Abstract
Objective:
To examine the association between sequence variants in genetic risk factors for age-related macular degeneration (AMD), and delayed rod-mediated dark adaptation (RMDA), the first functional biomarker for incident AMD, in older adults with normal macular health and early AMD.
Design:
Cross-sectional
Subjects:
Older adults aged ≥60 years in normal macular health (defined as both eyes at step 1 on the Age-Related Eye Disease 9-step AMD classification system) and those with AMD in one or both eyes (defined as steps 2–9).
Methods:
Single nucleotide polymorphisms were genotyped in the CFH and ARMS2 genes using a Taqman assay. RMDA was assessed in one eye after photobleach with targets centered at 5° on the inferior vertical meridian. Rate of dark adaptation was defined by rod intercept time (RIT), duration (minutes) required for sensitivity to reach a criterion sensitivity level in the latter half of the second component of rod recovery. Associations between CFH and ARMS2 polymorphisms and RMDA were adjusted for age and smoking.
Main Outcome Measure:
RIT.
Results:
The sample consisted of 543 participants having both genotype and RIT determination; 408 were in normal macular health and 135 had AMD, most having early AMD (124 of 135). For the combined sample, higher RIT (slower RMDA) was observed for both the A69S variant in ARMS2 and the Y402H variant in CFH (adjusted p=0.0001 and p=0.0023 respectively). For normal subjects the A69S variant in ARMS2 was associated with higher RIT (adjusted p=0.0011), whereas CFH Y402H was not (adjusted p=0.2175). For AMD cases, the A69S variant of ARMS2 and CFH Y402H were associated with higher RIT (adjusted p=0.0182 and p=0.0222 respectively). Those with a greater number of high-risk ARMS2 and CFH alleles had higher RIT, in both normal and AMD groups (adjusted p=0.0002 and p<0.0001 respectively).
Conclusions:
We report a novel association wherein older adults with high risk ARMS2 and CFH genotypes are more likely to have delayed RMDA, the first functional biomarker for incident early AMD. Before the AMD clinical phenotype is present, those in normal macular health with the ARMS2 A69S allele have delayed RMDA. Understanding ARMS2 function is a research priority.
Précis
In a large cohort of older adults (n=543), most with normal maculas, high risk ARMS2 and CFH genotypes associate with delayed rod-mediated dark adaptation, the first identified functional risk factor of incident age-related macular degeneration.
Age-related macular degeneration (AMD) is a leading cause of irreversible central vision loss in older persons worldwide,1 resulting from loss of photoreceptors, retinal pigment epithelium (RPE), and choriocapillaris endothelium in the setting of characteristic extracellular deposits.2 AMD progression can be slowed by dietary supplements at the intermediate stage, and the neovascular end-stage is partially managed with anti-angiogenic agents.3, 4 Genotyping of well-defined patient populations is one route to discover biologic underpinnings of early disease stages that if treated, could prevent progression to advanced disease.
Two of the strongest genetic associations for AMD are common polymorphisms at chromosome 1 and chromosome 10. The variant at chromosome 1 (rs1061170) results in a tyrosine to histidine change in codon 402 of the complement factor H (CFH) gene and is associated with increased risk of AMD in individuals, ranging from 2–7-fold depending on the studied population.5–8 The AMD-associated locus at chromosome 10q26 includes an amino acid-changing single nucleotide polymorphism (SNP) in the ARMS2 gene (rs10490924) that results in an alanine to serine substitution at codon 69.9, 10 Due to the physical proximity of ARMS2 and the protease-encoding gene HTRA1, and the shared variants in and between these genes on most haplotype blocks, unequivocally determining whether ARMS2 A69S or conservative polymorphisms in HTRA1 are responsible for AMD has been a major challenge. However, very large case-control studies on individuals with rare recombinations between these two genes indicate that variants in ARMS2 and not HTRA1 are responsible for the increased AMD risk at this locus.11 Most, but not all, studies suggest that while both Y402H in CFH and A69S in ARMS2 affect risk of AMD, they are not associated with progression to end-stage disease.12
In the human macula, rods outnumber cones.13 Delayed rod-mediated dark adaptation (RMDA) is the first functional biomarker identified for early AMD.14 We recently found that older adults with delayed RMDA despite normal macular health as assessed by color fundus photography have double the risk for incident AMD three years later.14 RMDA refers to the recovery of light sensitivity by rod photoreceptors after exposure to a bright light.15, 16 The speed of recovery is rate limited by retinoid concentration. This concentration, in turn, is a manifestation of the retinoid cycle, i.e., the process of eliminating products of light absorption from photoreceptor outer segments, recycling of the released retinoid to its original form (11-cis-retinal), and regenerating the visual photopigment opsin.15, 16 Changes in structures essential to the retinoid cycle including those in the choroid, the RPE-Bruch’s membrane complex, and the subretinal space are signature characteristics of aging and early AMD and thus are well positioned to impact the rate of RMDA. In contrast, cone photoreceptors additionally receive retinoids from Müller cells17, 18 and thus are less dependent on the supply route from the choroid.
The purpose of this study is to examine if older adults in normal macular health or with early AMD who have CFH or ARMS2 high-risk alleles are more likely to exhibit delayed RMDA, as compared to those without these high-risk alleles.
Methods
Study approval was obtained from the Institutional Review Boards of the University of Alabama at Birmingham (UAB) and the University of Iowa. Participants provided written informed consent to participate in the study. The research adhered to the tenets of the Declaration of Helsinki.
The study sample consisted of the baseline cohort of the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR) which was established to examine the association between delayed RMDA and AMD.14, 19 The sample was recruited from two primary care ophthalmology practices in the Callahan Eye Hospital at UAB since the study’s primary focus was on normal aging. Eligibility criteria for those in normal macular health were (1) age ≥ 60 years old; (2) normal macular health in both eyes as determined by 3-field color stereo color fundus photography (Carl Zeiss Meditec 450+ camera, Dublin CA) evaluated by an experienced grader masked to other study variables; each eye’s grade had to be 1 in the AREDS 9-step classification system;20 (3) No previous diagnoses of glaucoma, other retinal conditions, optic nerve conditions, corneal disease, diabetes, or neurological or psychiatric conditions known to impact vision. Eligibility criteria for those with AMD were identical to those for normal macular health except that one or both eyes were required to have a grade of 2–9 in the AREDS 9-step system.20 Using the AREDS 9-step system, early AMD was defined as grades 2 – 4, intermediate AMD as grades 5 – 8, and non-central geographic atrophy as grade 9.
Demographic characteristics (age, sex, race/ethnicity) and smoking status were collected through interview. Best-corrected visual acuity for each eye was assessed via the Electronic Visual Acuity tester21 (EVA; JAEB Center, Tampa FL) under photopic conditions and expressed as the logarithm of the minimum angle resolvable (logMAR). Rod-mediated dark adaptation was measured in one eye only of each participant because of time constraints in the protocol. The eye with better acuity was selected for testing. Dark adaptation was measured psychophysically using a computer-automated dark adaptometer (AdaptDx; MacuLogix, Middletown PA) as described previously.14, 22 Prior to testing, the eye was dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride so that a pupil diameter of ≥ 6 mm was achieved. Trial lenses were added as needed for the 30-cm viewing distance. The fellow eye was occluded with an opaque patch. The participant’s head was positioned in the forehead–chinrest of the adaptometer. An infrared camera positioned behind the fixation light displayed the eye on a monitor viewed by the examiner, who facilitated positioning of the test eye. The procedure began with a photo-bleach exposure to a flash (0.25 ms duration, 58,000 scotopic cd/m2 s intensity; equivalent 83% bleach23) while the participant focused on the fixation light. The photo-bleach flash, subtending 4°, was centered at 5° on the inferior vertical meridian (i.e. superior to the fovea on the retina), which was also the test target’s position for measuring light sensitivity. Threshold measurement for a 2° diameter, 500 nm circular target began 15 seconds after bleach offset. During threshold measurement, the participant was instructed to maintain fixation on the red fixation light and press a response button when a flashing target first became visible within the bleached area. Threshold was estimated using a three-down/one-up modified staircase estimate procedure described and continued at 30 second intervals for 20 minutes. Log thresholds were expressed as sensitivity in decibel (dB) units as a function of time from bleach offset. The rate of rod mediated dark adaptation is defined by the rod intercept time (RIT), defined as the duration (in minutes) required for sensitivity to reach a criterion level of 5.0 × 10−3 scotopic cd/m2 (3.0 log units of stimulus attenuation).22 This sensitivity level is located in the latter half of the second component of rod recovery.15 Test-retest reproducibility of RIT is high (r = 0.95).24 For participants who did not reach the RIT criterion level of sensitivity within the 40-minute testing protocol, RIT was set to 39 minutes for analysis purposes similar to previous convention.24, 25
Blood (8 mL) was collected by phlebotomy. Genomic DNA was extracted from buffy coats derived from EDTA-anticoagulated blood tubes using the Gentra PureGene method (Qiagen) following the manufacturer’s instructions. Resulting DNA was quantitated by UV spectroscopy and had OD260/280 ratios of 1.7–1.9. One hundred ng of total DNA was used in allele-specific Taqman assays using a microfluidic workstation (Fluidigm, San Francisco, CA). To detect potential sample swaps, forensic markers discriminating X and Y chromosomal amplimers were evaluated as part of this process and registered to self-reported sex.26, 27 The CFH Y402H and ARMS2 A69S alleles were genotyped on a microfluidic qPCR platform (Fluidigm, San Francisco, CA) using Taqman reagents. Allele-specific primers designed for the SNPs rs10490924, rs3750847, rs1061170 and rs1061147 were utilized. Agreement between rs10490924 and rs3750847 for ARMS2, and rs1061170 and rs1061147 for CFH, were required to make a definitive judgment on genotype.
Analysis of variance (ANOVA) and chi-square tests were used to compare demographic characteristics and smoking status among the genotype groups. RIT was compared among groups and according to the number of high risk alleles using ANOVA with adjustment for smoking status since preliminary analysis indicated that it was a confounder; age was also adjusted for. Because we were examining hypotheses about two genes (CFH and ARMS2), p-values <0.025 (0.05/2) were considered statistically significant.
Results
Of the 654 participants enrolled in ALSTAR, 543 had both RMDA and genotyping and thus served as the analysis sample for this study. Those participants who had both RMDA and genotyping had similar age and gender distributions to those who did not have both types of data (p = 0.486 and p = 0.560 respectively). Approximately 75% of participants in the analysis sample were in normal macular health, and 25% had AMD, most of whom had early AMD (Table 1). Average age of the sample was 69.3 years (standard deviation 6.0), 63.7% were female, and the vast majority (97.1%) were white of European origin. The mean visual acuity for the eye tested for RMDA was −0.004 logMAR (SD 0.11).
Table 1.
Percentage of participants with normal macular health and AMD in the sample (total N = 543)
| N | % of total N | |
|---|---|---|
| Normal macular health | 408 | 75.14 |
| AMD (regardless of severity)1 | 135 | 24.86 |
| Early | 124 | 22.84 |
| Intermediate | 10 | 1.84 |
| Non-central geographic atrophy |
1 | 0.18 |
Early AMD = AREDS grade 2 −4, intermediate AMD = AREDS grade 5 – 8, non-central geographic atrophy = AREDS grade 9
With respect to CFH alleles, 37.6% of subjects were homozygous for the low-risk allele (YY), 50.0% were heterozygous (HY), and 13.4% were homozygous for the high-risk allele (HH) (Table 2). With respect to ARMS2 alleles, 56.9% of subjects were homozygous for the low-risk allele (AA), 38.1% were heterozygous (AS), and 5.0% were homozygous for the high-risk allele (SS). The distributions of age, sex, and race/ethnicity did not differ among the CFH alleles nor among ARMS2 alleles. However, for both CFH and ARMS2, there was a significant difference among alleles with respect to smoking; for CFH, those individuals homozygous for the low-risk allele were more likely to be former/current smokers (p=0.0039); for ARMS2, those homozygous for the low-risk allele were more likely to be never-smokers (p=0.0013). The genotype frequencies observed in this cohort were similar to those reported for European populations in the Exome Aggregation Consortium (ExAC).28
Table 2.
Descriptive statistics on demographic characteristics and smoking status of sample
| CFH Alleles | ARMS2 Alleles | |||||||
|---|---|---|---|---|---|---|---|---|
| Low-risk homozygous |
Heterozygous | High-risk homozygous |
p-value | Low-risk homozygous |
Heterozygous | High-risk homozygous |
p-value | |
| YY | YH | HH | AA | AS | SS | |||
| N (%) | 204 (37.6) | 266 (50.0) | 73 (13.4) | 309 (56.9) | 207 (38.1) | 27 (5.0) | ||
| Age, mean (SD) | 69.7 (6.2) | 69.3 (6.0) | 68.5 (5.5) | 0.3278 | 69.4 (6.0) | 69.3 (6.1) | 68.8 (5.1) | 0.8819 |
| Female, n (%) | 128 (62.8) | 173 (65.0) | 45 (61.6) | 0.8107 | 192 (62.1) | 135 (65.2) | 19 (70.4) | 0.5908 |
| White, n (%) | 197 (96.6) | 260 (97.7) | 70 (95.9) | 0.7759 | 299 (96.8) | 202 (97.6) | 26 (96.3) | 0.2283 |
| Smoking, n (%) | ||||||||
| Current | 6 (2.9) | 19 (7.1) | 1 (1.4) | 0.0039 | 9 (2.9) | 17 (8.2) | 0 (0.0) | 0.0013 |
| Former | 101 (49.5) | 104 (39.1) | 23 (31.5) | 145 (46.9) | 68 (32.9) | 15 (55.6) | ||
| Never | 97 (47.6) | 143 (53.8) | 49 (67.1) | 155 (50.2) | 122 (58.9) | 12 (44.4) | ||
Table 3 (see also Figure 1) shows that for a combined sample of normal macular health and AMD participants, higher RIT (slower RMDA) was observed for both the CFH and ARMS2 heterozygous and high-risk genotypes (adjusted p = 0.0023 and p = 0.0001 respectively). These associations remained when the sample was restricted to the 527 participants who were white of European origin (adjusted p = 0.0085 and p < 0.0001 respectively). Even when cases not reaching a RIT within 40 minutes were deleted from the analysis, the associations between RIT and CFH and ARMS2 remained (adjusted p = 0.0086 and p = 0.0044 respectively). Figure 2 presents boxplots separately for normal and AMD participants showing the RIT distribution for CFH and ARMS2 genotypes. For normal macular health subjects, CFH genotype was not associated with RIT (adjusted p = 0.2175). In contrast, in normal subjects, ARMS2 genotype was associated with higher RIT (adjusted p = 0.0011). Figure 3 illustrates this effect by presenting dark adaptation plots from representative individual normal eyes, with heterozygous and high-risk homozygous individuals showing a slower time course of recovery than an eye with the low-risk homozygous genotype. When the AMD cases are considered separately, both the CFH and ARMS2 genotypes were significantly associated with RIT (adjusted p= 0.0222 and p = 0.0182 respectively).
Table 3.
For the total sample, rod intercept time (RIT) in minutes stratified by CFH and ARMS2 risk alleles. Larger RIT represents slower rod-mediated dark adaptation.
| Total Sample |
CFH | p-value | Adjusted p-value1 |
ARMS2 | p-value | Adjusted p-value1 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low-risk homozygous |
Hetero- zygous |
High-risk homozygous |
Low-risk homozygous |
Hetero- zygous |
High-risk homozygous |
||||||
| YY | YH | HH | AA | AS | SS | ||||||
| N | 204 | 266 | 73 | 309 | 207 | 27 | |||||
| Mean (standard deviation) |
11.1 (6.2) |
12.8 (8.2) |
13.8 (9.0) |
0.0148 | 0.0023 | 11.4 (6.5) |
13.1 (8.6) |
16.5 (10.6) |
0.0005 | 0.0001 | |
Adjusted for age and smoking
Figure 1.
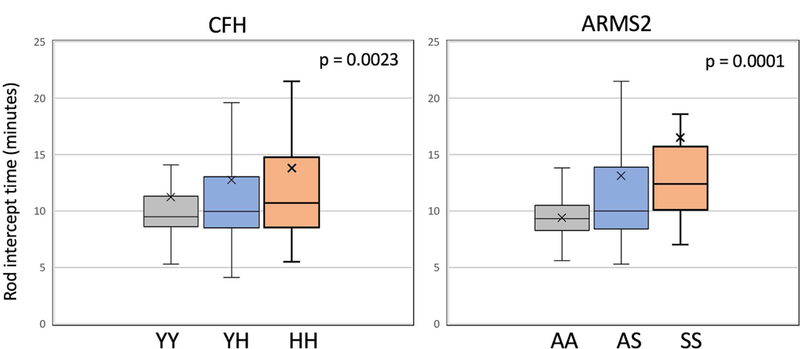
Box plots showing the distribution of RIT for CFH genotypes and for ARMS2 genotypes with combined data for all eyes in the study (with either normal macular health or with AMD). Associations are adjusted for age and smoking status. The box represents the middle two quartiles of the distribution; the lower whisker, the bottom quartile; the upper whisker, the highest quartile. The median is the horizontal line in each box and the “X” is the mean. Statistical outliers not shown.
Figure 2.
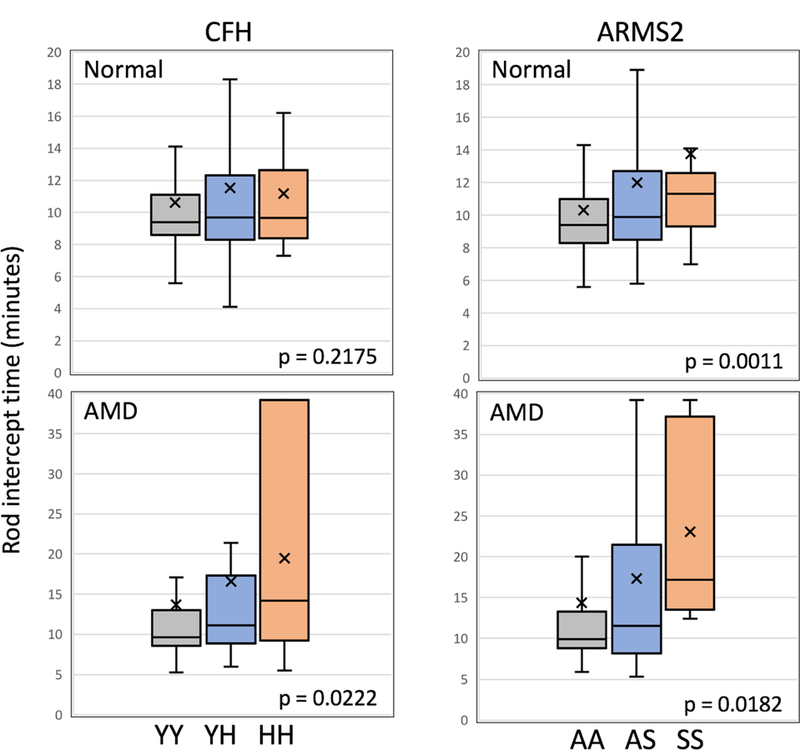
Box plots shown separately for normal macular health cases (upper panels) and those with AMD (lower panels) showing the distribution of RIT for CFH (left) and ARMS2 (right) genotypes. Associations are adjusted for age and smoking status. The box representation is as described in Figure 1. Note that ARMS2 A69S is associated with increased RIT in subjects in normal macular.
Figure 3.
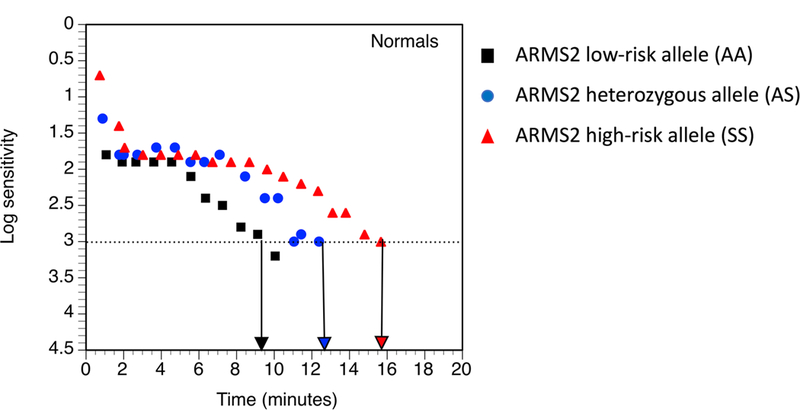
Representative rod-mediated dark adaptation plots (log sensitivity over time) from individual normal eyes with the low-risk (black squares), heterozygous (blue circles), and the high-risk, homozygous ARMS2 genotype (red triangles). Arrows pointing to the x-axis indicate the RIT for each eye. Note that the individual homozygous for the high-risk allele is slower to recovery sensitivity than the individual with the heterozygous genotype; and the latter is slower to recover than the individual homozygous for the low-risk allele.
The association between the number of high-risk alleles from CFH and ARMS2 and RIT is shown in Figure 4. Participants with a greater number of high-risk alleles had a longer RIT (adjusted p < 0.0001). This association was present for normal macular health cases and AMD cases analyzed separately (adjusted p = 0.0002 and p < 0.0001 respectively) (Table 4).
Figure 4.
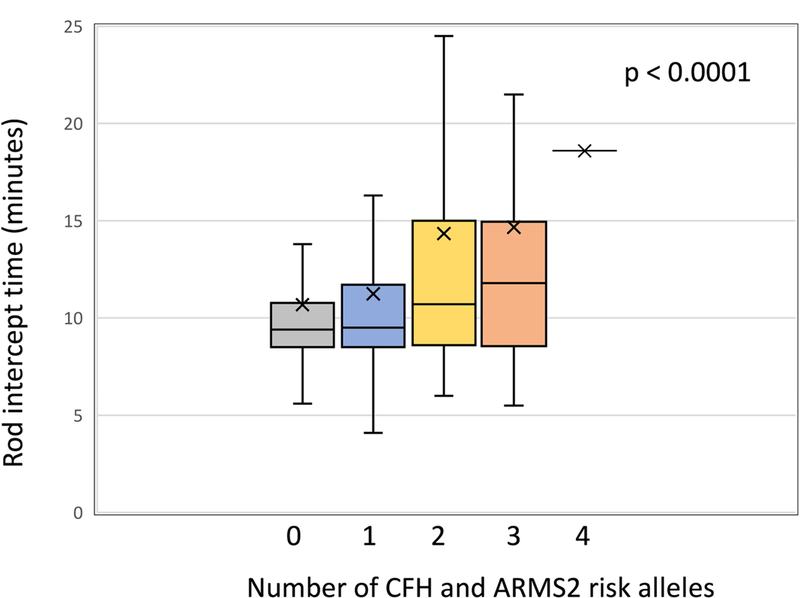
Box plots showing the distribution of RIT stratified by the number of CFH and ARMS2 risk alleles for the combined sample of eyes in normal eye health and those with AMD. Associations are adjusted for age and smoking status. The 4-allele group was not included in the statistical comparison of RIT among groups because there was only 1 eye in this group, however its RIT is plotted. The box representation is as described in Figure 1. Note that with increasing number of risk alleles, cases have slower rod-mediated sensitivity recovery (i.e, greater RIT).
Table 4.
Mean RIT (minutes) as a function of total number of risk alleles for CFH and ARMS2 stratified by normal and AMD eyes
| Normal macular health | AMD | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of alleles |
N | Mean (standard deviation) |
p-value | Adjusted p-value2 |
N | Mean (standard deviation) |
p-value | Adjusted p- value2 |
| 0 | 84 | 9.9 (2.8) |
0.0048 | 0.0002 | 32 | 12.8 (8.9) |
0.0038 | <0.0001 |
| 1 | 175 | 10.7 (4.7) |
49 | 13.2 (9.3) |
||||
| 2 | 118 | 12.5 (7.7) |
43 | 19.5 (12.8) |
||||
| 3 | 31 | 12.1 (5.8) |
11 | 22.2 (13.2) |
||||
| 41 | 0 | 1 | ||||||
There were no normal eyes having 4 risk alleles. There was one eye in the AMD group with 4 risk alleles. The RIT for this person was 18.6 minutes. The 4-allele category was not included in the comparison among groups for the AMD eyes.
Adjusted for age and smoking
Bee-swarm plots of the individual data are in Figures 5, 6, and 7 (available as online supplemental material at www.aaojournal.org.)
Discussion
For a combined sample of older adults with either normal macular health or AMD, we report a novel association between specific risk alleles for CFH and ARMS2, the two strongest common genetic risk factors for AMD, and delayed RMDA, the first functional biomarker for early AMD. The association appears to be stronger for ARMS2 than CFH. Interestingly, for the ARMS2 A69S polymorphism, delayed RMDA is observed in older adults in normal macular health before the clinical manifestation of AMD, as defined by color fundus photography. We also found that as the number of CFH or ARMS2 risk alleles a person has increases, so does their RIT, meaning more serious delays in RMDA. The number of homozygous individuals was low for CFH and ARMS2 (13.4% and 5.0%, respectively), as expected. Thus, our estimates for these alleles in this relatively young cohort are similar to those reported in very large population studies.28
The complement factor H (FH) protein is a major fluid-phase inhibitor of the alternative complement pathway. Its functions include docking at the pericellular extracellular matrix, sequestering activated C3 (C3b) to suppress binding with Factor B and acting as a cofactor for Factor I, which proteolytically cleaves and inactivates C3b. The effect of the Y402H variant (actually residue 384 of the mature FH protein)29 includes reduced affinity for extracellular matrix30 and lipid peroxidation products.31 Eyes with the Y402H genotype have increased abundance of the membrane attack complex, the effector protein of the complement cascade, likely contributing to choriocapillaris degeneration observed in early AMD.32–34 Functional studies have suggested a role of CFH in modulating lipoprotein binding to Bruch’s membrane.35 Yet there is also appreciable CFH expression in neurosensory retina, although not as high as in RPE and choroid,36, 37 and thus a role in signaling pathways impacting the photoreceptor support system cannot be excluded. Understanding the role of CFH in this setting requires a better understanding of which cells express this gene in the macula.
In contrast to FH protein, the function of the ARMS2 protein has been challenging to determine, due in part to low expression in adult tissues36 and the lack of an ortholog outside of non-human primates.38 ARMS2 RNA was first identified in placenta,10, 39 with only low levels detectable in other tissues except for testes (https://www.gtexportal.org/home/gene/ARMS2). ARMS2 protein has been immunolocalized to cytosol, mitochondria, extracellular matrix, and circulating leukocytes,40–43 discrepancies which remain to be reconciled. The relative lack of expression in adult retina, RPE and choroid36, 37 may suggest that, as in placenta, ARMS2 plays a developmental role for which the physiological consequences of the A69S polymorphism are not manifest until advanced age. Our finding that ARMS2 variants associate with visual function prior to the clinical manifestation of AMD in this cohort is consistent with this novel concept.
While much more needs to be learned about the function and pathophysiology of ARMS2, the association of the A69S polymorphism with delayed RMDA, even in asymptomatic or pre-symptomatic individuals in normal macular health, reveals a previously unappreciated role of this variant. RMDA assesses the efficiency of replenishing retinoids and loading of 11-cis retinal to the opsin molecule in rod outer segments, as established by a landmark quantitative model15, 16, 44 incorporating fundus reflectometry, visual cycle biochemistry, and clinical observations of sensitivity recovery after bleaching light in outer retinal disease. The A69S polymorphism could delay RMDA at one or more of several steps, including: extravasation and uptake of circulating vitamin A complexes through the choriocapillaris fenestrae or caveolae; diffusion across or binding to Bruch’s membrane; uptake of retinoids by the RPE; isomerization and/or oxidation of retinol; intracellular transport through RPE cell bodies and apical processes; trafficking through the interphotoreceptor matrix; and uptake/loading of 11-cis retinal onto the opsin molecule. A better understanding of ARMS2 function will be necessary to appreciate the molecular step(s) at which RMDA is impaired in individuals with high risk genotypes.
Our goal of seeking genetic associations for a functional phenotype (delayed RMDA) that appears early and involves cells and tissues that exhibit characteristic AMD pathology as disease progresses deserves comment. The original genome-wide association studies that highlighted the complement pathway, ARMS2, and lipid-related genes, among others, used patients at mixed disease stages including advanced AMD, diagnosed by clinical biomicroscopy or color fundus photography.5–7, 10, 12 In a large population-based cohort study on adults ≥ 85 years old, the CFH Y402H polymorphism was associated with worse visual acuity, whether binocular or for each eye measured separately.45 In a study of 43 clinically normal older adults, those with high-risk alleles of CFH, ARMS2, or HTRA1 were more likely to have reduced mesopic critical fusion frequency.46 To date, studies have not identified associations of CFH and ARMS2 with functional phenotypes of early AMD, although variants of CFH, LIPC and ABCA1 have been associated with different drusen morphologies.47–49 Recently, genes50, 51 associated with serum C3d-to-C3 ratio, a measure of complement activation, were found in 717 AMD cases and 831 controls. The relative contributions of intraocular and systemic mechanisms for complement genes remain to be discerned, although deposition of the membrane attack complex is largely choriocapillaris specific.52
Visual function testing is important for assessing the outcomes of treatments because quality of vision and visual task performance abilities in everyday life are how patients gauge treatment success. It is also an important means to gain mechanistic understanding of disease pathogenesis, because tests access specific neurophysiological processes within parallel channels of information flow within the precisely layered retina.53 Because differential rod and cone dysfunction is characteristic of disorders affecting photoreceptors, much can be learned from psychophysical tests performed at different background adaptation levels to assess photopic (cone-mediated), mesopic (rod- and cone-mediated), and scotopic (rod-mediated) vision. The 6-mm diameter human macula (as defined by neurobiology and by epidemiology) contains a cone-dominated fovea 0.8 mm in diameter in the center, surrounded by an annulus of rod-dominated perifovea. Further, rods in central macula die before cones in aging,54 as determined by accurate cell counts in flat-mounted tissues,54 and the longest-lasting photoreceptors in advanced AMD are cones.55–57 Thus, tests of rod- as well as cone-mediated vision are warranted in assessing macular health. RMDA assesses the dynamic time course of sensitivity recovery following a photobleach, which is mediated by the retinoid re-supply route and can be distinguished from tasks measured under steady-state background luminance conditions, such as scotopic sensitivity (e.g., as assessed in microperimetry) and low luminance acuity and low luminance deficit,58 which are mesopic (mediated by both rods and cones). Further, the methods just mentioned assay specific neurophysiologic mechanisms of the night vision system, including rod sensitivity, lateral interaction with cones, and rod signal convergence onto cone-driven pathways.59–61 Genes that influence these other visual functions in AMD will be sought in future studies.
Many aspects of AMD pathology were revealed first or best with optical coherence tomography (OCT), most notably subretinal drusenoid deposit (SDD), which is classified with soft drusen or omitted altogether from grading systems based on color fundus photography.62 Our model of RMDA impairment in AMD, initially based on age-changes in RPE-Bruch’s membrane complex63 now also incorporates the presence of SDD between photoreceptors and RPE in many AMD eyes and shown to particularly devastate the rate of RMDA.24, 25, 64 Like drusen, SDD also involves increased diffusion distance from the choriocapillaris and reduced transfer from RPE, plus direct cytotoxicity to photoreceptors, which the deposits contact. We previously assessed SDD prevalence in the ALSTAR cohort using multimodal imaging and criteria designed to capture early stages (i.e., by counting single deposits before obvious patterns typical of intermediate AMD), and by including OCT volumes centered on the optic nerve head, where SDD first appear.65 At this early stage of disease, age was a larger factor in delayed RMDA than was the presence of early SDD.66 Interestingly, as first pointed out in a sub-analysis of the Columbia Macular Genetics Study,67 ARMS2 A69S is more closely associated with the SDD phenotype than is CFH Y402H. More recently, a meta-analysis68 (6 studies totaling 346 patients) and direct genotyping of neovascular AMD patients69 (n=755) both confirm this association. Thus, it remains be determined if ARMS2’s influence on RMDA relates to incipient SDD or other AMD-relevant pathology, preferably in populations for which OCT-based imaging is available.
Strengths of this study include a large sample of normal and AMD eyes, as assessed by a standardized and accepted AMD classification system based on color fundus photography. This is the first study to examine the relationship between CFH or ARMS2 high-risk alleles and RMDA in normal older adults and those with AMD. Delayed RMDA in aging and AMD has strong biologic plausibility14, 70–72 and has been repeatedly demonstrated over 20 years.19, 24, 25, 63, 72–77 Limitations of this study are also acknowledged, which will be addressed in future research. The relationship between these high-risk alleles and other visual functions have not yet been evaluated, so specificity for RMDA remains to be determined. The analysis was limited to two genes (albeit two with the strongest association to AMD). The genotype-phenotype associations reported here have not been explicitly explored for SDD, an AMD pathology that accentuates RMDA delays24, 25 and the risk of progression to late-stage disease.78, 79
In this study, we established a link between the strongest genetic associations for AMD identified thus far, namely common polymorphisms at chromosome 1 and chromosome 10, and the first functional risk factor of incident AMD identified to date, namely delayed RMDA. This ARMS2 genotype-phenotype association emerges before the clinical phenotype of AMD is discernable, which has several implications. First, our results help refine the timeline of disease and in doing so direct attention toward stages when intervention could most effectively mitigate progression to end-stages. Second, we propose that when designing clinical trials, delayed RMDA together with genotype could identify patients most likely to progress during the study and thus those most likely to reveal an intervention effect. Third, because ARMS2 is strongly related to RMDA at pre-clinical stages of AMD, research directed towards understanding the function of this gene should be prioritized. ARMS2 is challenging to study due to its low expression in adults36 and exclusivity to Old World primates,38 yet we suggest that a focused effort will bring knowledge of value in reducing the public health burden of AMD.
Supplementary Material
Acknowledgments
This research was supported by NIH R01AG04212, R01EY027948, R01EY021470, 5R01EY026087, the National Center for Advanced Translational Sciences of NIH (UL1TR001417), the Dorsett Davis Discovery Fund, the Alfreda J. Schueler Trust, the EyeSight Foundation of Alabama, Research to Prevent Blindness, and the Macula Foundation. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement: Christine A. Curcio receives research funding from Hoffman LaRoche and Heidelberg Engineering. Cynthia Owsley is a patent holder on the apparatus used to measure dark adaptation in this study.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e16. [DOI] [PubMed] [Google Scholar]
- 2.Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet 2014;15:151–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol 2001;119:1417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT). Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards A, Ritter R, Abel K, et al. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308:421–4. [DOI] [PubMed] [Google Scholar]
- 7.Haines J, Hauser M, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308:419–21. [DOI] [PubMed] [Google Scholar]
- 8.Khanhadia S, Cipriani V, Yates JR, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology 2012;217:127–46. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsdottir J, Conley Y, Weeks D, et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet 2005;77:389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera A, Fisher SA, Keilhauer CN, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet 2005;14:3227–36. [DOI] [PubMed] [Google Scholar]
- 11.Grassmann F, Held IM, Weber BH, International AMD Genomics Consortium (IAMDGC). Recombinant haplotypes narrow the ARMS2/HTRA1 association signal for age-related macular degeneration. Genetics 2017;205:919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide associatio study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet 2016;48:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol 1990;292:497–523. [DOI] [PubMed] [Google Scholar]
- 14.Owsley C, McGwin G Jr, Clark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology 2016;123:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 2004;23:307–80. [DOI] [PubMed] [Google Scholar]
- 16.Lamb TD, Pugh EN Jr. Phototransduction, dark adaptation, and rhodopsin regeneration. Invest Ophthalmol Vis Sci 2006;47:5138–52. [DOI] [PubMed] [Google Scholar]
- 17.Mata NL, Radu RA, Clemmons RS, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: A novel pathway for visual-pigment regeneration in daylight. Neuron 2002;36:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res 2011;23:307–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owsley C, Huisingh C, Clark ME, et al. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res 2016;41:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration. AREDS Report No. 17. Arch Ophthalmol 2005;123:1484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol 2003;135:194–205. [DOI] [PubMed] [Google Scholar]
- 22.Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Bio Dis Infor 2008;1:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugh EN Jr. Rushton’s paradox: rod dark adaptation after flash photolysis. J Physiol 1975;248:413–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flamendorf J, Agrón E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology 2015;122:2053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laíns I, Miller JB, Park DH, et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology 2017;124:1340–52. [DOI] [PubMed] [Google Scholar]
- 26.Eng B, Ainsworth P, Waye JS. Anomalous migraton of PCR products using nondenaturing polyacrylamide gel electrophoresis: the amelogenin sex-typing system. J Forensic Sci 1994;39:1356–9. [PubMed] [Google Scholar]
- 27.National Institute of Standards and Technology, US Department of Commerce. Sex-typing markers https://strbase.nist.gov/sextype.htm 2007. Accessed 07.31.18.
- 28.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day AJ, Willis AC, Ripoche J, Sim RB. Sequence polymorphism of human complement factor H. Immunogenetics 1988;27:211–4. [DOI] [PubMed] [Google Scholar]
- 30.Clark SJ, Higman VA, Mulloy B, et al. His-384 allotypic variant of factor H associated with age-related macular degeneration has different heparin binding properties from the non-disease-associated form. J Biol Chem 2006;281:24713–20. [DOI] [PubMed] [Google Scholar]
- 31.Weismann D, Hartvigsen K, Lauer N, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011;478:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullins RF, Johnson MN, Faidley EA, et al. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng S, Whitmore SS, Sohn EH, et al. Molecular response of chorioretinal endothelial cells to complement injury: Implications for macular degeneration. J Pathol 2016;238:446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon JM, McLeod DS, Bhutto IA, et al. Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol 2016;134:1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by completment factor H. Proc Natl Acad Sci U S A 2015;112:E3034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Jia C, Kazmierkiewicz KL, et al. Comprehensive analysis of gene expression in human retina and supporting tissues. Hum Mol Genet 2014;23:4001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitmore SS, Wagner AH, DeLuca AP, et al. Transcriptomic analysis across nasal, temporal, and macular regions of human neural retina and RPE/choroid by RNA-Seq. Exp Eye Res 2014;129:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis PJ, Appukuttan B, Simmons E, et al. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Hum Mol Genet 2008;17:2673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaartokallio T, Cervera A, Kyllonen A, et al. Gene expression profiling of pre-eclamptic placentae by RNA sequencing. Sci Rep 2015;5:14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanda A, Chen W, Othman R, et al. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A 2007;104:16227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, Spencer KL, Court BL, et al. Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria. Invest Ophthalmol Vis Sci 2009;50:3084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Micklisch S, Lin Y, Jacob S, et al. Age-related macular degenerationi associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. J Neuroinflammation 2017;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kortvely E, Hauck SM, Duetsch G, et al. ARMS2 is a constitient of the extracellular matrix providing a link between familial and sporadic age-related macular degenerations. Invest Ophthalmol Vis Sci 2010;51:79–88. [DOI] [PubMed] [Google Scholar]
- 44.Lamb TD, Cideciyan AV, Jacobson SG, Pugh EN. Towards a molecular description of human dark adaptation. J Physiol 1998;506:88P. [Google Scholar]
- 45.Mooijaart SP, Koeijvoets KMC, Sijbrands EJG, et al. Complement Factor H polymorphism Y402H associates with inflammation, visual acuity, and cardiovascular mortality in the elderly population at large. Exp Gerontol 2007;42:1116–22. [DOI] [PubMed] [Google Scholar]
- 46.Feigl B, Cao D, Morris CP, Zele AJ. Persons with age-related maculopathy risk genotypes and clinically normal eyes have reduced mesopic vision. Invest Ophthalmol Vis Sci 2011;52:1145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrara D, Seddon JM. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthamol 2015;133:785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Asten F, Simmons M, Singhal A, et al. A deep phenotype association study reveals specific phenotype associations with genetic variants in age-related macular degeneration: Age-Related Eye Disease Study 2 (AREDS2) Report No. 14. Ophthalmology 2018;125:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Y, Reynolds R, Fagerness J, et al. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:4663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lores-Motta L, Paun CC, Corominas J, et al. Genome-wide association study reveals variants in CFH and CFHR4 associated with systematic complement activation. Ophthalmology 2018;125:1064–74. [DOI] [PubMed] [Google Scholar]
- 51.Wright AF, Barlow PN. Genome-wide association studies identify disease mechanisms in age-related macular degeneration. Ophthalmology 2018;125:962–4. [DOI] [PubMed] [Google Scholar]
- 52.Chirco KR, Tucker BA, Stone EM, Mullins RF. Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Exp Eye Res 2016;146:393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masland RH. The neuronal organization of the retina. Neuron 2012;76:266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis. Sci 1993;34:3278–96. [PubMed] [Google Scholar]
- 55.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci 1996;37:1236–49. [PubMed] [Google Scholar]
- 56.Schaal KB, Freund KB, Litts KM, et al. Outer retinal tubulation in advanced age-related macular degeneration: Optical coherence tomographic findings correspond to histology. Retina 2015;35:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Litts KM, Ach T, Hammack KM, et al. Quantitative analysis of outer retinal tubulation in age-related macular degeneration from spectral-domain optical coherence tomography and histology. Invest Ophthalmol Vis Sci 2016;57:2647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sunness JS, Rubin GS, Broman A, et al. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss resulting from geographic atrophy in age-related macular degeneration. Ophthalmology 2008;115:1480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca Fascicularis. J Physiol 1984;357:575–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boycott BB, Hopkins JM, Sperling HG. Cone connections of the horizontal cells of the rhesus monkey’s retina. Proc R Soc Lond B Biol Sci 1987;229:345–79. [DOI] [PubMed] [Google Scholar]
- 61.Beaudoin DL, Kupershtok M, Demb JB. Selective synaptic connections in the retinal pathway of night vision. J Comp Neurol 2017; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 62.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits a.k.a. pseudodrusen. Surv Ophthalmol 2018; Epub ahead of print. [DOI] [PubMed]
- 63.Steinmetz RL, Haimovici R, Jubb C, et al. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch’s membrane change. Br J Ophthalmol 1993;77:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ooto S, Suzuki M, Vongkulsiri S, et al. Multimodal visual function testing in eyes with nonexudatuve age-related macular degneration. Retina 2015;35:1726–34. [DOI] [PubMed] [Google Scholar]
- 65.Zarubina AV, Neely DC, Clark ME, et al. Prevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imaging. Ophthalmology 2016;123:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neely D, Zarubina AV, Clark ME, et al. Association between visual function and subretinal drusenoid deposits in normal and early age-related macular degeneration eyes. Retina 2017;37:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith RT, Merriam JE, Sohrab MA, et al. Complement factor H 402H variant and reticular macular disease. Arch Ophthalmol 2011;129:1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jabbarpoor Bonyadi MH, Yaseri M, Nikkhah H, et al. Association of risk genotypes of ARMS2/LOC387715 A69S and CFH Y402H with age-related macular degeneration with and without reticular pseudodrusen: a meta-analysis. Acta Ophthalmol 2017;96:e105–e10. [DOI] [PubMed] [Google Scholar]
- 69.Lin LY, Zhou Q, Hagstrom S, et al. Association of single-neuceltide polymorphisms in age-related macular degeneration with pseudodrusen: Secondary analysis of data from the Comparison of AMD Treatment Trials. JAMA Ophthalmol 2018;136:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curcio CA, Jackson GR, Owsley C. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci 2000;41:2015–8. [PubMed] [Google Scholar]
- 71.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev 2002;1:381–6. [DOI] [PubMed] [Google Scholar]
- 72.Owsley C, McGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest. Ophthalmol Vis Sci 2006;47:1310–8. [DOI] [PubMed] [Google Scholar]
- 73.Owsley C, Jackson GR, White MF, et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology 2001;108:1196–202. [DOI] [PubMed] [Google Scholar]
- 74.Dimitrov PN, Guymer RH, Zele AJ, et al. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci 2008;49:55–65. [DOI] [PubMed] [Google Scholar]
- 75.Jackson GR, Owsley C, McGwin G Jr. Aging and dark adaptation. Vision Res 1999;39:3975–82. [DOI] [PubMed] [Google Scholar]
- 76.Tahir HJ, Rodrigo-Diaz E, Parry NRA, et al. Slowed dark adaptation in older eyes; Effect of location. Exp Eye Res 2016;155:47–53. [DOI] [PubMed] [Google Scholar]
- 77.Cocce KJ, Stinnett SS, Luhmann UFO, et al. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am J Ophthalmol 2018;189:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steinberg JS, Gobel AP, Fleckenstein M, et al. Reticular drusen in eyes with high-risk characteristics for progression to late-stage age-related macular degeneration. Br J Ophthalmol 2015;99:1289–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


