Abstract
Purpose:
To determine exchange parameters for chemical exchange saturation transfer of phosphocreatine (PCrCEST) in phantoms and characterize PCrCEST in vivo in the muscle at different powers and fields.
Methods:
Exchange parameters were measured in PCr solutions using varying power at 15.2T. Z-spectra were analyzed using multi-pool Lorentzian fitting in the hindlimb using various powers at two different fields: 9.4 T and 15.2 T. Modulation of PCr signal in PCrCEST and 31P-MRS was observed in the mouse hindlimb before and after euthanasia.
Results:
The exchange rate of PCr at physiological pH in phantoms were confirmed to be in a much slower exchange regime compared to Cr: kex at pH 7.3 and below was less than 400 s−1. There was insufficient signal for detection of PCrCEST in the brain, but PCrCEST in the hindlimb was measured to be 2.98% ± .43 at a B1 of 0.47 μT at 15.2 T, which is 29% higher than 9.4 T values. PCrCEST at a B1 of 0.71 μT was not significantly different to that measured at a B1 of 0.47 μT. After euthanasia, PCrCEST signal dropped by 82.3% compared to an 85% decrease in PCr in 31P-MRS while CrCEST signal increased by 90.6%.
Conclusion:
PCrCEST is a technique with viable sensitivity in the muscle at high fields and shows promise for the study of metabolic dysfunction and cardiac systems.
Keywords: CEST, phosphocreatine, ultrahigh field, creatine, chemical exchange
Introduction
The phosphorylation of creatine plays a major role in governing the energetics of tissues that have high demands on energy such as the heart or skeletal muscles by storing high energy phosphates in the form of phosphocreatine (PCr) (1). In these tissues, adenosine-5’-triphosphate (ATP) is a required source of free energy to maintain functionality:
| [1] |
where the products are adenosine-5’-diphosphate (ADP), inorganic phosphate (Pi), free hydrogen (H+), and Gibb’s free energy (ΔG). These organs can operate despite the relatively low concentration of ATP due to the efficient activity of an enzyme known as creatine kinase (CK) which acts to actively replenish ATP during high energy demand (2). The reversible reaction responsible is as follows:
| [2] |
Insight into the metabolites involved in this process is important to the diagnosis and study of such diseases as cardiac afflictions (3–5), systemic metabolic changes in Huntington’s disease (6,7), and various other metabolic dysfunctions (8–10). Traditionally, the important metabolites of creatine phosphorylation, PCr and ATP, have been studied through Phosphorus Magnetic Resonance Spectroscopy (31P-MRS), however, this study is restricted by low resolution and long acquisition times due to the need for extensive signal averaging.
Chemical exchange (CE)-sensitive magnetic resonance imaging has been gaining ground as a non-invasive technique that can potentially image metabolites with particular labile protons (11–14) such as amides (15–19), amines (20–22), and hydroxyl groups (23,24). This is accomplished through on-resonance saturation (Chemical Exchange-sensitive Spin Lock [CESL]) or by off-resonance saturation (Chemical Exchange Saturation Transfer [CEST] or CESL) enabling imaging with sensitivity that would be difficult to accomplish by other spectroscopic means. Thus, a number of groups have held interest in imaging creatine (Cr) using CEST methods (25–28), since creatine has labile protons in its guanidyl group. However, a portion of this CEST signal will come from the guanidyl groups of mobile proteins (29–32). Conversely, targeting PCr in this phosphorylation process may be crucial for obtaining more specific information about this metabolic process.
In this study, we examine the potential of PCr CEST (PCrCEST) by i) examining chemical exchange characteristics in phantoms (e.g. power dependency and exchange rate), ii) testing and measuring the sensitivity of PCrCEST in vivo in the brain and muscle, iii) comparing PCrCEST sensitivity in the hindlimb at different B1 powers (0.47 μT vs 0.71 μT) and different field strengths (9.4 T vs 15.2 T), and iv) investigating dynamic changes that occur in PCrCEST before and after euthanasia by comparing Z-spectra, 31P-MRS, and 3-point measurement CEST maps.
Methods
Overall MR experiments
All MR experiments were primarily performed on a horizontal bore 15.2 T/11-cm Bruker Biospec while 31P-MRS and comparison study was performed on a horizontal bore Bruker Biospec 9.4 T/30-cm (Billerica, MA, USA). The 15.2T system has an actively shielded 6.0-cm-diameter gradient operating at a maximum strength of 100 gauss/cm with a rise time of 110 μs. The 9.4T system has an actively shielded 12.0-cm-diameter insert operating at a maximum gradient strength of 66 gauss/cm with a rise time of 141 μs. In both magnets, the magnetic field homogeneity was optimized by utilizing a protocol that calculated shim values based on a field map and then subsequently optimized by localized shimming over a ~20 × 20 × 4 mm3 volume in phantoms, a ~8 × 4 × 3 mm3 volume in the in vivo brain, or a ~7 × 7 × 4 mm3 volume in the in vivo hindlimb. B0 maps were obtained using the WASSR method (33). B1 maps were obtained by measuring signal nutation as was done in Vaughan et al. (34). A T1 map was obtained using a saturation-recovery sequence in both phantoms and in vivo, and a T2,0 map was obtained in vivo using an on-resonance spin-lock sequence at a high spin-lock power in order to reduce the chemical exchange component of transverse relaxation and measure an exchange independent T2 value (irradiation power = 106 μT and irradiation length = 50 ms (13)).
The chemical exchange-sensitive MR pulse sequence consists of a preparation module for chemical exchange contrast and an image readout. An off-resonance continuous wave pulse was used for CE preparation. Images were read-out by an RF-spoiled TurboFLASH sequence with a center-out phase-encoding scheme with parameters as follows: matrix size = 64 × 64, slice thickness = 2 mm, flip angle = 20.0 deg, GRE readout TR = 10.0 ms, TE = 1.90 ms, image TR = 24.0 s (phantom)/8.0 s (in vivo), and readout bandwidth = 50 kHz, comparable to the parameters used in our previous work (14).
For 31P-MRS at 9.4 T, an Image-Selected In vivo Spectroscopy (ISIS) volume localization sequence was used with a voxel that measured 284 μL for phantoms or 171 μL for the hindlimb in vivo. ISIS parameters were: TR = 2 seconds; NA = 1152 (144 ISIS cycles) preceded by 1 dummy cycle; 2.05 ms 180° adiabatic full passage inversion pulses (bandwidth = 98.7 ppm); 50 μs 90° square excitation pulse (bandwidth = 158.0 ppm); 8196 acquisition points. From the difference in chemical shift between the PCr peak and Pi peak, the pH was inferred using an equation introduced by Petroff et al. (35)
MR Experiments of Metabolite Phantoms
Metabolite solutions were prepared and transferred into 9-mm I.D. syringes and four phantoms were bundled together for imaging. All phantom experiments were performed at 37.2 ± 0.5°C with volume coil excitation and reception (3.5-cm ID for 15.2 T and a 4.0-cm ID dual tuned coil [1H and 31P] for 9.4 T) and a field of view of 50 mm × 50 mm.
Three sets of MRI phantom experiments were performed. Z-spectra were obtained using a CW irradiation pulse which was applied at 151 offsets with uneven intervals between Ω = ±6.0 ppm. As a reference (S0), images were obtained at Ω = 300 ppm.
Experiment I:
CEST MRI of Creatine Phosphorylation Associated Metabolites. Creatine (Cr), Adenosine Triphosphate (ATP), and Phosphocreatine (PCr) were prepared at the concentration of 50 mM in 1 × phosphate buffered saline (PBS) and titrated to a pH of 7.3. Z-spectra were taken at B1 = 0.47, 0.71, and 0.94 μT (ω1/2π = 20, 30 and 40 Hz) with Tirrad = 4.0 sec at 15.2 T and 31P MRS spectra were acquired at 9.4 T.
Experiment II:
CEST MRI of Phosphocreatine at Different Concentrations and Different Powers. PCr was prepared at the concentrations of 25, 50, 100, and 200 mM in PBS and titrated to a pH of 7.3. Z-spectra was taken at B1 = 0.24–2.35 μT (ω1/2π = 10–100 Hz) in 0.24 μT increments with Tirrad of 10 s.
Experiment III:
CEST MRI of Phosphocreatine at Different pH for Quantification of Exchange Parameters. PCr was prepared at the concentrations of 50 mM in PBS and titrated to a pH of 6.4, 6.7, 7.0 and 7.3. Z-spectra was taken at B1 = 0.24–2.35 μT (ω1/2π = 10–100 Hz) in 0.24 μT increments with a Tirrad of 10 s. Additionally, MTRasym data was acquired 2.6 ppm (+2.6 ppm, −2.6 ppm, and 300 ppm for reference)) using an extended Tirrad = 13 s to achieve steady-state saturation with an extended TR = 33 s to ensure full relaxation (36).
In vivo Animal Experiments
A total of 17 male C57BL/6 mice (weighing between 20 and 30 g) [6 for Z-spectra experiments, 2 for 31P-MRS, 3 for dynamic imaging, and 6 for PCr* and Cr* maps] were used with approval from the Institutional Animal Care and Use Committee of Sungkyunkwan University. Mice were anesthetized with isoflurane (5% for induction and 1.5–2.0% during MRI experiments) via a vaporizer with a mixture of O2 and N2 (30:70) and the rectal temperature was controlled at 37.2 ± 0.5°C using a water circulating pad. The brain was imaged first at 15.2 T, then their hindlimbs were imaged at 9.4 T and 15.2 T. Prior to imaging their hindlimbs at 15.2 T, the tail vein was cannulated. To obtain post-mortem data, a bolus of saturated KCl (50–80 μL) was injected through the tail vein. After euthanasia, rectal temperature continued to be monitored and controlled at 37.2 ± 0.5°C using the aforementioned water circulating pad.
For the brain, a volume excitation and 4 channel receiver array coil assembly was used for imaging with a field of view of 22 mm × 22 mm (resolution = 3.4 μm × 3.4 μm × 2 mm).
-
Z-spectra (n = 6) were obtained with B1 = 0.24, 0.47, and 0.71 μT (ω1/2π = 10, 20, and 30 Hz) and Tirrad = 4.0 sec at 151 offsets with uneven intervals between Ω = ±6.0 ppm. The selection of CEST parameters was based on exchange parameters and the region of linearity of the MTRasym in PCr phantoms. As a reference (S0), images were obtained at Ω = 300 ppm.
For the hindlimb, volume coil excitation and reception (3.5-cm ID for 15.2 T and a 4.0-cm ID dual tuned coil [1H and 31P] for 9.4 T) was used for imaging with a field of view of 32 mm × 32 mm (resolution = 5 μm × 5 μm × 2 mm).
Z-spectra (n = 6) were obtained with B1 = 0.47 and 0.71 μT (ω1/2π = 20 and 30 Hz) as well as Tirrad = 4.0 sec at 151 offsets with uneven intervals between Ω = ±6.0 ppm at 15.2 T. The selection of CEST parameters for these experiments was based on the spectra that showed sensitivity for PCr in the brain. Z-spectra were also taken from these same animals at 9.4 T while Z-spectra with B1 = 0.47 μT (ω1/2π = 20 Hz) was obtained additionally at approximately 30 minutes after euthanasia at 15.2 T. As a reference (S0), images were obtained at Ω = 300 ppm. On two additional animals, 31P-MRS was performed at 9.4 T before and at approximately 30 minutes after euthanasia.
To obtain CEST maps, additional experiments were performed at a higher resolution. The field of view and slice thickness were reduced to 25 mm × 25 mm and 1.5 mm, respectively, and the matrix size was increased to 96 × 96 to achieve a resolution of 2.6 μm × 2.6 μm × 1.5mm reducing voxel volume by a factor of 5. Three-point approaches using B1 = 0.47 μT (ω1/2π = 20 Hz) and Tirrad = 4.0 sec were adopted at Ω = 1.6 ppm, 1.9 ppm, 2.2 ppm and 300 ppm for Cr and Ω = 2.3 ppm, 2.6 ppm, 2.9 ppm and 300 ppm for PCr. Maps were acquired dynamically for three rodents with number of averages (NA) = 10 resulting in a temporal resolution of 5 minutes and 20 seconds until 30 minutes after euthanasia. Maps at static points for additional rodents (n=6) were acquired for spatial detail before euthanasia and at 30 minutes post-mortem using extensive signal averaging due to the loss in signal-to-noise from the increase in resolution [number of averages (NA) = 40].
Data Processing
All data were analyzed with in house developed Matlab code (MathWorks, Natick, Massachusetts, USA). Images with the exactly same parameters were averaged before further analysis. For Z-spectra analyses, regions of interest (ROI) were used. In phantoms, a ROI with minimal B0 inhomogeneity (<3 Hz) was selected from each sample; while in animal studies, small ROIs were drawn in the cortices, caudate putamen, or muscle of both hemispheres in the brain and large ROIs were drawn encompassing the muscle in the hindlimb. Exchange parameters were estimated for phantoms using the formulas for Quantification-of-exchange-by-saturation-power method (QUESP) introduced by McMahon et al (37). All data were reported as mean and standard deviation while statistical significance of differences was established using a student’s t-test with a significance set at or below 5%.
For full Z-spectra in vivo, the spectra was also fit to a multiple-pool Lorentzian function according to the method described in Windschuh et al (38). Initially, the spectrum excluding the PCr peak (2.3 ppm to 2.9 ppm) was fit to experimental Z-spectra using five pools (water, semi-solid magnetization transfer [MT], amide, guanidyl, NOE) with the parameters in Table 1 of the reference (38). The spectrum fitting was then fit again introducing a sixth PCr peak with the above-fitted five peak parameters being set as fixed. Then, peak intensities of PCrCEST and CrCEST were determined in the hindlimb. MTRasym curves were obtained from Z-spectra by (S(-Ω) – S(Ω))/S0. For CEST maps, Cr* and PCr* was computed by three-point analysis (17) as Cr* = [(S1.6ppm+S2.2ppm)/2 −S1.9ppm]/S0 and PCr* = [(S2.3ppm+S2.9ppm)/2 −S2.6ppm]/S0, respectively. Additionally, to examine effects due to changes in relaxation times after euthanasia, post-mortem spectra were adjusted for relaxation differences using ante-mortem relaxation values as a reference point. Adjusted spectra for analysis were determined using Eq. [12] from Jin et al.(13) by calculating Rex,CEST for the post-mortem case, then calculating adjusted spectra by using Rex,CEST with ante-mortem relaxation rates.
Results
CEST MRI of Creatine Phosphorylation Associated Metabolites (Phantom Experiment I)
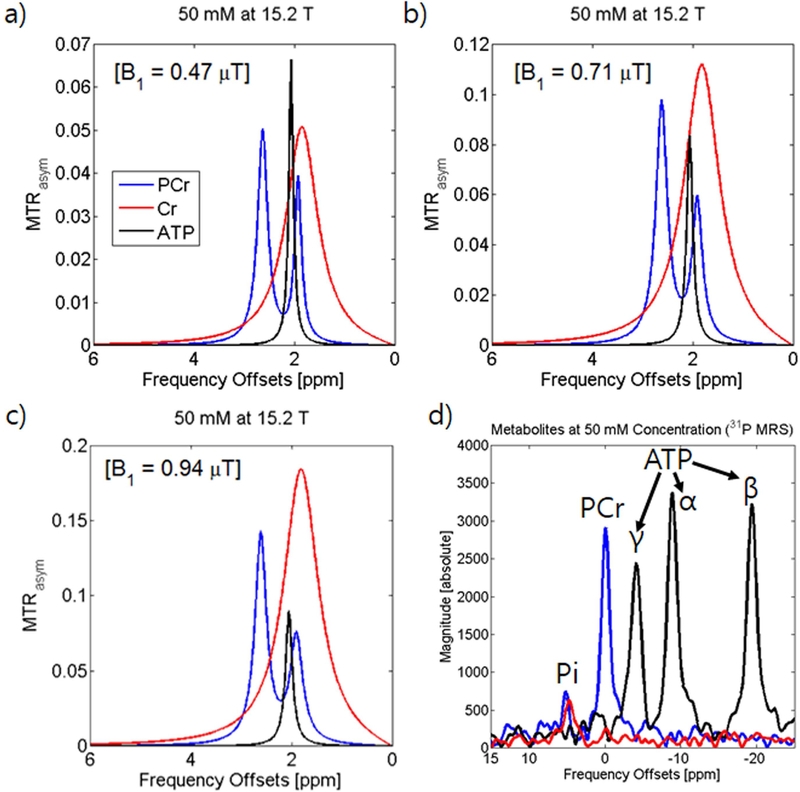
Fig. 1a depicts the MTRasym curves for 50 mM PCr, Cr, and ATP at pH of 7.3 with B1 = 0.47 μT and Tirrad = 4 sec. PCr has a major peak with higher sensitivity of 5% at Ω = 2.6 ppm and a second peak of ~3.9% at 1.9 ppm. ATP has a similar peak of 6.7% at Ω ~ 2.0 ppm while Cr exhibits a broad peak of 5.1% at Ω = 1.8 ppm. At this power, the peak at 2.6 ppm shows high specificity from the other peaks while at the peaks around 2.0 ppm greatly overlap resulting in contamination between Cr, PCr, and ATP. At the higher power of 0.71 μT (Fig. 1b), all four MTRasym peak intensities have increased. However, the increase in the faster exchanging Cr peak is significantly higher followed by the 2.6 ppm PCr peak, the 1.9 ppm PCr peak, and finally the ATP peak. At 0.94 μT power (Fig. 1c), the Cr peak becomes significantly more dominant and the overlap with the PCr peak at 2.6 ppm becomes significantly larger. Our CEST data of PCr and ATP were compared to 31P MRS spectra of the same samples acquired at 9.4 T (Fig. 1d). In 31P MRS data, the three phosphate groups of ATP (α, β, and γ) exhibit comparable height to the PCr peak. For Cr phantom, due to the absence of a phosphate group, there is no peak aside from the inorganic phosphate present in PBS.
Figure 1. Chemical Exchange Saturation Transfer MRI signals of Phosphocreatine phantoms.
MTRasym curves of creatine phosphorylation metabolites (50 mM PCr, Cr, and ATP in pH 7.3 PBS at 37°C) acquired at 15.2 T (a-c) at 0.47 μT, 0.71 μT, and 0.94 μT saturation power. 31P-MRS spectra acquired at 9.4 T (d).
CEST MRI of Phosphocreatine at Different pH/Concentrations and Different Powers (Phantom Experiment II and III)
To determine exchange rates of PCr labile protons to water protons, saturation power-dependent Z-spectra of PCr phantoms with different pH were obtained using a long saturation of 10 s (R1 = 0.28 sec−1). Fig. 2a depicts the MTRasym curves for PCr at 50 mM with a pH of 7.3 using varied saturation power. As seen in Fig 1a, two peaks are present from PCr and their sensitivity increases with saturation power. The increase in peak height is greater at lower powers and this increase diminishes as more power is applied. Saturation power-dependent Z-spectra of PCr phantoms with different concentration were obtained using a saturation duration of 4 s. Fig. 2b shows the concentration dependence of the peak at 2.6 ppm for PCr at pH 7.3 at different powers as will be used in vivo. PCr exhibits linear concentration dependence up to around 0.71 μT saturation power and begins to exhibit some non-linearity above that saturation power.
Figure 2. Chemical Exchange Saturation Transfer MRI signals of Phosphocreatine phantoms at 15.2 T.
MTRasym curves of 50 mM PCr in pH 7.3 PBS at 10 different saturation powers (0.24 μT −2.35 μT) with 4 s irradiation (a) and the MTRasym at 2.6 ppm of 4 different concentrations at these powers (b). MTRasym curves of 50 mM PCr in PBS at 0.47 μT with 10 s irradiation varying pH (c) and the MTRasym at 2.6 ppm at these pH with varied saturation power with 13 s irradiation (d).
The relationship between peak height and power is governed in part the exchange rate of PCr modulated by pH. Fig. 2c shows MTRasym curves for PCr at 50 mM at a fixed power of 0.47 μT. At this power, both peaks increase with increasing pH until pH 7.0. At pH 7.3, the height of both peaks start to decrease. Fig. 2d shows the power dependence of the peak at 2.6 ppm for 50 mM PCr at varying pH at steady state. Above B1 = 0.71 μT, PCr peak height increases as pH increases, while under 0.71 μT, the peak height at pH 7.3 is smaller than lower pH PCr peaks. This indicates that lower power is preferable when interested in slower exchanges over faster exchanges. QUESP was used to fit these curves and determine exchange parameters which yielded the following exchange rates: kex, pH6.4 = 142.3 ± 12.5 s−1; kex, pH6.7 = 193.6 ± 15.4 s−1; kex, pH7.0 = 202.5 ± 8.4 s−1; and kex, pH7.3 = 365.5 ± 21.5 s−1. The proton fraction was not fixed and there were slight discrepancies with the values for population: pb, pH6.4 = .0004; pb, pH6.7 = .0005; pb, pH7.0 = .0006; and pb, pH7.3 = .0006 with only pH 7.0 and pH 7.3 showing consistency.
PCrCEST MRI of the Mouse Brain vs. Muscle
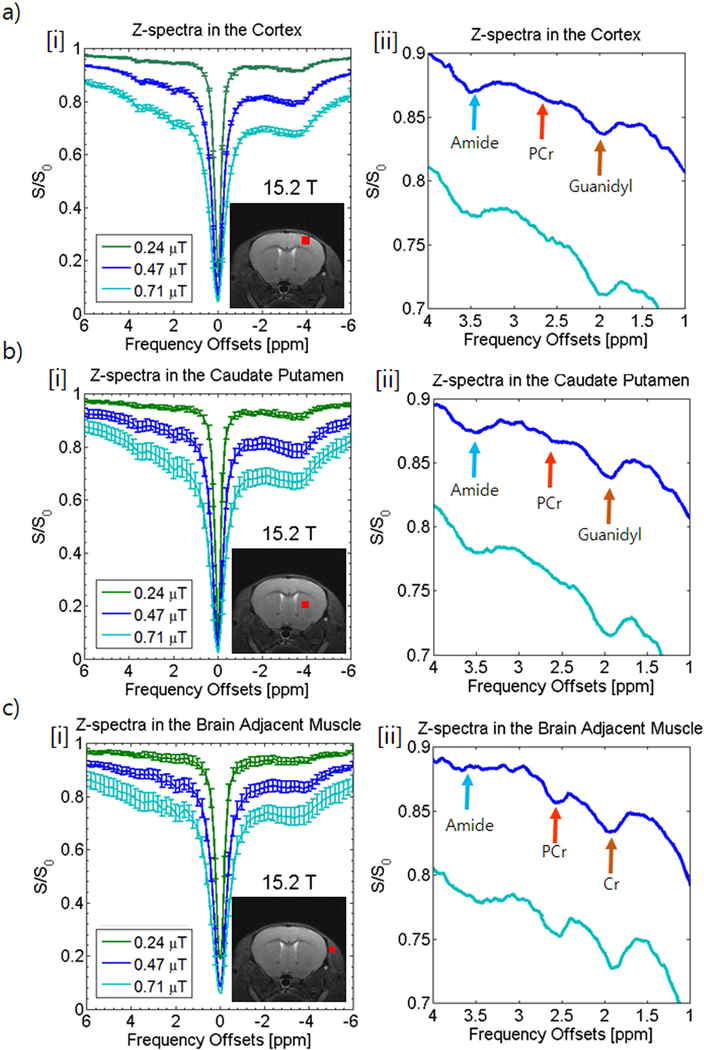
Z-spectra were acquired from the mouse brain at three different powers and ROIs were drawn in three different areas of the brain (cortex [Fig. 3a], caudate putamen [Fig. 3b], and the brain adjacent muscle [Fig. 3c]). Z-spectra [i] are shown with an enlargement [ii]. A large Nuclear Overhauser Enhancement peak is clearly visible on the negative end of the spectrum. Since peaks were difficult to determine on Z-spectra at 0.24 μT, enlargement focused on the 0.47 μT and 0.71 μT curves.
Figure 3. In vivo Z-spectra analysis at 15.2 T of the rat brain cortex (a), caudate putamen (b), and surrounding muscle (c) at various saturation powers (0.24 μT[green], 0.47 μT [blue], and 0.71 μT [turquoise]).
Z-spectra with error bars (i) shown with ROIs displayed in red in the anatomical image inset in each Z-spectrum. An enlarged view of the amide (blue arrow), PCr (orange arrow), and guanidyl peaks (brown arrow) are shown in [ii] for each Z-spectra.
The cortex (Fig. 3a) and the caudate putamen (Fig. 3b) show very strong amide and guanidyl peaks, however at 2.6 ppm there appears to be little to no peak with only the semblance of a peak in the caudate putamen spectrum at 0.47 μT saturation power. The signal level within the brain may be too low or the peak could be obfuscated by other overlapping sources such as the amide peak or direct water saturation.
Conversely, looking at the muscle adjacent to the brain (Fig. 3c), there are strong peaks at the guanidyl (comparable to the brain) and PCr resonances whereas the NOE peak on the opposite side of the spectrum and the amide peak is visibly weaker than in the brain. The lower level of amide and NOE sources seems to indicate a lower protein level which may mean more of the guanidyl peak in muscle can be attributed to Cr rather than mobile proteins.
PCrCEST Z-spectra of the Mouse Hindlimb and 31P MRS Spectroscopy
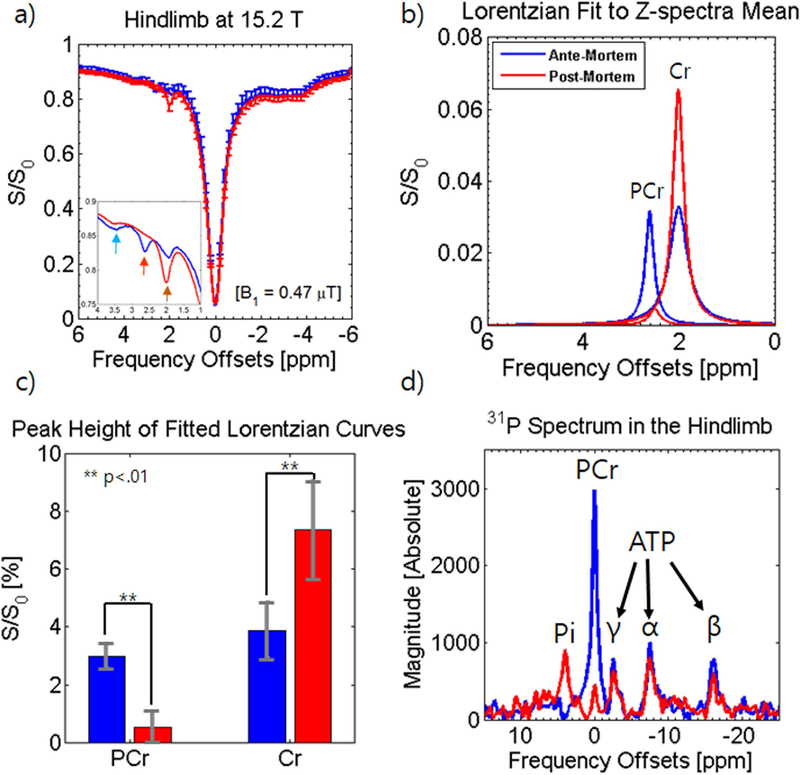
To further study phosphocreatine in the muscle, CEST data was acquired from the hindlimb in mice at 15.2T to observe saturation power dependency and at two fields (9.4 T and 15.2 T) to examine field dependency. An imaging slice was chosen to include the gastrocnemius, the soleus, as well as the tibia and its surrounding muscle while ROIs were chosen to encompass all muscles shown on the imaging slice. Fig. 4a shows the Z-spectra from the mouse hindlimb at 0.47 μT and 0.71 μT and Fig. 4b shows an enlarged view of the amide, Cr, and PCr peaks. A comparison of the PCr peak height as determined by 6-pool Lorentzian fitting (Fig. 4b inset) reveals that although the sensitivity is higher at 0.71 μT (PCr 0.47 μT = 2.98 ± 0.43% and PCr 0.71 μT = 3.35 ± .044%), this difference is not statistically significant whereas the increased influence of the semi-solid MT pool and direct water suppression is readily apparent. Consequentially, the power of 0.47 μT was chosen for further comparison. The similarity between the two spectra are apparent from the fitted PCr peak (Fig. 4c inset) and the appearance of the fit and residue (Fig. 4c solid and dashed lines).
Figure 4. In vivo Z-spectra analysis of the hindlimb with different powers (a-c) or with different field strength (d-f).
Z-spectra with error bars are shown comparing saturation powers of 0.47 μT (blue) or 0.71 μT (turquoise) with a 4 s duration at 15.2T (a) or at 9.4 T (red) or 15.2 T (blue) with a fixed power of 0.47 μT (d). Error bars are only shown on one side of the curve for better visualization. Expanded Z-spectra (b,e) at a range of 1.0 to 4.0 ppm inset with a statistical comparison of peak height from Lorentzian fitting. The multi-Lorentzian fit is shown (c,f) with the PCr peak inset and the residue overlaid on the fit in dashed lines. Blue arrows: amide; orange arrows: PCr; brown arrows: Cr.
Fig. 4d and 4e show the Z-spectra and enlarged view of amide, Cr, and PCr peaks at 9.4 T and 15.2 T using 0.47 μT saturation. The PCr peak at 15.2 T is significantly sharper with less overlap with the amide and Cr peaks which allows for better quantification. A comparison of the PCr peak height as determined by Lorentzian fitting (Fig. 4e inset) reveals that PCr quantization is significantly higher at 15.2 T than at 9.4 T (PCr 9.4 T = 2.12 ± 0.64% and PCr 15.2 T = 2.98 ± 0.43%) exhibiting a 41% higher signal at the higher field. The lower spectral resolution at 9.4 T resulted in more overlap between peaks which affected the quality of the fit (Fig. 4f). Other contributing factors to higher signal at 15.2T may include: higher T1 and T2 values (for the hindlimb, R1 = 0.52 s−1 and R2,0 = 31.3 s−1 at 9.4 T while R1 = 0.42 s−1 and R2,0 = 40.1 s−1 at 15.2 T), less direct saturation, and overall better separation from other peaks. Particularly for slower exchanges like PCr utilizing low saturation powers, R1 may have a significant effect on the sensitivity of the signal detected (39,40).
Fig. 5a shows the Z-spectra and the inset shows an enlarged view of the amide, Cr, and PCr peaks before and after euthanasia using 0.47 μT saturation. From first inspection, the lack of a significant difference between the NOE peak and the direct saturation imply there is little change in R1 and R2,0 after euthanasia which does seem true for measured R1 (R1 = 0.42 s−1 before euthanasia and R1 = 0.42 s−1 after euthanasia), however, data-fitting reveals a slight difference in R2,0 (R2,0 = 40.1 s−1 before euthanasia and R2,0 = 32.7 s−1 after euthanasia). While the fitted amide peak did decrease (36.8% from 4.32% to 2.74% [2.75% adjusted]), the more drastic changes came in the nearly complete decrease of the PCr peak and the increase in the Cr peak. To visualize this better, the Lorentzian fit to the PCr peak and Cr peak before and after euthanasia are displayed in Fig. 5b and a summary of these changes are presented in the bar graph in Fig. 5c. The PCr peak decreased significantly post-mortem exhibiting an 82.3% drop (from 2.98% to 0.52% [0.52% adjusted]) and Cr exhibited a significant increase of about 90.6% post-mortem (from 3.86% to 7.37% [7.37% adjusted]). The NOE peak showed a significantly smaller decrease (13% decrease) while the MT peak remained nearly unchanged (8.04% to 8.01% [8.1% adjusted]).
Figure 5. In vivo Z-spectra analysis of the hindlimb at 15.2 T and 31P-MRS at 9.4 T before and after euthanasia.
Z-spectra with error bars are shown before (blue) and after euthanasia (red) at 0.47 μT with a 4 s duration at 15.2T (a) and expanded Z-spectra at a range of 1.0 to 4.0 ppm are shown inset. Error bars are only shown on one side of the curve for better visualization. PCr and Cr peaks obtained from multi-pool Lorentzian fitting are shown before and after euthanasia (b) and a statistical analysis of peak height show significant differences (c). 31P-MRS spectra at 9.4T are shown overlaid before and after euthanasia (d).
Fig. 5d shows the overlay of the 31P MRS spectrum acquired in the hindlimb at 9.4T before and after euthanasia. The PCr peak is significantly higher than the three peaks of ATP and the difference in chemical shift between the Pi peak and PCr peak is 4.85 ppm which reveals a pH of 7.05. The post-mortem 31P MRS spectrum shows a dramatic decrease in the PCr peak losing 85% of detected phosphorus (confirmed by an 86.0% decrease in a second mouse). At 30 minutes to an hour after euthanasia when this data was acquired, there is not a great deal of change in the levels of ATP, dropping slightly compared to PCr. Conversely, there is an increase in the peak of Pi of about 251% of its value before euthanasia (195% increase in the second mouse) with a difference in chemical shift of 3.97 ppm which infers a pH of 6.41.
PCrCEST Maps of the Mouse Hindlimb
Fig. 6 shows exemplary image data acquired at high resolution in the mouse hindlimb. The ΔB0 and ΔB1 maps (Fig. 6a and Fig. 6b, respectively) show relative homogeneity throughout the hindlimb except for a large signal drop in the tibia as can be seen on the T2 weighted anatomical image (Fig. 6c) along with some degree of inhomogeneity in the small muscle in the adjacent area. Fig. 6d shows a dynamic time-course of the PCr* and Cr* signal in the hindlimb from 30 minutes before to 30 minutes post-euthanasia (n=3). Before euthanasia, the average signal of PCr* and Cr* fluctuate around the 2% level, comparable to the more highly averaged CEST maps (n=6), examples of which are shown in Fig. 6e[i] for PCr* and Fig. 6f[i] for Cr* (2.34 ± 0.49% for PCr* and 1.75 ± 0.25% for Cr*).
Figure 6. In vivo maps of PCr* and Cr* obtained before and after euthanasia at 15.2 T.
ΔB0 maps (a) and ΔB1 maps (b) show no specificity to anatomical structure aside from the tibia while anatomical maps taken using a T2-weighted RARE sequence (c) show various features of the hindlimb (Tib-Tibia; Fib-Fibula; SSV and GSV- small and great saphenous vein; PTV and ATV- posterior and anterior tibial veins). A dynamic time-course (d) shows PCr* and Cr* averaged over the entire hindlimb before euthanasia and until 30 minutes post-euthanasia and PCr* maps (e) and Cr* maps (f) were taken before [i] and after [ii] euthanasia.
In the static PCr* map before euthanasia (Fig. 6e[i]), some features which were also seen on the T2 weighted anatomical image (Fig.6c) can be noted. Large drops in PCr* signal are noticeable particularly in the area of the tibia, the great saphenous vein, as well as the anterior tibial vein which borders the interosseous membrane. Lesser drops in signal are seen in the areas of the small fibula bone as well as the posterior tibial vein. Conversely, the small saphenous vein area induces an area of artifacts which display as an increase in signal on the PCr* maps as well as on the Cr* maps (Fig. 6e[i] and Fig. 6f[i]).
After the induction of cardiac arrest, PCr* signal starts to drop rapidly while Cr* signal begins to increase (Fig. 6d). The rate of decrease in PCr* begins to reduce as it gets closer to 0%. This level is reached around 30 minutes post-euthanasia; at which point the increase in Cr* also tapers off into a peak around 6.5%. The static post-mortem images were acquired at this point. PCr* maps show a drastic drop in signal of about 109% to PCr* = −0.21 ± 0.05% (Fig. 6e[ii]), while Cr* maps (Fig. 6f[ii]) show a dramatic increase in signal of about 196% to a Cr* = 5.18 ± 1.70%.
Discussion
We have demonstrated that phosphocreatine can provide sufficient contrast to perform CEST quantification studies in the mouse hindlimb. From our measurements, we measured a sensitivity level of over 2% (at 15.2 T, 2.98%) which is above our previously published data (14) for Amide Proton Transfer* (APT*) in the rat brain at 15.2 T (2.87%). We calculated an exchange rate of around 202.5 ± 8.4 s−1 for pH 7.0, which was slightly higher than exchange rates (140 ± 60 s−1) calculated previously by Haris et al. (25) using line broadening at 9.4 T, though still significantly lower than the 950 ± 100 s−1 reported about creatine. Using our exchange rate with equation [2] in van Zijl et al. (12), the CEST signal can roughly be approximated to be around 2.27% which is similar to the values we have measured. While the resonance around 2 ppm was denoted as guanidyl or Cr protons, CEST signal at this frequency will involve guanidyl protons, ATP, and the second peak of PCr. At low powers such as 0.47 μT, the CEST signal will be mixed while an increase in power or a drop in pH will shift dominance at this resonance more toward the faster exchanging guanidyl protons such as Cr.
PCrCEST was investigated after euthanasia because the post-mortem exemplifies a strong example of dephosphorylation which can be easily and certainly enacted. Bertram et al. (41) described a study of changes in metabolite levels in a porcine post-mortem. In the first hour, while ATP was utilized increasing Pi levels, rapid creatine dephosphorylation replenished ATP allowing it to decrease at a slow pace while PCr levels dropped rapidly (rapid PCr depletion was also observed by in’t Zandt et al. in mice (42)). Furthermore, Becila et al. reports that muscle extracts taken from mice 1 hour post-mortem show a drop in pH from 7.0 to 6.67 post-mortem (43). These changes support what we have observed with PCrCEST and in 31P-MRS.
The consumption of PCr to replenish ATP as well as the drop in pH that we saw in our 31P-MRS data (pH 7.05 to pH 6.41) resulted in a drop in PCrCEST of 82.3% echoing the 85% decrease in the PCr peak in spectroscopy. Though temperature drop post-mortem could be a potential source of the decrease, the relatively small size of the mouse and the proximity of the temperature probe to the hindlimb of the mouse mitigates this concern. Estimating from our phantom data at pH 7.0 and pH 6.4, we would expect only a 16% decrease from pH with the rest of the drop being a result of phosphorylative changes. The 90.6% increase in the Cr peak as seen in CEST is similarly due to the shift toward slower exchange (30) from the drop in pH as well as the conversion of PCr to Cr from dephosphorylation. Due to both phosphorylative changes and pH differences, changes detected by PCrCEST were expected to be exaggerated in comparison to 31P-MRS due to the drop in pH post-mortem, however, this may have been offset by the fact that initially fitting to a 5-pool model underestimates PCr and overestimates Cr ante-mortem. Since the PCr signal is overlapped by CEST peaks at 2.0 ppm and 3.5 ppm the distribution of the Lorentzian fit between these peaks is partially arbitrary. We chose to approach this problem by uniformly underestimating the PCr peak by first explaining as much of the spectra as possible with a five pool Lorentzian fit (water, MT, NOE, amide, and Cr) before adding the PCr pool.
Quantitation of PCrCEST was limited by the proximity of PCr’s resonant frequency to other labile proton groups. While amide exchange may have comparatively slower exchange rates, amide sources are significantly higher in concentration in vivo. Zu et al. (44) has attempted to address this issue by exchange rate filtering using a technique known as Chemical Exchange Rotation Transfer (CERT), however, these methods may improve the specificity of the signal at the cost of signal to noise ratio (SNR). In the current study, we use the advantage of improved spectral resolution, as we have demonstrated previously (14), for enhanced quantitation through reduced overlap. At lower fields, quantitation may be possible with methods that may consider more aggregated signals.
PCrCEST proved to be unable to detect sufficient signal in the brain for accurate quantification. There only appeared to be a semblance of a peak in the caudate putamen at 0.47 μT power. This could be attributed to the low concentration of PCr in the brain compared to the muscle. Balaban et al. (45) reported that the rat brain contains a 4.7 mM concentration of PCr compared to 26.6 mM concentration in the rat leg muscle. This difference of over 560% could mean that the 2.98% signal detected in the hindlimb would be only 0.5% in the brain, comparable to the signal level in the hindlimb after euthanasia. This small semblance may be similar to the peak that Zaiss et al.(46) reported in the human brain at 9.4 T where it was suggested at this resonance that aromatic NOE (47) and N-acetyl aspartate may also be contributing factors to the peak. If the numerous sources have slightly differing chemical shifts, they could in some cases broaden the peak and increase the overlap hindering accurate quantitation.
These data suggest that PCrCEST tracks changes in PCr and can likely be applied to track the process of dephosphorylation during exercise. Muscle contraction exhibits many of the same changes that are observed after euthanasia: usage of ATP, depletion of PCr through dephosphorylation, and a similar pH drop (as seen in Liu et al.’s study using 31P-MRS (48); pH of PCr measured ~ 7.2 during rest and dropped to almost ~ 6.5 during contraction). This has particular implications particularly with regards to diagnosing mitochondrial defects, cardiac issues such as cardiac myopathy, as well as tracking treatment using PCr as protective therapeutics. In these applications, a dynamic approach may be important.
The temporal resolution at which we acquired was quite low (5 minutes and 20 seconds) due to averaging to maintain robustness of PCrCEST signal. Other groups commonly use echo planar imaging (EPI) readout to maintain sufficient contrast which may potentially reduce the needed signal averaging (without averaging, a single map requires at minimum 4 offsets. Given a TR of 8 second, this means a potential maximum resolution of 32 seconds). In our case (particularly in the mouse hindlimb), B0 inhomogeneities in the vicinity of the small mouse hindlimb made it difficult to acquire images without significant image distortion, so decreasing this distortion may be a potential challenge for these applications. To increase the temporal resolution further the saturation time and the recovery time would have to be reduced which would lead to incomplete saturation and prevent full recovery between TRs so a careful saturation scheme would have to be devised to optimize PCrCEST map sensitivity. Alternatively, many of the potential applications involve repetitive movement (muscle exertion or cardiac motion) which may allow images to be averaged across several repetitions to achieve robust PCrCEST imaging.
Conclusions
PCrCEST at ultrahigh fields is a viable technique for study in the muscle with sensitivity on par with APT. While, perhaps, not suitable for study in the brain, PCrCEST shows promise for the examination and diagnosis of dysfunction in energetics in the musculoskeletal and cardiac systems.
Acknowledgements
The authors thank Chanhee Lee for maintaining the 9.4 T and 15.2 T MRI systems. This work was supported by the Institute for Basic Science (IBS-R015-D1) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2016R1A2A1A05004952). Tao Jin is supported by NIH NS100703 (USA).
References
- 1.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. The Journal of biological chemistry 1955;217(1):409–427. [PubMed] [Google Scholar]
- 2.Davies RE. A Molecular Theory of Muscle Contraction: Calcium-Dependent Contractions with Hydrogen Bond Formation Plus Atp-Dependent Extensions of Part of the Myosin-Actin Cross-Bridges. Nature 1963;199:1068–1074. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96(7):2190–2196. [DOI] [PubMed] [Google Scholar]
- 4.Bakermans AJ, Abdurrachim D, van Nierop BJ, Koeman A, van der Kroon I, Baartscheer A, Schumacher CA, Strijkers GJ, Houten SM, Zuurbier CJ, Nicolay K, Prompers JJ. In vivo mouse myocardial (31)P MRS using three-dimensional image-selected in vivo spectroscopy (3D ISIS): technical considerations and biochemical validations. NMR in biomedicine 2015;28(10):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakermans AJ, Bazil JN, Nederveen AJ, Strijkers GJ, Boekholdt SM, Beard DA, Jeneson JAL. Human Cardiac (31)P-MR Spectroscopy at 3 Tesla Cannot Detect Failing Myocardial Energy Homeostasis during Exercise. Frontiers in physiology 2017;8:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schapira A, Lodi R. Assessment of in vitro and in vivo mitochondrial function in Friedreich’s ataxia and Huntington’s disease. Methods Mol Biol 2004;277:293–307. [DOI] [PubMed] [Google Scholar]
- 7.Lodi R, Schapira AH, Manners D, Styles P, Wood NW, Taylor DJ, Warner TT. Abnormal in vivo skeletal muscle energy metabolism in Huntington’s disease and dentatorubropallidoluysian atrophy. Annals of neurology 2000;48(1):72–76. [PubMed] [Google Scholar]
- 8.Pipinos II, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. Journal of vascular surgery 2000;31(5):944–952. [DOI] [PubMed] [Google Scholar]
- 9.Sithamparanathan S, Rocha MC, Parikh JD, Rygiel KA, Falkous G, Grady JP, Hollingsworth KG, Trenell MI, Taylor RW, Turnbull DM, Gorman GS, Corris PA. Skeletal muscle mitochondrial oxidative phosphorylation function in idiopathic pulmonary arterial hypertension: in vivo and in vitro study. Pulmonary circulation 2018;8(2):2045894018768290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreux PA, van Diemen MPJ, Heezen MR, Auwerx J, Rinsch C, Jan Groeneveld G, Singh A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Scientific reports 2018;8(1):8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guivel-Scharen V, Sinnwell T, Wolff SD, Balaban RS. Detection of proton chemical exchange between metabolites and water in biological tissues. J Magn Reson 1998;133(1):36–45. [DOI] [PubMed] [Google Scholar]
- 12.van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magnetic resonance in medicine 2011;65(4):927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin T, Autio J, Obata T, Kim SG. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magnetic resonance in medicine 2011;65(5):1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung JJ, Choi W, Jin T, Lee JH, Kim SG. Chemical-exchange-sensitive MRI of amide, amine and NOE at 9.4 T versus 15.2 T. NMR in biomedicine 2017;30(9). [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magnetic resonance in medicine 2003;50(6):1120–1126. [DOI] [PubMed] [Google Scholar]
- 16.Sun PZ, Cheung JS, Wang E, Lo EH. Association between pH-weighted endogenous amide proton chemical exchange saturation transfer MRI and tissue lactic acidosis during acute ischemic stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2011;31(8):1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magnetic resonance in medicine 2013;69(3):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo HY, Zhang Y, Lee DH, Hong X, Zhou J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 Tesla. Magnetic resonance in medicine 2016;75(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaiss M, Windschuh J, Goerke S, Paech D, Meissner JE, Burth S, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Ladd ME, Bachert P, Radbruch A. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magnetic resonance in medicine 2017;77(1):196–208. [DOI] [PubMed] [Google Scholar]
- 20.Jin T, Wang P, Zong X, Kim SG. Magnetic resonance imaging of the Amine-Proton EXchange (APEX) dependent contrast. NeuroImage 2012;59(2):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nature medicine 2012;18(2):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin T, Kim SG. Advantages of chemical exchange-sensitive spin-lock (CESL) over chemical exchange saturation transfer (CEST) for hydroxyl- and amine-water proton exchange studies. NMR in biomedicine 2014;27(11):1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haris M, Singh A, Cai K, Nath K, Crescenzi R, Kogan F, Hariharan H, Reddy R. MICEST: a potential tool for non-invasive detection of molecular changes in Alzheimer’s disease. Journal of neuroscience methods 2013;212(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin T, Mehrens H, Hendrich KS, Kim SG. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2014;34(8):1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haris M, Nanga RP, Singh A, Cai K, Kogan F, Hariharan H, Reddy R. Exchange rates of creatine kinase metabolites: feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR in biomedicine 2012;25(11):1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC, Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nature medicine 2014;20(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rerich E, Zaiss M, Korzowski A, Ladd ME, Bachert P. Relaxation-compensated CEST-MRI at 7 T for mapping of creatine content and pH--preliminary application in human muscle tissue in vivo. NMR in biomedicine 2015;28(11):1402–1412. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Barker PB, Weiss RG, van Zijl PCM, Xu J. Creatine and phosphocreatine mapping of mouse skeletal muscle by a polynomial and Lorentzian line-shape fitting CEST method. Magnetic resonance in medicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin T, Kim SG. The CEST effect of guanidine and hydroxyl protons can be used as a positive contrast in ischemia. Proceedings of International Society for Magnetic Resonance in Medicine; 2013; Salt Lake City, Utah, USA. p 2542. (Proceedings of International Society for Magnetic Resonance in Medicine). [Google Scholar]
- 30.Jin T, Wang P, Hitchens TK, Kim SG. Enhancing sensitivity of pH-weighted MRI with combination of amide and guanidyl CEST. NeuroImage 2017;157:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Zeng H, Xu X, Yadav NN, Cai S, Puts NA, Barker PB, Li T, Weiss RG, van Zijl PCM, Xu J. Investigation of the contribution of total creatine to the CEST Z-spectrum of brain using a knockout mouse model. NMR in biomedicine 2017;30(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang XY, Xie J, Wang F, Lin EC, Xu J, Gochberg DF, Gore JC, Zu Z. Assignment of the molecular origins of CEST signals at 2 ppm in rat brain. Magnetic resonance in medicine 2017;78(3):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magnetic resonance in medicine 2009;61(6):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magnetic resonance in medicine 2001;46(1):24–30. [DOI] [PubMed] [Google Scholar]
- 35.Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG. Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology 1985;35(6):781–788. [DOI] [PubMed] [Google Scholar]
- 36.Zaiss M, Angelovski G, Demetriou E, McMahon MT, Golay X, Scheffler K. QUESP and QUEST revisited - fast and accurate quantitative CEST experiments. Magnetic resonance in medicine 2018;79(3):1708–1721. [DOI] [PubMed] [Google Scholar]
- 37.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magnetic resonance in medicine 2006;55(4):836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Windschuh J, Zaiss M, Meissner JE, Paech D, Radbruch A, Ladd ME, Bachert P. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR in biomedicine 2015;28(5):529–537. [DOI] [PubMed] [Google Scholar]
- 39.Zong X, Wang P, Kim SG, Jin T. Sensitivity and source of amine-proton exchange and amide-proton transfer magnetic resonance imaging in cerebral ischemia. Magnetic resonance in medicine 2014;71(1):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaiss M, Xu J, Goerke S, Khan IS, Singer RJ, Gore JC, Gochberg DF, Bachert P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR in biomedicine 2014;27(3):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertram HC, Donstrup S, Karlsson AH, Andersen HJ, Stodkilde-Jorgensen H. Post mortem energy metabolism and pH development in porcine M. longissimus dorsi as affected by two different cooling regimes. A (31)P-NMR spectroscopic study. Magnetic resonance imaging 2001;19(7):993–1000. [DOI] [PubMed] [Google Scholar]
- 42.in ‘t Zandt HJ, de Groof AJ, Renema WK, Oerlemans FT, Klomp DW, Wieringa B, Heerschap A. Presence of (phospho)creatine in developing and adult skeletal muscle of mice without mitochondrial and cytosolic muscle creatine kinase isoforms. The Journal of physiology 2003;548(Pt 3):847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becila S, Herrera-Mendez CH, Coulis G, Labas R, Astruc T, Picard B, Boudjellal A, Pelissier P, Bremaud L, Ouali A. Postmortem muscle cells die through apoptosis. European Food Research and Technology 2010;231(3):485–493. [Google Scholar]
- 44.Zu Z, Lin EC, Louie EA, Jiang X, Lankford CL, Damon BM, Does MD, Gore JC, Gochberg DF. Chemical Exchange Rotation Transfer imaging of Phosphocreatine in Muscle Proceedings of International Society for Magnetic Resonance in Medicine; 2018; Paris, France. (Proceedings of International Society for Magnetic Resonance in Medicine). [Google Scholar]
- 45.Balaban RS, Kantor HL, Ferretti JA. In vivo flux between phosphocreatine and adenosine triphosphate determined by two-dimensional phosphorous NMR. The Journal of biological chemistry 1983;258(21):12787–12789. [PubMed] [Google Scholar]
- 46.Zaiss M, Schuppert M, Deshmane A, Herz K, Ehses P, Fullbier L, Lindig T, Bender B, Ernemann U, Scheffler K. Chemical exchange saturation transfer MRI contrast in the human brain at 9.4T. NeuroImage 2018;179:144–155. [DOI] [PubMed] [Google Scholar]
- 47.Jin T, Kim SG. In vivo saturation transfer imaging of nuclear overhauser effect from aromatic and aliphatic protons: implication to APT quantification. Proceedings of International Society for Magnetic Resonance in Medicine; 2013; Salt Lake City, Utah, USA. p 2528. (Proceedings of International Society for Magnetic Resonance in Medicine). [Google Scholar]
- 48.Liu M, Walter GA, Pathare NC, Forster RE, Vandenborne K. A quantitative study of bioenergetics in skeletal muscle lacking carbonic anhydrase III using 31P magnetic resonance spectroscopy. Proceedings of the National Academy of Sciences of the United States of America 2007;104(1):371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]