Abstract
The significance of developing host-modulating personalized therapies to counteract the growing threat of antimicrobial resistance is well-recognized because such resistance cannot be overcome using microbe-centered strategies alone. Immune host defenses must be finely controlled during infection to balance pathogen clearance with unwanted inflammation-induced tissue damage. Thus, an ideal antimicrobial treatment would enhance bactericidal activity while preventing neutrophilic inflammation, which can induce tissue damage. We report that disrupting the inositol hexakisphosphate kinase 1 (Ip6k1) gene or pharmacologically inhibiting IP6K1 activity using the specific inhibitor TNP [N2-(m-(trifluoromethyl)benzyl) N6-(p-nitrobenzyl)purine] efficiently and effectively enhanced host bacterial killing but reduced pulmonary neutrophil accumulation, minimizing the lung damage caused by both Gram-positive and Gram-negative bacterial pneumonia. IP6K1-mediated inorganic polyphosphate (polyP) production by platelets was essential for infection-induced neutrophil-platelet aggregate (NPA) formation and facilitated neutrophil accumulation in alveolar spaces during bacterial pneumonia. IP6K1 inhibition reduced serum polyP levels, which regulated NPAs by triggering the bradykinin pathway and bradykinin-mediated neutrophil activation. Thus, we identified a mechanism that enhances host defenses while simultaneously suppressing neutrophil-mediated pulmonary damage in bacterial pneumonia. IP6K1 is, therefore, a legitimate therapeutic target for such disease.
INTRODUCTION
We recently investigated the role of higher inositol pyrophosphates, which are ubiquitous and have diverse cellular functions, in neutrophils (1). We revealed that diphosphoinositol pentakisphosphate (IP7) inhibits PtdIns(3,4,5)P3-mediated plasma membrane translocation of pleckstrin homology domain–containing proteins. Through this mechanism, IP7 acts as a key modulator of PtdIns(3,4,5)P3-mediated neutrophil functions such as phagocytosis, reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–mediated reactive oxygen species (ROS) production, and bacterial killing. Inositol hexakisphosphate kinase 1 (IP6K1) appears to be the main enzyme responsible for IP7 production in neutrophils; as a result, IP6K1-deficient neutrophils exhibit increased PtdIns(3,4,5)P3 signaling, enhanced phagocytic and bactericidal capacity, and elevated NADPH oxidase–mediated superoxide production.
Here, we further investigated IP6K1’s role in neutrophil function in bacterial pneumonia models. We found that IP6K1 expression in platelets was required for lipopolysaccharide (LPS)–induced formation of the neutrophil-platelet aggregates (NPAs) and essential for neutrophil accumulation in the alveolar spaces during bacterial pneumonia (2–6). IP6K1 function in NPA formation was mainly mediated by inorganic polyphosphate (polyP), which regulated NPA by triggering the bradykinin pathway and bradykinin-mediated neutrophil activation.
RESULTS
IP6K1 disruption leads to enhanced bacterial killing, reduced neutrophil accumulation, and alleviates lung damage in bacterial pneumonia
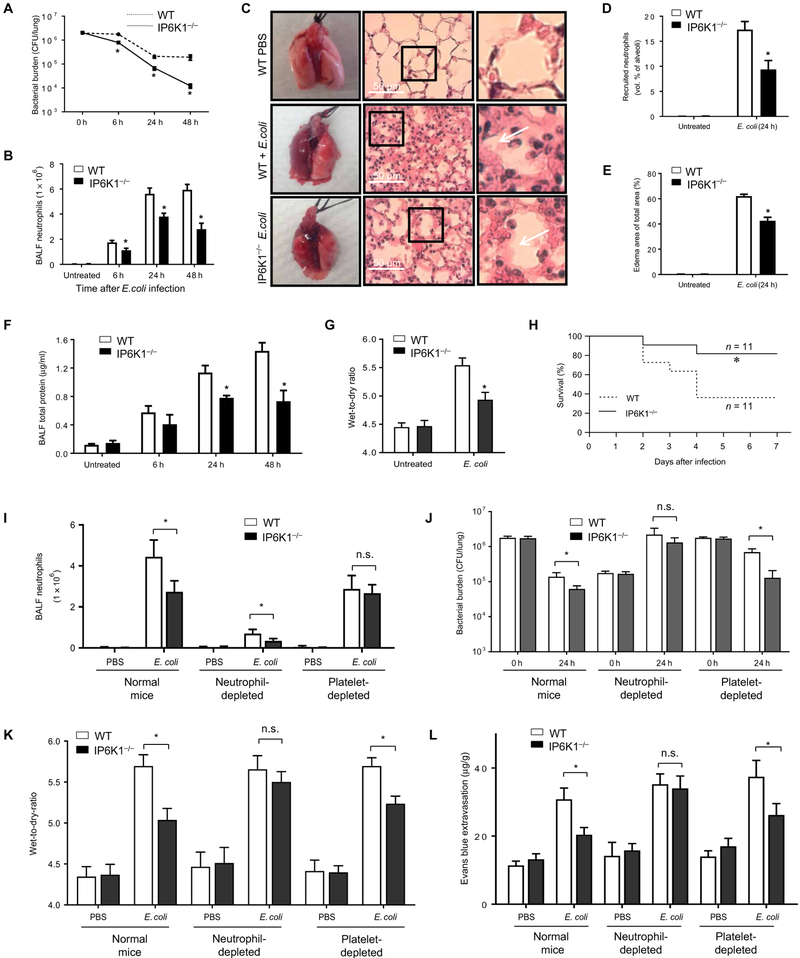
Disruption of IP6K1 in neutrophils up-regulates PtdIns(3,4,5)P3 signaling in a mouse bacteria-induced peritonitis model, enhancing bacterial killing by the host (1). Similarly, bacterial clearance was also enhanced in IP6K1-deficient mice in a bacteria-induced pneumonia model (Fig. 1). Bacterial pneumonia was induced by intratracheal instillation of Escherichia coli, a common Gram-negative pathogen. The lung bacterial burden, as measured by colony-forming units (CFU), was reduced by up to 1 log10 CFU per lung compared to wild-type (WT) mice at each time point examined (Fig. 1A). We assessed the number of alveolar macrophages in unchallenged mice and the amount of surfactant protein A, surfactant protein B, cathelin-related antimicrobial peptide, and β-defensin 2 in the lungs after the infection (fig. S1, A and B). No significant differences were detected between WT and IP6K1-deficient mice, consistent with the notion that the elevated bacterial killing observed in IP6K1-deficient mice may be mainly mediated by neutrophils.
Fig. 1. Disrupting IP6K1 enhances bacterial killing and reduces lung damage in bacterial pneumonia.
Mice were intratracheally instilled with 2 × 106 colony-forming units (CFU) of E. coli and euthanized at indicated time points. (A) Bacterial killing in inflamed lungs. Live bacteria were quantified as CFU per lung. The experiment was repeated three times, and the data were pooled and analyzed together. Data are means ± SEM (n ≥ 5 mice per group). (B) The numbers of recruited neutrophils in bronchoalveolar lavage fluid (BALF). All data are means ± SEM (n ≥ 5 mice per group). (C) Representative hematoxylin and eosin (H&E)–stained images of lung tissues show emigrated neutrophils and polymerized fibrin (arrows) in the pulmonary parenchyma. (D) Recruited neutrophils in alveolar spaces were quantified as volume fraction of the alveolar spaces using standard point-counting morphometry. Data are means ± SEM (n ≥ 5 mice per group). (E) Pulmonary edema formation was quantified as the percentage of edema area in the total parenchymal region. Data are means ± SEM (n ≥ 5 mice per group). (F) BALF total protein level. Data are means ± SEM (n = 5 mice per group). (G) Lung wet-to-dry ratio was measured at 24 hours after E. coli instillation. Data are means ± SEM (n = 5 mice per group). (H) Survival rates of E. coli–challenged wild-type (WT) and inositol hexakisphosphate kinase 1 (IP6K1)–deficient mice. Mice were intratracheally challenged with 5 × 106 live E. coli. Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. (I) The numbers of recruited neutrophils in BALF were measured at 24 hours after E. coli instillation. In untreated and platelet-depleted groups, mice were intratracheally instilled with 2 × 106 CFU of E. coli. In the neutrophil-depleted group, mice were intratracheally instilled with 2 × 105 CFU. Data are means ± SD (n = 5 mice per group). n.s., not significant. (J) Bacterial killing in inflamed lungs. Data are means ± SD (n = 5 mice per group). (K) Lung wet-to-dry ratio. Values are means ± SD (n = 5 mice per group). (L) Lung vascular permeability was evaluated by using Evans blue (EB) dye. Data are means ± SD (n = 5 mice per group). *P < 0.05 versus WT mice. Statistical analysis was performed using Student’s t test unless differently indicated.
Although IP6K1 did not regulate neutrophil recruitment in the peritonitis model (1), IP6K1 deficiency led to a reduction in pulmonary neutrophil accumulation in the pneumonia model (Fig. 1B). During bacterial pneumonia, the number of neutrophils in the bronchoalveolar lavage fluid (BALF) increased gradually, reaching more than 6 × 106 cells per lung 24 hours after bacterial instillation. Bacteria-induced neutrophil accumulation was substantially lower in IP6K1-deficient mice, with only 4 × 106 neutrophils recruited to each inflamed lung 24 hours after bacterial instillation (Fig. 1B). The number of emigrated neutrophils in alveolar spaces was also assessed by morphometry of lung tissue sections (7): Very few neutrophils were present in the alveolar air spaces of mice challenged with phosphate-buffered saline (PBS), but bacterial infection induced substantial neutrophil accumulation in alveolar air spaces (Fig. 1, C and D), with IP6K1 disruption decreasing the number of neutrophils in the alveolar air spaces in bacteria-challenged mice (Fig. 1, C and D). Similar to the peritonitis model (1), IP6K1 disruption did not alter the rate of apoptosis of recruited neutrophils (fig. S1, C and D), suggesting that apoptosis was not responsible for reduced neutrophil accumulation. In addition, the levels of proinflammatory cytokines/chemokines, including interleukin-1 (IL-1), IL-6, tumor necrosis factor–α, keratinocyte-derived chemokine, and macrophage inflammatory protein 2 (MIP2), in the BALF were the same between IP6K1-deficient and WT mice in bacterial pneumonia (fig. S2).
We next investigated whether enhanced bacterial killing and reduced neutrophil accumulation in IP6K1-deficient mice alleviated lung damage. Pulmonary edema is a well-characterized sign of lung inflammation and can be directly detected and measured in lung sections by microscopy and quantified by morphometry. Disrupting IP6K1 improved the histologic integrity of the lungs and reduced lung edema formation (Fig. 1E). Consistent with reduced edema formation, total protein levels in the BALF of IP6K1-deficient mice were much lower than in WT mice at each time point examined (Fig. 1F). In addition, the lung wet-to-dry ratio, which also measures the change in the capillary permeability, was reduced in infected IP6K1-deficient mice compared to WT mice (Fig. 1G). IP6K1 deficiency also increased the survival rate of bacteria-challenged mice in a more severe pneumonia model induced by a higher dose of live E. coli (Fig. 1H). Together, these results suggest that disrupting IP6K1 protects mice from bacterial infection–induced lung damage.
Neutrophils are key players in host defense and inflammation-elicited lung injury. Consistently, neutrophil depletion impaired host defense against E. coli infection (Fig. 1, I to K, and fig. S3, A to E); bacteria kept proliferating in neutropenic mice (Fig. 1J). Although peripheral blood neutrophil count was reduced to a similar level in neutrophil-depleted WT and IP6K1-deficient mice (fig. S3C), a reduction of neutrophil accumulation in the inflamed lungs was still detected in the IP6K1-deficient mice (Fig. 1I). Nevertheless, the difference in bacteria clearance (Fig. 1J), E. coli–elicited lung damage (Fig. 1, K and L, and fig. S3D), and the related death (fig. S3E) could not be detected between neutrophil-depleted WT and IP6K1KO mice anymore, suggesting that the lung damage in neutropenic mice was mainly mediated by a neutrophil-independent mechanism.
Numerous studies showed that platelets have the capacity to promote neutrophil accumulation (8–11). As previously reported (2–6), platelet depletion substantially reduced E. coli–induced neutrophil presence in the inflamed lungs (Fig. 1I). The reduced number of neutrophils observed in IP6K1-deficient mice was diminished in platelet-depleted mice, indicating that platelets contributed to IP6K1 function in E. coli–elicited pulmonary inflammation (Fig. 1I). Noticeably, although a similar number of neutrophils were recruited to the lungs of platelet-depleted IP6K1 knockout (KO) mice as platelet-depleted WT mice, the bacterial killing capability was elevated in these mice compared to platelet-depleted WT mice (Fig. 1J). This is in agreement with our previous observation that IP6K1-deficient neutrophils exhibit increased PtdIns(3,4,5)P3 signaling, enhanced phagocytic and bactericidal capacity, and elevated NADPH oxidase–mediated superoxide production (1). Consistently, E. coli–elicited lung damage (Fig. 1, K to L, and fig. S3D) and the related death (fig. S3E) were also reduced in platelet-depleted IP6K1-deficient mice. Together, our results demonstrate that both neutrophils and platelets contributed to IP6K1 function in E. coli–elicited bacterial pneumonia.
IP6K1 disruption alleviates lung damage in pneumonia induced by Gram-positive bacteria Staphylococcus aureus
NPA formation facilitates neutrophil accumulation in alveolar spaces in various types of acute lung inflammation, not just in E. coli–induced lung inflammation (2–5). Thus, we next explored whether IP6K1 disruption can also alleviate lung damage in pneumonia induced by Gram-positive bacteria. Pneumonia was induced by intratracheal instillation of S. aureus, a commonly used and clinically relevant Gram-positive coccal bacterium. Similar to what was observed in E. coli–induced pneumonia, the clearance of S. aureus was also enhanced in IP6K1-deficient mice (fig. S4). The lung bacterial burden was substantially reduced compared to WT mice 24 hours after bacterial instillation (fig. S4A). IP6K1 deficiency also led to a reduction in pulmonary neutrophil accumulation in the inflamed lungs (fig. S4B). Consistently, pneumonia-induced vascular leakage and lung damage, measured as total protein levels in the BALF and lung wet-to-dry ratio, in IP6K1-deficient mice was much less severe than in WT mice (fig. S4, C and D). In addition, the pneumonia-related mortality was reduced in the IP6K1-deficient mice (fig. S4E). Together, these results reveal that disrupting IP6K1 protects hosts from infection-induced lung damage in both Gram-negative and Gram-positive bacterial pneumonia.
IP6K1 disruption reduces neutrophil accumulation and alleviates lung damage in LPS-induced lung inflammation
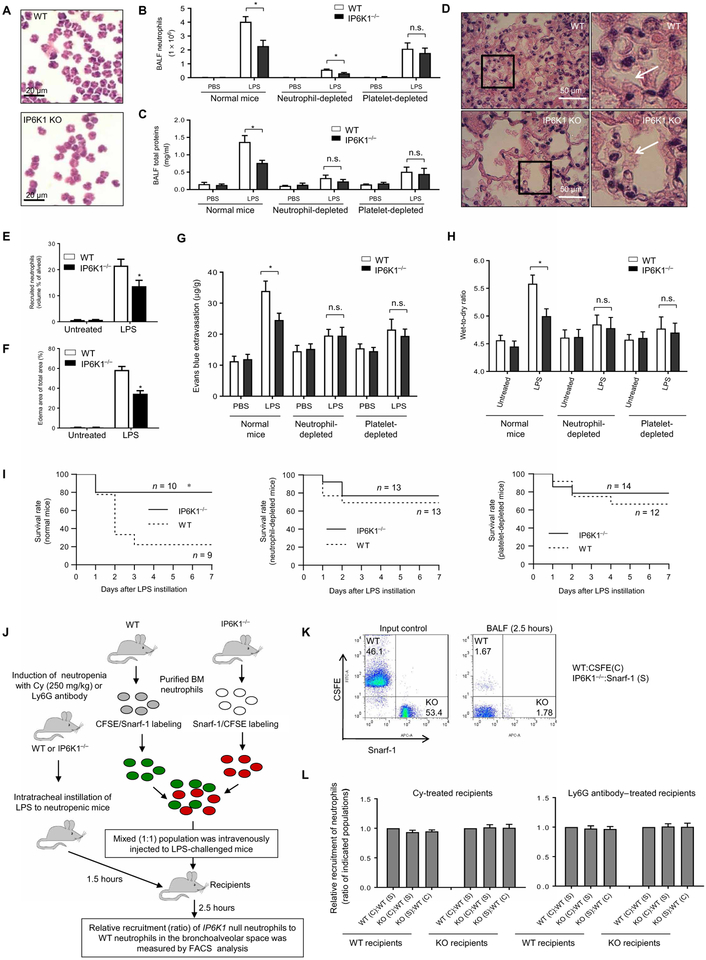
To directly assess whether IP6K1 disruption can reduce neutrophil accumulation independent of bacterial clearance, we examined neutrophils in an LPS-induced acute lung injury (ALI) model. IP6K1 disruption similarly diminished LPS-induced neutrophil accumulation in the inflamed lung as assessed by both quantification of BALF neutrophil numbers (Fig. 2, A and B). As expected, reduced neutrophil accumulation in the lungs substantially alleviated inflammation-induced lung damage. The total BALF protein level (Fig. 2C) and edema formation (Fig. 2, D and F) decreased in the lungs of IP6K1-deficient mice compared to WT mice. Finally, we directly assessed vascular permeability by using Evans blue, an albumin-binding dye (Fig. 2G), and by measuring lung wet-to-dry ratio (Fig. 2H). Infection-induced increases in pulmonary vascular permeability were partially suppressed in IP6K1-deficient mice. Consistently, ALI-related mortality was reduced in these mice (Fig. 2I). Thus, disrupting IP6K1 reduced neutrophil accumulation and alleviated lung injury independent of increased bactericidal effects.
Fig. 2. Disrupting IP6K1 inhibits neutrophil accumulation and reduces lung damage in LPS-induced lung inflammation.
Mice were intratracheally instilled with lipopolysaccharide (LPS; 5 mg/kg; E. coli O111:B4) and euthanized at the indicated time points. (A) Total cells in BALF were stained with a modified Wright-Giemsa stain. (B) The number of pulmonary neutrophils was counted using cytospin preparations. Data are means ± SD (n = 4 mice per group). (C) BALF total protein levels. Data are means ± SD (n = 4 mice per group). (D) Representative H&E staining of LPS-treated lung tissues. (E) Quantification of recruited neutrophils in alveolar spaces. Data are means ± SEM (n ≥ 5 mice per group). (F) Pulmonary edema formation quantified as the percentage of edema area in the total parenchymal region. Data are means ± SEM (n = 5 mice per group). (G) Lung vascular permeability was evaluated by using EB dye. Data are means ± SD (n = 5 mice per group). (H) Lung wet weight–to–dry weight ratio was measured at 24 hours after E. coli instillation. Data are means ± SD (n = 4 mice per group). (I) Survival rates of LPS-challenged WT and IP6K1-deficient mice. Mice were intratracheally challenged with LPS (10 mg/kg body weight). Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. (J) Schematic of the neutrophil-adoptive transfer assay. (K) Purified WT and IP6K1-deficient neutrophil mixture for adoptive transfer (input) and adoptively transferred circulating neutrophils. (L) Relative accumulation rates were quantified as ratios of adoptively transplanted IP6K1-null neutrophils to WT neutrophils in BALF. Data are means ± SD (n = 4 mice per group). *P < 0.05 versus WT mice. Statistical analysis was performed using Student’s t test unless differently indicated.
We next explored whether the reduced neutrophil accumulation in IP6K1-deficient mice is due to IP6K1 disruption in neutrophils. In IP6K1-deficient mice, IP6K1 expression is ablated in all cell types including endothelial cells, lymphocytes, platelets, and macrophages. Therefore, IP6K1 disruption may alter the overall inflammatory environment in the lungs to affect neutrophil function and accumulation indirectly. To circumvent this problem, we used an adoptive transfer assay to directly explore neutrophil trafficking (Fig. 2J). Purified Ip6k1-null neutrophils were labeled with green fluorescent dye 5-(and 6-) carboxyfluorescein diacetate succinimidyl esters (CFSEs), and WT neutrophils were labeled with a red fluorescent dye 5- (and 6-) chloromethyl SNARF-1 acetate or vice versa.
To increase the relative frequency of adoptively transferred neutrophils and improve detection sensitivity, we reduced numbers of endogenous neutrophils by treating recipients with cyclophosphamide, a chemotherapeutic drug, or a Ly6G antibody before challenging recipients with LPS (12). In this setup, the WT and KO neutrophils were isolated and prepared using an identical procedure before being mixed and studied in parallel such that accumulation of WT and KO neutrophils would occur under exactly the same conditions. The relative accumulation of neutrophils was calculated as the ratio of CFSE+ to Snarf-1+ cells in the BALF normalized to the ratio of these two populations in the peripheral blood. IP6K1 disruption did not alter neutrophil half-life in the peripheral blood, as shown by the CFSE+–to–Snarf-1+ cell ratio being similar to the input control (1:1). This ratio was also unaltered in the BALF, indicating that these two populations were similarly recruited to the lungs (Fig. 2, K and L). Adoptively transferred neutrophil presence in the inflamed lungs after LPS installation was also independent of the neutrophil staining method (Fig. 2L). Therefore, the reduced neutrophil accumulation observed in the IP6K1-deficient mice was not simply due to an intrinsic migration defect elicited by IP6K1 disruption in neutrophils. The experiments were conducted in both WT and IP6K1 KO recipient mice, and we observed essentially the same results (Fig. 2L).
IP6K1 in platelets is essential for efficient neutrophil-platelet aggregation
A recent study showed that IP6K1 critically regulates mammalian hemostasis by controlling inorganic polyP production in platelets: IP6K1-deficient platelets produced less polyP, slowing platelet aggregation and impairing platelet-mediated plasma clotting (13). Because reduced neutrophil accumulation in IP6K1-deficient mice was not caused by IP6K1 disruption in neutrophils, we hypothesized that IP6K1 regulates neutrophil presence in the inflamed lungs indirectly by controlling platelet function.
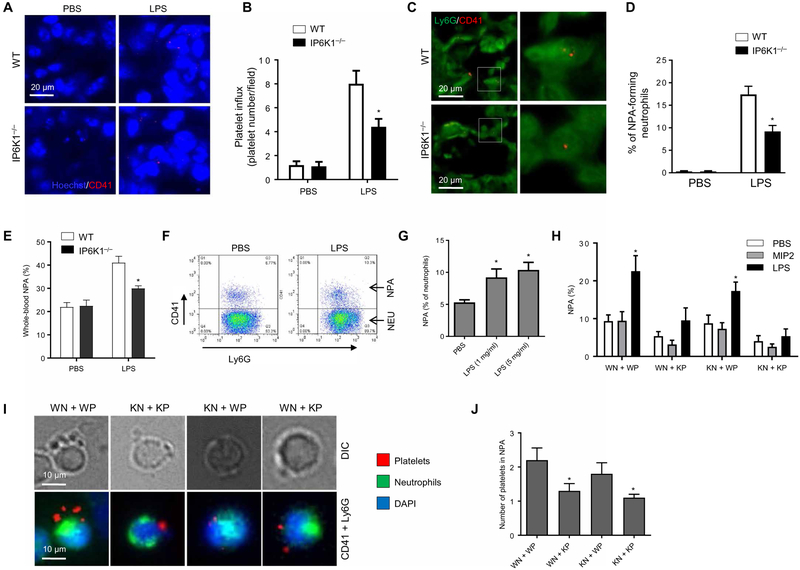
During lung inflammation, both neutrophils and platelets are sequestered in the pulmonary vasculature. Disruption of IP6K1 reduced not only neutrophil but also platelet accumulation in the lungs in LPS-induced lung inflammation (Fig. 3, A and B). The reduced number of CD41+ platelets was not caused by a decrease in surface expression of CD41 because Ip6k1-null platelets expressed the same amount of the platelet markers CD41 and CD61 (fig. S5, A and B). We next quantified NPAs in lung sections stained with Gr1 and CD41 antibodies. LPS-induced NPA formation was substantially lower in IP6K1-deficient mice compared to WT mice (Fig. 3, C and D). We also quantified NPAs in the peripheral blood in live animals. The percentage of NPA in the blood of LPS-challenged mice increased compared to that in untreated mice, but such increase was attenuated in the IP6K1-deficient mice (Fig. 3E).
Fig. 3. Disruption of IP6K1 in platelets suppresses NPA formation both ex vivo and in vivo in LPS-induced lung inflammation.
(A) Platelet accumulation in the lungs after intratracheal LPS challenge (5 mg/kg body weight). Lung sections were stained with CD41 antibody. Hoechst was used as a nuclear counterstain. (B) The platelet index in lung sections was expressed as the number of platelets (CD41+ cells) per field of view. At least five fields of view were randomly picked for each experiment, and the averages were used for the calculation. Data are means ± SEM (n = 10 mice total, data are pooled from three experiments). (C) Neutrophil-platelet aggregates (NPAs) in the inflamed lungs. The lung sections were costained with Ly6G and CD41 antibodies. The colocalization of neutrophils (Ly6G+) and platelets (CD41+) indicated NPA formation. The right panel is an inset of the left. (D) NPA % was calculated as the percentage of Ly6G and CD41 double-positive cells (NPAs) among all Ly6G+ cells. Data are means ± SEM (n = 10 mice). (E) Fluorescence-activated cell sorting (FACS) analysis of NPA formation in peripheral blood. Data are means ± SEM (n = 4 mice). *P < 0.05 versus WT mice. (F) FACS analysis of ex vivo NPA formation. WT neutrophils and platelets were incubated with LPS (1 μg/ml) for 2 hours at 37°C. After incubation, cell mixtures were stained with CD11b, CD41, and Ly6G. The NPAs were CD41 and Ly6G double-positive on FACS. (G) NPA formation was calculated at the indicated LPS concentrations. Data are means ± SEM of four experiments. (H) NPA formation between WT or Ip6k1 knockout (KO) neutrophils and platelets. WT (WN) or Ip6k1-KO (KN) neutrophils and WT (WP) or Ip6k1-KO (KP) platelets were incubated in the presence of LPS (5 μg/ml) or macrophage inflammatory protein 2 (MIP2; 1 nM). Data are means ± SEM of ≥4 experiments. (I) NPA formation between fluorescently labeled neutrophils and platelets. Neutrophils were stained with calcein AM (5 μg/ml) for 10 min. Platelets were isolated from whole blood and stained with calcein red AM (5 μg/ml) for 10 min. The labeled neutrophils and platelets were incubated with LPS (5 μg/ml) for 2 hours at 37°C. Shown are representative images. (J) The number of platelets in each NPA. The experiment was repeated three times, and the data were pooled and analyzed together. Data are means ± SEM (n = 10 samples). *P < 0.05 versus WN + WP. Statistical analysis was performed using Student’s t test.
To directly assess the role of IP6K1 in NPA formation, we used an ex vivo system in which purified neutrophils and purified platelets underwent heterotypic aggregation in the presence of serum components before detection by fluorescence-activated cell sorting (Fig. 3F and fig. S6). Consistent with the in vivo data, LPS treatment increased NPA formation by nearly 100% (Fig. 3G). However, LPS-induced NPA formation did not occur between IP6K1-deficient neutrophils and IP6K1-deficient platelets, regardless of the LPS used (LPS from E. coli O111:B4 or LPS from E. coli O157:H7; fig. S7).
LPS-induced NPAs were completely abolished when WT neutrophils and Ip6k1-null platelets were incubated together but could still be formed when Ip6k1-null neutrophils and WT platelets were used (Fig. 3H). LPS-elicited NPA formation appeared to be specific; MIP2, a common proinflammatory cytokine, failed to induce the same NPA formation (Fig. 3I). We next assessed the number of platelets in each LPS-induced NPA by immunostaining and found that the number of platelets was reduced when platelet IP6K1 was disrupted (Fig. 3, I and J). Collectively, these findings demonstrate that platelet IP6K1 expression was essential for LPS-induced NPA formation in the inflamed lungs, suggesting that reduced neutrophil presence in the lungs observed in IP6K1-deficient mice may be due to IP6K1 disruption in platelets rather than in neutrophils. (1). We also assessed the bactericidal activity of neutrophil-platelet cocultures. Disruption of IP6K1 in neutrophils, but not in platelets, substantially diminished the bacterial killing capability (fig. S8), indicating that the elevated bactericidal activity in the IP6K1-deficient mice may be mediated by neutrophils.
IP6K1-mediated polyP production by platelets plays a critical role in LPS-induced formation of NPAs
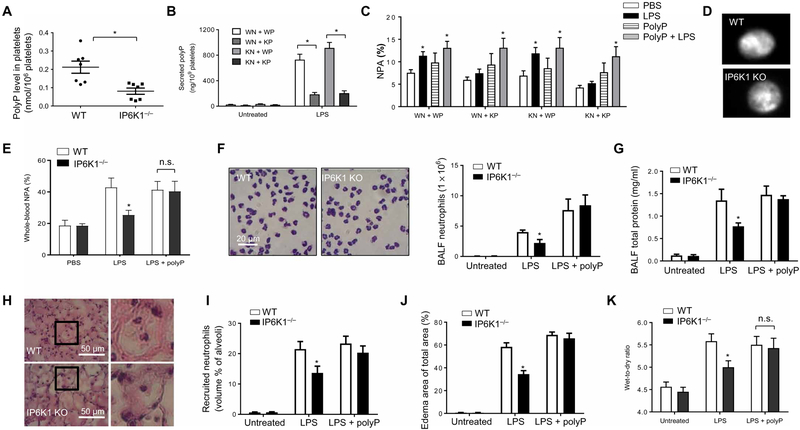
Upon stimulation, activated platelets release polyP into the extra-cellular space to regulate mammalian hemostasis (14–16). Consistent with a previous study (13), we found that IP6K1 could control platelet polyP production and that polyP levels were reduced in IP6K1-deficient platelets (Fig. 4A). LPS stimulation also augmented the level of secreted extracellular polyP in platelet-neutrophil coculture. IP6K1 disruption in platelets, but not in neutrophils, substantially suppressed LPS-induced polyP secretion (Fig. 4B). Accordingly, we hypothesized that the defective NPA formation observed in Ip6k1-null mice was due to impaired platelet polyP production. Confirming this hypothesis, the defective NPA formation between IP6K1-deficient platelets and WT or IP6K1-deficient neutrophils was rescued by the addition of polyP in the in vitro NPA-forming assay (Fig. 4C). The defective LPS-induced NPA formation was, therefore, likely to be mainly caused by reduced polyP levels in a system in which most of polyP was produced by platelets. Notably, polyP-mediated NPA formation still relied on LPS stimulation; polyP alone did not elicit NPA formation, suggesting that other LPS-induced signals were also required for efficient NPA formation. Furthermore, IP6K1 disruption only affected the amount of polyP produced by platelets with the granular localization of polyP remaining unaltered (Fig. 4D).
Fig. 4. IP6K1-mediated polyP production by platelets plays a critical role in LPS-induced NPA formation.
(A) Polyphosphate (polyP) levels in WT and Ip6k1−/− platelets. *P < 0.05 versus WT platelets. (B) LPS-induced polyP secretion in neutrophil-platelet cocultures. WT or Ip6k1-KO neutrophils (5 × 106) and WT or Ip6k1-KO platelets (1 × 109) were incubated in the presence of LPS (5 μg/ml) for 2 hours at 37°C. PolyP levels in the supernatants were measured. Data are means ± SEM of four experiments. (C) NPA formation in the presence of polyP analyzed as described in Fig. 3F. Data are means ± SEM of five experiments. *P < 0.05 versus cells treated with phosphate-buffered saline (PBS). (D) Subcellular localization of polyP in unstimulated platelets. PolyP was stained with 4′,6-diamidino-2-phenylindole (DAPI). DAPI-polyP fluoresces yellow when viewed under ultraviolet light. (E) FACS analysis of NPA formation in peripheral blood. Shown are percentages of neutrophils (Ly6G+) forming NPAs in whole blood. Data are means ± SEM (n = 5 mice per group). *P < 0.05 versus WT mice. (F) Neutrophil accumulation to the inflamed lungs in polyP-treated mice. Data are means ± SEM (n = 5 mice per group). (G) BALF total protein levels. Data are means ± SEM (n ≥ 5 mice per group). (H) H&E staining of lung tissues. The right panels are insets of left panels. (I) Recruited neutrophils in alveolar spaces were quantified as volume fraction of the alveolar spaces using standard point-counting morphometry. Data are means ± SEM (n ≥ 5 mice per group). (J) Pulmonary edema formation was quantified as the percentage of edema area in the total parenchymal region. Data are means ± SEM (n ≥ 5 mice per group). *P < 0.05 versus WT mice. (K) Lung wet weight–to–dry weight ratio was measured at 24 hours after LPS instillation. Data are means ± SEM (n = 4 mice per group). *P < 0.05 versus WT mice. Statistical analysis was performed using Student’s t test.
Consistent with the in vitro results, injection of polyP into IP6K1-deficient mice restored LPS-induced NPA formation in the peripheral blood (Fig. 4E) and pulmonary neutrophil numbers (Fig. 4, F, H, and I) to the same as that in WT mice. PolyP treatment also increased pulmonary vascular permeability and neutrophils in Ip6k1-KO mice. The difference between the WT and KO mice was diminished when polyP was applied (Fig. 4, F to I). Consistent with this, polyP-treated IP6K1-deficient mice displayed the same amount of LPS-induced lung damage as polyP-treated WT mice (Fig. 4, J and K). Therefore, IP6K1-mediated polyP production by platelets appears to play a critical role in LPS-induced NPA formation. Reduced polyP production contributed to the decreased neutrophil accumulation and alleviated the lung damage observed in IP6K1-deficient mice. Noticeably, PolyP treatment could still enhance neutrophil presence in the absence of NPA, indicating the existence of an NPA-independent mechanism (fig. S9).
PolyP regulates bradykinin pathway and bradykinin-mediated neutrophil activation
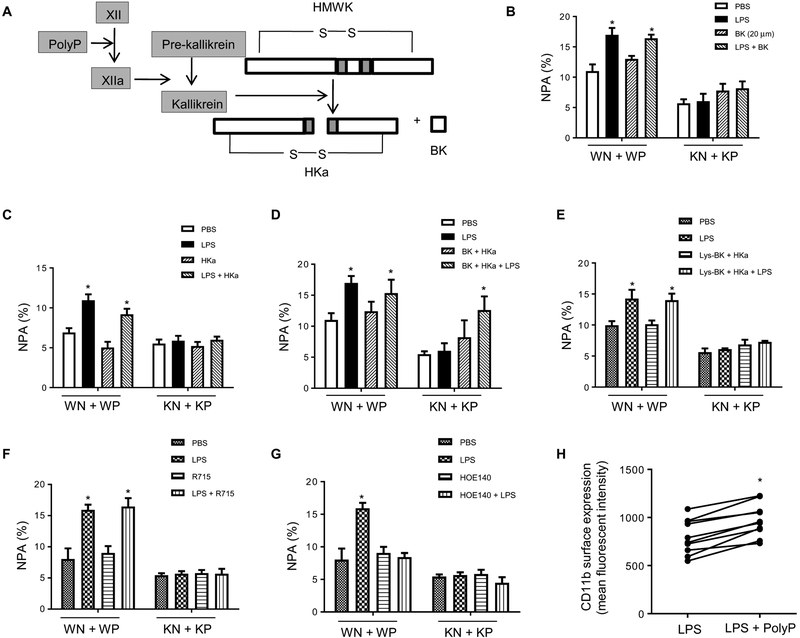
We next explored the mechanism by which polyP regulates NPA formation. It has previously been shown that platelet-derived polyP can trigger the plasma protease factor XII–dependent contact activation system to drive inflammatory reactions (14, 15, 17). PolyP directly binds to and activates factor XII, leading to proteolysis of high–molecular weight kininogen (HMWK) by kallikrein and the release of the inflammatory mediator bradykinin and cleaved HMWK (HKa; Fig. 5A). Both HKa and bradykinin were required for LPS-induced NPA formation. Neither bradykinin (Fig. 5B) nor HKa alone (Fig. 5C) rescued the defective NPA formation elicited by IP6K1 disruption in platelets. When both bradykinin and HKa were applied exogenously, the LPS-induced NPA between IP6K1-deficient platelets and WT or IP6K1-deficient neutrophils was restored (Fig. 5D).
Fig. 5. PolyP regulates NPA formation through the bradykinin pathway.
(A) Schematic of polyP activation in the bradykinin pathway. HMWK (or HK) high–molecular weight kininogen; BK, bradykinin; HKa, cleaved HMWK. (B) NPA formation in the presences or absence of bradykinin (20 μM). Neutrophils were pretreated with bradykinin for 5 min and then incubated with WT or IP6K1-deficient platelets. Data are means ± SD of three experiments. *P < 0.05 versus cells treated with PBS. (C) NPA formation in the presence or absence of HKa (1 μg/ml). Data are means ± SEM of ≥3 experiments. (D) NPA formation in the presence or absence of both bradykinin and HKa. Data are means ± SEM of three experiments. (E) NPA formation in the presence or absence of HKa and Lys-BK (1 μM), a B1 receptor agonist. Data are means ± SEM of four experiments. (F) NPA formation in the presence or absence of a BK1 receptor inhibitor. Neutrophils were pretreated with BK2 receptor inhibitor R715 (8 μM) for 5 min. Data are means ± SEM of four experiments. (G) NPA formation in the presence or absence BK2 receptor inhibitor. Neutrophils were pretreated with BK2 receptor inhibitor HOE140 (150 nM) for 5 min. Data are means ± SEM of four experiments. (H) Surface expression of adhesion molecule CD11b on neutrophils. WT neutrophils and platelets were treated with polyP for 2 hours, and CD11b surface levels were detected by FACS. *P < 0.05 versus cells treated with LPS alone (n = 10 mice per group). Statistical analysis was performed using Student’s t test.
Bradykinin exerts its function via G protein–coupled bradykinin receptors. There are two bradykinin receptors, B1 and B2; bradykinin is the major B2 receptor agonist, whereas the B1 receptor is mainly activated by des-Arg9-BK, a bradykinin metabolite. B1 receptor expression is known to be up-regulated under inflammatory conditions and to mediate neutrophil migration elicited by cytokines such as IL-1β (18, 19). However, treatment of a neutrophil-platelet mixture with HKa and the B1 receptor–specific agonist Lys-BK could not restore NPA formation between IP6K1-deficient platelets and neutrophils (Fig. 5E), suggesting that the B1 receptor may not be critical for LPS-induced NPA formation. To definitively determine the receptor type mediating NPA formation, we treated neutrophil-platelet mixtures with specific B1 and B2 receptor antagonists. HOE140, a B2 receptor antagonist, inhibited LPS-induced NPA formation (Fig. 5F), whereas R715, a B1 receptor antagonist, failed to do so (Fig. 5G), confirming that bradykinin’s effect on NPA formation was mediated via B2 receptors. As a well-known proinflammatory factor and neutrophil activator, bradykinin specifically increases surface expression of the CD11b and CD18 adhesion molecules on neutrophils (20). The same effect was also detected in polyP-stimulated neutrophil and platelet cell mixtures (Fig. 5H and fig. S10). HKa can also promote platelet-neutrophil interactions by bridging CD11b on neutrophils and its receptor glycoprotein Ib (GPIb) on platelets (21), thus providing a potential mechanism for LPS-induced and polyP-mediated NPA formation.
Treatment with the IP6K1 inhibitor TNP alleviates pulmonary inflammation and bacterial pneumonia–associated lung damage
IP6K1 facilitates NPA formation by maintaining high polyP levels in platelets, thereby providing a mechanism to control neutrophil accumulation during lung infection and inflammation. It also suggests that IP6K1 could be a legitimate therapeutic target in infection and inflammation-induced lung injury, as shown by the Ip6k1-KO mouse model. Thus, we next investigated whether a specific IP6K1 inhibitor TNP [N2-(m-(trifluoromethyl)benzyl) N6-(p-nitrobenzyl)purine] (22) could also alleviate lung damage in a mouse bacterial pneumonia model. We found that both mouse and human neutrophils treated with TNP showed substantially enhanced PtdIns(3,4,5)P3 signaling and elevated PtdIns(3,4,5)P3-mediated cellular functions [fig. S11 (1)].
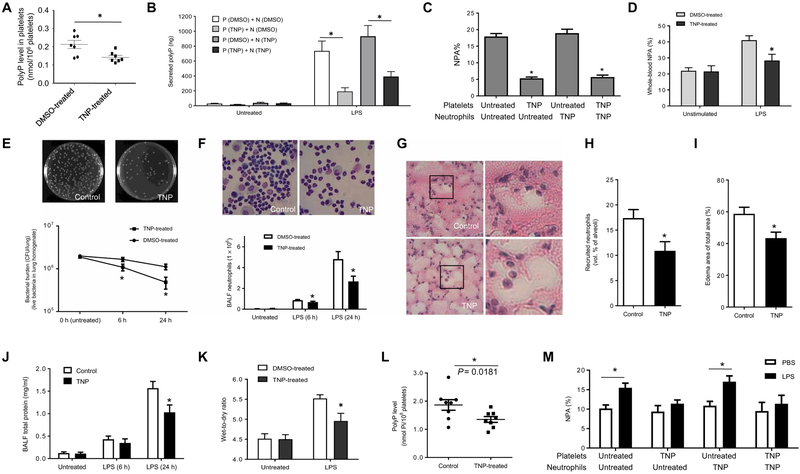
TNP treatment efficiently reduced polyP production in platelets, with platelets isolated from TNP-treated mice displaying reduced intracellular polyP levels and LPS-elicited polyP secretion compared to those isolated from dimethyl sulfoxide–treated controls (Fig. 6, A and B). As a result, LPS-induced ex vivo NPA formation and infection-induced NPA formation in the lungs of live mice were inhibited by TNP treatment (Fig. 6, C and D). Consistent with the results observed in IP6K1-deficient mice, TNP-treated mice exhibited much improved bactericidal activity (Fig. 6E), and neutrophil accumulation in the inflamed lungs was suppressed as assessed by both BALF neutrophil counts (Fig. 6F) and morphometric analysis of lung sections (Fig. 6, G and H). Consequently, TNP treatment alleviated inflammation-induced lung damage: There was less edema formation (Fig. 6I), and the total BALF protein levels were decreased (Fig. 6J) in TNP-treated mice compared to those treated with PBS alone. Finally, the measurement of lung wet-to-dry ratio directly confirmed a reduction in pulmonary vascular permeability in TNP-treated mice (Fig. 6K). Thus, inhibiting IP6K1 with TNP efficiently and effectively reduced neutrophil accumulation and alleviated lung injury.
Fig. 6. Treatment with IP6K1 inhibitor TNP alleviates pulmonary inflammation and lung damage associated with bacterial pneumonia.
(A) PolyP abundance in platelets. Mice were treated with TNP [N2-(m-(trifluoromethyl)benzyl) N6-(p-nitrobenzyl)purine] or dimethyl sulfoxide (DMSO) alone for 10 days (20 mg/kg body weight, once a day). The experiment was repeated three times, and the data were pooled and analyzed together. Data are means ± SEM (n = 7 mice per group). (B) LPS-induced polyP secretion in neutrophil-platelet cocultures. Neutrophils (5 × 106) isolated from DMSO (N-DMSO)– or TNP (N-TNP)–treated mice and platelets (1 × 109) isolated from DMSO (P-DMSO)– or TNP (P-TNP)–treated mice were incubated in the presence of LPS (5 μg/ml) for 2 hours at 37°C. Data are means ± SEM of four experiments. (C) LPS-induced NPA formation. Neutrophils and platelets were isolated from DMSO (untreated) and TNP-treated mice and incubated with LPS (1 μg/ml) for 2 hours at 37°C. Data are means ± SEM of five experiments. (D) Percentage of NPAs in whole blood. DMSO- and TNP-treated mice were intratracheally instilled with LPS (5 mg/kg). NPA formation was analyzed at 24 hours after the LPS instillation. Data are means ± SEM (n = 4 mice per group). (E) Bacterial killing in inflamed lungs. Data are means ± SEM (n ≥ 5 mice per group). (F) Neutrophil accumulation in the inflamed lungs. Data are means ± SEM (n ≥ 5 mice per group). (G) Representative H&E staining of lung tissues. (H) Recruited neutrophils in alveolar spaces were quantified as volume fraction of the alveolar space using standard point-counting morphometry. Data are means ± SEM (n = 9 mice per group). (I) Pulmonary edema formation was quantified as the percentage of edema area in the total parenchymal region using. Data are means ± SEM (n = 9 mice per group). (J) Lung vascular permeability was evaluated by BALF total protein level. Data are means ± SEM (n ≥ 5 mice per group). (K) Lung wet weight–to– dry weight ratio was measured at 24 hours after E. coli instillation. Data are means ± SD (n = 4 mice per group). (L) PolyP levels in human platelets. Human platelets were treated with TNP (20 μM) at room temperature for 4 days. Data are means ± SEM (n = 8 donors per group). (M) NPA formation between human neutrophils and platelets. NPA% was calculated as the percentage of CD66+, CD16+, and CD41 triple-positive cells (NPAs) among all CD66+ and CD16+ cells. Data are means ± SEM of five experiments. *P < 0.05. Statistical analysis was performed using Student’s t test.
To examine whether inhibition of IP6 kinase can alter LPS-induced NPA formation between human primary neutrophils and platelets, we treated human platelets and/or neutrophils with the IP6K-selective inhibitor TNP. Consistent with the results observed in mice, human platelets treated with TNP exhibited reduced polyP levels (Fig. 6L). Consequently, LPS-induced NPA formation was suppressed between human primary neutrophils and platelets treated with TNP. This effect was mainly mediated by IP6K inhibition in platelets because NPA formation between TNP-treated neutrophils and untreated platelets was unaltered (Fig. 6M). These results suggest that IP6K also plays a role in regulating NPA formation between human neutrophils and platelets.
DISCUSSION
By producing IP7, IP6K1 negatively regulates PtdIns(3,4,5)P3 signaling. Thus, IP6K1 disruption in mouse neutrophils elevates PtdIns(3,4,5)P3 signaling and enhances various PtdIns(3,4,5)P3-mediated neutrophil functions such as phagocytosis and ROS production (1). Because up-regulated PtdIns(3,4,5)P3 signaling also augments neutrophil accumulation (23, 24), the fact that IP6K1 disruption suppressed neutrophil migration to inflamed lungs in the pneumonia model is somewhat surprising. We show that this effect is mediated by a platelet-mediated but PtdIns(3,4,5)P3 signaling–independent mechanism. Numerous studies showed that platelets have the capacity to promote neutrophil accumulation (8–11). Neutrophil accumulation in inflamed lungs is controlled by a unique mechanism that critically involves platelets (25). NPA formation promotes neutrophil accumulation in the alveolar spaces during acute lung inflammation. In addition, endovascular NPAs may directly damage the pulmonary capillary endothelium, exaggerating lung injury. In a mouse transfusion-related acute lung injury model (2) and a mouse LPS-induced (3) or acid-induced acute respiratory distress syndrome (4, 5) model, both neutrophils and platelets sequester in the pulmonary vasculature. Platelet depletion or treatments that disrupt NPAs can substantially reduce inflammation-induced lung damage (2–6). A recent study showed that IP6K1 is a novel regulator of mammalian hemostasis via the control of inorganic polyP production by platelets. IP6K1-deficient mice have reduced platelet polyP levels, slower platelet aggregation, and impaired platelet-mediated plasma clotting (13). Here, we demonstrate that the defective neutrophil accumulation in the lungs in IP6K1-deficient mice is mainly caused by IP6K1 disruption in platelets but not in neutrophils.
IP6K1 disruption leads to reduced neutrophil accumulation in the lungs in bacterial pneumonia. One obvious explanation for reduced neutrophil accumulation is that the augmented neutrophil killing and resulting bacterial clearance accelerates the resolution of pulmonary inflammation, in turn causing fewer neutrophils. However, the amount of proinflammatory cytokines in the inflamed lungs is unaltered in the IP6K1-deficient mice. In addition, disruption of IP6K1 does not alter neutrophil apoptosis during lung inflammation; thus, the reduced neutrophil accumulation is not due to accelerated death. We conducted an adoptive transfer assay to directly explore neutrophil trafficking and accumulation. Adoptively transferred WT and IP6K1-deficient neutrophils were recruited to the inflamed lung at a similar rate, suggesting that the reduced neutrophil accumulation observed in the IP6K1-deficient mice was not simply due to an intrinsic migration defect elicited by IP6K1 disruption in neutrophils. As previously reported, platelet depletion could reduce LPS-induced lung damage. The reduced neutrophil accumulation and lung injury observed in the IP6K1-deficient mice was diminished in platelet-depleted mice, indicating that platelets contributed to IP6K1 function in LPS-elicited pulmonary inflammation and injury. The reduced neutrophil accumulation in IP6K1-deficient mice is likely due to decreased polyP production by IP6K1-deficient platelets because this defect can be rescued by polyP treatment. Consistent with this, polyP-treated IP6K1-deficient mice displayed the same amount of LPS-induced lung damage as that of polyP-treated WT mice. Therefore, IP6K1-mediated polyP production by platelets appears to play a critical role in lung inflammation. We further demonstrate that polyP regulates neutrophil accumulation by triggering the bradykinin pathway and bradykinin-mediated neutrophil activation.
PolyP treatment rescued impaired LPS-induced NPA formation between IP6K1-deficient platelets and neutrophils, suggesting that IP6K1’s function in NPA formation is mediated by polyP. Consistently, treatment of IP6K1-deficient mice with polyP restored LPS-induced pulmonary neutrophil accumulation to the same levels as that of WT mice. However, treatment with polyP was not sufficient for NPA formation; LPS stimulation was required for this IP6K1-mediated process. Thus, other LPS-dependent factors are also involved in NPA formation. One mechanism by which polyP regulates NPA formation is through factor XII activation and the subsequent generation of bradykinin and HKa. Here, both HKa and bradykinin were required for LPS-induced NPA formation, with bradykinin specifically increasing surface expression of adhesion molecule Mac-I on neutrophils and HKa presumably promoting NPAs by bridging Mac-1 (CD11b/CD18) on neutrophils to its receptor GPIb on platelets (21). HKa is also reported to be able to interact with Mac-1 and block Mac-1–dependent leukocyte adhesion to endothelial cells (26, 27).
IP6K1 facilitates NPA formation by maintaining high polyP levels in platelets. Here, we hypothesize that the reduced polyP production contributed to the decreased NPA formation, diminished neutrophil accumulation, and alleviated lung damage observed in IP6K1-deficient mice. However, although the concept of NPA has been around for over a decade, there is still no direct evidence showing that NPAs mediate neutrophil accumulation. Some studies show that there is no preferential pulmonary sequestration of NPA in local (28) or systemic (29) inflammation. Here, we demonstrate that polyP produced by platelets is essential for neutrophil accumulation. PolyP regulates neutrophil accumulation by triggering the bradykinin pathway. As a well-known proinflammatory factor and neutrophil activator, bradykinin specifically increases surface expression of the CD11b and CD18 adhesion molecules on neutrophils (20) and thus may enhance neutrophil accumulation independent of NPA. A previous report by Asaduzzaman et al. (29) also showed that platelets play a key role in regulating neutrophil infiltration in the lung via up-regulation of Mac-1 (CD11b/CD18) in sepsis induced by cecal ligation and puncture. In platelet-depleted mice, LPS-induced NPA formation was suppressed with no difference detected between WT and IP6K1-deficient mice. However, polyP treatment could still enhance neutrophil accumulation in the absence of NPA, indicating existence of an NPA-independent mechanism.
MATERIALS AND METHODS
Study design
The research objective of our study was to determine how IP6K1, through polyP production by platelets, regulates neutrophil accumulation in bacterial pneumonia. To achieve this objective, we undertook various approaches, including measurement of BALF neutrophil count, bacterial killing assay, hematoxylin and eosin staining, and assessment of pulmonary edema formation, to analyze neutrophil accumulation, host defense, and neutrophil-mediated pulmonary damage in bacterial pneumonia. For animal studies, 10- to 14-week-old mice were used. The Children’s Hospital Animal Care and Use Committee approved and monitored all animal procedures. The effect of IP6K1 disruption or the specific IP6K inhibitor TNP on phenotype was assessed by investigators who were blind for genotype and treatment. To perform reliable statistical analysis, at least three independent experiments were conducted for each data shown in the manuscript, unless differently indicated in the figure legends. These numbers were chosen based on power analyses and previous experience in our laboratory. Primary data are located in table S1.
Neutrophil depletion with Ly6G antibody
Neutrophil depletion with Ly6G antibody was carried out as previously described (30). Briefly, WT and IP6K1-deficient mice were intraperitoneally injected with a single dose of anti-mouse Ly6G antibody (400 μg/kg; clone 1A8, BioLegend). The antibody was administered intraperitoneally to obtain a sustained depletion over the first 48 hours of the experiment. Differential white blood cell count using Wright-Giemsa staining was performed to confirm that the neutrophil depletion was successful (peripheral blood neutrophil count was reduced by >85%) (30).
Platelet depletion in WT and IP6K1-deficient mice
Mice were intravenously injected with a single dose of platelet-depleting antibody (2 μg/g bodyweight, diluted in 100 μl of sterile PBS; anti-GPIb/CD42b, Emfret Analytics). The peripheral blood platelet counts were assessed at indicated time points.
Neutrophil-platelet aggregation
Mouse and human neutrophils were isolated and purified as previously described (31). Whole blood was collected into acid citrate dextrose tubes and platelet-rich plasma was isolated by centrifuging twice at 100g for 5 min. Neutrophils (0.5 × 106) and platelets (5 × 106) were incubated for 2 hours at 37°C with LPS in 1 ml of Tyrode’s buffer (Sigma) supplemented with 1% bovine serum albumin. After incubation, mouse neutrophils and platelets were stained with CD11b, CD41, and Ly6G and analyzed by flow cytometry to detect NPAs. Human neutrophils and platelets were stained with CD66, CD16, and CD41. NPA% was calculated as the percentage of CD66+, CD16+, and CD41 triple-positive cells (NPAs) among all CD66+ and CD16+ cells.
Statistical analysis
Survival rates were analyzed using the Kaplan-Meier survival curves and log-rank test. Other values were compared using Student’s t test. Data were presented as means (±SD). Statistical significance was determined using a two-tailed paired t test for Figs. 5H and 6L, and a two-tailed unpaired t test was used for other comparisons. All calculations were performed using GraphPad Prism 6.0 software for Windows (GraphPad Software). Most experiments were repeated at least three times, and the data were pooled and analyzed together. Differences were considered significant when the P value was < 0.05.
Supplementary Material
Acknowledgments:
We thank L. Silberstein, J. Manis, and L. Chai for helpful discussions. Cell sorting was performed at the Harvard Stem Cell Institute/Diabetes Research Center Flow Core.
Funding:Y.X. was supported by grants from the National Basic Research Program of China (2015CB964903), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016–12M-1–003 and 2017-I2M-1–015), and the Chinese National Natural Science Foundation (31471116). H.R.L. was supported by NIH grants (R01AI103142, R01HL092020, and P01 HL095489) and a grant from the Flight Attendant Medical Research Institute (CIA 123008). A.C. was supported by NIH grant R01DK103746.
Footnotes
Competing interests: The authors declare that they have no competing financial interests.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/10/435/eaal4045/DC1
Materials and Methods
Fig. S1. Disruption of IP6K1 does not alter alveolar macrophage number, expression of surfactant proteins/antimicrobial peptides, and neutrophil apoptosis during lung inflammation.
Fig. S2. Disruption of IP6K1 does not affect the production of proinflammatory cytokines/chemokines.
Fig. S3. E. coli–induced pneumonia in untreated, neutrophil-depleted, and platelet-depleted mice.
Fig. S4. Disrupting IP6K1 enhances bacterial killing and reduces lung damage in S. aureus–induced pneumonia.
Fig. S5. IP6K1-deficient platelets expressed the same amount of platelet markers CD41 and CD61.
Fig. S6. Flow cytometry analysis of ex vivo NPA formation.
Fig. S7. NPA formation triggered by LPS from E. coli O157:H7.
Fig. S8. In vitro killing of bacteria by neutrophil-platelet coculture.
Fig. S9. PolyP can also enhance neutrophil accumulation via an NPA-independent mechanism.
Fig. S10. Surface expression of adhesion molecules on neutrophils.
Fig. S11. The level of PtdIns(3,4,5)P3 signaling, assessed by phospho-Akt, is elevated in TNP-treated mice.
Table S1. Primary data.
REFERENCES AND NOTES
- 1.Prasad A, Jia Y, Chakraborty A, Li Y, Jain SK, Zhong J, Roy SG, Loison F, Mondal S, Sakai J, Blanchard C, Snyder SH, Luo HR, Inositol hexakisphosphate kinase 1 regulates neutrophil function in innate immunity by inhibiting phosphatidylinositol-(3,4,5)-trisphosphate signaling. Nat. Immunol 12, 752–760 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA, Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J. Clin. Invest 119, 3450–3461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR, Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood 124, 2625–2634 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdulnour R-EE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD, Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. U.S.A. 111, 16526–16531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarbock A, Singbartl K, Ley K, Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest 116, 3211–3219 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asaduzzaman M, Rahman M, Jeppsson B, Thorlacius H, P-selectin glycoprotein-ligand-1 regulates pulmonary recruitment of neutrophils in a platelet-independent manner in abdominal sepsis. Br. J. Pharmacol 156, 307–315 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, Ye K, Luo HR, Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 113, 4930–4941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laschke MW, Dold S, Menger MD, Jeppsson B, Thorlacius H, Platelet-dependent accumulation of leukocytes in sinusoids mediates hepatocellular damage in bile duct ligation-induced cholestasis. Br. J. Pharmacol 153, 148–156 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Hundelshausen P, Weber C, Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res 100, 27–40 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Singbartl K, Forlow SB, Ley K, Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J. 15, 2337–2344 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Jurk K, Kehrel BE, Platelets: Physiology and biochemistry. Semin. Thromb. Hemost 31, 381–392 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Prasad A, Jia Y, Roy SG, Loison F, Mondal S, Kocjan P, Silberstein LE, Ding S, Luo HR, Pretreatment with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670 augments the efficacy of granulocyte transfusion in a clinically relevant mouse model. Blood 117, 6702–6713 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Shukla D, Suman K, Lakshmi BJ, Manorama R, Kumar S, Bhandari R, Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood 122, 1478–1486 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T, Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139, 1143–1156 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae J-S, Lee W, Rezaie AR, Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J. Thromb. Haemost 10, 1145–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrissey JH, Choi SH, Smith SA, Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 119, 5972–5979 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SA, Morrissey JH, Polyphosphate: A new player in the field of hemostasis. Curr. Opin. Hematol 21, 388–394 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean PG, Ahluwalia A, Perretti M, Association between kinin B1 receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J. Exp. Med 192, 367–380 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahluwalia A, Perretti M, Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1 beta in vivo in the mouse. J. Immunol 156, 269–274 (1996). [PubMed] [Google Scholar]
- 20.Figueroa CD, Matus CE, Pavicic F, Sarmiento J, Hidalgo MA, Burgos RA, Gonzalez CB, Bhoola KD, Ehrenfeld P, Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun. 21, 289–304 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Chavakis T, Santoso S, Clemetson KJ, Sachs UJH, Isordia-Salas I, Pixley RA, Nawroth PP, Colman RW, Preissner KT, High molecular weight kininogen regulates platelet-leukocyte interactions by bridging Mac-1 and glycoprotein Ib. J. Biol. Chem 278, 45375–45381 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty A, The inositol pyrophosphate pathway in health and diseases. Biol. Rev. Camb. Philos. Soc 10.1111/brv.12392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarraj B, Massberg S, Li Y, Kasorn A, Subramanian K, Loison F, Silberstein LE, von Andrian U, Luo HR, Myeloid-specific deletion of tumor suppressor PTEN augments neutrophil transendothelial migration during inflammation. J. Immunol 182, 7190–7200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondal S, Subramanian KK, Sakai J, Bajrami B, Luo HR, Phosphoinositide lipid phosphatase SHIP1 and PTEN coordinate to regulate cell migration and adhesion. Mol. Biol. Cell 23, 1219–1230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarbock A, Polanowska-Grabowska RK, Ley K, Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Rev. 21, 99–111 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Sheng N, Fairbanks MB, Heinrikson RL, Canziani G, Chaiken IM, Mosser DM, Zhang H, Colman RW, Cleaved high molecular weight kininogen binds directly to the integrin CD11b/CD18 (Mac-1) and blocks adhesion to fibrinogen and ICAM-1. Blood 95, 3788–3795 (2000). [PubMed] [Google Scholar]
- 27.Chavakis T, Kanse SM, Pixley RA, May AE, Isordia-Salas I, Colman RW, Preissner KT, Regulation of leukocyte recruitment by polypeptides derived from high molecular weight kininogen. FASEB J. 15, 2365–2376 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K, Kondo R, Wang Q, Doerschuk CM, Neutrophil cytoskeletal rearrangements during capillary sequestration in bacterial pneumonia in rats. Am. J. Respir. Crit. Care Med. 174, 689–698 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asaduzzaman M, Lavasani S, Rahman M, Zhang S, Braun OÖ, Jeppsson B, Thorlacius H, Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit. Care Med. 37, 1389–1396 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Kwak H-J, Liu P, Bajrami B, Xu Y, Park S-Y, Nombela-Arrieta C, Mondal S, Sun Y, Zhu H, Chai L, Silberstein LE, Cheng T, Luo HR, Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity 42, 159–171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loison F, Zhu H, Karatepe K, Kasorn A, Liu P, Ye K, Zhou J, Cao S, Gong H, Jenne DE, Remold-O’Donnell E, Xu Y, Luo HR, Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J. Clin. Invest 124, 4445–4458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.