Abstract
There is controversy regarding the utility of left ventricular (LV) mechanics assessed by feature-tracking (FT)-SSFP, a readily implementable technique in clinical practice. In particular, whether LV mechanics assessed by FT-SSFP predicts outcomes in subjects with heart failure (HF) with reduced ejection fraction (HFrEF), with preserved ejection fraction (HFpEF), or without HF is unknown. We aimed to assess whether LV mechanics measured with FT-SSFP cine MRI predicts adverse outcomes. We prospectively enrolled 612 adults without HF (n=402), with HF with reduced ejection fraction (HFrEF; n=113), or HFpEF (n=97) and assessed LV strain using FT-SSFP cine MRI. Over a median follow-up of 39.5 months, 75 participants had a HF admission, and 85 died. In Cox proportional hazards models, lower global longitudinal (Standardized Hazard Ratio: 1.56, 95% CI=1.22 to 2.00, p=0.0004), circumferential (Standardized HR: 1.46, 95% CI=1.08 to 1.95, p=0.0123), and radial strain (Standardized HR: 0.59, 95% CI=0.43–0.83, p=0.0019) were independently associated with the composite endpoint, after adjustment for HF status, LV ejection fraction (LVEF), age, sex, ethnicity, body mass index, systolic and diastolic blood pressure, hypertension, diabetes, coronary artery disease and glomerular filtration rate. Furthermore, global longitudinal strain stratified the risk of adverse outcomes across tertiles better than LVEF. In analyses that included only participants with a preserved LVEF, systolic radial, circumferential and longitudinal strain were independently predictive of adverse outcomes. We conclude that LV longitudinal, circumferential and radial strain measured using FT-SSFP cine MRI (a readily implementable technique in clinical practice) predict the risk of adverse events, independently of LVEF.
Keywords: Strain, magnetic resonance imaging, HFpEF, outcomes
Cardiac magnetic resonance (CMR)-tagging, current “gold standard” method for the measurement of myocardial strain,1 requires additional sequences for data acquisition and dedicated post-processing. Feature-tracking CMR, applied directly on standard balanced steady-state free-precession (bSSFP) LV cine images, requires no additional imaging sequences, and is increasingly studied in research settings.1–6 The prognostic significance of some systolic and diastolic strain-based measures has been studied in population-based samples of asymptomatic adults using speckle-tracking echocardiography and various ad-hoc CMR-based methods.7–11
In isolated samples of participants with Heart failure with preserved ejection fraction (HFpEF) or HF with reduced ejection fraction (HFrEF), LV systolic strain parameters measured using speckle-tracking echocardiography have been studied as predictors of adverse outcomes.12–15 However, the prognostic value of LV deformation measured derived using CMR-based methods remains largely unexplored.16 In this study, we aimed to study the prognostic significance of systolic and diastolic strain measures as well as LV torsion parameters, derived using feature-tracking CMR, among adults without HF, with HFpEF and HFrEF.
Methods
We prospectively enrolled 612 adults without HF (n=402), with HF with reduced ejection fraction (HFrEF; n=113), or HFpEF (n=97) referred for a CMR study at the Corporal Michael J. Crescenz Philadelphia VA Medical Center (CMJC VAMC). The CMJC VAMC Institutional Review Board approved the protocol, and all participants provided written informed consent.
HFrEF was defined as symptomatic HF in the presence of a LV ejection fraction (LVEF) <50%. HFpEF was defined as: (1) New York Heart Association (NYHA) Class II-IV symptoms consistent with HF, in the absence of significant aortic or mitral stenosis; (2) LVEF >50%; (3) a mitral E wave to annular tissue e’ ratio >14,17 or at least 2 of the following: (a) a mitral E wave to tissue annular e’ ratio >8; (b) treatment with a loop diuretic to control HF symptoms; (c) LA volume index >34 mL/m2 of body surface area (BSA)18; (d) NT-pro B-type natriuretic peptide level >200 pg/mL; (e) LV mass index >149 g/m2 in men and 122 g/m2 in women (as measured by CMR).
We excluded potential participants based on the following criteria: (1) Claustrophobia; (2) Presence of implanted medical devices or metallic devices in the body; (3) Significant arrhythmias (eg., atrial fibrillation, or flutter) at the time of enrollment, which may compromise the validity of study measurements; (4) Other conditions that would make the study measurements less accurate or unreliable (i.e., inability to perform an adequate breath hold for CMR acquisitions.
Indications for the CMR included assessment of ventricular function and/or wall motion (30.4%), assessment of myocardial ischemia (22.7%), assessment of the presence/pattern of delayed enhancement (10%), simultaneous assessment of aortic dimensions and cardiac function (7.8%), severe diastolic dysfunction (8.2%), evaluation for ruling out cardiac thrombus (5.9%), myocardial viability (2.6%), quantification of mitral regurgitation (1.3%), or miscellaneous indications (11.1%).
At baseline, a CMR study was performed, which was used for analyses of LV mechanics. The incidence of death or hospitalization for HF was ascertained over a median period of 39.5 months (interquartile range=23.2–62.6). All study participants underwent a cardiac MRI using a 1.5 Tesla (T) whole body MRI scanner (Avanto or Espree, Siemens, Malvern, Pennsylvania, USA) equipped with a phase-array cardiac coil. LV volumes and EF were determined using balanced steady-state free-precession (SSFP) cine imaging. Typical parameters were as follows: TR=30.6 ms; TE=1.3 ms; Phases=30; Slice thickness=8 mm; Matrix size=192×192; Parallel image (IPAT) factor=2.
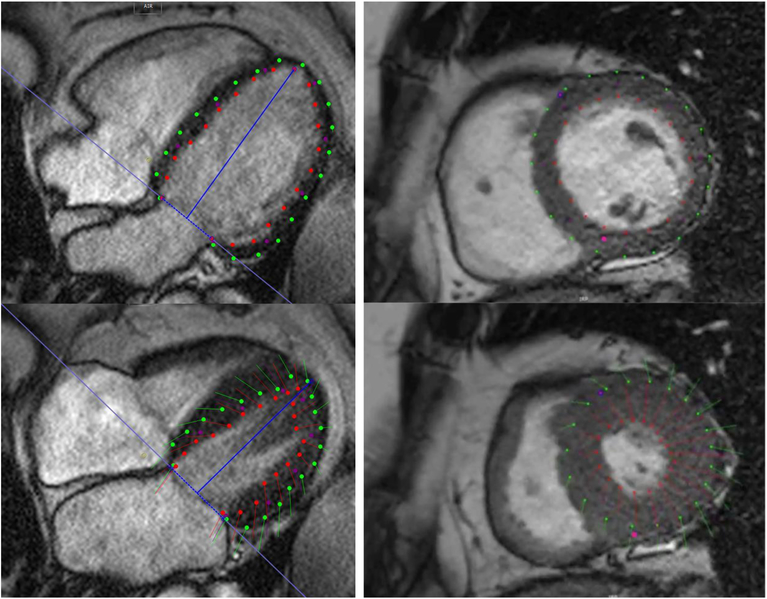
Global myocardial LV strain was measured from integrated long and short-axis and axis cine images, using feature tracking (CMR42 software; Circle CVI, Calgary, Alberta, Canada). LV endocardial and epicardial borders were manually traced along all views using LV enddiastole as the point of reference. The RV insertion points and mitral annular plane were specified. An automated tracking algorithm was applied and tracking of LV segments, as well as the mitral annular plane, was confirmed. Manual adjustments were performed as needed to optimize LV wall tracking. Basal, mid-ventricular, and apical short-axis images, as well as LV long-axis images were analyzed for global circumferential, radial and longitudinal strain measurements (Figure 1). Among repeated analyses in 10 cases, coefficients of variation for peak systolic radial, circumferential and longitudinal strain were <5%; Intra and inter-observer coefficients of variation for peak systolic, early diastolic and late diastolic strain rates ranged between 5–10%.
Figure 1:
Representative example of feature tracking. The top panels show diastolic phases, whereas the bottom panels show the tracking at end-systole in a representative 4-chamber view (left) and mid-ventricular short-axis view (right). See also online videos 1 and 2.
We first compared baseline clinical characteristics of patients without HF, HFrEF and HFpEF. Logarithmic transformation of continuous variables was applied as needed to improve the normality of the distribution. We used chi-square tests for categorical variables and analysis of variance (ANOVA) with post-hoc pairwise comparisons with Bonferroni correction for continuous variables. The prognostic value of various LV strain and torsion parameters was assessed using Cox proportional hazards regression, to obtain standardized hazard ratios (HR) and 95% confidence intervals (CIs). Multivariable models were adjusted for LVEF, HF status, age, sex, African-American ethnicity, BMI, systolic and diastolic blood pressure, diabetes, coronary artery disease and estimated glomerular filtration rate. Statistical significance was defined as a 2-tailed P value<0.05. All probability values presented are 2-tailed. Statistical analyses were performed using the Matlab statistics and machine learning toolbox (the Mathworks; Natwick, MA, USA) and SPSS for Windows v22 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of study participants are presented in Table 1. The majority of study participants were of Caucasian or African-American ethnicity. There was a high prevalence of hypertension in all groups. Participants with HFrEF and HFpEF were slightly older than participants without HF and were more often African-Americans. Body mass index and the prevalence of obstructive sleep apnea were higher in the HFpEF group, whereas the prevalence of coronary artery disease was highest in the HFrEF group. Diabetes mellitus was more common in both HF groups, as compared to participants without HF.
Table 1.
General Characteristics of Study Participants
| Heart Failure Status | ||||
|---|---|---|---|---|
| Variable | None (n=402) | HFrEF (n=113) |
HFpEF (n=97) |
P value |
| Age (years) | 60.6 (59.5 to 61.7) | 65.6 (63.3 to 67.8) | 63.6 (61.3 to 66) | <0.0001 |
| Men | 373 (93.25%) | 111 (98.23%) | 88 (90.72%) | 0.0615 |
| White | 213 (53.25%) | 42 (37.17%) | 40 (41.24%) | 0.003 |
| Black | 168 (42.00%) | 69 (61.06%) | 54 (55.67%) | 0.003 |
| Other race | 19 (4.75%) | 2 (1.77%) | 3 (3.09%) | 0.003 |
| Hypertension | 303 (76.13%) | 96 (84.96%) | 90 (92.78%) | 0.0004 |
| Coronary artery disease | 128 (32.16%) | 60 (53.10%) | 36 (37.11%) | 0.0003 |
| Diabetes mellitus | 174 (43.72%) | 63 (55.75%) | 61 (63.54%) | 0.0007 |
| Obstructive sleep apnea | 93 (23.48%) | 22 (19.47%) | 36 (37.11%) | 0.0069 |
| Glomerular filtration rate (ml/min) | 78 (74.3 to 81.6) | 70.2 (63.9 to 76.5) | 72.9 (65.8 to 79.9) | 0.0753 |
| Low density lipoprotein cholesterol (mg/dL) | 84.1 (78.3 to 89.9) | 74.2 (64.1 to 84.3) | 85.6 (73.4 to 97.8) | 0.1949 |
| Mitral A velocity (cm/s) | 71.3 (68.3 to 74.3) | 67.7 (62.7 to 72.6) | 73.5 (67.8 to 79.1) | 0.2687 |
| Mitral E velocity (cm/s) | 67.2 (64.7 to 69.7) | 67.8 (63.4 to 72.1) | 75.9 (70.8 to 81) | 0.0054 |
| Deceleration Time (ms) | 214 (205 to 222) | 186 (173 to 198) | 217 (202 to 232) | 0.0005 |
| Lateral e’ (cm/s) | 9.24 (8.82 to 9.67) | 7.01 (6.48 to 7.55) | 8.28(7.61 to 8.96) | <0.0001 |
| Septal e’ (cm/s) | 7.37 (7.04 to 7.7) | 5.41 (5.01 to 5.82) | 6.33 (5.82 to 6.83) | <0.0001 |
| E/e’ | 8.2 (7.8 to 8.7) | 11.1 (10.2 to 12.1) | 10.8 (9.8 to 11.7) | <0.0001 |
| Left ventricular end-diastolic volume (ml) | 147 (143 to 152) | 207 (194 to 220) | 153 (143 to 163) | <0.0001 |
| Left ventricular end-diastolic volume index (ml/m2) | 69.4 (67.2 to 71.5) | 99.8 (93.8 to 105.8) | 67 (62.6 to 71.3) | <0.0001 |
| Left ventricular stroke volume (ml) | 80.9 (78.3 to 83.5) | 69 (64.8 to 73.3) | 94.5 (88.3 to 100.8) | <0.0001 |
| Left ventricular ejection fraction (%) | 54.9 (53.5 to 56.3) | 33.4 (31.7 to 35) | 61.8 (58.4 to 65.2) | <0.0001 |
| Medication Use | ||||
| β-Blocker | 190 (47.62%) | 94 (83.19%) | 70 (72.16%) | <0.0001 |
| Aspirin | 233 (58.40%) | 88 (77.88%) | 64 (65.98%) | 0.0006 |
| Clopidogrel | 35 (8.79%) | 21 (18.58%) | 12 (12.37%) | 0.0132 |
| Angiotensin converting enzyme inhibitor | 179 (44.86%) | 70 (61.95%) | 60 (61.86%) | 0.0003 |
| Angiotensin receptor blockers | 38 (9.52%) | 17 (15.04%) | 18 (18.56%) | 0.0265 |
| Furosemide | 15 (3.77%) | 67 (59.29%) | 65 (67.01%) | <O.0001 |
| Spironolactone | 14 (3.51%) | 15 (13.27%) | 8 (8.25%) | 0.0004 |
| Statin | 261 (65.41%) | 89 (78.76%) | 69 (71.13%) | 0.0224 |
| Calcium-channel blocker | 102 (25.56%) | 28 (24.78%) | 38 (39.18%) | 0.0204 |
Continuous variables are presented as mean (95% confidence interval) values. Categorical variables are presented by number (proportion). HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction
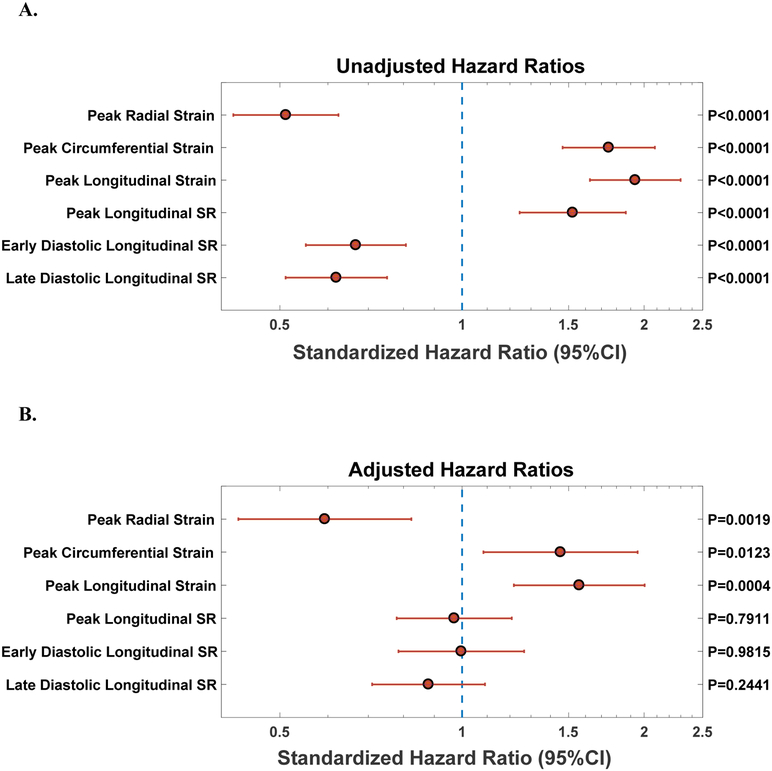
Over a median duration of follow-up of 39.5 months, 75 participants (12.3%) had HF admissions and 85 (13.9%) died. A total of 137 (22.4%) participants experienced the composite end point of HF admission or all-cause death. Figure 2A depicts the unadjusted associations of LV strain with the composite outcome. In these analyses, all measures of systolic strain (longitudinal, circumferential, radial), longitudinal strain rate, and late diastolic strain rate were strongly associated with adverse outcomes. Of note, hazard ratios less than 1 for radial strain, longitudinal diastolic strain rate (early and late), peak torsion and torsion rate would imply that lower function is associated with worse prognosis. On the other hand, hazard ratios greater than 1 for systolic longitudinal strain (and strain rate), circumferential strain, and diastolic untorsion rate (early and late) imply that lower function is associated with worse prognosis.
Figure 2. (A) Unadjusted Standardized Hazard Ratios for Indices of LV deformation as Predictors of Incident Death or HF admission. (B) Adjusted Standardized Hazard Ratios for Indices of LV deformation as Predictors of Incident Death or HF admission.
Adjusted for LVEF, heart failure status, age, sex, ethnicity, body mass index, systolic and diastolic blood pressure, diabetes, coronary artery disease and glomerular filtration rate.
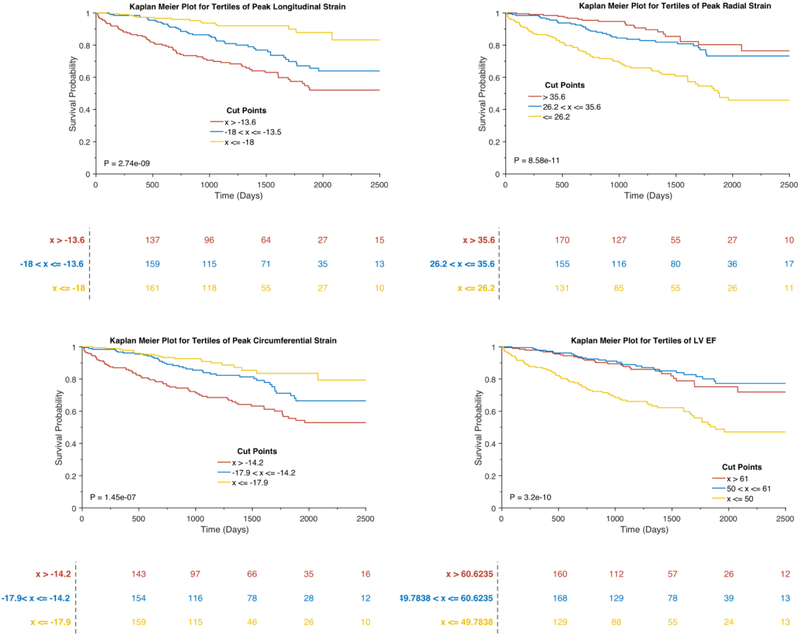
Figure 2B depicts the adjusted associations between LV strain and the composite outcome in Cox-proportional hazards models. In these models, lower peak radial strain was associated with an increased risk of death or HF hospitalization (Standardized HR: 0.59, 95% CI 0.43–0.83, p=0.0019). Similarly, higher (i.e., less negative) peak circumferential (Standardized HR: 1.46, 95% CI 1.08 to 1.95, p=0.0123) and longitudinal strain (Standardized HR: 1.56, 95% CI 1.22 to 2.00, p=0.0004) were also independently associated with adverse outcomes. Multivariable adjustment attenuated the association of longitudinal systolic and diastolic strain rates with adverse outcomes. Figure 3 depicts the Kaplan Meier curves for tertiles of longitudinal, circumferential, radial strain, as well as tertiles of LVEF in relation to the composite outcome of HF hospitalization or death. Peak longitudinal strain stratified the risk of adverse outcomes across tertiles, whereas LVEF was only associated with adverse outcomes in the lowest tertile (LVEF<50%), without differences between the top 2 tertiles.
Figure 3.
Kaplan Meier Survival Plots for tertiles of longitudinal, radial, circumferential strain, and LVEF.
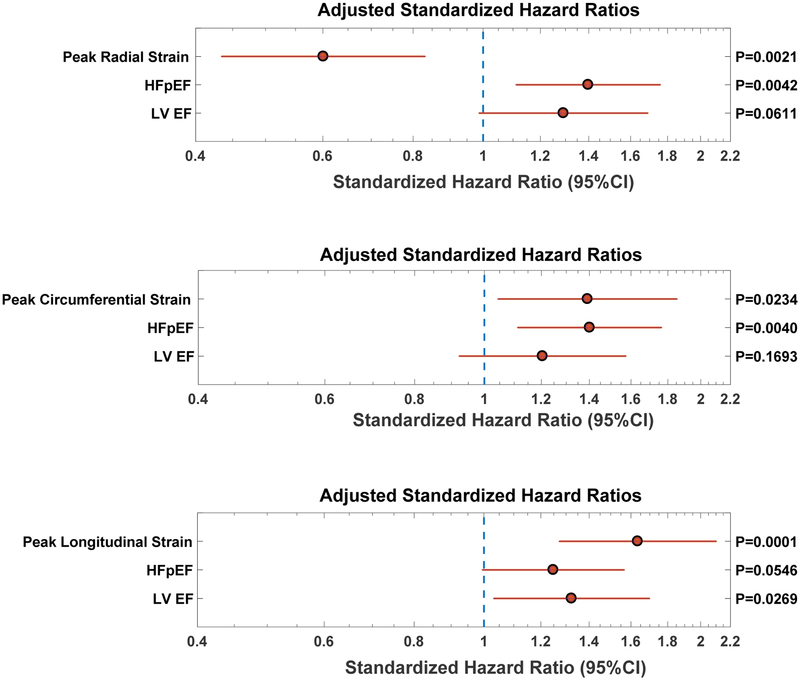
To assess the value of strain measures as sensitive parameters of myocardial dysfunction when the LV EF is preserved, we performed additional analyses including only participants with a preserved EF (≥50%, including subjects with HFpEF and without HF) to study the association between strain measures with adverse outcomes. Figure 4 depicts the results of these analyses, which were adjusted for presence of HFpEF and LVEF. In these models, all three systolic strain measures (radial, circumferential, and longitudinal strain) were independently associated with adverse outcomes independently of LV EF and the presence of HFpEF.
Figure 4. Predictive value of radial (top panel), circumferential strain (mid-panel) and longitudinal strain (bottom panel), among subjects with preserved LVEF (≥50%).
Models are adjusted for LVEF, the presence of heart failure with preserved ejection fraction (HFpEF), age, sex, ethnicity, body mass index, systolic and diastolic blood pressure, hypertension, diabetes, coronary artery disease and glomerular filtration rate. In these models, all measures of LV strain were independently predictive of incident hospitalized HF and death.
Discussion
We prospectively studied a relatively large sample of participants with HFpEF, HFrEF and no HF, and performed assessments of LV mechanics using feature-tracking CMR techniques. We found that radial, circumferential and longitudinal LV systolic strain measures were independently associated with HF hospitalization and death over a median follow-up of 39.5 months, in models that adjusted for HF status, LVEF, as well as other potential demographic and clinical confounders. LV longitudinal strain stratified the risk of adverse outcomes better than LV EF. Furthermore, among patients with preserved EF, strain measures independently predicted the composite outcome. Interestingly, in this subgroup with preserved EF, a greater (rather than a lower) LVEF tended to predict the composite outcome. Our findings demonstrate that feature tracking CMR, which has the potential to be readily applicable in current clinical practice, provides useful prognostic information, and can be leveraged to discriminate the risk of adverse outcomes, especially in the presence of preserved LVEF. Future studies should investigate whether LV strain measured by feature tracking CMR can be used to aid monitoring disease and therapeutic status in HF.
Due to the logistical limitations of myocardial tagging (dedicated acquisition time and dedicated post-processing), feature-tracking has emerged as a reliable alternative for LV strain measurement.1,4,19 With increasing adoption of CMR in clinical practice, especially for patients with HFpEF, LV strain measured using feature-tracking could provide clinically useful prognostic information without increasing acquisition times.20 The prognostic significance of LV systolic strain measured using speckle-tracking echocardiography has been studied in isolated samples of HFpEF or HFrEF.12–15 However, the association of CMR-based LV deformation measures with adverse prognosis in HF, specifically HFpEF, has not been explored previously. In a convenience sample of 539 patients undergoing CMR, Mordi et al. measured circumferential strain using myocardial tagging.21 They observed that the prognostic value of circumferential strain in predicting adverse cardiovascular events (all-cause mortality, HF hospitalization, and aborted sudden cardiac death) was incremental to LVEF and myocardial scarring. Interestingly, they did not measure longitudinal strain or strain rate in their study, to minimize acquisition time. This highlights a limitation of myocardial tagging and an important advantage of feature-tracking for assessments of LV mechanics with clinically-applicable imaging protocols. Buss et al. studied 210 subjects with dilated non-ischemic cardiomyopathy using feature-tracking-derived systolic strain measures and observed that global longitudinal strain, but not circumferential or radial strain, was associated with adverse cardiovascular outcomes, independent of LVEF.16 Similarly, in a retrospective analysis of 364 participants (>80% with cardiovascular comorbidities), all measures of LV strain (derived using feature-tracking) were associated with adverse events (cardiac death, aborted cardiac death, HF hospitalization) with LV transverse strain (a surrogate for radial strain) being the strongest predictor.22 In a community-based sample of 1768 participants, impaired circumferential strain (by myocardial tagging), was independently and incrementally associated with increased risk of incident HF and atherosclerotic cardiovascular events.10 In contrast to prior studies, our study was designed to assess the prognostic utility of LV deformation measurements by CMR feature-tracking among subjects with HFpEF, HFrEF and no HF. Furthermore, we performed comprehensive feature-tracking analyses of LV function using radial, circumferential and longitudinal systolic strain, as well as longitudinal systolic, early and late diastolic longitudinal strain rate. Our findings demonstrate that LV strain is more robust than LVEF in predicting adverse outcomes in participants with and without HF, particularly when LVEF is preserved. Interestingly, in our analyses, peak longitudinal strain stratified the risk of adverse outcomes across tertiles in the overall sample, whereas an increased risk of adverse outcomes was seen only among participants in the lowest tertile of LVEF (which corresponded to an LVEF of ~50% in our sample). In secondary analyses that included only participants with a preserved LVEF (≥50%) systolic strain measures (radial, circumferential, and longitudinal strain), but not a lower LVEF, were independently associated with adverse outcomes. These findings are in line with prior speckle-tracking echocardiography-based analyses in which the global longitudinal strain was identified as a predictor of adverse outcomes in the participants with HFpEF, independent of LVEF.12,23 The fact that a higher, and not a lower EF, tended to be associated with adverse outcomes in these adjusted models is likely due to the fact that endocardial EF is greater with greater degrees of concentric remodeling, for any given amount of fiber shortening.
We note that the lack of association of strain rates with adverse outcomes in adjusted analyses should be interpreted with caution, as they were derived from the rate of change in strain using feature-tracking on cine CMR and thus limited by the temporal resolution of our acquisitions (30 frames/cardiac cycle); our findings regarding strain rates should not be extrapolated to techniques in which strain rates are directly/primarily measured or those derived from time derivatives of strain measures obtained with greater temporal resolution.
Our study has several limitations. First, our study sample was enrolled from a Veterans Affairs medical center. Therefore, although racially diverse, our sample was largely composed mainly of older males. Our findings should be validated in samples with greater gender diversity and in younger adults, as well as pediatric populations. Second, due to the limited number of events in three groups (HFpEF, HFrEF, and patients without heart failure), our analyses were not powered to measure the prognostic significance of CMR strain measures in each group separately. Third, neither longitudinal strain rate, nor diastolic strain rates were associated with adverse outcomes in our sample. However, these findings should be interpreted with caution, as discussed above. Finally, we did not assess the prognostic value of strain, independent of circulating biomarkers, particularly B-type natriuretic peptide and/or NT-pro-BNP, which requires further study.
In conclusion, we demonstrate significant associations between longitudinal, circumferential, and radial strain and adverse outcomes, after adjustment for potential clinical and demographic confounders, as well as LVEF. LV strain measurements using feature-tracking have widespread potential to be incorporated in the clinical workflow, in which standard LV cine CMR acquisitions are performed. This relatively simple strategy provides useful prognostic information without the need for additional acquisition time. Future studies should assess whether feature-tracking derived LV strain is useful for implementing or monitoring therapeutic interventions.
Supplementary Material
Acknowledgments
Disclosures: J.A.C. has received consulting honoraria from Bristol-Myers Squibb, OPKO Healthcare, Fukuda-Denshi, Microsoft, Ironwood Pharmaceuticals, Sanifit, Pfizer, Vital Labs, Merck and Bayer. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb, Microsoft and CVRx Inc., and device loans from AtCor Medical. Other authors have no disclosures.
Sources of Funding: This study was supported by NIH grants R56HL-124073-01A1 (J.A.C), R01 HL 121510-01A1 (J.A.C), 5-R21-AG-043802-02 (J.A.C) and a VISN-4 research grant from the department of Veterans Affairs (J.A.C)
The author hereby grants the American Journal of Cardiology rights to the manuscript, and the full copyright
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scatteia A, Baritussio A, Bucciarelli-Ducci C. Strain imaging using cardiac magnetic resonance. Heart Fail Rev 2017;22:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowallick JT, Lamata P, Hussain ST, Kutty S, Steinmetz M, Sohns JM, Fasshauer M, Staab W, Unterberg-Buchwald C, Bigalke B, Lotz J, Hasenfuss G, Schuster A. Quantification of left ventricular torsion and diastolic recoil using cardiovascular magnetic resonance myocardial feature tracking. PLoS One 2014;9:e109164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maret E, Todt T, Brudin L, Nylander E, Swahn E, Ohlsson JL, Engvall JE. Functional measurements based on feature tracking of cine magnetic resonance images identify left ventricular segments with myocardial scar. Cardiovasc Ultrasound 2009;7 10.1186/1476-7120-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ, Ferro CJ, Young AA, Townend JN, Leyva F, Steeds RP. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging 2015;41:1000–1012. [DOI] [PubMed] [Google Scholar]
- 5.Schuster A, Stahnke VC, Unterberg-Buchwald C, Kowallick JT, Lamata P, Steinmetz M, Kutty S, Fasshauer M, Staab W, Sohns JM, Bigalke B, Ritter C, Hasenfuss G, Beerbaum P, Lotz J. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: Intervendor agreement and considerations regarding reproducibility. Clin Radiol 2015;70:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claus P, Omar AM, Pedrizzetti G, Sengupta PP, Nagel E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc Imaging 2015;8:1444–1460. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sorensen T, Biering-Sorensen SR, Olsen FJ, Sengelov M, Jorgensen PG, Mogelvang R, Shah AM, Jensen JS. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging 2017;10:e005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambale-Venkatesh B, Armstrong AC, Liu CY, Donekal S, Yoneyama K, Wu CO, Gomes AS, Hundley GW, Bluemke DA, Lima JA. Diastolic function assessed from tagged MRI predicts heart failure and atrial fibrillation over an 8-year follow-up period: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2014;15:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Solomon SD, Benjamin EJ, Vasan RS. Distinct Aspects of Left Ventricular Mechanical Function Are Differentially Associated With Cardiovascular Outcomes and All-Cause Mortality in the Community. J Am Heart Assoc 2015;4:e002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida AL, Wu CO, Gomes AS, Bluemke DA, Lima JA. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail 2014;16:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015;132:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 2009;54:618–624. [DOI] [PubMed] [Google Scholar]
- 14.Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK, Borlaug BA, Chirinos JA, St John Sutton M, Cappola TP, Ky B. Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc 2014;3:e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K, Biering-Sorensen T. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc Imaging 2015;8:1351–1359. [DOI] [PubMed] [Google Scholar]
- 16.Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T, Frankenstein L, Steen H, Meder B, Giannitsis E, Katus HA, Korosoglou G. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:307–315. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 19.Obokata M, Nagata Y, Wu VC, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M. Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging 2016;17:525–532. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Chang SM, Nabi F, Shah DJ, Estep JD. Cardiac Imaging in Patients With Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 21.Mordi I, Bezerra H, Carrick D, Tzemos N. The Combined Incremental Prognostic Value of LVEF, Late Gadolinium Enhancement, and Global Circumferential Strain Assessed by CMR. JACC Cardiovasc Imaging 2015;8:540–549. [DOI] [PubMed] [Google Scholar]
- 22.Yang LT, Yamashita E, Nagata Y, Kado Y, Oshima S, Otsuji Y, Takeuchi M. Prognostic value of biventricular mechanical parameters assessed using cardiac magnetic resonance feature-tracking analysis to predict future cardiac events. J Magn Reson Imaging 2017;45:1034–1045. [DOI] [PubMed] [Google Scholar]
- 23.Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography 2015;32:71–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.