Key points
An opioid-associated amnestic syndrome has emerged as a unique clinical entity, with distinct findings on magnetic resonance imaging (MRI) indicating bilateral hippocampal injury.
Fentanyl, a synthetic opioid, has been implicated in opioid-associated amnestic syndrome in cases of prescription and nonprescription opioid use, possibly through toxic–metabolic injury to limbic structures.
Early resolution of key neuroimaging findings, and inability to detect synthetic opioids on standard toxicology screens necessitate early use of MRI and testing for synthetic opioids in cases of amnesia after overdose.
Greater awareness of opioid-associated amnestic syndrome and careful use of opioid medications are needed to improve recognition and prevention of this syndrome.
A 63-year-old woman with a history of multiple sclerosis and chronic musculoskeletal pain was found unresponsive in bed by her husband at about 1100 h. A year prior, the patient had been prescribed fentanyl patches for postoperative pain, which she did not use. Her usual daily medications included hydromorphone 9 mg 3 times per day, zopiclone 7.5 mg every night at bedtime and pregabalin 75 mg twice per day, all of which had been taken at the same dose at which they had been used for the last year, except for the hydromorphone. In the weeks before her presentation to hospital, the patient had not been taking her hydromorphone as prescribed because of adverse effects. At about 2300 h the evening before her arrival in the emergency department, the patient had reported increasing pain and applied three 12 μg fentanyl patches, doubling her daily morphine equivalent dose by the time she was found unresponsive 12 hours later. Her husband performed 10 minutes of cardiopulmonary resuscitation without checking for a pulse. When paramedics arrived, the patient had a pulse of 91 beats/min and was in normal sinus rhythm, with a blood pressure reading of 94/57 mm Hg and oxygen saturation of 84%. She awoke after naloxone infusion in the emergency department. Toxicology screening was positive for opiates only. Routine serology testing showed mild transient elevation in transaminase, troponin and creatine kinase levels. Over the following days, the patient showed profound anterograde amnesia and was unable to recall daily autobiographical details, despite meticulously recording them in a journal (Figure 1).
Figure 1:
Journaling as written by a 63-year-old woman with opioid-associated amnestic syndrome. A page from her notepad shows meticulous journaling of daily events and suggests the patient had insight into her amnestic syndrome. Afterward, she had no recollection of these documented events or her journaling of them.
Neurologic examination identified anisocoria and mild spastic paraparesis, which were unchanged from a previously documented examination 3 years earlier. Her Montreal Cognitive Assessment score was 19/30: she lost 3 points for date, month and day; 5 points on delayed recall despite intact immediate recall; and 1 point on each of trails, cube and repetition. No prior cognitive testing was available for comparison. Gadolinium-enhanced magnetic resonance imaging (MRI) of the head performed on day 7 showed striking bilateral gyriform hippocampal hyperintensity on T2-weighted imaging with corresponding restricted diffusivity on diffusion-weighted imaging (Figure 2), in addition to white-matter lesions compatible with multiple sclerosis that were stable on comparison with previous imaging. Electroencephalography done the same day showed no epileptiform activity. The patient’s cerebrospinal fluid profile was unremarkable, and results of polymerase chain reaction testing for herpes simplex virus were negative. Results of testing for arbovirus and syphilis, and a routine paraneoplastic antibody panel were also negative.
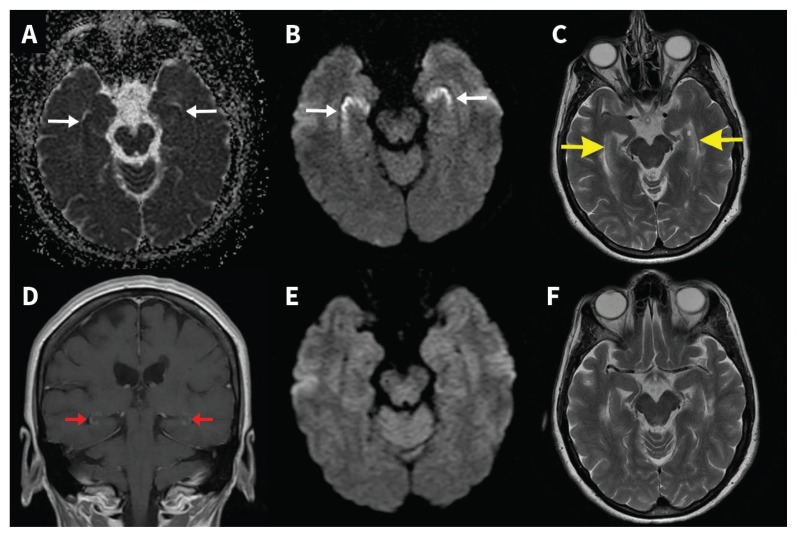
Figure 2:
Serial magnetic resonance imaging (MRI) in opioid-associated amnestic syndrome. Gadolinium-enhanced MRI on day 7 shows bilateral gyriform hippocampal apparent diffusion coefficient hypointensity (A) with corresponding hyperintensity on diffusion-weighted imaging (B), indicating restricted hippocampal diffusivity (white arrows). Hippocampal T2 hyperintensity (C; yellow arrows) along with subtle hippocampal gadolinium enhancement (D; red arrows) is also seen. Follow-up MRI on day 12 (performed without gadolinium despite request) shows resolution of hippocampal restricted diffusion (E) and resolution of T2 hyperintensity (F).
Magnetic resonance imaging of the head on day 12 showed complete resolution of the previously seen hippocampal diffusion restriction and corresponding T2 hyperintensities. Neuropsychological assessment showed mild executive impairment (felt to be in keeping with her known burden of subcortical demyelination) and severe anterograde amnesia. After discharge, the patient moved with her husband across the country to be closer to their children. A follow-up phone interview with the patient’s husband 4 months later confirmed persistent severe amnestic impairment in a novel environment that required constant monitoring by the family.
Discussion
A case series and subsequent alert by the Massachusetts Department of Public Health identified 13 cases of an opioid-associated amnestic syndrome between 2012 and 2016.1 Patients had acute-onset amnesia and evidence of opioid use on history or toxicology. All patients had a single MRI study by day 8 that showed bilateral hippocampal restricted diffusion, in keeping with infarction.2 Findings on our patient’s initial MRI were consistent with those in previous cases in the Massachusetts area.
After cerebral infarction, restricted diffusion may resolve within 8–14 days; however, T2 hyperintensity typically persists for at least 1 month.2 Serial MRI in our patient showed complete resolution of the early hippocampal diffusion restriction and T2 hyperintensity within 12 days. Although hypoxic ischemic injury from hypotension and respiratory depression in the context of opioid overdose potentially contributes to hippocampal injury in opioid-associated amnestic syndrome,3 the progression of changes on T2- and diffusion-weighted imaging seen in this case, is not characteristic of typical cerebral infarction or ischemia.
A recent letter by Barash and colleagues described 4 patients with opioid-associated amnestic syndrome and a history of heroin use who tested positive for fentanyl and norfentanyl, a fentanyl metabolite.4 In 2 patients, fentanyl was the only drug detected. Although none of the 4 patients were known to have used fentanyl, the toxicology findings were consistent with the presence of fentanyl in heroin, an increasingly common finding. 4 Although the precise role that our patient’s prescription medications (including another opioid, hydromorphone) played in her clinical presentation cannot be delineated in this case, no medication was taken in excess of the routinely prescribed dose. Our findings support the hypothesis that fentanyl has a direct role in the development of opioid-associated amnestic syndrome, although this is controversial.5
The hippocampi and deep grey nuclei are metabolically demanding structures and are vulnerable to a variety of ischemic, toxic and metabolic insults;3 however, the exact mechanism of the characteristic bilateral hippocampal injury in opioid-associated amnestic syndrome has not been shown. A rodent model of opioid neurotoxicity has been described, in which it was found that incomplete ischemia may be worsened by hippocampal hyperexcitability at the time of overdose,6 but this has not been reliably reproduced in subsequent experiments.7 In contrast, fentanyl has been shown to exert a direct toxic effect on the hippocampus and associated limbic structures of intubated rats,8 and as such potentially contributes to the development of opioid-associated amnestic syndrome even in the absence of profound hippocampal ischemia. Fentanyl use during general anesthesia has been associated with numerous reported cases of severe global amnesia during the postoperative period in absence of perioperative hemodynamic instability or any other demonstrable etiology.9–11 Interestingly, no case report identified fentanyl as a potential precipitant of acute amnestic syndrome, despite data from rodent models. This indicates that opioid-associated amnestic syndrome is potentially underrecognized in various clinical settings.
Several important clinical conclusions may be drawn from this case. First is the importance of testing for synthetic opioids in patients with a history of opioid use, as has been described by Barash and colleagues.4 Second, this case should alert clinicians to the possibility of an opioid-associated amnestic syndrome in cases of acute global amnesia and opioid overdose, including cases of prescription-fentanyl overdose. Finally, given the resolution of diffusion restriction observed in this case, early use of diffusion-weighted imaging may improve diagnosis in select patients with acute amnesia.
The current case of opioid-associated amnestic syndrome after use of prescription fentanyl suggests that a contaminant or adulterant is not a necessary factor in the pathophysiology of this syndrome.5 Previous reports of the syndrome occurred in the United States; this report should alert clinicians in Canada and internationally who prescribe or administer opioids to opioid-associated amnestic syndrome and the potential role of fentanyl in its emergence.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: Ryan Taylor contributed to project conception through literature review, figure preparation and drafting of the case report. Adrian Budhram contributed to project conception and data acquisition (neuropsychological testing, acquisition of journal pages and informed consent) and revised the manuscript for important intellectual content. Donald Lee contributed to the conception of the work by providing expert imaging interpretation and contributed critical radiological commentary on the manuscript and revision of the work. Seyed Mirsattari contributed to conception of the work by providing clinical expertise as the consulting neurologist and provided critical revision of the work for intellectual content. All authors gave final approval of this version of the manuscript, and all are in agreement to be accountable for all aspects of the work.
References
- 1.Barash JA, Somerville N, DeMaria A. Cluster of an unusual amnestic syndrome — Massachusetts, 2012–2016. MMWR Morb Mortal Wkly Rep 2017;66: 76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lansberg MG, Thijs VN, Brien MWO, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol 2001;22:637–44. [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Gholipour T, Colorado RA, et al. Bilateral hippocampal restricted diffusion: same picture many causes. J Neuroimaging 2017;27:300–5. [DOI] [PubMed] [Google Scholar]
- 4.Barash JA, Ganetsky M, Boyle KL, et al. Acute amnestic syndrome associated with fentanyl overdose [letter]. N Engl J Med 2018;378:1157–8. [DOI] [PubMed] [Google Scholar]
- 5.Dutton RP. More on acute amnestic syndrome associated with fentanyl overdose [letter]. N Engl J Med 2018;378:2247. [DOI] [PubMed] [Google Scholar]
- 6.Kofke WA, Garman RH, Garman R, et al. Opioid neurotoxicity: fentanyl-induced exacerbation of cerebral ischemia in rats. Brain Res 1999;818:326–34. [DOI] [PubMed] [Google Scholar]
- 7.Ammon-Treiber S, Stolze D, Höllt V. Differential effects of mu-opioid receptor agonists in a hippocampal hypoxia/hypoglycemia model. Brain Res 2007; 1183:60–5. [DOI] [PubMed] [Google Scholar]
- 8.Kofke WA, Garman RH, Stiller RL, et al. Opioid neurotoxicity: fentanyl dose-response effects in rats. Anesth Analg 1996;83:1298–306. [DOI] [PubMed] [Google Scholar]
- 9.Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist 2012;18:216–8. [DOI] [PubMed] [Google Scholar]
- 10.Galipienzo J, Lablanca MS, Zannin I, et al. Amnesia global transitoria en el contexto de una anestesia general. Rev Esp Anestesiol Reanim. Sociedad Española de Anestesiología. Spanish J Anesthesiol Resuscitation 2012;59:335–8. [DOI] [PubMed] [Google Scholar]
- 11.Wood T, Donegan J. Transient global amnesia following general anesthesia. Anesthesiology 1985;62:807–9. [DOI] [PubMed] [Google Scholar]