Abstract
Background:
Sex differences in cannabis use disorder (CUD) and its treatment have been identified. Women report more severe withdrawal and have shown worse treatment outcomes. Ovarian hormones are implicated in these differences and research suggests that exogenous progesterone may be an effective pharmacotherapy.
Methods:
The current randomized, placebo-controlled, feasibility trial tested a novel multimodal methodology for administering exogenous progesterone during acute cannabis withdrawal. Eight heavy cannabis using women received micronized progesterone (200mg bid) (n = 3) or matching placebo (n = 5) during the early follicular phase of their menstrual cycle over a 5-day study period while abstaining from cannabis. Laboratory visits (days 1 and 5) included biological and self-report assessments, while home-based procedures (days 2 – 4) included ambulatory assessments, video data capture and tele-drug testing, and biological assessments. Primary outcomes were medication adherence and salivary hormone levels, and the exploratory outcome was cannabis withdrawal severity.
Results:
Medication adherence rates were high as assessed via self-report (100.0%) and video data capture (98.0%). Salivary progesterone levels differed between groups over time (p < 0.027) and the progesterone group achieved levels within the normal range during the luteal phase in healthy adults. All tele-drug tests were negative confirming cannabis abstinence and there was an indication (p = 0.07) of reduced cannabis craving among participants receiving progesterone.
Conclusion:
More effective and sex-based treatments for cannabis use disorder are needed. The current study provides a novel multimodal methodology with low participant burden for investigating new medications for cannabis withdrawal. Clinical trials of progesterone for cannabis withdrawal may be warranted.
1.0. Background
Sex and gender differences in the behavioral, biological, and clinical correlates of substance use disorders are myriad (Becker et al., 2017), yet, there is a dearth of sex/gender-informed treatments. A growing literature suggests that ovarian hormones play an important role in these differences and therefore may be an effective treatment option (Moran-Santa Maria et al., 2014). Evidence from preclinical, clinical, and human laboratory studies has shown that estradiol enhances drug sensitivity and related behavior, while progesterone attenuates drug sensitivity and behavior (Anker et al., 2007; Evans & Foltin, 2010; Feltenstein & See, 2007; Frye, 2007; Hecht et al., 1999; Larson et al., 2007; Mello, 2010; Roberts et al., 1989; Saladin et al., 2015). As such, recent clinical trials have investigated exogenous progesterone as a potential pharmacologic intervention for substance use disorders (Evans & Foltin, 2006; Fox et al., 2013; Sofuoglu et al., 2001; 2004; 2011).
Developing effective treatments for cannabis use disorder (CUD) has been particularly challenging, and given recent legalization efforts in the U.S. and worldwide, there is an increasing need for more effective treatments (Sahlem et al., 2018). Pharmacotherapy trials for CUD have been largely negative (Sherman & McRae-Clark, 2016) and evidence exists that women fare worse than men in cannabis cessation trials (McRae-Clark et al., 2015), even when they are highly motivated to quit (Sherman et al., 2016). This may be due in part to the finding that women report more severe and impairing cannabis withdrawal symptoms than men (Copersino et al., 2010; Herrmann et al., 2015; Sherman et al., 2017), which is associated with relapse (Davis et al., 2016). Cannabis withdrawal is an important treatment target (Balter et al., 2014; Copeland & Pokorski, 2016) and exogenous progesterone may be a viable sex-specific treatment option.
Progesterone is a sex hormone involved in ovulation and parturition, released primarily during the luteal phase of the menstrual cycle. For over 60 years it has been used to treat medical disorders including ovarian failure, amenorrhea, premenstrual symptoms, uterine bleeding, menopausal symptoms, and for contraception (de Lignieres, 1999; Simon, 1995). Micronized progesterone offers a specific pharmacological agent to investigate the effects of this hormone without the additional adverse effects of synthetic derivatives. Safety and tolerability of micronized progesterone has been established in numerous studies with nicotine and cocaine-dependent individuals (Evans & Foltin, 2006; Fox et al., 2013; Reed et al., 2011; Sofuoglu et al., 2001; 2002; 2004; 2011), and multiple clinical studies in these populations have achieved stable levels of plasma progesterone comparable to normal luteal phase levels (2–20 ng/ml) using twice daily administration of 200mg micronized progesterone (Fox et al., 2013; Sofuoglu et al., 2001; 2011).
Building on preclinical and human laboratory evidence, recent clinical trials have shown that women in the luteal phase of the menstrual cycle, when progesterone is high, showed decreased subjective effects of cocaine (Evans, Haney, & Foltin, 2002; Sofuoglu et al., 1999) and amphetamines (Justice et al., 1999) compared to women in the follicular phase, when progesterone is low. In addition, a study of female smokers randomly assigned to quit during the luteal phase had lower relapse rates than those assigned to quit during the follicular phase (Allen et al., 2008). In an effort to advance cannabis treatment development, the current pilot study tested a novel multimodal methodology to investigate the feasibility of administering exogenous progesterone for cannabis withdrawal in women. The multimodal study design combined ambulatory assessment, human laboratory, and clinical trials methodologies. The primary outcomes were medication adherence and salivary progesterone levels. The exploratory outcome was self-reported cannabis withdrawal. It was hypothesized that participants receiving progesterone would demonstrate elevated progesterone levels and reduced cannabis withdrawal severity compared to placebo participants.
2.0. Materials and Methods
2.1. Participants and General Procedures.
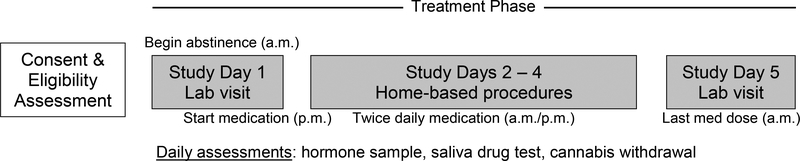
Heavy cannabis-using women age 18–45 with regular menstrual cycles (25 to 35 days) were recruited from the greater Charleston area between October 2017 and April 2018. Following an initial screening visit participants were randomly assigned to receive micronized progesterone or matching placebo over a consecutive 5-day period during the early follicular phase of their menstrual cycle and asked to abstain from cannabis during that time. Following screening and within 3 days of menses onset, participants returned to the laboratory and underwent lab visit 1 (day 1 abstinence) which included biochemical and subjective assessments. Study procedures on days 2–4 were completed at home and participants returned to the lab on day 5 for repeat assessments. Cannabis withdrawal was assessed twice daily, concurrent with medication adherence. See Figure 1 for study design and timeline.
Figure 1.
Study design and timeline.
Additional inclusion criteria included 1) cannabis use > 4 days per week for the past year, 2) consent to remain abstinent from alcohol for 12 hours prior to study visits and all other drugs other than nicotine for the duration of the study, and 3) consent to random assignment. Participants were excluded if they were pregnant, nursing, amenorrhoeic, or using oral contraceptives; had a history of major medical illnesses including liver diseases, abnormal vaginal bleeding, suspected or known malignancy, thrombophlebitis, deep vein thrombosis, pulmonary embolus, clotting or bleeding disorders, heart disease, diabetes, history of stroke or other medical conditions that the physician investigator deems as contraindicated for the patient to be in the study; reported regular use of psychotropic medication (antidepressants, antipsychotics, or anxiolytics) and recent/current psychiatric diagnosis and treatment for Axis I disorders including major depression, bipolar affective disorder, schizophrenia or panic disorder; were a suicidal or homicidal risk; had a known allergy to progesterone or peanuts (vehicle for micronized progesterone); were unwilling or unable to maintain abstinence from alcohol 12 hours prior to study visits, and all other drugs other than cannabis or nicotine for the duration of the study; met DSM-5 criteria for moderate to severe substance use disorder (other than nicotine, cannabis, or alcohol) within the past year. A medical history and physical examination were completed to assess for medical exclusions. Participants meeting inclusion criteria and no exclusion criteria were scheduled to complete study procedures. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and received approval from the Medical University of South Carolina Institutional Review Board (MUSC IRB).
2.2. Eligibility Assessment
Potential participants presented to the Addiction Sciences Division for informed consent and eligibility assessment. The Mini-International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1998) is a brief structured interview and was used to assess exclusionary psychiatric and substance use diagnoses. Participants provided a urine sample, which was first tested for pregnancy, and if negative, for substance use. Urine drug screens were performed using the One Step Multi-Drug Test Dip Card (Drugconfirm™) a lateral flow chromatographic immunoassay for the qualitative detection of drug or drug metabolite in the urine at the following cutoffs (ng/ml): cocaine (300), amphetamines (1000), methamphetamine (1000), THC (50), opiates (2000), and benzodiazepines (300). Cannabis use during the past year, and all other substance use for the past month, was then assessed using the Timeline Follow-Back (TLFB; Sobell & Sobell, 1992), a calendar-based instrument designed to assess daily substance. A medical history and physical exam was then conducted. After all inclusion criteria and no exclusion criteria were satisfied, participants were randomly assigned to receive either micronized progesterone 200mg bid or matching placebo. Participants provided expected date of menses onset based on menstrual history so study personnel could follow-up prior to scheduling day 1 visit.
2.3. Menstrual Tracking Phase.
Following the screening visit, participants were asked to track their menstrual cycle and contact the clinic upon menses onset. As a reminder, study personnel reached out to participants three days prior to expected onset to discuss scheduling. Participants began the study within three days of menses onset in order to ensure study completion within the early follicular phase. Day 1 always occurred on Monday, Thursday, or Friday in order to ensure an in-person lab visit on day 5. Participants were asked to abstain from cannabis beginning on the morning of day 1.
2.4. Treatment Phase.
2.4.1. Micronized progesterone (Prometrium 200mg).
Prometrium 200mg or matching placebo dosing began at 9:00 p.m. on study day 1 to minimize sedation. On study days 2–4 participants took their medication at 9:00 a.m. and 9:00 p.m. and on day 5 the final dose was taken at 9:00 a.m. prior to the final lab visit. All capsules were prepared by the MUSC Investigational Drug Services. Medication adherence was monitored using mobile technology. Following a text message prompt, participants recorded themselves taking their morning and evening medication doses and submitted the videos to research staff via the university’s secure electronic data capture system. Participants were trained on medication adherence procedures during lab visit 1. All videos were reviewed by research staff to confirm medication adherence. Self-report adherence was also assessed.
2.4.2. Salivary hormone sampling.
Salivary hormone samples were collected using IBL SaliCap Set and progesterone was measured using progesterone luminescence immunoassay (IBL International GMBH). Day 1 hormone sample was collected in the laboratory and participants were trained on how to properly collect saliva using the passive-drool method. Hormone samples on days 2 – 4 were collected at home, stored in the participant’s freezer, and returned to the lab on day 5 using travel ice packs to minimize thawing. Day 5 hormone sample was collected in the lab.
2.4.2. Cannabis abstinence and withdrawal.
The Cannabis Withdrawal Scale (CWS; Allsop et al., 2011) was used to assess cannabis withdrawal symptoms. The CWS is a 19-item self-report measure that assesses cannabis withdrawal symptoms in the past 24 hours (sample items: I felt restless; I had a headache; I had been imagining being stoned) on a 10-point Likert scale (0=Not at All to 10=Extremely). The CWS was administered once on days 1 and 5, and twice daily on days 2 – 4 as part of the survey link that was sent via text message for medication adherence. Day 1 assessment occurred on the evening of study day 1 (day 1 abstinence) prior to their first medication dose, and was used as baseline assessment. Withdrawal was assessed in the morning and evening to capture diurnal variation, thus, participants were asked to respond in reference to the past 12 hours. Cannabis abstinence was confirmed using self-report, daily saliva drug testing, and urine cannabinoid levels. Self-report was conducted using Timeline Follow-Back procedures. Salivary drug testing was conducted using 5 Panel SalivaConfirm™ Oral Fluid Multi-Drug Screening Kit, which can detect THC metabolites in saliva at a cutoff of 25 ng/ml for up to 12 hours after the most recent use. Salivary testing occurred in person on days 1 and 5, and at home on days 2 – 4. On day 1, participants were trained to self-administer saliva drug screen and on days 2 – 4 they scheduled a Skype/FaceTime call with study personnel and completed drug screen procedures via teleconference. Adverse events were also assessed during this call.
2.5. Data Analytic Plan
The primary aim of the study was to test the feasibility of exogenous progesterone administration in cannabis using women during acute withdrawal. Medication adherence rates using mobile adherence procedures were collected and expected to be at or above generally acceptable rates for medication trials (90%) (Shiovitz et al. 2016). Salivary progesterone levels in the progesterone group were expected to be elevated within the normal range found during the luteal phase (Gandara et al., 2007). The exploratory aim concerned the efficacy of exogenous progesterone in attenuating cannabis withdrawal symptoms in female cannabis users. Cannabinoid levels were compared from day 1 to day 5 to confirm self-reported abstinence and daily saliva drug test results. Hypotheses were tested using a random effects linear mixed model for repeated measures; this methodology allows the estimation of the variance associated with a random intercept for each subject and can accommodate partially missing data (Brown and Prescott, 1999). In addition to the primary analyses, contrasts of pertinent baseline characteristics were performed between groups. All analyses are considered preliminary in nature and should be replicated by a larger study. All statistical analyses were conducted using IBM SPSS v.24.
3.0. Results
3.1. Participants
Sixteen heavy cannabis using women were screened for participation. Of the 13 who met initial screening criteria, 10 met all inclusion and no exclusion criteria and were enrolled into the study. One participant was lost during the menses tracking phase and another dropped out after study day 1, resulting in an 80% retention rate. Participants (Progesterone = 3; Placebo = 5) were 75% Caucasian, M(SD) age was 22.2 (2.6), and reported using cannabis on 27.5 of the past 30 days prior to screening, on average (see Table 1). Though not required for inclusion, all participants tested positive for cannabis at screening and had used at least once on the day before screening. Two participants reported smoking cigarettes, but both were in the placebo condition so between-group contrasts could not be performed for this variable at baseline. Six of the eight participants met DSM-5 CUD criteria (3 of 5 in the placebo group, and 3 of 3 in the progesterone group).
Table 1.
Descriptive characteristics for full sample and by condition.
| Full Sample (N = 8) | PROG (n = 3) | PBO (n = 5) | p-value | |
|---|---|---|---|---|
| AgeM (SD) | 22.2 (2.6) | 21.7 (1.5) | 22.6 (3.3) | 0.667 |
| Race N (%) | 0.049 | |||
| African-American | 2 (25) | 2 (66.7) | 0 (0) | |
| Caucasian | 6 (75) | 1 (33.3) | 5 (100) | |
| Education N (%) | 0.237 | |||
| Some college | 6 (75) | 3 (100) | 3 (60.0) | |
| College degree | 2 (25) | 0 (0) | 2 (40.0) | |
| Cannabis sessions per day (30 day TLFB) M (SD) | 1.72 (0.92) | 1.73 (0.68) | 1.72 (1.11) | 0.986 |
| Cannabis use days (past 30) M(SD) | 27.5 (5.15) | 30.0 (0.00) | 26.3 (6.25) | 0.324 |
| Standard drinks per day (30 day TLFB) M (SD) | 0.71 (0.73) | 0.63 (0.80) | 0.75 (0.77) | 0.841 |
Note: PROG = progesterone condition, PBO = placebo condition.
3.2. Feasibility
3.2.1. Adherence and tolerability.
For adherence results the participant who dropped out after visit 1 was included as intent-to-treat. Progesterone adherence was assessed via self-report and video data capture. Each participant was provided eight doses of medication or matching placebo resulting in a total of 72 instances of medication administration (9 participants x 8 doses). Self-reported adherence rates were high (64/72, 88%) and all 8 participants who completed the study reported 100% adherence. Similarly, video capture adherence rates were high (63/72, 87.5%), completers demonstrated 98% adherence, with the one missing dose being a video upload malfunction. Adherence did not differ between treatment conditions. Progesterone was well-tolerated as only one adverse event was reported: a participant reported genitourinary discomfort (“like my bladder is full”) that fully resolved.
3.2.2. Progesterone levels.
Salivary progesterone levels were compared within and between groups over the five day study period using random effects linear mixed modeling. There was a main effect of treatment (F = 22.55, p = 0.001) and time (F = 4.48, p = 0.01), and a significant treatment (progesterone vs. placebo) by time (day 1 to day 5) interaction (F = 3.50, p = 0.027), indicating that progesterone levels increased as expected from day 1 to day 5 in the progesterone group compared to placebo (see Figure 2). Post-hoc pairwise comparison revealed no difference in baseline (day 1) progesterone levels between groups (p = 0.926).
Figure 2. Progesterone levels (pg/ml) by treatment condition and time.
Note: Salivary progesterone levels across treatment conditions during the five-day study period. The treatment x time interaction (F = 3.50, p = 0.027) demonstrates increased progesterone levels among participants receiving exogenous progesterone compared to placebo. Post-hoc comparisons revealed no group differences on Day 1 prior to first medication dose (p = 0.926), and significant differences on Days 2–5 (all p-values < 0.01).
3.3. Cannabis Abstinence and Withdrawal
3.3.1. Cannabis abstinence.
Cannabis abstinence was confirmed using self-report, saliva drug testing, and cannabinoid levels. All participants reported abstinence for the duration of study procedures. Likewise, 40/40 saliva samples were negative for THC as assessed via tele-drug testing procedures. Urine cannabinoid levels decreased by approximately 56% from day 1 to day 5 (582.21ng/ml to 258.07ng/ml, p=0.061), and groups did not differ over time (treatment by time, p=0.36), supporting the self-report and saliva drug testing data on cannabis abstinence.
3.3.2. Cannabis withdrawal.
Cannabis withdrawal was compared within and between groups over the five day study period using random effects linear mixed modeling. Models were adjusted for baseline withdrawal assessed on study day 1 (day 1 abstinence) prior to initiating study medication. There was no significant effect of treatment or time on overall cannabis withdrawal score (p > 0.13 for both effects), and the interaction of treatment by time was also non-significant (p = 0.382). When considering the cannabis craving component of withdrawal independently, the treatment by time interaction showed a statistical trend (F = 2.21, p = 0.07) with progesterone participants showing a numerically greater reduction in craving over time compared to placebo. All participants self-reported abstinence during the 5-day study, which was confirmed by negative salivary drug tests conducted via teleconference.
4.0. Discussion
The current study investigated the feasibility of exogenous progesterone administration for cannabis withdrawal in heavy cannabis-using women. The primary aims were to test a novel methodology for administration and adherence monitoring, and whether progesterone administration would achieve elevated salivary progesterone levels relative to placebo. Cannabis withdrawal was also assessed daily and compared between groups.
Study findings demonstrate feasibility of a novel multimodal methodology for exogenous progesterone administration in cannabis-using women. Combining human laboratory visits (days 1 and 5) with home-based procedures (days 2 – 4) participants were highly adherent to study procedures. Participants demonstrated high rates of medication adherence via video data capture, which was confirmed using bioluminescence immunoassay testing showing elevated progesterone levels among participants receiving micronized progesterone compared to placebo. Salivary progesterone levels were comparable to those observed during the luteal phase in healthy women (Gandara et al., 2007). To assess cannabis abstinence and withdrawal, two forms of ambulatory assessment were used. Following text message prompts, participants completed cannabis withdrawal self-report surveys twice-daily (9:00 a.m. and 9:00 p.m.) yielding real-time data for withdrawal symptomotology; in future large-scale clinical trials diurnal variation could be tested as withdrawal severity may vary by time of day. Second, in addition to self-report, cannabis abstinence was confirmed via live tele-drug testing procedures once daily during home-based procedures (days 2 – 4). Together these findings support the feasibility of a multimodal methodology with low participant burden for exogenous progesterone administration during acute cannabis withdrawal.
Another notable methodological success was the initiation of study procedures during the early follicular phase when progesterone levels are low. Day 1 progesterone levels were similar between groups and comparable to those during the early follicular phase in healthy women, suggesting that the menses tracking phase was feasible and effective. Progesterone has been shown to attenuate drug-seeking behavior and drug effect in preclinical and clinical studies (see Moran Santa-Maria et al., 2014 for review), and smokers randomized to quit during the luteal phase compared to the follicular phase had a significantly lower relapse rate after 30 days (Allen et al., 2008). Successful administration and elevation of progesterone levels is essential for any future trials investigating the efficacy of progesterone for cannabis withdrawal. Importantly, this methodology may be applied to other pharmacotherapy trials for cannabis withdrawal.
The study also offers preliminary evidence for the efficacy of progesterone in reducing cannabis craving in female cannabis users. While this finding was not statistically significant, it provides support for future fully-powered clinical trials of progesterone for cannabis withdrawal. Cannabis craving is associated with relapse (Davis et al., 2016) and treatments that target unique aspects of cannabis withdrawal may be important to improving cannabis treatment outcomes, similar to recommendations in nicotine treatment development research (Shiffman et al., 2004).
Results should be interpreted in light of important limitations. Principle of these is the small sample size. While the sample size limited statistical power to detect and interpret results, earlier human laboratory studies of progesterone effects in cocaine-dependent populations had similarly small samples sizes (N = 5, Sofuoglu et al., 2002; N = 10, Sofuoglu et al., 2004). Second, a longer study duration would allow more detailed assessment of cannabis withdrawal symptomology and progesterone effects. Cannabis withdrawal generally peaks 2–6 days post-cessation, but acute effects can last up to 14 days (Budney et al., 2003). Relatedly, two participants did not meet DSM-5 CUD criteria which may have impacted withdrawal related outcome measures, though cannabis use characteristics were similar for all participants. Third, variable dosing was not included in our study design so we cannot attest to the most efficacious dose for cannabis using women. Last, while we only collected saliva drug tests once a day, which detects use within the past 12 hours, the reduction in quantitative urinary cannabinoids was consistent with abstinence. Despite these limitations, the current study demonstrates the feasibility of exogenous progesterone administration for cannabis withdrawal in women using a novel multimodal methodology. Fully-powered clinical trials should consider variable dosing and longer study duration.
Highlights.
A novel multimodal methodology for the treatment of cannabis withdrawal was tested (82)
It is feasible to administer exogenous progesterone during acute cannabis withdrawal (84)
Salivary progesterone was appropriately elevated compared to placebo (68)
Progesterone may attenuate cannabis withdrawal-induced craving in women (71)
Acknowledgments
Funding sources:
This work was supported by the National Institutes of Health [NIDA grants K23DA045099, K24DA038240].
Footnotes
Disclosure statement:
All authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SS, Bade T, Center B, Finstad D, & Hatsukami D (2008). Menstrual phase effects on smoking relapse. Addiction, 103(5), 809–821. doi: 10.1111/j.1360-0443.2008.02146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, & Budney AJ (2011). The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend, 119(1–2), 123–129. doi: 10.1016/j.drugalcdep.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, & Carroll ME (2007). Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol, 15(5), 472–480. doi: 10.1037/1064-1297.15.5.472 [DOI] [PubMed] [Google Scholar]
- Balter RE, Cooper ZD, & Haney M (2014). Novel Pharmacologic Approaches to Treating Cannabis Use Disorder. Curr Addict Rep, 1(2), 137–143. doi: 10.1007/s40429-014-0011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, & Reed BG (2017). Sex differences, gender and addiction. J Neurosci Res, 95(1–2), 136–147. doi: 10.1002/jnr.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, & Prescott R (1999). Applied Mixed Models in Medicine. New York, NY: Wiley & Sons Inc. [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, & Hughes JR (2003). The time course and significance of cannabis withdrawal. J Abnorm Psychol, 112(3), 393–402. [DOI] [PubMed] [Google Scholar]
- Copeland J, & Pokorski I (2016). Progress toward pharmacotherapies for cannabis-use disorder: an evidence-based review. Subst Abuse Rehabil, 7, 41–53. doi: 10.2147/SAR.S89857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, … Gorelick DA (2010). Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. The American Journal of Drug and Alcohol Abuse, 36(6), 311–319. doi: 10.3109/00952990.2010.503825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP, Smith DC, Morphew JW, Lei X, & Zhang S (2016). Cannabis Withdrawal, Posttreatment Abstinence, and Days to First Cannabis Use Among Emerging Adults in Substance Use Treatment: A Prospective Study. J Drug Issues, 46(1), 64–83. doi: 10.1177/0022042615616431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lignieres B (1999). Oral micronized progesterone. Clin Ther, 21(1), 41–60; discussion 41–42. doi: 10.1016/s0149-2918(00)88267-3 [DOI] [PubMed] [Google Scholar]
- Evans SM, & Foltin RW (2006). Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology, 31(3), 659–674. doi: 10.1038/sj.npp.1300887 [DOI] [PubMed] [Google Scholar]
- Evans SM, & Foltin RW (2010). Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav, 58(1), 13–21. doi: 10.1016/j.yhbeh.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, & Foltin RW (2002). The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl), 159(4), 397–406. doi: 10.1007/s00213-001-0944-7 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, & See RE (2007). Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend, 89(2–3), 183–189. doi: 10.1016/j.drugalcdep.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, & Sinha R (2013). The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology, 38(9), 1532–1544. doi: 10.1016/j.psyneuen.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA (2007). Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav, 86(2), 209–219. doi: 10.1016/j.pbb.2006.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara BK, Leresche L, & Mancl L (2007). Patterns of salivary estradiol and progesterone across the menstrual cycle. Ann N Y Acad Sci, 1098, 446–450. doi: 10.1196/annals.1384.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, & Spear LP (1999). Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol, 35(2), 136–145. [PubMed] [Google Scholar]
- Herrmann ES, Weerts EM, & Vandrey R (2015). Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Experimental and Clinical Psychopharmacology, 23(6), 415–421. doi: 10.1037/pha0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, & de Wit H (1999). Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl), 145(1), 67–75. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, & Carroll ME (2007). Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol, 15(5), 461–471. doi: 10.1037/1064-1297.15.5.461 [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, … Norton J (2015). Buspirone treatment of cannabis dependence: A randomized, placebo-controlled trial. Drug Alcohol Depend, 156, 29–37. doi: 10.1016/j.drugalcdep.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK (2010). Hormones, nicotine, and cocaine: clinical studies. Horm Behav, 58(1), 57–71. doi: 10.1016/j.yhbeh.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Flanagan J, & Brady K (2014). Ovarian hormones and drug abuse. Curr Psychiatry Rep, 16(11), 511. doi: 10.1007/s11920-014-0511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Evans SM, Bedi G, Rubin E, & Foltin RW (2011). The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm Behav, 59(2), 227–235. doi: 10.1016/j.yhbeh.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, & Vickers GJ (1989). The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl), 98(3), 408–411. [DOI] [PubMed] [Google Scholar]
- Sahlem GL, Tomko RL, Sherman BJ, Gray KM, & McRae-Clark AL (2018). Impact of cannabis legalization on treatment and research priorities for cannabis use disorder. Int Rev Psychiatry, 1–10. doi: 10.1080/09540261.2018.1465398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, & Gray KM (2015). Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob Res, 17(4), 398–406. doi: 10.1093/ntr/ntu262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59 Suppl 20, 22–33; [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, & McRae-Clark AL (2016). Gender differences in cannabis use disorder treatment: Change readiness and taking steps predict worse cannabis outcomes for women. Addict Behav, 60, 197–202. doi: 10.1016/j.addbeh.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, & McRae-Clark AL (2016). Treatment of Cannabis Use Disorder: Current Science and Future Outlook. Pharmacotherapy, 36(5), 511–535. doi: 10.1002/phar.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, Baker NL, Sonne SC, Killeen TK, Cloud K, & Gray KM (2017). Gender differences among treatment-seeking adults with cannabis use disorder: Clinical profiles of women and men enrolled in the achieving cannabis cessation-evaluating N-acetylcysteine treatment (ACCENT) study. Am J Addict, 26(2), 136–144. doi: 10.1111/ajad.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D, Craving S. W. G. o. t. A. o., & Withdrawal in Clinical, T. (2004). Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res, 6(4), 599–614. doi: 10.1080/14622200410001734067 [DOI] [PubMed] [Google Scholar]
- Shiovitz TM, Bain EE, McCann DJ, Skolnick P, Laughren T, Hanina A, & Burch D (2016). Mitigating the Effects of Nonadherence in Clinical Trials. J Clin Pharmacol, 56(9), 1151–1164. doi: 10.1002/jcph.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA (1995). Micronized progesterone: vaginal and oral uses. Clin Obstet Gynecol, 38(4), 902–914. [PubMed] [Google Scholar]
- Sobell L, & Sobell M (1992). Timeline Follow-Back In Litten R & Allen J (Eds.), Measuring Alcohol Consumption (pp. 41–72): Humana Press. [Google Scholar]
- Sofuoglu M, Babb DA, & Hatsukami DK (2001). Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav, 69(1–2), 299–304. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, & Hatsukami DK (2002). Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav, 72(1–2), 431–435. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, & Hatsukami DK (1999). Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol, 7(3), 274–283. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, & Kosten TR (2004). Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav, 78(4), 699–705. doi: 10.1016/j.pbb.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, & Mooney M (2011). Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology, 36(1), 123–132. doi: 10.1016/j.psyneuen.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Forray A, Nich C, Carroll KM, Hine C, Merry BC, … Sofuoglu M (2014). Progesterone Reduces Cocaine Use in Postpartum Women with a Cocaine Use Disorder: A Randomized,Double-Blind Study. Lancet Psychiatry, 1(5), 360–367. doi: 10.1016/S2215-0366(14)70333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]