells (data not shown).
FIG. 3.
CD39 is expressed on human CD4+ Tregs with high Foxp3 expression. Resting, ex vivo PBMC were analyzed for expression of surface CD25, CD39, CD73, and intracellular CTLA4 and Foxp3. (A, B) Cells were stained for surface expression of CD4, CD25, and CD39. (C) Cells were permeabilized with the Foxp3/Transcription Factor Staining Buffer Set and stained for intracellular Foxp3 expression. Dot plots show intracellular expression of Foxp3 on CD39+ versus CD39− subsets of CD4+CD25−, CD4+CD25hi, and CD4+CD25med/lo T cell populations. (D) Cells were stained for surface expression of CD4, CD25, CD39, and CD73. Quadrant analyses show coexpression of surface CD39 and CD73 on CD4+CD25−, CD4+CD25+, and CD4+CD25hi T cell populations. CD4+CD25+ T cells include both CD4+CD25hi and CD4+CD25med/lo T cells. Dot plots show surface expression of CD73 and coexpression of CD39 and CD73 on CD4+CD25−, CD4+CD25+, and CD4+CD25hi T cell populations. (E) Cells were stained for surface expression of CD4, CD25, CD39, and intracellular CTLA4. Quadrant analyses show coexpression of surface CD39 and intracellular CTLA4 on CD4+CD25−, CD4+CD25+, and CD4+CD25hi T cell populations. Dot plots show intracellular expression of CTLA4 on CD39+ versus CD39− subsets of CD4+CD25−, CD4+CD25+, and CD4+CD25hi T cell populations. For (A) data from 1 representative experiment are shown in histogram. Gray area is FMO control; for (B, C) n = 12 different donors, for (D, E) data from 1 representative experiment are shown in quadrant analyses and n = 8 (D) and n = 9 (E) different donors. Data are expressed as the mean ± SEM. Statistical analysis was performed using repeated measures ANOVA with Tukey's post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. FMO, fluorescence minus 1.
As CTLA-4 is thought to be important for Treg cell function, we investigated the expression of surface and intracellular CTLA-4 on ex vivo isolated PBMC. CD4+CD25hi Tregs have low (7.3 ± 2.4), although significantly higher, surface CTLA-4 expression than CD4+CD25− (0.03 ± 0.1; P < 0.04) T cells. CD4+CD25hiCD39+ Tregs expressed significantly more intracellular CTLA-4 (83% ± 10%) compared to CD4+CD25hiCD39− Tregs (55% ± 20%; P < 0.001) (Fig. 3E). The phenotyping data indicate that CD39+ Tregs have a higher expression of Foxp3 and intracellular CTLA-4 but similar expression of CD45RO to CD39− Tregs.
CCR6 and IL-23R are expressed on the surface of suppressive CD39+ Treg cells following activation
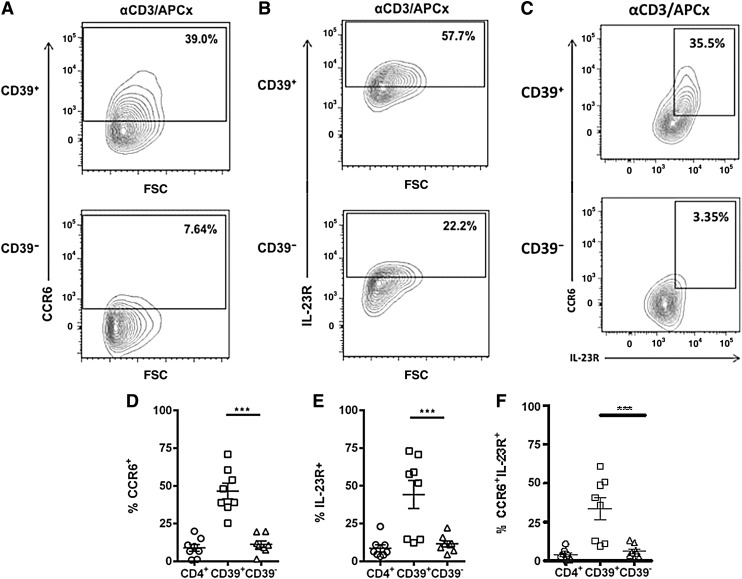
Evidence from studies on murine Tregs shows that upregulation of chemokine receptors is critical for the ability of Tregs to suppress specific types of immune responses. In addition, 1 study classified human Foxp3+ human Tregs as Th1-like, Th2-like, and Th17-like Treg subsets based on their expression of subset-associated chemokine receptors and transcription factors (Duhen and others 2012). Since CD39+ Tregs suppress in vitro IL-17 secretion, we next investigated the expression of CCR6 and IL-23R on CD4+CD25hiCD39+ and CD4+CD25hiCD39− Tregs. CCR6 expression was very low on resting, ex vivo human CD4+CD25hi Tregs (2.9% ± 1.3%) and CD4+CD25− T cells (1.6% ± 0.5%). CD39+ Tregs markedly upregulated CCR6 expression following activation (P < 0.001) in comparison to the CD39− Treg cells (Fig. 4A, D). Furthermore, CD39+ Tregs strongly upregulated IL-23R expression on activation (P < 0.01) (Fig. 4B, E). CCR6 and IL-23R were coexpressed on significantly more CD39+ Tregs then CD39− Tregs (P < 0.001) (Fig. 4C, F). CD39− Tregs and CD4+ Teffs expressed low levels of either CCR6 or IL-23R or their coexpression following activation (Fig. 4D–F). These findings show that the CD39+ Tregs that suppress IL-17 production express the Th17-associated surface markers CCR6 and IL-23R.
FIG. 4.
Human CD39+ Tregs express CCR6 and IL-23R following activation. CD4+CD25− Teffs, CD39+ Tregs, and CD39− Tregs were collected by FACS and cultured alone in the presence of plate-bound anti-CD3 and irradiated allogeneic APCs for 6 days. Following activation, cells were stained with fluorochrome-conjugated antibodies to CD4, CD25, CD39, CCR6, and IL-23R, and flow cytometry was used to analyze surface expression of CCR6 (A, D), IL-23R (B, E), and CCR6 and IL-23R coexpression (C, F) on activated CD4+CD25− Teffs, CD4+CD25hiCD39+ Tregs, and CD4+CD25hiCD39− Tregs. Contour plots in (A–C) show 1 representative donor out of 8 different donors. Data are expressed as the mean ± SEM. Statistical analysis was performed using repeated measures 1-way ANOVA with Tukey's post hoc test. ***P < 0.001.
Stat3 phosphorylation contributes to CD39+ Treg-mediated suppression of IL-17
As Tregs may be specialized to suppress a specific CD4+ T cell subset by expressing the hallmark transcription factor of that specific Th subset (Chaudhry and others 2009; Koch and others 2009; Zheng and others 2009), we analyzed phosphorylation of the Th17- and Th1-associated transcription factors Stat3 and Stat1, in CD4+ Teffs and CD39+ Tregs activated alone and when cocultured together. CD4+ Teffs expressed similar levels of pStat3 and pStat1 regardless of whether they were activated alone or in coculture with CD39+ Tregs (Fig. 5A, C, and D). CD39+ Tregs activated alone phosphorylated Stat3, and slightly higher levels of pStat3 were seen following coculture with CD4+ Teffs. CD39+ Tregs activated alone phosphorylated Stat1, and significantly lower levels of pStat1 were seen following coculture with CD4+ Teffs (Fig. 5B–D). Analysis of the ratio of pStat3/pStat1 showed a significant (P < 0.05) preference toward Stat3 phosphorylation in the CD39+ Tregs following coculture with CD4+ Teffs, whereas the pStat3/pStat1 ratio did not significantly differ in the Teffs regardless of whether these cells were cultured alone or in coculture with CD39+ Tregs (Fig. 5E). These data suggest that the relative levels of pStat3 and pStat1 may contribute to the functional suppression mediated by CD39+ Tregs.
FIG. 5.
Levels of Stat3 and Stat1 phosphorylation in CD39+ Tregs. CD4+CD25− Teffs and CD4+CD25hiCD39+ Tregs were collected by FACS and cultured alone or in Teff:Treg cocultures at a 1:1 ratio for 6 days in the presence of plate-bound anti-CD3 and irradiated allogeneic APCs. (A–E) Following 6 days of activation, cells were harvested and stained for surface expression of CD3, CD4, and CD25 as well as intracellular expression of pStat3 and pStat1 as described (Purvis and others 2014). Expression of pStat3 and pStat1, as well as the pStat3/pStat1 ratio, was determined using flow cytometric analysis of Teffs and Tregs collected from alone and coculture conditions. Histograms in (A, B) show data from 1 representative donor. Gray area is FMO control. MFI of Stat1 and Stat3 for 4 donors from 3 independent experiments is shown in (C–E). Statistical analysis was performed using paired Student's t-test (C–E) *P < 0.05. FMO, fluorescence minus 1; MFI, mean fluorescence intensity.
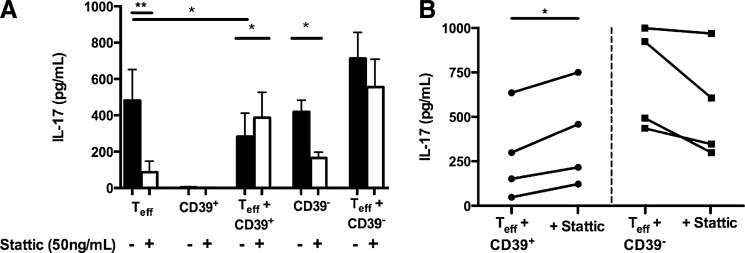
As Stat3 is associated with IL-17 production, we investigated the role of Stat3 activation in the production and suppression of IL-17. Sorted CD4+ CD25− Teffs, CD39+ Tregs, and CD39− Tregs were pretreated with the Stat3 inhibitor Stattic V for 16 h before activation. Stattic-treated and untreated CD39+ and CD39− Tregs were cultured alone and in 1:1 cocultures with untreated CD4+ CD25− Teffs and activated with plate-bound anti-CD3 and irradiated allogeneic APCs. In the absence of Stattic treatment, CD4+CD25hiCD39+ T cells significantly (P < 0.02) suppressed IL-17 production of CD4+CD25− Teffs, whereas CD4+CD25hiCD39− T cells slightly enhanced although not significantly IL-17 production when cultured with CD4+CD25− Teffs (Fig. 6A). IL-17 production by CD4+ Teffs cultured in the absence of Tregs was significantly inhibited (P < 0.01) by Stattic pretreatment (Fig. 6A). Likewise, production of IL-17 by the CD39− Tregs activated in the absence of CD4+CD25− Teffs was significantly (P < 0.02) inhibited by Stattic treatment (Fig. 6A). In contrast, inhibition of Stat3 activation in the CD39+ Tregs resulted in a significant (P < 0.02) increase in IL-17 in cocultures of CD4+CD25− Teffs and Stattic-pretreated CD39+ Tregs (Fig. 6A, B). We also observed a slight but not significant decrease in IL-17 production in cocultures of CD4+CD25− Teffs and Stattic-pretreated CD39− Tregs (Fig. 6A, B). The magnitude of reversal of IL-17 suppression by Stattic varied between donors, with the increase in IL-17 production ranging from 18% to 150%. These findings suggest that human CD39+ Tregs inhibit IL-17 in a Stat3-dependent mechanism, as activation of Stat3 within these cells is required for suppression of IL-17.
FIG. 6.
Inhibition of Stat3 activation in the CD39+ Tregs reverses their ability to suppress IL-17. CD4+CD25− Teffs, CD4+CD25hiCD39+ Tregs, and CD4+CD25hiCD39− Treg cells were collected by FACS and incubated with the Stat3 inhibitor Stattic V (50 ng/mL) for 16 h at 37°C. Stattic-treated and untreated CD4+CD25hiCD39+ Tregs and CD4+CD25hiCD39− Tregs were then cultured alone or at a 1:1 ratio with untreated CD4+CD25− Teffs for 6 days in the presence of plate-bound anti-CD3 and irradiated allogeneic APCs. Stattic-treated and untreated CD4+CD25− Teffs were cocultured alone under the same conditions. Supernatants were collected on day 6 and IL-17 production was measured by ELISA (A, B). Black bars represent Stattic untreated cultures, white bars represent Stattic treated cultures. Data represent the mean ± SEM for 4 different donors from 3 independent experiments. Statistical analysis was performed using repeated measures 1-way ANOVA with Tukey's post hoc test (A) or paired Student's t-test (B). *P < 0.02, **P < 0.01.
Discussion
Human Tregs are a phenotypically and functionally heterogeneous population of CD4+ T cells. Classifying diverse Treg populations and understanding the mechanisms they use to regulate polarized immune responses are important for the development of disease-specific Treg-based immunotherapy. Murine Tregs express canonical CD4+ T effector cell-associated transcription factors to control polarized Th1-, Th2-, and Th17-driven immune responses (Chaudhry and others 2009; Koch and others 2009; Zheng and others 2009). In this study, we examined the molecular basis underlying the phenotypic and functional diversity of human Tregs. We demonstrate for the first time that human CD4+CD25hiCD39+ Tregs, which suppress IL-17 production by CD4+ Teff cells, express the Th17-associated surface markers CCR6 and IL-23R. Moreover, phosphorylation of Stat3 and Stat1 by CD4+CD25hiCD39+ Tregs contributes to their suppressive effect. Suppression of IL-17 by CD4+CD25hiCD39+ Tregs occurs via a Stat3-dependent mechanism as inhibition of Stat3 activation in the CD39+ Tregs reverses their ability to suppress IL-17. CD4+CD25hiCD39− Tregs are not endowed with the ability to inhibit IL-17, as they do not upregulate CCR6 or IL-23R, and furthermore, they secrete IL-17. Our study suggests that CD4+CD25hiCD39+CCR6+IL-23R+Foxp3+ Tregs represent human Th17-specific regulatory T cells (Treg17).
In searching for “signature” Treg markers, we observed that expression of the ectonucleotidase CD39 was largely limited to the CD25hi subset of CD4+ T cells, with highly variable expression between donors, as previously reported (Fletcher and others 2009; Dwyer and others 2010). In contrast, nearly all CD4+CD25hiFoxp3+ Treg cells were reported positive for CD39 (Mandapathil and others 2010). We found that CD4+CD25hiCD39+ Tregs expressed the highest levels of intracellular Foxp3 and CTLA-4 in comparison to CD4+CD25hiCD39− Tregs. Only a small percentage of CD25hi Tregs coexpressed surface CD39 and CD73, in agreement with other human studies (Borsellino and others 2007; Dwyer and others 2010; Rissiek and others 2015).
To assess the suppressive capacity of these Treg populations, we used FACS-sorted CD4+ Teffs and Tregs activated with plate-bound CD3 and irradiated allogeneic APC (Borsellino and others 2007; Fletcher and others 2009; Miyara and others 2009; Duhen and others 2012). We observed that CD4+CD25hiCD39+ and CD4+CD25hiCD39− T cells have distinct suppressive capabilities. CD4+CD25hiCD39+ Tregs have the ability to suppress the proliferative response, as well as IL-17 and IFN-γ production by CD4+ Teffs, whereas CD4+CD25hiCD39− Tregs only suppress IFN-γ production by CD4+ Teffs. These results suggest that Treg cell subsets use different mechanisms for suppressing Teff proliferation and cytokine production, as has been reported in the murine system (Sojka and Fowell 2011). One study showed that both CD4+CD25hiCD39+ and CD4+CD25hiCD39− Tregs suppressed proliferation and IFN-γ production and that the CD4+CD25hiCD39+ Tregs inhibited IL-17 production (Fletcher and others 2009).
The concept of lineage-specific Tregs is supported by studies demonstrating that mouse Tregs expressing T-bet inhibit Th1 cells, IRF4-expressing Tregs inhibit Th2 cell function, and pStat3-expressing Tregs inhibit Th17-mediated inflammation (Chaudhry and others 2009; Koch and others 2009; Zheng and others 2009). Whether specialization and differentiation of human Tregs occur has not been shown and is important to determine since differences exist between mouse and human Tregs regarding subset specificities and expression of markers. We have demonstrated that expression of CCR6 and IL-23R was specifically upregulated on CD39+ Tregs following activation. These data suggest that CD4+CD25hiCD39+CCR6+IL-23R+ Tregs may be specifically primed to interact with Th17 cells, as they may migrate with Th17 cells to sites of inflammation. Murine models have shown that CCR6-expressing Tregs migrate with Th17 cells to sites of inflammation (Yamazaki and others 2008; Turner and others 2010). Tregs that lacked surface expression of CCR6 resulted in impaired renal trafficking in an animal model of glomerulonephritis (Kluger and others 2014). Furthermore, expression of IL-23R on these Tregs may allow for their expansion at sites of inflammation, as IL-23 is important for Th17 cell expansion and survival (Wilson and others 2007; Liu and Rohowsky-Kochan 2008). Likewise, expression of the IL-23R on the Tregs may deprive Th17 cells of essential survival factors. To our knowledge, this is the first report demonstrating that coexpression of CCR6 and IL-23R is upregulated on human Tregs.
In addition to specific expression of Th17 surface markers on the CD39+ Tregs, our studies suggest that the relative levels of pStat3 and pStat1 may be important in inhibiting Th17 function. The increase in pStat3, in conjunction with the significant decrease in pStat1 in CD39+ Tregs cocultured with CD4+ Teffs, results in a significant increase in the pStat3 to pStat1 ratio. These data are in agreement with studies of mouse Tregs that report increased pStat3 in IL-17-suppressing Tregs (Koch and others 2009; Kluger and others 2014). Further studies using small interfering RNA silencing of pStat3 and pStat1 are warranted to confirm the role of these factors in CD39+ Treg cell-mediated suppression. Moreover, it remains to be determined which Stat-inducing cytokines are involved. No IL-6 or IL-23 was detected in the activated CD4+ Teff and CD39+ Treg cocultures and slight increases in IL-10 were seen (data not shown).
The functional role of pStat3 in mediating the suppressive function of CD39+ Tregs was demonstrated by inhibiting Stat3 activation in CD39+ Tregs. A salient finding of our study is the demonstration that suppression of IL-17 by CD4+CD25hiCD39+ Tregs occurs via a Stat3-dependent mechanism, as inhibition of Stat3 activation in the CD39+ Tregs reverses their ability to suppress IL-17. Treatment with a Stat3 inhibitor resulted in decreased IL-17 production by CD4+ Teffs and by CD4+CD25hiCD39− T cells, whereas suppression of IL-17 production by CD39+ Tregs was significantly reversed on treatment of Tregs with the Stat3 inhibitor. Hence, Stat3 activation is involved in IL-17 production as well as in suppressing IL-17 by Tregs. Our findings are in agreement with murine data describing that lineage-specific Treg17 cells expressed pStat3 for Treg cell suppression of IL-17 production (Chaudhry and others 2011; Kluger and others 2014, 2016). CCR6 expression on human Tregs also appears dependent on Stat3, as patients with dominant-negative Stat3 mutations have greatly reduced CCR6 levels (Kluger and others 2014). Further studies are needed to characterize the mechanism by which activation of Stat3 in CD39+ Tregs endows them with the ability to suppress Th17 responses. Unpublished data from our laboratory found that the immunosuppressive cytokine IL-35 does not appear to be involved.
Our studies differ from those of Goodman and others (2011), who reported that IL-6 inhibits Treg cell function and that the IL-6-mediated loss of Treg suppression requires phosphorylation of Stat3. They showed that the ability of Tregs to suppress proliferation of CD4+ Teffs in response to CD3 and CD28 crosslinking is restored when Stat3 is inhibited. Only Treg-mediated suppression of the proliferative response was examined. We specifically studied CD39+ Tregs and their ability to inhibit IL-17 production by Teff cells stimulated with anti-CD3 and APCs. No exogenous IL-6 was added nor was any IL-6 detected in our culture system.
Distinct subsets of human Foxp3+ human Tregs that phenotypically mirror effector Th cells were reported. Th1-like, Th2-like, and Th17-like Treg subsets were identified based on their expression of subset-associated chemokine receptors and mRNA expression of the transcription factors T-bet, GATA-3, and RORγt, respectively (Duhen and others 2012). No differences in mRNA expression of IRF4 and Stat3 were seen among the different Treg subsets. Although this study indicated a role for Treg expression of a Th17-associated transcription factor, the specificity and function of these cells and mechanism of suppression remain to be elucidated.
As Th17 cells are implicated in various autoimmune diseases, Th17-specific CD39+ Tregs may be utilized as treatment for these conditions. Due to the heterogeneity of this population, Treg cell transplantation has not yet been effective. We have shown that while CD4+CD25hiCD39+ Tregs suppressed Teff proliferation and production of IL-17, the CD4+CD25hi T cell subset that lacks CD39 expression proliferated and produced IL-17. If CD4+CD25hi Tregs were used to treat a Th17-skewed autoimmune disease such as multiple sclerosis (MS), production of IL-17 by CD4+CD25hiCD39− Tregs may counteract the beneficial effects of the CD39+ Tregs. Thus, since Tregs display a degree of plasticity, it is crucial to understand the complex interplay of the transcription factors in controlling Treg suppressive versus pathogenic function. It will be important to further investigate whether the impaired suppressive activity of CD4+CD25hiCD39+ Tregs in MS patients is associated with a dysfunction in Stat3 (Fletcher and others 2009). In the future, targeting of T helper cell subset-specific Tregs may be utilized to reduce specific types of inflammation in humans.
Acknowledgments
We thank the members of the NJMS Flow Cytometry Core Facility specifically, Tammy Galenkamp and Hong Liu for performing the cell sorting. We acknowledge the assistance of Jianfeng Wang in the preparation of all the figures. This work was supported by the National Institutes of Health, National Institute of Neurological Disorders, and Stroke T32 grant 5T32NS051157 and by a fellowship from Rutgers University, Graduate School for Biomedical Sciences (to J.R.M.-B.) as well as by a grant from the Foundation of UMDNJ and Dean's Biomedical Research Support Grant (C.M.R.-K.).
Author Disclosure Statement
No competing financial interests exist.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646 [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. 2002. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med 196:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. 2009. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A 106:8635–8640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Passerini L, Gambineri E, Minyue D, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. 2006. Defective regulatory and effector T cell functions in patients with Foxp3 mutations. J Clin Invest 116:1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. 2001. CD4+CD25high regulatory cells in human peripheral blood. J Immunol 167:1245–1253 [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christine J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations in FOXP3. Nat Genet 27:20–21 [DOI] [PubMed] [Google Scholar]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110:1225–1232 [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko S, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder. Nat Genet 27:68–73 [DOI] [PubMed] [Google Scholar]
- Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, Höfer T. 2010. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A 107:3058–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. 2011. Cutting edge: human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol 186:6661–6666 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. 2009. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326:986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich J-M, Jack RS, Wunderlich FT, Brüning JC, Müller W, Rudensky AY. 2011. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34:566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Pillai MR, Chaturvedi V, Vignali DA. 2009. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol 182:6121–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450:566–569 [DOI] [PubMed] [Google Scholar]
- de la Rosa M, Rutz S, Dorninger H, Scheffold A. 2004. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 34:2480–2488 [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. 2001. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 193:1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. 2012. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector TH cells. Blood 119:4430–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. 2010. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 10:2410–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. 2007. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A 104:17034–17039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KHG. 2009. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183:7602–7610 [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Cunha RA, Svenningsson P. 2003. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr Top Med Chem 3:413–426 [DOI] [PubMed] [Google Scholar]
- Goodman WA, Young AB, McCormick TS, Cooper KD, Levine AD. 2011. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J Immunol 186:3336–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. 2004. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21:589–601 [DOI] [PubMed] [Google Scholar]
- Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. 2007. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods 319:41–52 [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061 [DOI] [PubMed] [Google Scholar]
- Huang S, Apasov S, Koshiba M, Sitkovsky M. 1997. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90:1600–1610 [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O'Connor W, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. 2011. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34:554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. 2001. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 193:1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. 2005. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood 105:2877–2886 [DOI] [PubMed] [Google Scholar]
- Kluger MA, Luig M, Wegscheid C, Goerke B, Paust HJ, Brix SR, Yan I, Mittrücker HW, Hagl B, Renner ED, Tiegs G, Wiech T, Stahl RA, Panzer U, Steinmetz OM. 2014. Stat3 programs Th17-specific regulatory T cells to control GN. J Am Soc Nephrol 25:1291–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MA, Melderis S, Nosko A, Goerke B, Luig M, Meyer MC, Turner JE, Meyer-Schwesinger C, Wegscheid C, Tiegs G, Stahl RA, Panzer U, Steinmetz OM. 2016. Treg17 cells are programmed by Stat3 to suppress Th17 responses in systemic lupus. Kidney Int 89:158–166 [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen HJ, Fasse E, Joosten I. 2005. CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol 174:7573–7583 [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 196:1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. 2006. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25:455–471 [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. 2007. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26:579–591 [DOI] [PubMed] [Google Scholar]
- Lipkowitz S, Greene WC, Rubin AL, Novogrodsky A, Stenzel KH. 1984. Expression of receptors for interleukin 2: Role in the commitment of T lymphocytes to proliferate. J Immunol 132:31–37 [PubMed] [Google Scholar]
- Liu H, Rohowsky-Kochan C. 2008. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol 180:7948–7957 [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. 2010. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem 285:7176–7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandapathil M, Lang S, Gorelik E, Whiteside TL. 2009. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol 346:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911 [DOI] [PubMed] [Google Scholar]
- Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE. 2005. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 66:13–20 [DOI] [PubMed] [Google Scholar]
- Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. 2006. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177:2765–2769 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Strober W. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbala W, Wei X-Q, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. 2007. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37:3021–3029 [DOI] [PubMed] [Google Scholar]
- Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. 2006. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 118:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onish Y, Fehervari Z, Yamaguchi T, Sakaguchi S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A 105:10113–10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis HA, Anderson AE, Young DA, Isaacs JD, Hilkens CM. 2014. A negative feedback loop mediated by STAT3 limits human Th17 responses. J Immunol 193:1142–1150 [DOI] [PubMed] [Google Scholar]
- Read S, Malmström V, Powrie F. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissiek A, Baumann I, Cuapio A, Mautner A, Kolster M, Arck PC, Dodge-Khatami A, Mittrücker HW, Koch-Nolte F, Haag F, Tolosa E. 2015. The expression of CD39 on regulatory T cells is genetically driven and further upregulated at sites of inflammation. J Autoimmun 58:12–20 [DOI] [PubMed] [Google Scholar]
- Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. 2005. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 201:1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). J Immunol 155:1151–1164 [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka DK, Fowell DJ. 2011. Regulatory T cells inhibit acute IFN-γ synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc Natl Acad Sci U S A 108:18336–18341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. 2000. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 192:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J-E, Paust H-J, Steinmetz OM, Peters A, Riedel J-H, Erhardt A, Wegscheid C, Velden J, Fehr S, Mittrücker H-W, Tiegs G, Stahl RAK, Panzer U. 2010. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol 21:974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voo KS, Wang Y-H, Santori FR, Boggiano C, Wang Y-H, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu Y-J. 2009. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 106:4793–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27:18–20 [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron J-C, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8:950–957 [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. 2008. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181:8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZJ, Zhou Q, Zhang JC, Li X, Wu C, Qin SM, Xin JB, Shi HZ. 2011. CD39+ regulatory T cells suppress generation and differentiation of Th17 cells in human malignant pleural effusion via a LAP-dependent mechanism. Respir Res 12:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, DeRoos P, Kim JM, Chu T-T, Corcoran L, Treuting P, Klein U, Rudensky AY. 2009. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458:351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]